User login
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, was first described in 1964. 1 Since then, several subtypes of SS have been recognized, including classic or idiopathic, which typically follows an acute viral illness; cancer related, typically in the form of a paraneoplastic syndrome; and drug induced. 2 Drug-induced SS is defined by the following: (1) an abrupt onset of painful erythematous plaques or nodules; (2) histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; (3) pyrexia above 38 ° C; (4) temporal relationship between drug and clinical presentation or temporally related recurrence after rechallenge; and (5) temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. 3 All 5 criteria must be met to make a diagnosis of drug-induced SS. Since these criteria were established by Walker and Cohen, 3 various drugs have been identified as causative agents, including antibiotics, antiepileptics, antiretrovirals, antineoplastic agents, antipsychotics, oral contraceptives, nonsteroidal anti-inflammatory agents, and retinoids. 4 W e present a rare case of SS caused by dupilumab, a monoclonal antibody therapy, used in the treatment of severe eosinophilic asthma and atopic dermatitis.
Case Report
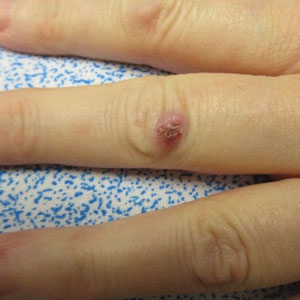
A 53-year-old woman presented with painful skin lesions, arthralgia, fever, and leukocytosis following initiation of dupilumab. She had a history of adult-onset, severe, persistent eosinophilic asthma, as well as chronic rhinosinusitis with nasal polyps, plaque psoriasis, and hypertrophic cardiomyopathy. She started mepolizumab 3 years prior to the current presentation for persistently uncontrolled asthma with a baseline peripheral eosinophil count of 860 cells/µL. After 3 years of minimal response to mepolizumab, she was started on dupilumab. Within 2 weeks of the first dose of dupilumab, she started experiencing bilateral knee pain. She subsequently developed daily fevers (temperature, 38.3 °C to 39.4 °C), fatigue, and pain in the back of the neck and head. After the second dose of dupilumab, she started experiencing painful skin lesions on the bilateral knuckles, elbows, and abdomen (Figure 1). She had difficulty using her hands and walking secondary to intense arthralgia involving the bilateral finger joints, elbows, and knees. Her primary care physician obtained a laboratory evaluation, which revealed an elevated total white blood cell count of 20×103/mm3 (reference range, 4–11×103/mm3) with 27.5% neutrophils and severely elevated eosinophils above her baseline to 57.3% with an absolute eosinophil count of 11,700 cells/µL (reference range, <400 cells/µL). Further assessment revealed an elevated erythrocyte sedimentation rate of 64 mm/h (reference range, 0–30 mm/h) and C-reactive protein level of 34 mg/dL (reference range, ≤0.80 mg/dL), with negative antinuclear antibody, rheumatoid factor, antineutrophilic cytoplasmic antibody, and Lyme antibody. IgG, IgA, and IgM levels were within reference range, and the IgE level was not elevated above her baseline. She had normal serum tryptase, and a peripheral D816V c-KIT mutation was not detected. She was subsequently hospitalized for further evaluation, at which time there was no fever or localizing infectious signs or symptoms. An infectious evaluation including urinalysis; respiratory swab for adenovirus, coronaviruses, human metapneumovirus, rhinovirus/enterovirus, influenza A and B, parainfluenza viruses, respiratory syncytial virus, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; Lyme serology; and a computed tomography (CT) scan of the chest, abdomen, and pelvis revealed no evidence of infection. A parasite evaluation was ordered but was not performed. There was no evidence of malignancy on CT of the chest, abdomen, and pelvis or CT of the head without contrast. A lumbar puncture was considered but was ultimately deferred.

