User login
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs. The pathogenesis of DRESS syndrome is still unknown. Immunological factors such as a defect in detoxification of culprit drugs and infections seem to be involved. The most commonly associated drugs are anticonvulsants and sulfonamides, but dapsone, allopurinol, and minocycline also have been reported to be associated with DRESS syndrome.1
Although therapies for postmenopausal osteoporosis are considered to be safe from cutaneous side effects, there have been several reported cases of DRESS syndrome associated with strontium ranelate.2 Strontium ranelate is not used in the United States; nevertheless, some US patients may be taking this drug as an alternative to the current US Food and Drug Administration–approved drugs for osteoporosis. We report a case of DRESS syndrome in a woman who developed an extensive maculopapular rash, eosinophilia, dyspnea, bilateral cervical lymphadenopathy, and reactivation of Epstein-Barr virus (EBV) with liver damage 3 weeks after administration of strontium ranelate for postmenopausal osteoporosis. Approximately 6 months after total remission of skin conditions, the patient developed autoimmune hepatitis.
Case Report
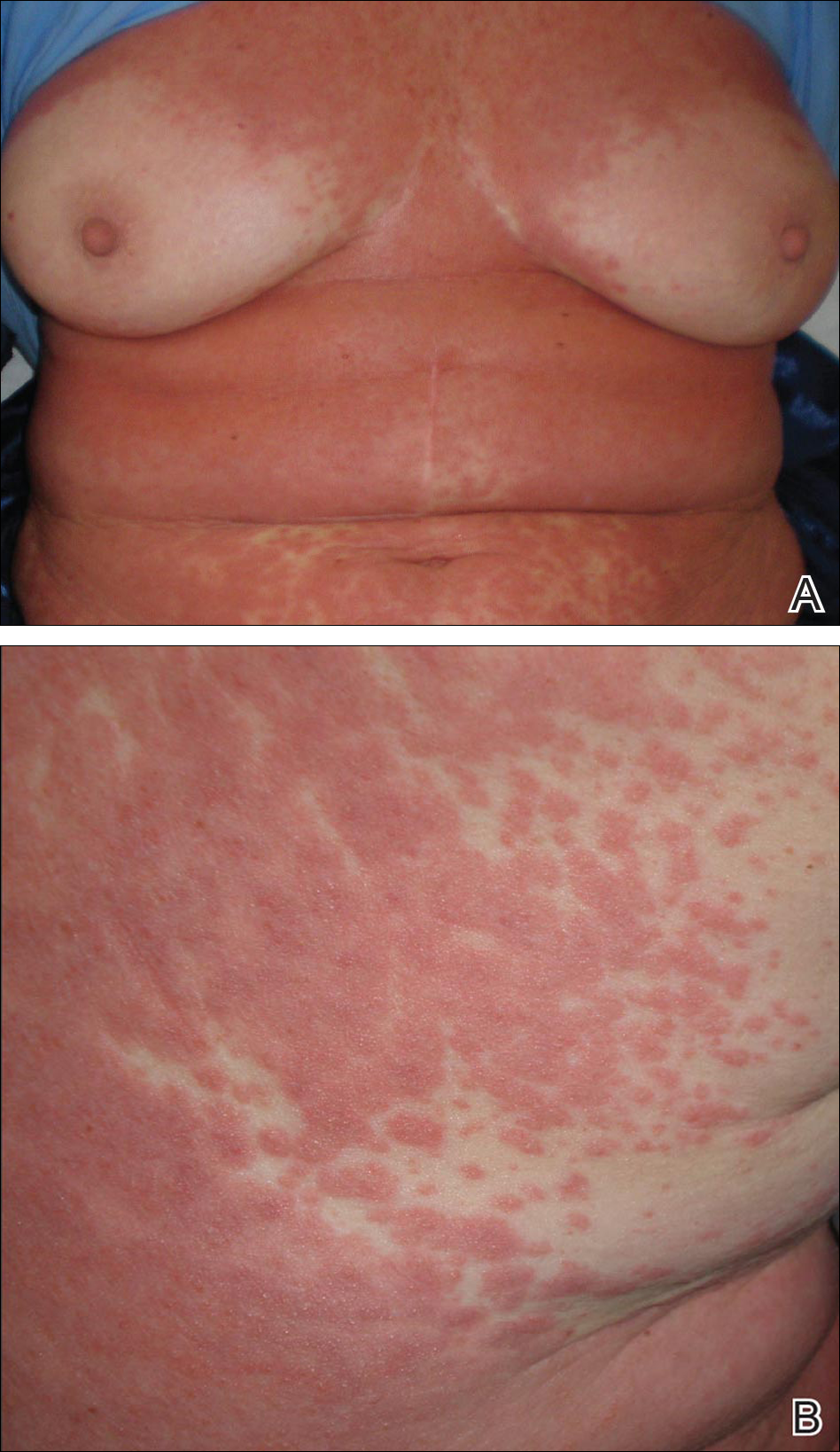
A 64-year-old woman presented to the emergency department with dyspnea, fever (temperature, 38.5°C), and a generalized rash that had developed a few days prior. The patient reported that she was previously in good health and had no prior allergic episodes. She had been taking strontium ranelate for 3 weeks to treat postmenopausal osteoporosis and reported no other medication use. The patient was hospitalized because of worsening symptoms. Physical examination revealed a pruritic maculopapular rash involving the trunk, arms, and legs (Figure 1) with facial edema, mild inspiratory as well as expiratory dyspnea, and wheezing on all lung fields. An enlarged soft liver (6–7 cm from the right costal arch) and cervical bilateral lymphadenopathy were found.
