User login
Emergency physicians (EPs) are expected to have a thorough working knowledge of life-threatening pathologic conditions that may typically be seen in an ED only once in a year or even once in a career. Deep space infections of the neck, recognized and described since the time of Galen in the second century, are one such entity.1
These infections are twice as common in males as in females. Although the patient age ranges from 11 months to 91 years, the average age of presentation is in the third decade of life. Although the incidence of deep space neck infections has plummeted with the advent of antibiotics and improvements in dental hygiene, they still occur with some regularity and when they do, are associated with high morbidity and occasionally mortality. Rapid diagnosis and effective treatment depend on a high degree of clinical suspicion and an understanding of the need for and type of timely intervention.
Infections that spread along the fascial planes and spaces of the head and neck can be either superficial or deep space infections. Superficial neck infections do not significantly differ from other superficial skin infections, and are frequently characterized by erythema with central induration and respond well to simple incision and drainage. In contrast, deep neck space infections are difficult to diagnose in their early stages. Occurring in a potential space bounded by the deep cervical fascia, deep space neck infections lack outward, grossly visualized physical findings. Whereas a “typical” abscess may lead to external rupture, the tough neck fascia prevents outward extension and leads to rapid deep spread.2 So what initially may begin as a simple dental infection, minor trauma, or upper respiratory illness, may progress to abscess formation in the deep neck spaces with the potential for significant complications, such as descending mediastinitis, jugular vein thrombosis, or acute airway collapse.3 Most of the current literature on deep space neck infections is based on retrospective studies published in surgery journals; however, these patients often initially present to the ED.
Incidence
Based on literature reports, the source of deep space neck infection in adults is identified in 30% to 90% of cases.4 Infections of the submandibular space are the most common site, and are caused by an odontogenic in up to 85% of cases, with 65% attributable to dental caries.5 In 68% of cases, the lower third molar was the involved tooth.5 Deep space neck infection can also result from laceration of the floor of the mouth, mandibular fracture, tumor, lymphadenitis, sialadenitis, patient injection of intravenous (IV) drugs, systemic infection, hematogenous spread of infection, and foreign body ingestion.4,6 In adults, branchial sinuses, thyroglossal duct cysts, tuberculosis, and malignancies can masquerade as infections or can present with secondary infection.7-9 In children, acute tonsillitis and pharyngitis remain the most commonly identified inciting conditions.9
Larawin et al10 reported the incidence of infection in children and adults in a retrospective study of 103 cases occurring at a teaching hospital. In this study, Ludwig’s angina was seen in 37% of cases, submandibular space infection in 27%, masticator space infection in 13%, parapharyngeal abscess in 11%, parotid space abscess 6%, retropharyngeal abscess in 5%, and the prevertebral in 1%.10 The predominant site of neck abscesses in children was in the retropharyngeal or parapharyngeal spaces, followed by the anterior or posterior triangle.11,12 While most deep neck infections will involve a single anatomic space, two or more spaces were involved in nearly 30% of patients in one retrospective study.6
Presentation
In adults, the most common presenting complaint in deep space neck infection is odynophagia, affecting 83.9% of patients. Other symptoms include dysphagia (71%), fever (67.7%), neck pain (54.8%), swelling (45.2%), trismus (38.7%), and respiratory distress (9.7%).10 Children and geriatric patients (age 65 years or older) tend to have a more subtle presentation. Pediatric patients are seldom able to verbalize their symptoms and their history commonly includes recent upper respiratory infection with fever, neck mass or swelling, and difficulty eating and drinking.11
As increased disease severity is linked to lack of preventive dental and primary care and delayed presentation, patients with severe infections tend to be of low socioeconomic status.7,13,14 Immunosuppression and the use of multiple medications have also been correlated with increased rates of infection.2 Deep space infections in diabetic patients, who have greater susceptibility to infection and are likely to be older at diagnosis, tend to be more severe and result in more serious complications, prolonged hospital stays, and higher mortality.1 Additionally, the bacterial flora may be unusual in diabetic patients, making culture and sensitivity more important for directed antimicrobial therapy.8 Interestingly, in one review, a seasonal distribution of cases was found with the highest incidence in autumn (46.2%) and far fewer cases in winter (15.6%).6
Pathophysiology and Microbiology
Deep space neck infections often begin as cellulitis adjacent to the primary source of infection and may progress to abscess formation. Abscess may also directly arise from perforation of the lymph node capsule. Fascial layers initially confine both cellulitis and abscess. Further spread tends to involve adjacent or communicating compartments.15,16
In the pre-antibiotic era, the most common organism associated with deep space neck infection was Staphylococcus aureus. Now, due to drug resistance and microbial flora change, these infections are most commonly associated with aerobic streptococcal species and nonstreptococcal anaerobes.4Streptococcus viridans is the predominant organism in adult neck infection (43.7%), with Klebsiella pneumoniae slightly more prevalent in diabetic patients (56.1%).8 The standard of care is to presume polymicrobial infection and to provide empiric coverage for both aerobic and anaerobic infection. Any condition that reduces the blood supply to an affected area (eg, trauma, foreign body, malignancy, surgery, edema, shock, vascular disease) creates a hypoxic environment—ideal for anaerobic infection.2
Anatomy
For successful management of patients with deep space neck infections, the provider must have a working knowledge of neck anatomy, common etiologies, typical presentations, and potential complications.3 Several classification systems are currently used to describe specific deep neck spaces. Essentially, there are three major clinically important spaces between the deep cervical fascia: the parapharyngeal, submental and submandibular, and retropharyngeal spaces.