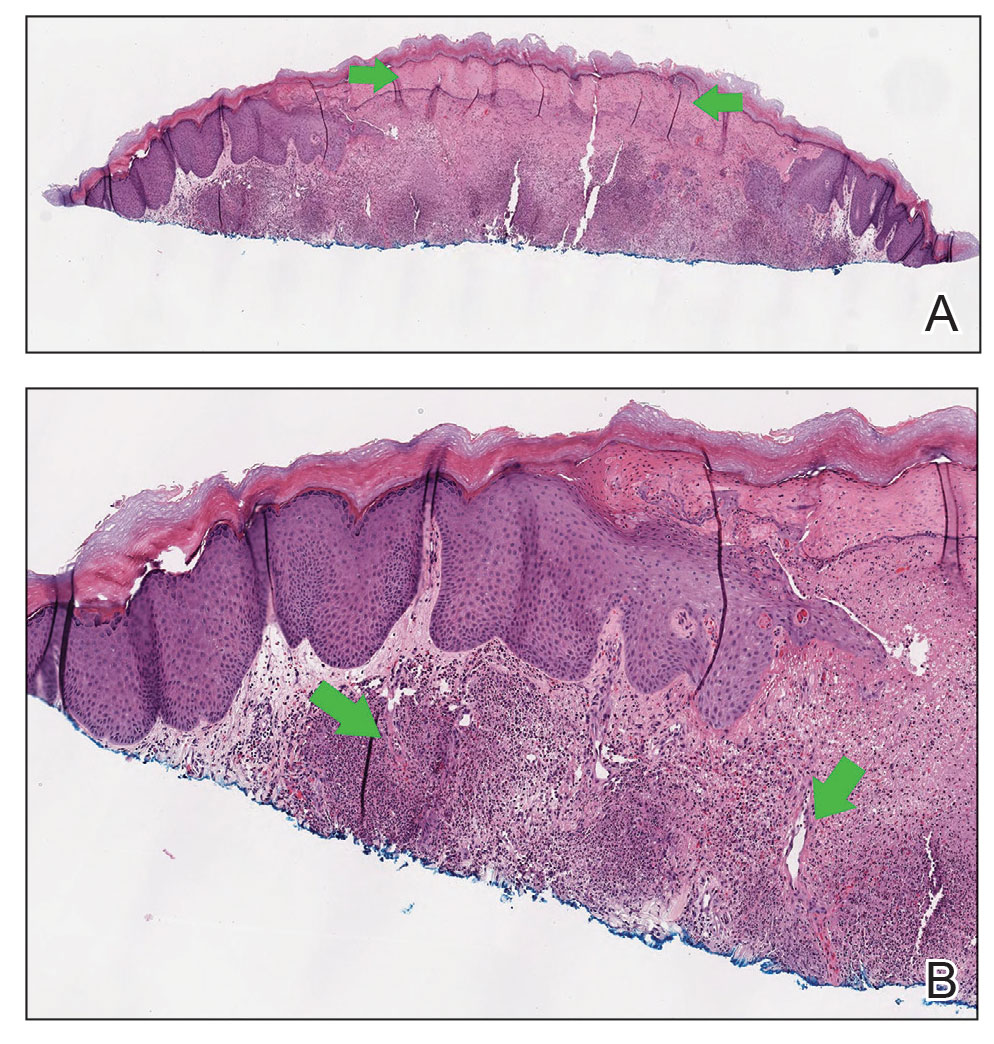
At the current presentation, the patient was following up in the dermatology clinic shortly after discharge. The lesions on the fingers and arms were described by the dermatologist as deep, erythematous, 0.5-cm bullous papules. The differential diagnosis at this time included a cutaneous or systemic infection, vasculitis, drug eruption, or cutaneous manifestation of an autoimmune condition. A shave biopsy of a skin lesion on the right hand demonstrated epidermal necrosis with a dense dermal neutrophilic infiltrate consistent with a neutrophilic dermatosis (Figure 2). There was no evidence of leukocytoclastic vasculitis. The histologic differential diagnosis included cutaneous infection, neutrophilic dermatosis of the hand, and SS. Special stains for infectious organisms including Gram, Grocott methenamine-silver, and auramine-rhodamine stains were negative for bacterial, fungal, and mycobacterial organisms, ruling out cutaneous infection. A diagnosis of drug-induced SS was made based on the histologic findings, diffuse distribution of the lesions, negative infectious evaluation, lack of underlying malignancy or autoimmune conditions, and onset following initiation of dupilumab.
Dupilumab was discontinued, and the patient was started on prednisone with rapid improvement in the symptoms. She underwent a slow taper of the prednisone over approximately 2 months with a slow downtrend of eosinophils. She was transitioned to a different biologic agent, benralizumab, with no further recurrence of the rash or arthralgia.