A chest radiograph detected a slight increase of the peribronchial thickening with interstitial involvement at the bilateral basal and perihilar levels, and an ultrasound of the chest confirmed the presence of many enlarged cervical bilateral lymph nodes between 2 and 4 cm in diameter.
Laboratory tests revealed the following values: leukocytosis (21,390/μL [reference range, 4500–11,000/μL]) with eosinophilia (27% [reference range, 2.7%]; 5780/μL [reference range, 0–450/μL]), elevated C-reactive protein (20 mg/L [reference range, 0.08–3.1 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–20 mm/h]), a reactivation of EBV confirmed by simultaneous seropositivity to early antigen IgM and EBV nuclear antigen, liver damage with notable increases in liver function tests (aspartate aminotransferase, 51 U/L [reference range, 10–30 U/L]); alanine aminotransferase, 104 U/L [reference range 10–40 U/L]); γ-glutamyltransferase, 52 U/L [reference range, 2–30 U/L]), and no thyroid dysfunction.
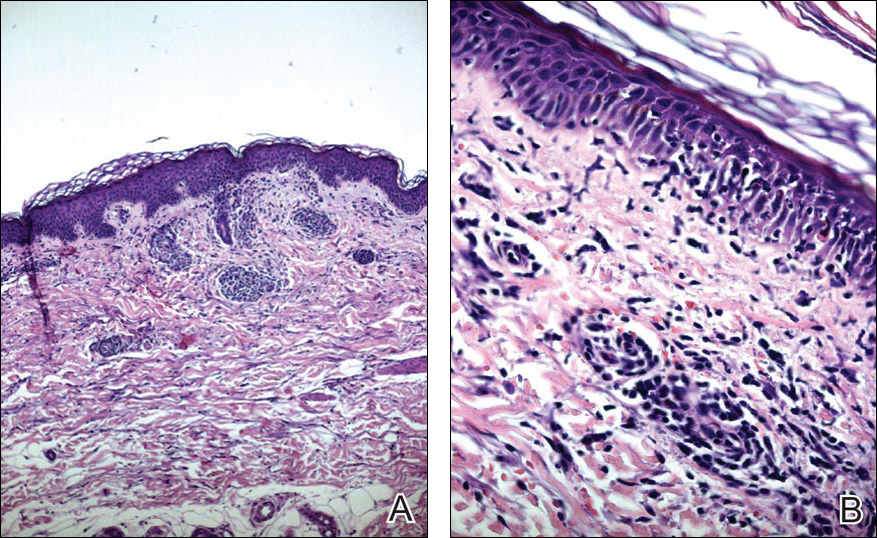
Blood and urine cultures; antinuclear antibodies; and serology for hepatitis A, B, and C virus, as well as herpes simplex virus type 6 (HHV-6), chlamydia, Mycoplasma, and cytomegalovirus (CMV) were all negative. Histologic examination after skin biopsy showed keratinocytes with spongiosis, intraepidermal eosinophilic infiltration, suffusion of red blood cells with perivascular granulocytes, and lymphocyte inflammatory infiltrate (Figure 2).
A diagnosis of DRESS syndrome was made on the basis of the following clinical data supported by laboratory findings: generalized maculopapular rash, eosinophilia, lung involvement with dyspnea, bilateral cervical lymphadenopathy, and liver damage, as well as an identified reactivation of EBV and onset of symptoms 3 weeks after treatment with strontium ranelate.
The patient was given intravenous methylprednisolone 120 mg once daily for 1 week in gradually decreasing doses. Three weeks of steroid therapy were necessary to obtain the first good results. Improvement of the patient’s clinical condition was considerably slow. Fever and rash gradually disappeared and the patient was discharged with oral corticosteroids. In the 2 months after starting systemic corticosteroid therapy, the lesions had not progressed and all other clinical symptoms improved. A slow but notable regression of the skin reaction was observed.
In a subsequent checkup approximately 8 months following initial presentation, the patient developed autoimmune hepatitis. There was a notable increase in liver enzymes and serum immunoglobulin content as well as positivity of antinuclear antibodies (1:160) and antimitochondrial antibodies (1:160). A liver biopsy was performed and confirmed the histologic pattern of autoimmune hepatitis. Thyroid function was reevaluated, but no other autoimmune disease was identified.
The patient was given another dose of steroids (prednisolone 25 mg daily). Liver function normalized within 1 month (aspartate aminotransferase levels went from 195 U/L to 21 U/L; alanine aminotransferase went from 324 U/L to 21 U/L; γ-glutamyltransferase went from 268 U/L to 63 U/L). The patient is currently taking a maintenance dose of prednisolone 5 mg and has normal liver function.
Comment
Uses of Strontium Ranelate
Strontium ranelate is recommended for reducing the risk for fracture in postmenopausal women 70 years and older with a bone mineral density T-score of –3.0 or lower (ie, primary prevention) as well as for the treatment of morphometric vertebral fracture in established postmenopausal osteoporosis (ie, secondary prevention). Strontium ranelate has a dual action that includes increasing bone formation and reducing bone resorption, leading to rebalancing of bone remodeling in favor of bone formation. Strontium ranelate was shown to increase the recruitment and activity of osteoblastic cells and to inhibit the recruitment and activity of osteoclasts.2 The recommended dose of oral strontium ranelate is 2 g once daily.
