User login
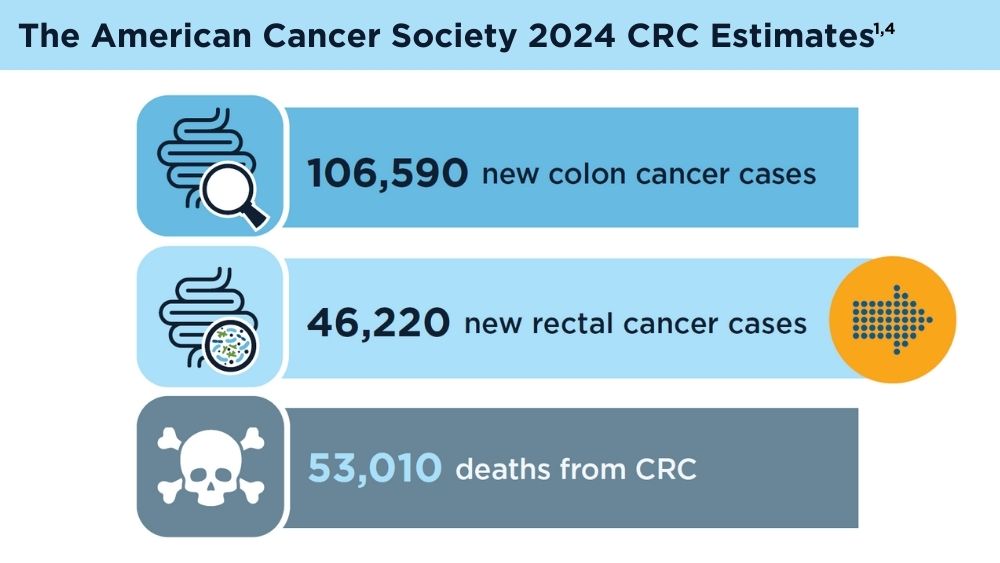
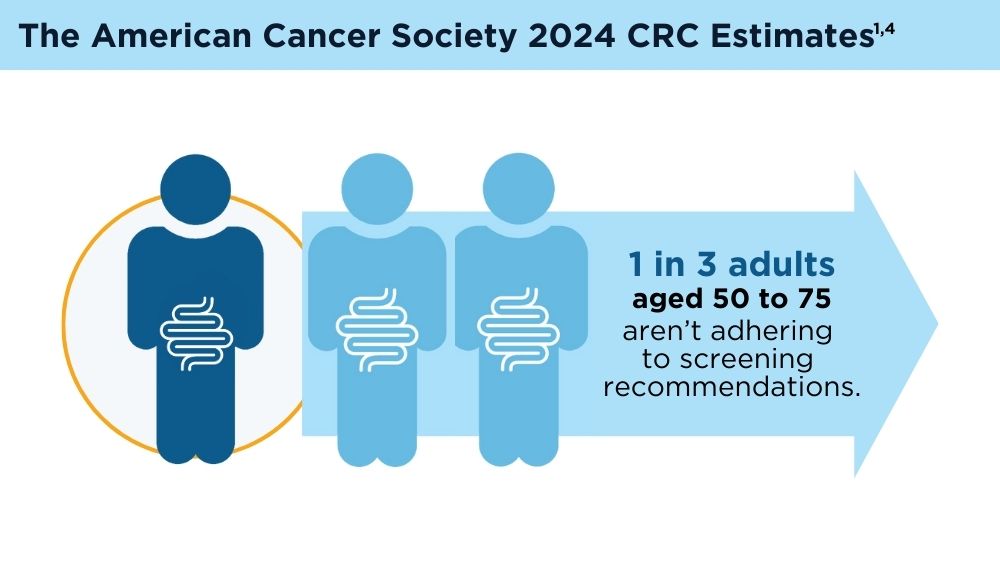
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
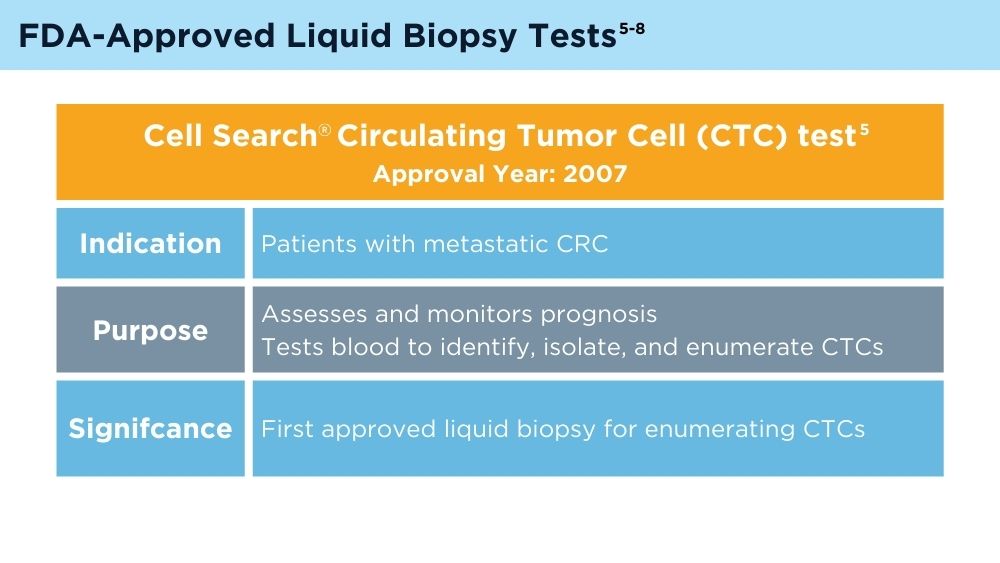
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
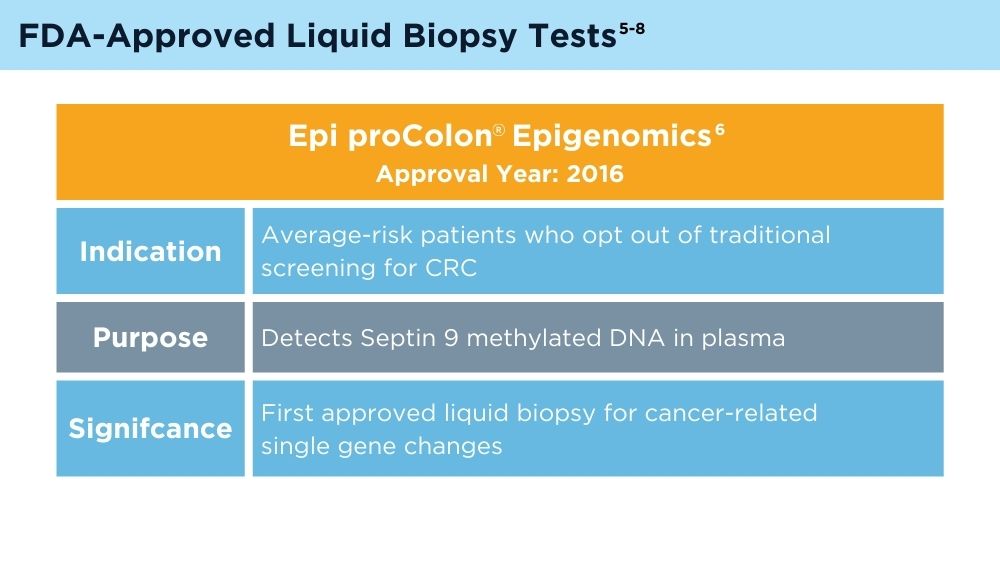
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937