User login
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
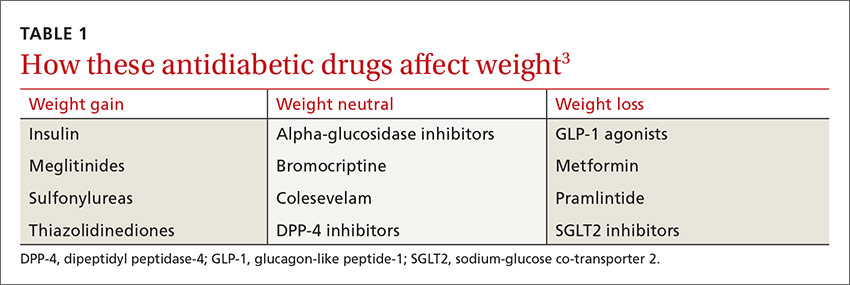
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
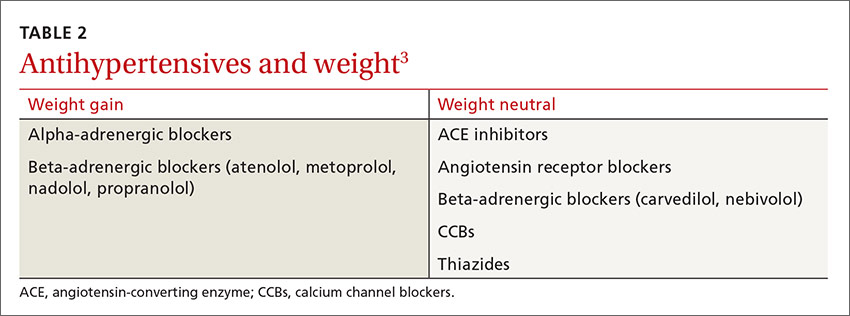
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
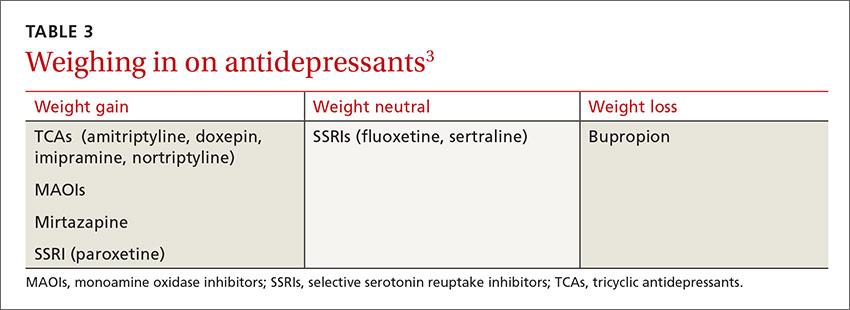
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; kph2001@med.cornell.edu.
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; kph2001@med.cornell.edu.
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; kph2001@med.cornell.edu.
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.