Comment
Dupilumab is a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. By blocking IL-4Rα, dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE. Currently, dupilumab is approved to treat refractory forms of moderate to severe asthma characterized by an eosinophilic phenotype or with corticosteroid-dependent asthma, moderate to severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis. The most common adverse events (incidence ≥1%) are injection-site reactions, oropharyngeal pain, and eosinophilia.5 Interestingly, our patient did exhibit a high degree of eosinophilia; however, she met all criteria for drug-induced SS, and the skin biopsy was not consistent with an eosinophilic process. Notably, the peripheral neutrophils were not elevated. Neutrophilia often is seen in classic SS but is not required for a diagnosis of drug-induced SS. Rare cases of dupilumab-associated arthritis and serum sickness–like reaction have been described,6-8 but our patient’s presentation was distinct, given other described signs, symptoms, and skin biopsy results. Histopathology results were not consistent with leukocytoclastic vasculitis, a potential mimicker of SS. Although the infectious and paraneoplastic evaluation was not exhaustive, the negative imaging from head to pelvis, the lack of recurrence of skin lesions, and the laboratory abnormalities after dupilumab discontinuation supported the conclusion that the culprit was not an infection or underlying malignancy. She had not started any other medications during this time frame, leaving dupilumab as the most likely offending agent. The mechanism for this reaction is not clear. It is possible that inhibition of IL-4 and IL-13 in the T helper 2 (TH2) cell pathway may have led to upregulated IL-17–mediated inflammation9 as well as a neutrophilic process in the skin, but this would not explain the concurrent peripheral eosinophilia that was noted. Further studies are needed to investigate the pathophysiology of SS.
Conclusion
We report a rare case of dupilumab-induced SS. Corticosteroids accompanied by cessation of the medication proved to be an effective treatment.
- Sweet RB. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Jackson K, Bahna SL. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev Clin Immunol. 2020;16:311-319.
- Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181:1068-1070.
- de Wijs LEM, van der Waa JD, de Jong PHP, et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45:262-263.
- Treudler R, Delaroque N, Puder M, et al. Dupilumab-induced serum sickness-like reaction: an unusual adverse effect in a patient with atopic eczema. J Eur Acad Dermatol Venereol. 2021;35:E30-E32.
- Guenova E, Skabytska Y, Hoetzenecker W, et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112:2163-2168.
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, was first described in 1964. 1 Since then, several subtypes of SS have been recognized, including classic or idiopathic, which typically follows an acute viral illness; cancer related, typically in the form of a paraneoplastic syndrome; and drug induced. 2 Drug-induced SS is defined by the following: (1) an abrupt onset of painful erythematous plaques or nodules; (2) histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; (3) pyrexia above 38 ° C; (4) temporal relationship between drug and clinical presentation or temporally related recurrence after rechallenge; and (5) temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. 3 All 5 criteria must be met to make a diagnosis of drug-induced SS. Since these criteria were established by Walker and Cohen, 3 various drugs have been identified as causative agents, including antibiotics, antiepileptics, antiretrovirals, antineoplastic agents, antipsychotics, oral contraceptives, nonsteroidal anti-inflammatory agents, and retinoids. 4 W e present a rare case of SS caused by dupilumab, a monoclonal antibody therapy, used in the treatment of severe eosinophilic asthma and atopic dermatitis.