Side Effects of Strontium Ranelate
In a 3-year study of side effects associated with strontium ranelate, severe reactions were described in 23% of the reported adverse effects in 844 patients.3 In this study, cardiovascular effects, particularly thromboembolism, and DRESS syndrome were the most frequent side effects. Since its introduction in the market, at least 16 cases of DRESS syndrome related to strontium ranelate use have been reported in Europe, including 2 fatal cases.2 Two deaths have been reported to be associated with this drug,2 which was the basis of the warning document by the European Medicines Agency regarding the risk for strontium ranelate inducing DRESS syndrome.4
Development of DRESS Syndrome
The most common agents involved in DRESS syndrome are anticonvulsants, sulfonamides, dapsone, minocycline, allopurinol, and gold salts, as well as celecoxib, antituberculosis drugs, nonsteroidal anti-inflammatory drugs, antibiotics, calcium channel blockers, and antiretroviral drugs.5,6 The mortality rate of DRESS syndrome is 10%.6
The pathophysiology of DRESS syndrome is still unclear. Altered drug metabolism, genetic predisposition, and concomitant infection or reactivation of bacterial or viral infection (eg, HHV-6, EBV, CMV, human immunodeficiency virus, influenza, viral hepatitis) could be factors leading to development of DRESS. Autoimmune or connective-tissue diseases also have been suggested to increase the risk.7
Clinicians should suspect DRESS syndrome in any patient developing a rash 3 to 6 weeks after starting drug therapy. This disorder often starts with fever (temperature >38°C) and includes cutaneous symptoms such as generalized rash that may progress to exfoliative dermatitis. There usually is involvement of one or several internal organs with the development of hepatitis; interstitial pneumonia; interstitial nephritis; myopericarditis; myositis; pancreatitis; thyroiditis; and hematological abnormalities, primarily eosinophilia or atypical lymphocytosis. Facial edema and lymphadenopathy also may be present. A skin biopsy can confirm the clinical diagnosis of DRESS syndrome but is not specific because cutaneous histologic patterns often show a lymphocytic infiltrate that sometimes mimics cutaneous lymphoma. Other diseases that DRESS syndrome may mimic include Stevens-Johnson syndrome and toxic epidermal necrolysis as well as Kawasaki disease, Still disease, acute viral infections, idiopathic hypereosinophilic syndrome, and lymphoma, which should be excluded from the differential diagnosis.8
Diagnosis of DRESS Syndrome
There is no gold standard for the diagnosis of DRESS syndrome. In our case, the diagnosis of DRESS syndrome was based on the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions to Drugs) score as described by Kardaun et al,9 which grades DRESS syndrome cases as excluded (<2 points), possible (2–3 points), probable (4–5 points), or definite (>5 points) based on the following clinical criteria: fever (temperature >38.5°C; from a minimum of –1 point if absent to a maximum of 0 points if present); enlarged lymph nodes (from a minimum of 0 points if absent to a maximum of 1 point if present); eosinophilia (0 points if absent, 1 point if 10%–19% or 700–1500 μL, 2 points if ≥20% or >1500 μL); atypical lymphocytes (from a minimum of 0 points if absent to maximum of 1 point if present); skin involvement with rash (1 point if >50% of body surface area is involved, 1 point if there is a maculopapular rash, 1 point if skin biopsy suggests DRESS syndrome); organ involvement (1 point each for liver, kidneys, lungs, muscle/heart, pancreas, and other organs); resolution in at least 15 days (from a minimum of –1 point if absent to maximum of 0 points if present); and evaluation of other potential causes measuring antinuclear antibodies, blood culture, and serology for hepatitis virus (A–C), chlamydia, and Mycoplasma (1 point if 3 or more are negative and none positive). Virus reactivation also should be considered a main characteristic of DRESS syndrome. Therefore, its prevalence is not homogenous, so the absence of viral reactivation cannot be considered exclusion criteria. Several case reports and a few well-documented series have evidenced markers of virus reactivation in many cases of DRESS. Herpes simplex virus 6, CMV, and EBV are the most frequently reactivated.
The total RegiSCAR score of 8 in our case was taken as a definite indication of DRESS syndrome (temperature, 38.5°C [0 points]; enlarged lymph nodes [1 point]; eosinophilia, ≥20% or >1500 μL [2 points]; skin involvement with >50% body surface area involved [1 point] with a maculopapular rash [1 point] and histopathologic findings suggesting DRESS syndrome [1 point]; lung and liver involvement [2 points]). The causative drug was identified by carefully collecting the patient’s medication history and by evaluating clinical outcome characterized by improved skin and systemic symptoms after discontinuation of strontium ranelate.
Because of the high morbidity of DRESS syndrome, it needs to be diagnosed effectively and must be considered in the differential for any patient developing the triad of skin rash, hypereosinophilia, and systemic symptoms, as well as several other side effects when taking strontium ranelate.10
Therapies for DRESS Syndrome
Treatment of DRESS syndrome has not yet been standardized. Prompt withdrawal of the causative drug is the only mandatory activity in the treatment of DRESS syndrome. Systemic corticosteroids may be needed for organ or life-threatening disease, though the efficacy is controversial because it may result in activation of HHV-6, which in turn is probably involved in the pathogenesis of DRESS syndrome.