The parapharyngeal (or lateral pharyngeal), located in the upper neck, extends superior to the hyoid bone. The parotid gland and mandible (lateral) are located in this space and are bound by the pretracheal fascia of the visceral compartment and the superficial fascia (which appears as an inverted cone). The submental and submandibular triangles contain the second space, located between the mucosa of the floor of the mouth and superficial layer of the deep fascia. Lastly, the retropharyngeal space running from the base of the skull to the posterior mediastinumis is bordered posteriorly by the prevertebral fascia and anteriorly by the posterior portion of the pretracheal fascia.4,15,16
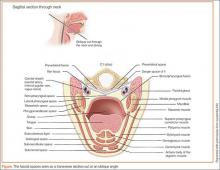
A knowledge of cervical fascia anatomy is critical to understanding the likely source, predicting the extent of the progression of infection as well as aiding in the choice of medical versus surgical treatment.4 From a purely anatomic standpoint, deep space neck infections follow the path of least resistance, penetrating the nearest and thinnest cortical bone and tracking along the fascial planes in the neck and face.8 The tough connective tissue of the deep cervical fascia in the neck, which is divided into superficial, middle, and deep layers, prevents the egress of pus toward the skin. As a result, infections will alternatively descend toward the mediastinum, ascend to the lateral pharynx and masticator spaces, or expand to the point of airway obstruction. (See the Figure for a sagittal illustration of the fascial spaces.)
Ludwig’s Angina
While the aim of this article is for comprehensive knowledge of deep space neck infections, it is worth turning attention to one condition that has high name recognition and is representative of the discussion. Ludwig’s angina was first described in 1836 by German physician Wilhelm Frederick von Ludwig as a rapidly and frequently fatal progressive gangrenous cellulitis and edema of the soft tissues of the neck and floor of the mouth, which he considered a “morbid entity.”17
Although the term “Ludwig’s” is often loosely applied to deep space neck infections, it should be limited to those infections which are bilateral and involve the submandibular space (including both the sublingual and submylohyoid spaces). Prior to the advent of antibiotics, swelling frequently led to respiratory obstruction and death; thus, the term angina was added to the description (angina coming from the word angere, meaning “to strangle.”4,18,19
Diagnosis
Physical Examination
When evaluating a patient with a suspected deep space neck infection, an orderly evaluation should be conducted following the basic principles of a thorough physical examination, including visualization, palpation, and percussion.4 Careful examination alone may obviate the need for imaging prior to consultation and result in decreased time to surgical intervention.
Vital Signs. Patients with deep space neck infections are often quite ill, some with associated shock. As is the case with all presentations to the ED, the physical examination should always begin by documenting the patient’s blood pressure, pulse, temperature, peripheral perfusion by assessment of capillary refill time, skin temperature, degree of moisture/dryness, and oxygen saturation.
General Observations. Note the overall level of patient comfort and position. A seated patient leaning forward in the sniffing position is an ominous sign, and placing such a patient supine may lead to complete airway collapse.
Mouth. Visually assess the uvula, tonsils, and posterior pharynx for symmetry, color, edema, and presence of exudate. Poor oral intake is the norm for these patients. Comment on whether the oral mucosa is moist or dry, and assess for sublingual edema, tongue elevation, and pooling of oral secretions. Closely inspect gums and teeth for gingival disease, dental caries, fractures, and purulent drainage, with extra consideration for lower molars as a frequent source. Palpate to assess fluctuance, induration, or draining sinuses; crepitus may signal involvement of a gas-producing organism. Percussion of teeth may elicit pain from the involved nerve root. Trismus and limited mouth opening should give one pause in attempting orotracheal intubation, as this finding is associated with a difficult airway.
Neck. Examine the skin for swelling, erythema, ecchymosis, pustules, or “pointing” infection. Palpate the anterior and posterior triangles. Hesitation with range of motion may indicate retro-involvement. Auscultate for bruits and note the appearance of unilateral jugular venous distension; jugular vein thrombosis is associated with infections in these spaces. Palpate the trachea to assess whether the position is midline or deviated. Consider additional causes for the patient’s clinical condition such as branchial cyst, thyroid carcinoma, and aneurysm.
Chest. Observe the patient’s respiratory rate, use of accessory muscles, presence of retractions, and overall work of breathing. Auscultate, as descending infections leading to mediastinitis have been associated with pleural effusion, empyema, and pneumonia.
Laboratory Studies
As in the case of most infections, no single laboratory test provides definitive diagnosis of deep space neck infection. Consensus recommendations advise obtaining a complete blood count, basic metabolic panel, blood cultures, and coagulation studies, while considering measures of inflammatory markers.
A study by Bakir et al6 found that 56% of patients with diagnosed deep space neck infection had white blood cell counts higher than 10,000 cells/mm3. Although the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are typically elevated, specificity of both is lacking. In a review by Boscolo-Rizzo, et al20 of patients with deep space neck infections, 100% had a “pathologic” elevation in the ESR, with an average value of 48.3 mm/h. In this study, CRP was also consistently elevated.20
In patients with diabetes, glycemic control is important in the treatment of infection. Impaired neutrophil bactericidal function is associated with poor blood glucose control and neutrophil bactericidal function will likely improve as blood glucose control improves.1 Although blood cultures are typically obtained in patients with critical illness, the value of cultures has been questioned. Some reports indicate that culture and sensitivity data do not lead to change in antibiotic selection or treatment. Therefore, the data clearly are not comprehensive enough for a formal recommendation.8,9
Imaging
The literature supports radiologic evaluation to identify the location, extension, and characteristics of deep space neck infections.6 Although computed tomography (CT) is by far the modality of choice and the one most widely used for diagnosing these infections, ultrasonography has gained acceptance as the sole imaging procedure. While CT is less expensive than magnetic resonance imaging (MRI) and is more readily available, quickly acquired, and useful to localize abscesses in the head and neck, as well as other structural abnormalities,4 it is not as effective as ultrasound in differentiating abscesses from cellulitis.
