User login
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
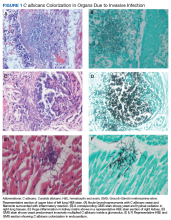
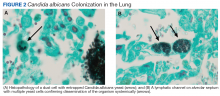
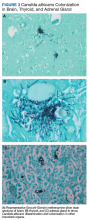
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.