Conclusion
This case confirms that strontium ranelate should be considered a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome. Case reports underline the importance of recognition of cutaneous adverse reactions in patients undergoing treatment of postmenopausal osteoporosis. The prognosis is good with immediate recognition followed by immediate and permanent withdrawal of the drug, along with hospitalization and systemic corticosteroids when necessary. The possibility of developing autoimmune hepatitis as a part of DRESS syndrome related to strontium ranelate has been reported,11 usually months after the acute episode.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Le Merlouette M, Adamski H, Dinulescu M, et al. Strontium ranelate–induced DRESS syndrome. Ann Dermatol Venereol. 2011;138:124-128.
- Jonville-Bera AP, Autret-Leca E. Adverse drug reactions of strontium ranelate (Protelos®) in France. Presse Med. 2011;40:453-462.
- Assessment report for Protelos and Osseor. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000560/WC500131789.pdf). Published May 25, 2012. Accessed May 9, 2016.
- Breathnach S. Drug rash eosinophilia and systemic symptoms (DRESS) syndrome. types of clinical reaction: drug reaction. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 4. 8th ed. Hoboken, NJ: Oxford Wiley-Blackwell Publications; 2010:75.26.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723-732.
- Telles Rudge de Aquino R, Vieitas Vergueiro CS, Ruffolo Magliari ME, et al. Sulfasalazine-induced DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Sao Paulo Med J. 2008;126:225-226.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Pernicova I, Middleton ET, Aye M. Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert [published online September 20, 2008]. Osteoporos Int. 2008;19:1811-1812.
- Kinyó A, Belsö N, Nagy N, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205-206.
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs. The pathogenesis of DRESS syndrome is still unknown. Immunological factors such as a defect in detoxification of culprit drugs and infections seem to be involved. The most commonly associated drugs are anticonvulsants and sulfonamides, but dapsone, allopurinol, and minocycline also have been reported to be associated with DRESS syndrome.1
Although therapies for postmenopausal osteoporosis are considered to be safe from cutaneous side effects, there have been several reported cases of DRESS syndrome associated with strontium ranelate.2 Strontium ranelate is not used in the United States; nevertheless, some US patients may be taking this drug as an alternative to the current US Food and Drug Administration–approved drugs for osteoporosis. We report a case of DRESS syndrome in a woman who developed an extensive maculopapular rash, eosinophilia, dyspnea, bilateral cervical lymphadenopathy, and reactivation of Epstein-Barr virus (EBV) with liver damage 3 weeks after administration of strontium ranelate for postmenopausal osteoporosis. Approximately 6 months after total remission of skin conditions, the patient developed autoimmune hepatitis.
Case Report
A 64-year-old woman presented to the emergency department with dyspnea, fever (temperature, 38.5°C), and a generalized rash that had developed a few days prior. The patient reported that she was previously in good health and had no prior allergic episodes. She had been taking strontium ranelate for 3 weeks to treat postmenopausal osteoporosis and reported no other medication use. The patient was hospitalized because of worsening symptoms. Physical examination revealed a pruritic maculopapular rash involving the trunk, arms, and legs (Figure 1) with facial edema, mild inspiratory as well as expiratory dyspnea, and wheezing on all lung fields. An enlarged soft liver (6–7 cm from the right costal arch) and cervical bilateral lymphadenopathy were found.

A chest radiograph detected a slight increase of the peribronchial thickening with interstitial involvement at the bilateral basal and perihilar levels, and an ultrasound of the chest confirmed the presence of many enlarged cervical bilateral lymph nodes between 2 and 4 cm in diameter.
Laboratory tests revealed the following values: leukocytosis (21,390/μL [reference range, 4500–11,000/μL]) with eosinophilia (27% [reference range, 2.7%]; 5780/μL [reference range, 0–450/μL]), elevated C-reactive protein (20 mg/L [reference range, 0.08–3.1 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–20 mm/h]), a reactivation of EBV confirmed by simultaneous seropositivity to early antigen IgM and EBV nuclear antigen, liver damage with notable increases in liver function tests (aspartate aminotransferase, 51 U/L [reference range, 10–30 U/L]); alanine aminotransferase, 104 U/L [reference range 10–40 U/L]); γ-glutamyltransferase, 52 U/L [reference range, 2–30 U/L]), and no thyroid dysfunction.
Blood and urine cultures; antinuclear antibodies; and serology for hepatitis A, B, and C virus, as well as herpes simplex virus type 6 (HHV-6), chlamydia, Mycoplasma, and cytomegalovirus (CMV) were all negative. Histologic examination after skin biopsy showed keratinocytes with spongiosis, intraepidermal eosinophilic infiltration, suffusion of red blood cells with perivascular granulocytes, and lymphocyte inflammatory infiltrate (Figure 2).

A diagnosis of DRESS syndrome was made on the basis of the following clinical data supported by laboratory findings: generalized maculopapular rash, eosinophilia, lung involvement with dyspnea, bilateral cervical lymphadenopathy, and liver damage, as well as an identified reactivation of EBV and onset of symptoms 3 weeks after treatment with strontium ranelate.