A combination of clinical evaluation and CT findings led to 89% accuracy in identifying drainable collections of pus, with high sensitivity (95%) and specificity (80%).4,16 Yet even with contrast enhancement, the false-positive rate of CT in evaluating surgical drainage remains above 10%. In patients with deep space abscess, enhanced-contrast CT results were useful for determining which patients might avoid surgical drainage. Nearly half of patients who met defined criteria responded well to medical therapy alone and had rapid improvement.21
Ultrasound can be performed at the bedside, which may improve time to diagnosis, initiation of medical management, and admission.22 Furthermore, ultrasound can be used to direct an aspirating needle, and involves no radiation. This modality is particularly useful in pediatric patients and in peritonsillar-upper parapharyngeal infections to avoid incision and drainage. While ultrasound is more accurate than CT in differentiating drainable abscesses, it can rarely discern deeper neck spaces.16
Soft tissue definition is better visualized with MRI than CT and avoids radiation exposure to the patient. However, MRI requires patient participation and often has a longer acquisition time. In a patient with a potential airway obstruction, the use of MRI in the acute setting is limited. Once the patient is stabilized and the airway is secured, MRI may be advantageous for those with a high suspicion of neurovascular involvement.4,16
Complications
Even in the modern antibiotic era, life-threatening complications, namely, airway compromise, jugular vein thrombosis, mediastinal involvement, pneumonia, septic shock, and intracranial extensions may develop due to delays in diagnosis and treatment.7,10 Rare reports of cavernous sinus thrombosis have even been described.23 Abscesses left untreated can rupture spontaneously into the pharynx, leading to aspiration.15 In one study of 20 patients with descending necrotizing mediastinitis (DNM) as a severe complication of odontogenic infections, 30.4% of patients died as a result of septic shock and multiorgan failure.24 Descending necrotizing mediastinitis is associated with chest pain, dyspnea, fever, and significant toxemia. When DNM is suspected, CT imaging should be continued inferiorly into the chest, and a thoracic surgeon should be consulted.
Predictors of complications include patients older than age 65 years (OR, 6.12; 95% CI, 1.63-22.89), diabetes mellitus (OR, 9.0; 95% CI, 2.08-38.95), other comorbidities (OR, 5.44; 95% CI, 1.72-17.17), multiple space involvement (OR, 10.80; 95% CI, 2.59-44.97), and anterior visceral space involvement (larynx, thyroid, trachea, cervical esophagus) (OR, 26.80; 95% CI, 5.95-120.76).20 The size, shape, and dimensions of the anterior visceral space plays a key role in determining airway obstruction as well as the spread of infection to the anterior mediastinum.20
Treatment and Management
Deep neck infections are treated with airway control, IV antibiotics, and, if necessary, surgical drainage.20 Although surgical drainage has been the mainstay of treatment for decades, newer studies suggest that patients who have abscesses less than 3.0 cm, are in stable clinical condition, and lack “danger” space involvement, may do well with antimicrobial therapy alone. Patients tolerating this approach tend to have shorter hospital stays with decreases in overall cost of treatment. However, prompt consultation for diagnosed or presumed deep space neck infection is key. In one study, more than 90% of the patients required surgical intervention. Without rapid medical/surgical intervention, patients tend to progress to critical status.3 Thus, the general consensus is for admission to a tertiary care center under the supervision of the head and neck surgical team.
Antibiotic Therapy
Due to the predictable makeup of the majority of polymicrobial neck infections, most patients can be treated empirically with antibiotic regimens that include clindamycin and β lactams alone or in combination with metronidazole. In odontogenic infections, nearly 20% of isolated species were penicillin resistant5; only 4% were resistant to clindamycin.8 Empiric antibiotic coverage must consider aerobic and anaerobic pathogens that synthesize β lactamase.3 Second- or third-generation cephalosporins such as cefoxitin or ceftriaxone, also are effective. Alternatively, a penicillin and β-lactamase inhibitor combination such as ampicillin-sulbactam provides appropriate coverage of predicted flora. De-escalation of antibiotic regimen is typical following return of culture and sensitivity testing.
Other Medical Therapy
Additional medical management should include initiation of IV fluid resuscitation. Until patients have demonstrated stability with positive clinical trajectory, the possibility of impending airway compromise should be considered and patients should be placed on a “nothing by mouth” status.
The debate is ongoing over use of corticosteroids, with controversy stemming from the anti-inflammatory and immunosuppressant effects of this intervention. Consensus recommendations are for dexamethasone 10 mg as an initial dose with 4 mg every 6 hours for 48 hours to aid in decreasing edema and chemical decompression with a goal of airway protection and improved antibiotic tissue penetration.24
Airway Management
Airway compromise can progress rapidly and is the most immediate, life-threatening complications encountered in the management of deep space neck infections. Direct compression of the airway may result from either displacement of the tongue posteriorly or secondary to laryngeal edema. Patients should be presumed to have a difficult airway and present challenges even when in a relatively controlled operating-room environment. In several studies, up to 75% of patients with Ludwig’s angina required tracheostomy. In a study reviewing 20 attempts at oral intubation, 11 were unsuccessful with failed airways requiring emergent tracheostomy. In one of the few prospective studies, airway management was accomplished by endotracheal intubation in over 90% of patients aided by a near equal number of fiberoptic and laryngoscopic approaches.5
There are multiple options for airway management, including close clinical observation, endotracheal intubation (fiberoptic or direct), and surgery. There is a lack of data in the literature to help determine the best method.25 A decision to observe the airway, perform intubation, or obtain tracheostomy must be made on an individual basis, considering the advantages and disadvantages of each.4,20
Observation. If initial evaluation reveals no impending airway compromise, close observation of the airway in an ICU setting is appropriate. The main complications of observation without mechanical intervention are unrecognized impending airway loss, risk of over sedation and loss of airway, or extension of infection and edema leading to asphyxiation. The benefit is that there is no mechanical intervention, which carries inherent risks.
Direct Endotracheal Intubation. The advantages of endotracheal intubation include the speed at which airway control can be achieved, and that it is a nonsurgical procedure. Disadvantages include the potential for failed intubation, inability to bypass upper airway obstruction, requirement for mechanical ventilation, and subglottic stenosis.