Case Report
A 53-year-old woman presented with painful skin lesions, arthralgia, fever, and leukocytosis following initiation of dupilumab. She had a history of adult-onset, severe, persistent eosinophilic asthma, as well as chronic rhinosinusitis with nasal polyps, plaque psoriasis, and hypertrophic cardiomyopathy. She started mepolizumab 3 years prior to the current presentation for persistently uncontrolled asthma with a baseline peripheral eosinophil count of 860 cells/µL. After 3 years of minimal response to mepolizumab, she was started on dupilumab. Within 2 weeks of the first dose of dupilumab, she started experiencing bilateral knee pain. She subsequently developed daily fevers (temperature, 38.3 °C to 39.4 °C), fatigue, and pain in the back of the neck and head. After the second dose of dupilumab, she started experiencing painful skin lesions on the bilateral knuckles, elbows, and abdomen (Figure 1). She had difficulty using her hands and walking secondary to intense arthralgia involving the bilateral finger joints, elbows, and knees. Her primary care physician obtained a laboratory evaluation, which revealed an elevated total white blood cell count of 20×103/mm3 (reference range, 4–11×103/mm3) with 27.5% neutrophils and severely elevated eosinophils above her baseline to 57.3% with an absolute eosinophil count of 11,700 cells/µL (reference range, <400 cells/µL). Further assessment revealed an elevated erythrocyte sedimentation rate of 64 mm/h (reference range, 0–30 mm/h) and C-reactive protein level of 34 mg/dL (reference range, ≤0.80 mg/dL), with negative antinuclear antibody, rheumatoid factor, antineutrophilic cytoplasmic antibody, and Lyme antibody. IgG, IgA, and IgM levels were within reference range, and the IgE level was not elevated above her baseline. She had normal serum tryptase, and a peripheral D816V c-KIT mutation was not detected. She was subsequently hospitalized for further evaluation, at which time there was no fever or localizing infectious signs or symptoms. An infectious evaluation including urinalysis; respiratory swab for adenovirus, coronaviruses, human metapneumovirus, rhinovirus/enterovirus, influenza A and B, parainfluenza viruses, respiratory syncytial virus, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; Lyme serology; and a computed tomography (CT) scan of the chest, abdomen, and pelvis revealed no evidence of infection. A parasite evaluation was ordered but was not performed. There was no evidence of malignancy on CT of the chest, abdomen, and pelvis or CT of the head without contrast. A lumbar puncture was considered but was ultimately deferred.

At the current presentation, the patient was following up in the dermatology clinic shortly after discharge. The lesions on the fingers and arms were described by the dermatologist as deep, erythematous, 0.5-cm bullous papules. The differential diagnosis at this time included a cutaneous or systemic infection, vasculitis, drug eruption, or cutaneous manifestation of an autoimmune condition. A shave biopsy of a skin lesion on the right hand demonstrated epidermal necrosis with a dense dermal neutrophilic infiltrate consistent with a neutrophilic dermatosis (Figure 2). There was no evidence of leukocytoclastic vasculitis. The histologic differential diagnosis included cutaneous infection, neutrophilic dermatosis of the hand, and SS. Special stains for infectious organisms including Gram, Grocott methenamine-silver, and auramine-rhodamine stains were negative for bacterial, fungal, and mycobacterial organisms, ruling out cutaneous infection. A diagnosis of drug-induced SS was made based on the histologic findings, diffuse distribution of the lesions, negative infectious evaluation, lack of underlying malignancy or autoimmune conditions, and onset following initiation of dupilumab.

Dupilumab was discontinued, and the patient was started on prednisone with rapid improvement in the symptoms. She underwent a slow taper of the prednisone over approximately 2 months with a slow downtrend of eosinophils. She was transitioned to a different biologic agent, benralizumab, with no further recurrence of the rash or arthralgia.
Comment
Dupilumab is a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. By blocking IL-4Rα, dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE. Currently, dupilumab is approved to treat refractory forms of moderate to severe asthma characterized by an eosinophilic phenotype or with corticosteroid-dependent asthma, moderate to severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis. The most common adverse events (incidence ≥1%) are injection-site reactions, oropharyngeal pain, and eosinophilia.5 Interestingly, our patient did exhibit a high degree of eosinophilia; however, she met all criteria for drug-induced SS, and the skin biopsy was not consistent with an eosinophilic process. Notably, the peripheral neutrophils were not elevated. Neutrophilia often is seen in classic SS but is not required for a diagnosis of drug-induced SS. Rare cases of dupilumab-associated arthritis and serum sickness–like reaction have been described,6-8 but our patient’s presentation was distinct, given other described signs, symptoms, and skin biopsy results. Histopathology results were not consistent with leukocytoclastic vasculitis, a potential mimicker of SS. Although the infectious and paraneoplastic evaluation was not exhaustive, the negative imaging from head to pelvis, the lack of recurrence of skin lesions, and the laboratory abnormalities after dupilumab discontinuation supported the conclusion that the culprit was not an infection or underlying malignancy. She had not started any other medications during this time frame, leaving dupilumab as the most likely offending agent. The mechanism for this reaction is not clear. It is possible that inhibition of IL-4 and IL-13 in the T helper 2 (TH2) cell pathway may have led to upregulated IL-17–mediated inflammation9 as well as a neutrophilic process in the skin, but this would not explain the concurrent peripheral eosinophilia that was noted. Further studies are needed to investigate the pathophysiology of SS.