The patient was given intravenous methylprednisolone 120 mg once daily for 1 week in gradually decreasing doses. Three weeks of steroid therapy were necessary to obtain the first good results. Improvement of the patient’s clinical condition was considerably slow. Fever and rash gradually disappeared and the patient was discharged with oral corticosteroids. In the 2 months after starting systemic corticosteroid therapy, the lesions had not progressed and all other clinical symptoms improved. A slow but notable regression of the skin reaction was observed.
In a subsequent checkup approximately 8 months following initial presentation, the patient developed autoimmune hepatitis. There was a notable increase in liver enzymes and serum immunoglobulin content as well as positivity of antinuclear antibodies (1:160) and antimitochondrial antibodies (1:160). A liver biopsy was performed and confirmed the histologic pattern of autoimmune hepatitis. Thyroid function was reevaluated, but no other autoimmune disease was identified.
The patient was given another dose of steroids (prednisolone 25 mg daily). Liver function normalized within 1 month (aspartate aminotransferase levels went from 195 U/L to 21 U/L; alanine aminotransferase went from 324 U/L to 21 U/L; γ-glutamyltransferase went from 268 U/L to 63 U/L). The patient is currently taking a maintenance dose of prednisolone 5 mg and has normal liver function.
Comment
Uses of Strontium Ranelate
Strontium ranelate is recommended for reducing the risk for fracture in postmenopausal women 70 years and older with a bone mineral density T-score of –3.0 or lower (ie, primary prevention) as well as for the treatment of morphometric vertebral fracture in established postmenopausal osteoporosis (ie, secondary prevention). Strontium ranelate has a dual action that includes increasing bone formation and reducing bone resorption, leading to rebalancing of bone remodeling in favor of bone formation. Strontium ranelate was shown to increase the recruitment and activity of osteoblastic cells and to inhibit the recruitment and activity of osteoclasts.2 The recommended dose of oral strontium ranelate is 2 g once daily.
Side Effects of Strontium Ranelate
In a 3-year study of side effects associated with strontium ranelate, severe reactions were described in 23% of the reported adverse effects in 844 patients.3 In this study, cardiovascular effects, particularly thromboembolism, and DRESS syndrome were the most frequent side effects. Since its introduction in the market, at least 16 cases of DRESS syndrome related to strontium ranelate use have been reported in Europe, including 2 fatal cases.2 Two deaths have been reported to be associated with this drug,2 which was the basis of the warning document by the European Medicines Agency regarding the risk for strontium ranelate inducing DRESS syndrome.4
Development of DRESS Syndrome
The most common agents involved in DRESS syndrome are anticonvulsants, sulfonamides, dapsone, minocycline, allopurinol, and gold salts, as well as celecoxib, antituberculosis drugs, nonsteroidal anti-inflammatory drugs, antibiotics, calcium channel blockers, and antiretroviral drugs.5,6 The mortality rate of DRESS syndrome is 10%.6
The pathophysiology of DRESS syndrome is still unclear. Altered drug metabolism, genetic predisposition, and concomitant infection or reactivation of bacterial or viral infection (eg, HHV-6, EBV, CMV, human immunodeficiency virus, influenza, viral hepatitis) could be factors leading to development of DRESS. Autoimmune or connective-tissue diseases also have been suggested to increase the risk.7
Clinicians should suspect DRESS syndrome in any patient developing a rash 3 to 6 weeks after starting drug therapy. This disorder often starts with fever (temperature >38°C) and includes cutaneous symptoms such as generalized rash that may progress to exfoliative dermatitis. There usually is involvement of one or several internal organs with the development of hepatitis; interstitial pneumonia; interstitial nephritis; myopericarditis; myositis; pancreatitis; thyroiditis; and hematological abnormalities, primarily eosinophilia or atypical lymphocytosis. Facial edema and lymphadenopathy also may be present. A skin biopsy can confirm the clinical diagnosis of DRESS syndrome but is not specific because cutaneous histologic patterns often show a lymphocytic infiltrate that sometimes mimics cutaneous lymphoma. Other diseases that DRESS syndrome may mimic include Stevens-Johnson syndrome and toxic epidermal necrolysis as well as Kawasaki disease, Still disease, acute viral infections, idiopathic hypereosinophilic syndrome, and lymphoma, which should be excluded from the differential diagnosis.8
Diagnosis of DRESS Syndrome
There is no gold standard for the diagnosis of DRESS syndrome. In our case, the diagnosis of DRESS syndrome was based on the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions to Drugs) score as described by Kardaun et al,9 which grades DRESS syndrome cases as excluded (<2 points), possible (2–3 points), probable (4–5 points), or definite (>5 points) based on the following clinical criteria: fever (temperature >38.5°C; from a minimum of –1 point if absent to a maximum of 0 points if present); enlarged lymph nodes (from a minimum of 0 points if absent to a maximum of 1 point if present); eosinophilia (0 points if absent, 1 point if 10%–19% or 700–1500 μL, 2 points if ≥20% or >1500 μL); atypical lymphocytes (from a minimum of 0 points if absent to maximum of 1 point if present); skin involvement with rash (1 point if >50% of body surface area is involved, 1 point if there is a maculopapular rash, 1 point if skin biopsy suggests DRESS syndrome); organ involvement (1 point each for liver, kidneys, lungs, muscle/heart, pancreas, and other organs); resolution in at least 15 days (from a minimum of –1 point if absent to maximum of 0 points if present); and evaluation of other potential causes measuring antinuclear antibodies, blood culture, and serology for hepatitis virus (A–C), chlamydia, and Mycoplasma (1 point if 3 or more are negative and none positive). Virus reactivation also should be considered a main characteristic of DRESS syndrome. Therefore, its prevalence is not homogenous, so the absence of viral reactivation cannot be considered exclusion criteria. Several case reports and a few well-documented series have evidenced markers of virus reactivation in many cases of DRESS. Herpes simplex virus 6, CMV, and EBV are the most frequently reactivated.