Tracheostomy. This surgical intervention allows for the bypass of upper airway obstruction. It is a very secure airway, there is less need for sedation and mechanical ventilation, and it allows for earlier transfer out of the critical care unit.25 Tracheostomy carries inherent risks such as pneumothorax, bleeding, subglottic stenosis, fistula formation, and unsightly scarring. Training and comfort with airway management procedures, as well as available hospital resources such as anesthesiology, fiberoptic equipment, and critical care resources, also have an impact.
Conventional endotracheal intubation is often difficult in patients with deep space neck infections, and direct laryngoscopy may precipitate acute airway collapse. Blind nasal intubation carries a considerable risk of damage to the swollen and fragile pharyngeal mucosa, with bleeding, abscess perforation, and complete upper airway obstruction as possible outcomes. Tracheostomy while the patient is awake remains the gold standard in airway management for deep space neck infections, but achieving adequate anesthesia can be problematic. Patients are frequently ill, panic stricken, and hypoxic.2,26
Fiberoptic Intubation. In the awake patient, fiberoptic intubation overcomes some of the problems associated with tracheostomy. The procedure requires the patient to sit in front of the operator. The head-up position prevents the tongue and pharynx from collapsing backwards and obstructing the view. However, this technique is not without risk. A good understanding of the equipment, proficiency in its use, and knowledge on how to best preserve airway patency is mandatory. Anecdotal surveys suggest that many anesthetists and emergency care providers do not feel confident using fiberoptic scopes due to lack of training and infrequent chances for exposure.2 This is a key skill that the ED must learn and practice to maintain.
Conclusion
Despite the significant decrease in the incidence of deep space neck infections, these infections still do occur as a spectrum of pathology, with many patients initially presenting to the ED. Since these infections often lack outward visual findings on physical examination, bedside ultrasound and consultation with an otolaryngologist should be obtained as soon as an abscess is suspected. Timely diagnosis and intervention are essential to prevent life-threatening complications such as airway compromise, jugular vein thrombosis, mediastinal involvement, pneumonia, septic shock, and intracranial extensions. In all cases of deep space neck infection, the EP should anticipate an early critical course.
Dr Leigh is a clinical associate in the Mayo Clinic department of emergency medicine and Mayo Clinic Health System Hospitals, Rochester, Minnesota. Dr Bellew is a second-year emergency medicine resident at the Mayo Clinic, Rochester, Minnesota. Dr Wangsgard is a simulation fellow at the Mayo Clinic department of emergency medicine, Rochester, Minnesota. Dr Cabrera is an assistant professor in the department of emergency medicine at the Mayo Clinic, Rochester, Minnesota.
- Chen MK, Wen YS, Chang CC, Lee HS, Huang MT, Hsiao HC. Deep neck infections in diabetic patients. Am J Otolaryngol. 2000;21(3):169-173.
- Karkos PD, Leong SC, Beer H, Apostolidou MT, Panarese A. Challenging airways in deep neck space infections. Am J Otolaryngol. 2007;28(6):415-418.
- Daramola OO, Flanagan CE, Maisel RH, Odland RM. Diagnosis and treatment of deep neck space abscesses. Otolaryngol Head Neck Surg. 2009;141(1):123-130.
- Osborn TM, Assael LA, Bell RB. Deep space neck infection: principles of surgical management. Oral Maxillofac Surg Clin North Am. 2008;20(3):353-365.
- Flynn TR, Shanti RM, Levi MH, Adamo AK, Kraut RA, Trieger N. Severe odontogenic infections, part 1: prospective report. J Oral Maxillofac Surg. 2006;64(7):1093-1103.
- Bakir S, Tanriverdi MH, Gün R, et al. Deep neck space infections: a retrospective review of 173 cases. Am J Otolaryngol. 2012;33(1):56-63.
- Lee JK, Kim HD, Lim SC. Predisposing factors of complicated deep neck infection: an analysis of 158 cases. Yonsei Med J. 200728;48(1):55-62.
- Caccamese JF Jr, Coletti DP. Deep neck infections: clinical considerations in aggressive disease. Oral Maxillofac Surg Clin North Am. 2008;20(3):367-380.
- Ridder GJ, Technau-Ihling K, Sander A, Boedeker CC. Spectrum and management of deep neck space infections: an 8-year experience of 234 cases. Otolaryngol Head Neck Surg. 2005;133(5):709-714.
- Larawin V, Naipao J, Dubey SP. Head and neck space infections. Otolaryngol Head Neck Surg. 2006;135(6):889-893.
- Songu M, Demiray U, Adibelli ZH, Adibelli H.Bilateral deep neck space infection in the paediatric age group: a case report and review of the literature. Acta Otorhinolaryngol Ital. 2010;31(3):190-193.
- Coticchia JM, Getnick GS, Yun RD, Arnold JE. Age-, site-, and time-specific differences in pediatric deep neck abscesses. Arch Otolaryngol Head Neck Surg. 2004;130(2):201-207.
- Agarwal AK, Ashwani Sethi, Deepika Sethi, Sumit Mrig, Shamit Chopra. Role of socioeconomic factors in deep neck abscess: A prospective study of 120 patients. Br J Oral Maxillofac Surg. 2007;45(7):553-555.
- Meher R, Jain A, Sabharwal A, Gupta B, Singh I, Agarwal AK. Deep neck abscess: a prospective study of 54 cases. J Laryngol Otol. 2005;119(4):299-302.
- Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62(12):1545-1550.
- Maroldi R, Farina D, Ravanelli M, Lombardi D, Nicolai P. Emergency imaging assessment of deep neck space infections. Semin Ultrasound CT MR. 2012;33(5):432-442.
- Thomas TT. Ludwig’s augina. An anatomical, clinical, and statistical study. Ann Surg. 1908;47(2): 161-183.