Conclusion
We report a rare case of dupilumab-induced SS. Corticosteroids accompanied by cessation of the medication proved to be an effective treatment.
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, was first described in 1964. 1 Since then, several subtypes of SS have been recognized, including classic or idiopathic, which typically follows an acute viral illness; cancer related, typically in the form of a paraneoplastic syndrome; and drug induced. 2 Drug-induced SS is defined by the following: (1) an abrupt onset of painful erythematous plaques or nodules; (2) histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; (3) pyrexia above 38 ° C; (4) temporal relationship between drug and clinical presentation or temporally related recurrence after rechallenge; and (5) temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. 3 All 5 criteria must be met to make a diagnosis of drug-induced SS. Since these criteria were established by Walker and Cohen, 3 various drugs have been identified as causative agents, including antibiotics, antiepileptics, antiretrovirals, antineoplastic agents, antipsychotics, oral contraceptives, nonsteroidal anti-inflammatory agents, and retinoids. 4 W e present a rare case of SS caused by dupilumab, a monoclonal antibody therapy, used in the treatment of severe eosinophilic asthma and atopic dermatitis.
Case Report
A 53-year-old woman presented with painful skin lesions, arthralgia, fever, and leukocytosis following initiation of dupilumab. She had a history of adult-onset, severe, persistent eosinophilic asthma, as well as chronic rhinosinusitis with nasal polyps, plaque psoriasis, and hypertrophic cardiomyopathy. She started mepolizumab 3 years prior to the current presentation for persistently uncontrolled asthma with a baseline peripheral eosinophil count of 860 cells/µL. After 3 years of minimal response to mepolizumab, she was started on dupilumab. Within 2 weeks of the first dose of dupilumab, she started experiencing bilateral knee pain. She subsequently developed daily fevers (temperature, 38.3 °C to 39.4 °C), fatigue, and pain in the back of the neck and head. After the second dose of dupilumab, she started experiencing painful skin lesions on the bilateral knuckles, elbows, and abdomen (Figure 1). She had difficulty using her hands and walking secondary to intense arthralgia involving the bilateral finger joints, elbows, and knees. Her primary care physician obtained a laboratory evaluation, which revealed an elevated total white blood cell count of 20×103/mm3 (reference range, 4–11×103/mm3) with 27.5% neutrophils and severely elevated eosinophils above her baseline to 57.3% with an absolute eosinophil count of 11,700 cells/µL (reference range, <400 cells/µL). Further assessment revealed an elevated erythrocyte sedimentation rate of 64 mm/h (reference range, 0–30 mm/h) and C-reactive protein level of 34 mg/dL (reference range, ≤0.80 mg/dL), with negative antinuclear antibody, rheumatoid factor, antineutrophilic cytoplasmic antibody, and Lyme antibody. IgG, IgA, and IgM levels were within reference range, and the IgE level was not elevated above her baseline. She had normal serum tryptase, and a peripheral D816V c-KIT mutation was not detected. She was subsequently hospitalized for further evaluation, at which time there was no fever or localizing infectious signs or symptoms. An infectious evaluation including urinalysis; respiratory swab for adenovirus, coronaviruses, human metapneumovirus, rhinovirus/enterovirus, influenza A and B, parainfluenza viruses, respiratory syncytial virus, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; Lyme serology; and a computed tomography (CT) scan of the chest, abdomen, and pelvis revealed no evidence of infection. A parasite evaluation was ordered but was not performed. There was no evidence of malignancy on CT of the chest, abdomen, and pelvis or CT of the head without contrast. A lumbar puncture was considered but was ultimately deferred.