The total RegiSCAR score of 8 in our case was taken as a definite indication of DRESS syndrome (temperature, 38.5°C [0 points]; enlarged lymph nodes [1 point]; eosinophilia, ≥20% or >1500 μL [2 points]; skin involvement with >50% body surface area involved [1 point] with a maculopapular rash [1 point] and histopathologic findings suggesting DRESS syndrome [1 point]; lung and liver involvement [2 points]). The causative drug was identified by carefully collecting the patient’s medication history and by evaluating clinical outcome characterized by improved skin and systemic symptoms after discontinuation of strontium ranelate.
Because of the high morbidity of DRESS syndrome, it needs to be diagnosed effectively and must be considered in the differential for any patient developing the triad of skin rash, hypereosinophilia, and systemic symptoms, as well as several other side effects when taking strontium ranelate.10
Therapies for DRESS Syndrome
Treatment of DRESS syndrome has not yet been standardized. Prompt withdrawal of the causative drug is the only mandatory activity in the treatment of DRESS syndrome. Systemic corticosteroids may be needed for organ or life-threatening disease, though the efficacy is controversial because it may result in activation of HHV-6, which in turn is probably involved in the pathogenesis of DRESS syndrome.
Conclusion
This case confirms that strontium ranelate should be considered a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome. Case reports underline the importance of recognition of cutaneous adverse reactions in patients undergoing treatment of postmenopausal osteoporosis. The prognosis is good with immediate recognition followed by immediate and permanent withdrawal of the drug, along with hospitalization and systemic corticosteroids when necessary. The possibility of developing autoimmune hepatitis as a part of DRESS syndrome related to strontium ranelate has been reported,11 usually months after the acute episode.
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs. The pathogenesis of DRESS syndrome is still unknown. Immunological factors such as a defect in detoxification of culprit drugs and infections seem to be involved. The most commonly associated drugs are anticonvulsants and sulfonamides, but dapsone, allopurinol, and minocycline also have been reported to be associated with DRESS syndrome.1
Although therapies for postmenopausal osteoporosis are considered to be safe from cutaneous side effects, there have been several reported cases of DRESS syndrome associated with strontium ranelate.2 Strontium ranelate is not used in the United States; nevertheless, some US patients may be taking this drug as an alternative to the current US Food and Drug Administration–approved drugs for osteoporosis. We report a case of DRESS syndrome in a woman who developed an extensive maculopapular rash, eosinophilia, dyspnea, bilateral cervical lymphadenopathy, and reactivation of Epstein-Barr virus (EBV) with liver damage 3 weeks after administration of strontium ranelate for postmenopausal osteoporosis. Approximately 6 months after total remission of skin conditions, the patient developed autoimmune hepatitis.
Case Report
A 64-year-old woman presented to the emergency department with dyspnea, fever (temperature, 38.5°C), and a generalized rash that had developed a few days prior. The patient reported that she was previously in good health and had no prior allergic episodes. She had been taking strontium ranelate for 3 weeks to treat postmenopausal osteoporosis and reported no other medication use. The patient was hospitalized because of worsening symptoms. Physical examination revealed a pruritic maculopapular rash involving the trunk, arms, and legs (Figure 1) with facial edema, mild inspiratory as well as expiratory dyspnea, and wheezing on all lung fields. An enlarged soft liver (6–7 cm from the right costal arch) and cervical bilateral lymphadenopathy were found.

A chest radiograph detected a slight increase of the peribronchial thickening with interstitial involvement at the bilateral basal and perihilar levels, and an ultrasound of the chest confirmed the presence of many enlarged cervical bilateral lymph nodes between 2 and 4 cm in diameter.
Laboratory tests revealed the following values: leukocytosis (21,390/μL [reference range, 4500–11,000/μL]) with eosinophilia (27% [reference range, 2.7%]; 5780/μL [reference range, 0–450/μL]), elevated C-reactive protein (20 mg/L [reference range, 0.08–3.1 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–20 mm/h]), a reactivation of EBV confirmed by simultaneous seropositivity to early antigen IgM and EBV nuclear antigen, liver damage with notable increases in liver function tests (aspartate aminotransferase, 51 U/L [reference range, 10–30 U/L]); alanine aminotransferase, 104 U/L [reference range 10–40 U/L]); γ-glutamyltransferase, 52 U/L [reference range, 2–30 U/L]), and no thyroid dysfunction.