- Moreland LW, Corey J, McKenzie R. Ludwig’s Angina: Report of a Case and Review of the Literature. Arch Intern Med. 1988;148(2)461-466.
- Wang LF, Kuo WR, Tsai SM, Huang KJ. Characterizations of life-threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol. 2003;24(2)111-117.
- Boscolo-Rizzo P, Da Mosto MC. Submandibular space infection: a potentially lethal infection. Int J Infect Dis. 2009;13(3):327-333.
- Boscolo-Rizzo P, Marchiori C, Zanetti F, Vaglia A, Da Mosto MC. Conservative management of deep neck abscesses in adults: the importance of CECT findings. Otolaryngol Head Neck Surg. 2006;135(6):894-899.
- Gaspari RJ. Bedside ultrasound of the soft tissue of the face: a case of early Ludwig’s angina. J Emerg Med. 2006;31(3):287-291.
- Feldman DP, Picerno NA, Porubsky ES. Cavernous sinus thrombosis complicating odontogenic parapharyngeal space neck abscess: a case report and discussion. Otolaryngol Head Neck Surg. 2000;123(6):744-745.
- Roccia F, Pecorari GC, Oliaro A, et al. Ten years of descending necrotizing mediastinitis: management of 23 cases. J Oral Maxillofac Surg. 2007;65(9):1716-1724.
- Potter JK, Herford AS, Ellis E 3rd. Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002;60(4):349-354.
- Neff SP, Merry AF, Anderson B. Airway management in Ludwig’s angina. Anesth Intensive Care. 1999;27(6):659-661.
Emergency physicians (EPs) are expected to have a thorough working knowledge of life-threatening pathologic conditions that may typically be seen in an ED only once in a year or even once in a career. Deep space infections of the neck, recognized and described since the time of Galen in the second century, are one such entity.1
These infections are twice as common in males as in females. Although the patient age ranges from 11 months to 91 years, the average age of presentation is in the third decade of life. Although the incidence of deep space neck infections has plummeted with the advent of antibiotics and improvements in dental hygiene, they still occur with some regularity and when they do, are associated with high morbidity and occasionally mortality. Rapid diagnosis and effective treatment depend on a high degree of clinical suspicion and an understanding of the need for and type of timely intervention.
Infections that spread along the fascial planes and spaces of the head and neck can be either superficial or deep space infections. Superficial neck infections do not significantly differ from other superficial skin infections, and are frequently characterized by erythema with central induration and respond well to simple incision and drainage. In contrast, deep neck space infections are difficult to diagnose in their early stages. Occurring in a potential space bounded by the deep cervical fascia, deep space neck infections lack outward, grossly visualized physical findings. Whereas a “typical” abscess may lead to external rupture, the tough neck fascia prevents outward extension and leads to rapid deep spread.2 So what initially may begin as a simple dental infection, minor trauma, or upper respiratory illness, may progress to abscess formation in the deep neck spaces with the potential for significant complications, such as descending mediastinitis, jugular vein thrombosis, or acute airway collapse.3 Most of the current literature on deep space neck infections is based on retrospective studies published in surgery journals; however, these patients often initially present to the ED.
Incidence
Based on literature reports, the source of deep space neck infection in adults is identified in 30% to 90% of cases.4 Infections of the submandibular space are the most common site, and are caused by an odontogenic in up to 85% of cases, with 65% attributable to dental caries.5 In 68% of cases, the lower third molar was the involved tooth.5 Deep space neck infection can also result from laceration of the floor of the mouth, mandibular fracture, tumor, lymphadenitis, sialadenitis, patient injection of intravenous (IV) drugs, systemic infection, hematogenous spread of infection, and foreign body ingestion.4,6 In adults, branchial sinuses, thyroglossal duct cysts, tuberculosis, and malignancies can masquerade as infections or can present with secondary infection.7-9 In children, acute tonsillitis and pharyngitis remain the most commonly identified inciting conditions.9
Larawin et al10 reported the incidence of infection in children and adults in a retrospective study of 103 cases occurring at a teaching hospital. In this study, Ludwig’s angina was seen in 37% of cases, submandibular space infection in 27%, masticator space infection in 13%, parapharyngeal abscess in 11%, parotid space abscess 6%, retropharyngeal abscess in 5%, and the prevertebral in 1%.10 The predominant site of neck abscesses in children was in the retropharyngeal or parapharyngeal spaces, followed by the anterior or posterior triangle.11,12 While most deep neck infections will involve a single anatomic space, two or more spaces were involved in nearly 30% of patients in one retrospective study.6
Presentation
In adults, the most common presenting complaint in deep space neck infection is odynophagia, affecting 83.9% of patients. Other symptoms include dysphagia (71%), fever (67.7%), neck pain (54.8%), swelling (45.2%), trismus (38.7%), and respiratory distress (9.7%).10 Children and geriatric patients (age 65 years or older) tend to have a more subtle presentation. Pediatric patients are seldom able to verbalize their symptoms and their history commonly includes recent upper respiratory infection with fever, neck mass or swelling, and difficulty eating and drinking.11
As increased disease severity is linked to lack of preventive dental and primary care and delayed presentation, patients with severe infections tend to be of low socioeconomic status.7,13,14 Immunosuppression and the use of multiple medications have also been correlated with increased rates of infection.2 Deep space infections in diabetic patients, who have greater susceptibility to infection and are likely to be older at diagnosis, tend to be more severe and result in more serious complications, prolonged hospital stays, and higher mortality.1 Additionally, the bacterial flora may be unusual in diabetic patients, making culture and sensitivity more important for directed antimicrobial therapy.8 Interestingly, in one review, a seasonal distribution of cases was found with the highest incidence in autumn (46.2%) and far fewer cases in winter (15.6%).6
Pathophysiology and Microbiology
Deep space neck infections often begin as cellulitis adjacent to the primary source of infection and may progress to abscess formation. Abscess may also directly arise from perforation of the lymph node capsule. Fascial layers initially confine both cellulitis and abscess. Further spread tends to involve adjacent or communicating compartments.15,16
In the pre-antibiotic era, the most common organism associated with deep space neck infection was Staphylococcus aureus. Now, due to drug resistance and microbial flora change, these infections are most commonly associated with aerobic streptococcal species and nonstreptococcal anaerobes.4Streptococcus viridans is the predominant organism in adult neck infection (43.7%), with Klebsiella pneumoniae slightly more prevalent in diabetic patients (56.1%).8 The standard of care is to presume polymicrobial infection and to provide empiric coverage for both aerobic and anaerobic infection. Any condition that reduces the blood supply to an affected area (eg, trauma, foreign body, malignancy, surgery, edema, shock, vascular disease) creates a hypoxic environment—ideal for anaerobic infection.2
Anatomy
For successful management of patients with deep space neck infections, the provider must have a working knowledge of neck anatomy, common etiologies, typical presentations, and potential complications.3 Several classification systems are currently used to describe specific deep neck spaces. Essentially, there are three major clinically important spaces between the deep cervical fascia: the parapharyngeal, submental and submandibular, and retropharyngeal spaces.