At the current presentation, the patient was following up in the dermatology clinic shortly after discharge. The lesions on the fingers and arms were described by the dermatologist as deep, erythematous, 0.5-cm bullous papules. The differential diagnosis at this time included a cutaneous or systemic infection, vasculitis, drug eruption, or cutaneous manifestation of an autoimmune condition. A shave biopsy of a skin lesion on the right hand demonstrated epidermal necrosis with a dense dermal neutrophilic infiltrate consistent with a neutrophilic dermatosis (Figure 2). There was no evidence of leukocytoclastic vasculitis. The histologic differential diagnosis included cutaneous infection, neutrophilic dermatosis of the hand, and SS. Special stains for infectious organisms including Gram, Grocott methenamine-silver, and auramine-rhodamine stains were negative for bacterial, fungal, and mycobacterial organisms, ruling out cutaneous infection. A diagnosis of drug-induced SS was made based on the histologic findings, diffuse distribution of the lesions, negative infectious evaluation, lack of underlying malignancy or autoimmune conditions, and onset following initiation of dupilumab.

Dupilumab was discontinued, and the patient was started on prednisone with rapid improvement in the symptoms. She underwent a slow taper of the prednisone over approximately 2 months with a slow downtrend of eosinophils. She was transitioned to a different biologic agent, benralizumab, with no further recurrence of the rash or arthralgia.
Comment
Dupilumab is a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. By blocking IL-4Rα, dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE. Currently, dupilumab is approved to treat refractory forms of moderate to severe asthma characterized by an eosinophilic phenotype or with corticosteroid-dependent asthma, moderate to severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis. The most common adverse events (incidence ≥1%) are injection-site reactions, oropharyngeal pain, and eosinophilia.5 Interestingly, our patient did exhibit a high degree of eosinophilia; however, she met all criteria for drug-induced SS, and the skin biopsy was not consistent with an eosinophilic process. Notably, the peripheral neutrophils were not elevated. Neutrophilia often is seen in classic SS but is not required for a diagnosis of drug-induced SS. Rare cases of dupilumab-associated arthritis and serum sickness–like reaction have been described,6-8 but our patient’s presentation was distinct, given other described signs, symptoms, and skin biopsy results. Histopathology results were not consistent with leukocytoclastic vasculitis, a potential mimicker of SS. Although the infectious and paraneoplastic evaluation was not exhaustive, the negative imaging from head to pelvis, the lack of recurrence of skin lesions, and the laboratory abnormalities after dupilumab discontinuation supported the conclusion that the culprit was not an infection or underlying malignancy. She had not started any other medications during this time frame, leaving dupilumab as the most likely offending agent. The mechanism for this reaction is not clear. It is possible that inhibition of IL-4 and IL-13 in the T helper 2 (TH2) cell pathway may have led to upregulated IL-17–mediated inflammation9 as well as a neutrophilic process in the skin, but this would not explain the concurrent peripheral eosinophilia that was noted. Further studies are needed to investigate the pathophysiology of SS.
Conclusion
We report a rare case of dupilumab-induced SS. Corticosteroids accompanied by cessation of the medication proved to be an effective treatment.
- Sweet RB. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Jackson K, Bahna SL. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev Clin Immunol. 2020;16:311-319.
- Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181:1068-1070.
- de Wijs LEM, van der Waa JD, de Jong PHP, et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45:262-263.
- Treudler R, Delaroque N, Puder M, et al. Dupilumab-induced serum sickness-like reaction: an unusual adverse effect in a patient with atopic eczema. J Eur Acad Dermatol Venereol. 2021;35:E30-E32.
- Guenova E, Skabytska Y, Hoetzenecker W, et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112:2163-2168.
- Sweet RB. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Jackson K, Bahna SL. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev Clin Immunol. 2020;16:311-319.
- Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181:1068-1070.
- de Wijs LEM, van der Waa JD, de Jong PHP, et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45:262-263.
- Treudler R, Delaroque N, Puder M, et al. Dupilumab-induced serum sickness-like reaction: an unusual adverse effect in a patient with atopic eczema. J Eur Acad Dermatol Venereol. 2021;35:E30-E32.
- Guenova E, Skabytska Y, Hoetzenecker W, et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112:2163-2168.
Practice Points
- Prescribers of dupilumab should be aware that Sweet syndrome is a potential adverse reaction.
- Sweet syndrome should be suspected if there is abrupt onset of painful erythematous plaques or nodules accompanied by pyrexia following injection of dupilumab. Biopsy of the nodules should be obtained to confirm the diagnosis.
- Systemic corticosteroids with cessation of dupilumab are effective treatments.
- Following treatment, dupilumab should not be reinitiated, and alternative therapies should be used.