Blood and urine cultures; antinuclear antibodies; and serology for hepatitis A, B, and C virus, as well as herpes simplex virus type 6 (HHV-6), chlamydia, Mycoplasma, and cytomegalovirus (CMV) were all negative. Histologic examination after skin biopsy showed keratinocytes with spongiosis, intraepidermal eosinophilic infiltration, suffusion of red blood cells with perivascular granulocytes, and lymphocyte inflammatory infiltrate (Figure 2).

A diagnosis of DRESS syndrome was made on the basis of the following clinical data supported by laboratory findings: generalized maculopapular rash, eosinophilia, lung involvement with dyspnea, bilateral cervical lymphadenopathy, and liver damage, as well as an identified reactivation of EBV and onset of symptoms 3 weeks after treatment with strontium ranelate.
The patient was given intravenous methylprednisolone 120 mg once daily for 1 week in gradually decreasing doses. Three weeks of steroid therapy were necessary to obtain the first good results. Improvement of the patient’s clinical condition was considerably slow. Fever and rash gradually disappeared and the patient was discharged with oral corticosteroids. In the 2 months after starting systemic corticosteroid therapy, the lesions had not progressed and all other clinical symptoms improved. A slow but notable regression of the skin reaction was observed.
In a subsequent checkup approximately 8 months following initial presentation, the patient developed autoimmune hepatitis. There was a notable increase in liver enzymes and serum immunoglobulin content as well as positivity of antinuclear antibodies (1:160) and antimitochondrial antibodies (1:160). A liver biopsy was performed and confirmed the histologic pattern of autoimmune hepatitis. Thyroid function was reevaluated, but no other autoimmune disease was identified.
The patient was given another dose of steroids (prednisolone 25 mg daily). Liver function normalized within 1 month (aspartate aminotransferase levels went from 195 U/L to 21 U/L; alanine aminotransferase went from 324 U/L to 21 U/L; γ-glutamyltransferase went from 268 U/L to 63 U/L). The patient is currently taking a maintenance dose of prednisolone 5 mg and has normal liver function.
Comment
Uses of Strontium Ranelate
Strontium ranelate is recommended for reducing the risk for fracture in postmenopausal women 70 years and older with a bone mineral density T-score of –3.0 or lower (ie, primary prevention) as well as for the treatment of morphometric vertebral fracture in established postmenopausal osteoporosis (ie, secondary prevention). Strontium ranelate has a dual action that includes increasing bone formation and reducing bone resorption, leading to rebalancing of bone remodeling in favor of bone formation. Strontium ranelate was shown to increase the recruitment and activity of osteoblastic cells and to inhibit the recruitment and activity of osteoclasts.2 The recommended dose of oral strontium ranelate is 2 g once daily.
Side Effects of Strontium Ranelate
In a 3-year study of side effects associated with strontium ranelate, severe reactions were described in 23% of the reported adverse effects in 844 patients.3 In this study, cardiovascular effects, particularly thromboembolism, and DRESS syndrome were the most frequent side effects. Since its introduction in the market, at least 16 cases of DRESS syndrome related to strontium ranelate use have been reported in Europe, including 2 fatal cases.2 Two deaths have been reported to be associated with this drug,2 which was the basis of the warning document by the European Medicines Agency regarding the risk for strontium ranelate inducing DRESS syndrome.4
Development of DRESS Syndrome
The most common agents involved in DRESS syndrome are anticonvulsants, sulfonamides, dapsone, minocycline, allopurinol, and gold salts, as well as celecoxib, antituberculosis drugs, nonsteroidal anti-inflammatory drugs, antibiotics, calcium channel blockers, and antiretroviral drugs.5,6 The mortality rate of DRESS syndrome is 10%.6
The pathophysiology of DRESS syndrome is still unclear. Altered drug metabolism, genetic predisposition, and concomitant infection or reactivation of bacterial or viral infection (eg, HHV-6, EBV, CMV, human immunodeficiency virus, influenza, viral hepatitis) could be factors leading to development of DRESS. Autoimmune or connective-tissue diseases also have been suggested to increase the risk.7
Clinicians should suspect DRESS syndrome in any patient developing a rash 3 to 6 weeks after starting drug therapy. This disorder often starts with fever (temperature >38°C) and includes cutaneous symptoms such as generalized rash that may progress to exfoliative dermatitis. There usually is involvement of one or several internal organs with the development of hepatitis; interstitial pneumonia; interstitial nephritis; myopericarditis; myositis; pancreatitis; thyroiditis; and hematological abnormalities, primarily eosinophilia or atypical lymphocytosis. Facial edema and lymphadenopathy also may be present. A skin biopsy can confirm the clinical diagnosis of DRESS syndrome but is not specific because cutaneous histologic patterns often show a lymphocytic infiltrate that sometimes mimics cutaneous lymphoma. Other diseases that DRESS syndrome may mimic include Stevens-Johnson syndrome and toxic epidermal necrolysis as well as Kawasaki disease, Still disease, acute viral infections, idiopathic hypereosinophilic syndrome, and lymphoma, which should be excluded from the differential diagnosis.8
Diagnosis of DRESS Syndrome
There is no gold standard for the diagnosis of DRESS syndrome. In our case, the diagnosis of DRESS syndrome was based on the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions to Drugs) score as described by Kardaun et al,9 which grades DRESS syndrome cases as excluded (<2 points), possible (2–3 points), probable (4–5 points), or definite (>5 points) based on the following clinical criteria: fever (temperature >38.5°C; from a minimum of –1 point if absent to a maximum of 0 points if present); enlarged lymph nodes (from a minimum of 0 points if absent to a maximum of 1 point if present); eosinophilia (0 points if absent, 1 point if 10%–19% or 700–1500 μL, 2 points if ≥20% or >1500 μL); atypical lymphocytes (from a minimum of 0 points if absent to maximum of 1 point if present); skin involvement with rash (1 point if >50% of body surface area is involved, 1 point if there is a maculopapular rash, 1 point if skin biopsy suggests DRESS syndrome); organ involvement (1 point each for liver, kidneys, lungs, muscle/heart, pancreas, and other organs); resolution in at least 15 days (from a minimum of –1 point if absent to maximum of 0 points if present); and evaluation of other potential causes measuring antinuclear antibodies, blood culture, and serology for hepatitis virus (A–C), chlamydia, and Mycoplasma (1 point if 3 or more are negative and none positive). Virus reactivation also should be considered a main characteristic of DRESS syndrome. Therefore, its prevalence is not homogenous, so the absence of viral reactivation cannot be considered exclusion criteria. Several case reports and a few well-documented series have evidenced markers of virus reactivation in many cases of DRESS. Herpes simplex virus 6, CMV, and EBV are the most frequently reactivated.