The parapharyngeal (or lateral pharyngeal), located in the upper neck, extends superior to the hyoid bone. The parotid gland and mandible (lateral) are located in this space and are bound by the pretracheal fascia of the visceral compartment and the superficial fascia (which appears as an inverted cone). The submental and submandibular triangles contain the second space, located between the mucosa of the floor of the mouth and superficial layer of the deep fascia. Lastly, the retropharyngeal space running from the base of the skull to the posterior mediastinumis is bordered posteriorly by the prevertebral fascia and anteriorly by the posterior portion of the pretracheal fascia.4,15,16
A knowledge of cervical fascia anatomy is critical to understanding the likely source, predicting the extent of the progression of infection as well as aiding in the choice of medical versus surgical treatment.4 From a purely anatomic standpoint, deep space neck infections follow the path of least resistance, penetrating the nearest and thinnest cortical bone and tracking along the fascial planes in the neck and face.8 The tough connective tissue of the deep cervical fascia in the neck, which is divided into superficial, middle, and deep layers, prevents the egress of pus toward the skin. As a result, infections will alternatively descend toward the mediastinum, ascend to the lateral pharynx and masticator spaces, or expand to the point of airway obstruction. (See the Figure for a sagittal illustration of the fascial spaces.)
Ludwig’s Angina
While the aim of this article is for comprehensive knowledge of deep space neck infections, it is worth turning attention to one condition that has high name recognition and is representative of the discussion. Ludwig’s angina was first described in 1836 by German physician Wilhelm Frederick von Ludwig as a rapidly and frequently fatal progressive gangrenous cellulitis and edema of the soft tissues of the neck and floor of the mouth, which he considered a “morbid entity.”17
Although the term “Ludwig’s” is often loosely applied to deep space neck infections, it should be limited to those infections which are bilateral and involve the submandibular space (including both the sublingual and submylohyoid spaces). Prior to the advent of antibiotics, swelling frequently led to respiratory obstruction and death; thus, the term angina was added to the description (angina coming from the word angere, meaning “to strangle.”4,18,19
Diagnosis
Physical Examination
When evaluating a patient with a suspected deep space neck infection, an orderly evaluation should be conducted following the basic principles of a thorough physical examination, including visualization, palpation, and percussion.4 Careful examination alone may obviate the need for imaging prior to consultation and result in decreased time to surgical intervention.
Vital Signs. Patients with deep space neck infections are often quite ill, some with associated shock. As is the case with all presentations to the ED, the physical examination should always begin by documenting the patient’s blood pressure, pulse, temperature, peripheral perfusion by assessment of capillary refill time, skin temperature, degree of moisture/dryness, and oxygen saturation.
General Observations. Note the overall level of patient comfort and position. A seated patient leaning forward in the sniffing position is an ominous sign, and placing such a patient supine may lead to complete airway collapse.
Mouth. Visually assess the uvula, tonsils, and posterior pharynx for symmetry, color, edema, and presence of exudate. Poor oral intake is the norm for these patients. Comment on whether the oral mucosa is moist or dry, and assess for sublingual edema, tongue elevation, and pooling of oral secretions. Closely inspect gums and teeth for gingival disease, dental caries, fractures, and purulent drainage, with extra consideration for lower molars as a frequent source. Palpate to assess fluctuance, induration, or draining sinuses; crepitus may signal involvement of a gas-producing organism. Percussion of teeth may elicit pain from the involved nerve root. Trismus and limited mouth opening should give one pause in attempting orotracheal intubation, as this finding is associated with a difficult airway.
Neck. Examine the skin for swelling, erythema, ecchymosis, pustules, or “pointing” infection. Palpate the anterior and posterior triangles. Hesitation with range of motion may indicate retro-involvement. Auscultate for bruits and note the appearance of unilateral jugular venous distension; jugular vein thrombosis is associated with infections in these spaces. Palpate the trachea to assess whether the position is midline or deviated. Consider additional causes for the patient’s clinical condition such as branchial cyst, thyroid carcinoma, and aneurysm.
Chest. Observe the patient’s respiratory rate, use of accessory muscles, presence of retractions, and overall work of breathing. Auscultate, as descending infections leading to mediastinitis have been associated with pleural effusion, empyema, and pneumonia.
Laboratory Studies
As in the case of most infections, no single laboratory test provides definitive diagnosis of deep space neck infection. Consensus recommendations advise obtaining a complete blood count, basic metabolic panel, blood cultures, and coagulation studies, while considering measures of inflammatory markers.