The total RegiSCAR score of 8 in our case was taken as a definite indication of DRESS syndrome (temperature, 38.5°C [0 points]; enlarged lymph nodes [1 point]; eosinophilia, ≥20% or >1500 μL [2 points]; skin involvement with >50% body surface area involved [1 point] with a maculopapular rash [1 point] and histopathologic findings suggesting DRESS syndrome [1 point]; lung and liver involvement [2 points]). The causative drug was identified by carefully collecting the patient’s medication history and by evaluating clinical outcome characterized by improved skin and systemic symptoms after discontinuation of strontium ranelate.
Because of the high morbidity of DRESS syndrome, it needs to be diagnosed effectively and must be considered in the differential for any patient developing the triad of skin rash, hypereosinophilia, and systemic symptoms, as well as several other side effects when taking strontium ranelate.10
Therapies for DRESS Syndrome
Treatment of DRESS syndrome has not yet been standardized. Prompt withdrawal of the causative drug is the only mandatory activity in the treatment of DRESS syndrome. Systemic corticosteroids may be needed for organ or life-threatening disease, though the efficacy is controversial because it may result in activation of HHV-6, which in turn is probably involved in the pathogenesis of DRESS syndrome.
Conclusion
This case confirms that strontium ranelate should be considered a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome. Case reports underline the importance of recognition of cutaneous adverse reactions in patients undergoing treatment of postmenopausal osteoporosis. The prognosis is good with immediate recognition followed by immediate and permanent withdrawal of the drug, along with hospitalization and systemic corticosteroids when necessary. The possibility of developing autoimmune hepatitis as a part of DRESS syndrome related to strontium ranelate has been reported,11 usually months after the acute episode.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Le Merlouette M, Adamski H, Dinulescu M, et al. Strontium ranelate–induced DRESS syndrome. Ann Dermatol Venereol. 2011;138:124-128.
- Jonville-Bera AP, Autret-Leca E. Adverse drug reactions of strontium ranelate (Protelos®) in France. Presse Med. 2011;40:453-462.
- Assessment report for Protelos and Osseor. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000560/WC500131789.pdf). Published May 25, 2012. Accessed May 9, 2016.
- Breathnach S. Drug rash eosinophilia and systemic symptoms (DRESS) syndrome. types of clinical reaction: drug reaction. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 4. 8th ed. Hoboken, NJ: Oxford Wiley-Blackwell Publications; 2010:75.26.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723-732.
- Telles Rudge de Aquino R, Vieitas Vergueiro CS, Ruffolo Magliari ME, et al. Sulfasalazine-induced DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Sao Paulo Med J. 2008;126:225-226.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Pernicova I, Middleton ET, Aye M. Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert [published online September 20, 2008]. Osteoporos Int. 2008;19:1811-1812.
- Kinyó A, Belsö N, Nagy N, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205-206.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Le Merlouette M, Adamski H, Dinulescu M, et al. Strontium ranelate–induced DRESS syndrome. Ann Dermatol Venereol. 2011;138:124-128.
- Jonville-Bera AP, Autret-Leca E. Adverse drug reactions of strontium ranelate (Protelos®) in France. Presse Med. 2011;40:453-462.
- Assessment report for Protelos and Osseor. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000560/WC500131789.pdf). Published May 25, 2012. Accessed May 9, 2016.
- Breathnach S. Drug rash eosinophilia and systemic symptoms (DRESS) syndrome. types of clinical reaction: drug reaction. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 4. 8th ed. Hoboken, NJ: Oxford Wiley-Blackwell Publications; 2010:75.26.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723-732.
- Telles Rudge de Aquino R, Vieitas Vergueiro CS, Ruffolo Magliari ME, et al. Sulfasalazine-induced DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Sao Paulo Med J. 2008;126:225-226.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Pernicova I, Middleton ET, Aye M. Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert [published online September 20, 2008]. Osteoporos Int. 2008;19:1811-1812.
- Kinyó A, Belsö N, Nagy N, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205-206.
Practice Points
- Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs.
- Strontium ranelate should be considered as a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome.