A study by Bakir et al6 found that 56% of patients with diagnosed deep space neck infection had white blood cell counts higher than 10,000 cells/mm3. Although the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are typically elevated, specificity of both is lacking. In a review by Boscolo-Rizzo, et al20 of patients with deep space neck infections, 100% had a “pathologic” elevation in the ESR, with an average value of 48.3 mm/h. In this study, CRP was also consistently elevated.20
In patients with diabetes, glycemic control is important in the treatment of infection. Impaired neutrophil bactericidal function is associated with poor blood glucose control and neutrophil bactericidal function will likely improve as blood glucose control improves.1 Although blood cultures are typically obtained in patients with critical illness, the value of cultures has been questioned. Some reports indicate that culture and sensitivity data do not lead to change in antibiotic selection or treatment. Therefore, the data clearly are not comprehensive enough for a formal recommendation.8,9
Imaging
The literature supports radiologic evaluation to identify the location, extension, and characteristics of deep space neck infections.6 Although computed tomography (CT) is by far the modality of choice and the one most widely used for diagnosing these infections, ultrasonography has gained acceptance as the sole imaging procedure. While CT is less expensive than magnetic resonance imaging (MRI) and is more readily available, quickly acquired, and useful to localize abscesses in the head and neck, as well as other structural abnormalities,4 it is not as effective as ultrasound in differentiating abscesses from cellulitis.
A combination of clinical evaluation and CT findings led to 89% accuracy in identifying drainable collections of pus, with high sensitivity (95%) and specificity (80%).4,16 Yet even with contrast enhancement, the false-positive rate of CT in evaluating surgical drainage remains above 10%. In patients with deep space abscess, enhanced-contrast CT results were useful for determining which patients might avoid surgical drainage. Nearly half of patients who met defined criteria responded well to medical therapy alone and had rapid improvement.21
Ultrasound can be performed at the bedside, which may improve time to diagnosis, initiation of medical management, and admission.22 Furthermore, ultrasound can be used to direct an aspirating needle, and involves no radiation. This modality is particularly useful in pediatric patients and in peritonsillar-upper parapharyngeal infections to avoid incision and drainage. While ultrasound is more accurate than CT in differentiating drainable abscesses, it can rarely discern deeper neck spaces.16
Soft tissue definition is better visualized with MRI than CT and avoids radiation exposure to the patient. However, MRI requires patient participation and often has a longer acquisition time. In a patient with a potential airway obstruction, the use of MRI in the acute setting is limited. Once the patient is stabilized and the airway is secured, MRI may be advantageous for those with a high suspicion of neurovascular involvement.4,16
Complications
Even in the modern antibiotic era, life-threatening complications, namely, airway compromise, jugular vein thrombosis, mediastinal involvement, pneumonia, septic shock, and intracranial extensions may develop due to delays in diagnosis and treatment.7,10 Rare reports of cavernous sinus thrombosis have even been described.23 Abscesses left untreated can rupture spontaneously into the pharynx, leading to aspiration.15 In one study of 20 patients with descending necrotizing mediastinitis (DNM) as a severe complication of odontogenic infections, 30.4% of patients died as a result of septic shock and multiorgan failure.24 Descending necrotizing mediastinitis is associated with chest pain, dyspnea, fever, and significant toxemia. When DNM is suspected, CT imaging should be continued inferiorly into the chest, and a thoracic surgeon should be consulted.
Predictors of complications include patients older than age 65 years (OR, 6.12; 95% CI, 1.63-22.89), diabetes mellitus (OR, 9.0; 95% CI, 2.08-38.95), other comorbidities (OR, 5.44; 95% CI, 1.72-17.17), multiple space involvement (OR, 10.80; 95% CI, 2.59-44.97), and anterior visceral space involvement (larynx, thyroid, trachea, cervical esophagus) (OR, 26.80; 95% CI, 5.95-120.76).20 The size, shape, and dimensions of the anterior visceral space plays a key role in determining airway obstruction as well as the spread of infection to the anterior mediastinum.20
Treatment and Management
Deep neck infections are treated with airway control, IV antibiotics, and, if necessary, surgical drainage.20 Although surgical drainage has been the mainstay of treatment for decades, newer studies suggest that patients who have abscesses less than 3.0 cm, are in stable clinical condition, and lack “danger” space involvement, may do well with antimicrobial therapy alone. Patients tolerating this approach tend to have shorter hospital stays with decreases in overall cost of treatment. However, prompt consultation for diagnosed or presumed deep space neck infection is key. In one study, more than 90% of the patients required surgical intervention. Without rapid medical/surgical intervention, patients tend to progress to critical status.3 Thus, the general consensus is for admission to a tertiary care center under the supervision of the head and neck surgical team.
Antibiotic Therapy
Due to the predictable makeup of the majority of polymicrobial neck infections, most patients can be treated empirically with antibiotic regimens that include clindamycin and β lactams alone or in combination with metronidazole. In odontogenic infections, nearly 20% of isolated species were penicillin resistant5; only 4% were resistant to clindamycin.8 Empiric antibiotic coverage must consider aerobic and anaerobic pathogens that synthesize β lactamase.3 Second- or third-generation cephalosporins such as cefoxitin or ceftriaxone, also are effective. Alternatively, a penicillin and β-lactamase inhibitor combination such as ampicillin-sulbactam provides appropriate coverage of predicted flora. De-escalation of antibiotic regimen is typical following return of culture and sensitivity testing.
Other Medical Therapy
Additional medical management should include initiation of IV fluid resuscitation. Until patients have demonstrated stability with positive clinical trajectory, the possibility of impending airway compromise should be considered and patients should be placed on a “nothing by mouth” status.
The debate is ongoing over use of corticosteroids, with controversy stemming from the anti-inflammatory and immunosuppressant effects of this intervention. Consensus recommendations are for dexamethasone 10 mg as an initial dose with 4 mg every 6 hours for 48 hours to aid in decreasing edema and chemical decompression with a goal of airway protection and improved antibiotic tissue penetration.24
Airway Management
Airway compromise can progress rapidly and is the most immediate, life-threatening complications encountered in the management of deep space neck infections. Direct compression of the airway may result from either displacement of the tongue posteriorly or secondary to laryngeal edema. Patients should be presumed to have a difficult airway and present challenges even when in a relatively controlled operating-room environment. In several studies, up to 75% of patients with Ludwig’s angina required tracheostomy. In a study reviewing 20 attempts at oral intubation, 11 were unsuccessful with failed airways requiring emergent tracheostomy. In one of the few prospective studies, airway management was accomplished by endotracheal intubation in over 90% of patients aided by a near equal number of fiberoptic and laryngoscopic approaches.5
There are multiple options for airway management, including close clinical observation, endotracheal intubation (fiberoptic or direct), and surgery. There is a lack of data in the literature to help determine the best method.25 A decision to observe the airway, perform intubation, or obtain tracheostomy must be made on an individual basis, considering the advantages and disadvantages of each.4,20
Observation. If initial evaluation reveals no impending airway compromise, close observation of the airway in an ICU setting is appropriate. The main complications of observation without mechanical intervention are unrecognized impending airway loss, risk of over sedation and loss of airway, or extension of infection and edema leading to asphyxiation. The benefit is that there is no mechanical intervention, which carries inherent risks.
Direct Endotracheal Intubation. The advantages of endotracheal intubation include the speed at which airway control can be achieved, and that it is a nonsurgical procedure. Disadvantages include the potential for failed intubation, inability to bypass upper airway obstruction, requirement for mechanical ventilation, and subglottic stenosis.
Tracheostomy. This surgical intervention allows for the bypass of upper airway obstruction. It is a very secure airway, there is less need for sedation and mechanical ventilation, and it allows for earlier transfer out of the critical care unit.25 Tracheostomy carries inherent risks such as pneumothorax, bleeding, subglottic stenosis, fistula formation, and unsightly scarring. Training and comfort with airway management procedures, as well as available hospital resources such as anesthesiology, fiberoptic equipment, and critical care resources, also have an impact.
Conventional endotracheal intubation is often difficult in patients with deep space neck infections, and direct laryngoscopy may precipitate acute airway collapse. Blind nasal intubation carries a considerable risk of damage to the swollen and fragile pharyngeal mucosa, with bleeding, abscess perforation, and complete upper airway obstruction as possible outcomes. Tracheostomy while the patient is awake remains the gold standard in airway management for deep space neck infections, but achieving adequate anesthesia can be problematic. Patients are frequently ill, panic stricken, and hypoxic.2,26
Fiberoptic Intubation. In the awake patient, fiberoptic intubation overcomes some of the problems associated with tracheostomy. The procedure requires the patient to sit in front of the operator. The head-up position prevents the tongue and pharynx from collapsing backwards and obstructing the view. However, this technique is not without risk. A good understanding of the equipment, proficiency in its use, and knowledge on how to best preserve airway patency is mandatory. Anecdotal surveys suggest that many anesthetists and emergency care providers do not feel confident using fiberoptic scopes due to lack of training and infrequent chances for exposure.2 This is a key skill that the ED must learn and practice to maintain.
Conclusion
Despite the significant decrease in the incidence of deep space neck infections, these infections still do occur as a spectrum of pathology, with many patients initially presenting to the ED. Since these infections often lack outward visual findings on physical examination, bedside ultrasound and consultation with an otolaryngologist should be obtained as soon as an abscess is suspected. Timely diagnosis and intervention are essential to prevent life-threatening complications such as airway compromise, jugular vein thrombosis, mediastinal involvement, pneumonia, septic shock, and intracranial extensions. In all cases of deep space neck infection, the EP should anticipate an early critical course.
Dr Leigh is a clinical associate in the Mayo Clinic department of emergency medicine and Mayo Clinic Health System Hospitals, Rochester, Minnesota. Dr Bellew is a second-year emergency medicine resident at the Mayo Clinic, Rochester, Minnesota. Dr Wangsgard is a simulation fellow at the Mayo Clinic department of emergency medicine, Rochester, Minnesota. Dr Cabrera is an assistant professor in the department of emergency medicine at the Mayo Clinic, Rochester, Minnesota.
Emergency physicians (EPs) are expected to have a thorough working knowledge of life-threatening pathologic conditions that may typically be seen in an ED only once in a year or even once in a career. Deep space infections of the neck, recognized and described since the time of Galen in the second century, are one such entity.1
These infections are twice as common in males as in females. Although the patient age ranges from 11 months to 91 years, the average age of presentation is in the third decade of life. Although the incidence of deep space neck infections has plummeted with the advent of antibiotics and improvements in dental hygiene, they still occur with some regularity and when they do, are associated with high morbidity and occasionally mortality. Rapid diagnosis and effective treatment depend on a high degree of clinical suspicion and an understanding of the need for and type of timely intervention.
Infections that spread along the fascial planes and spaces of the head and neck can be either superficial or deep space infections. Superficial neck infections do not significantly differ from other superficial skin infections, and are frequently characterized by erythema with central induration and respond well to simple incision and drainage. In contrast, deep neck space infections are difficult to diagnose in their early stages. Occurring in a potential space bounded by the deep cervical fascia, deep space neck infections lack outward, grossly visualized physical findings. Whereas a “typical” abscess may lead to external rupture, the tough neck fascia prevents outward extension and leads to rapid deep spread.2 So what initially may begin as a simple dental infection, minor trauma, or upper respiratory illness, may progress to abscess formation in the deep neck spaces with the potential for significant complications, such as descending mediastinitis, jugular vein thrombosis, or acute airway collapse.3 Most of the current literature on deep space neck infections is based on retrospective studies published in surgery journals; however, these patients often initially present to the ED.
Incidence
Based on literature reports, the source of deep space neck infection in adults is identified in 30% to 90% of cases.4 Infections of the submandibular space are the most common site, and are caused by an odontogenic in up to 85% of cases, with 65% attributable to dental caries.5 In 68% of cases, the lower third molar was the involved tooth.5 Deep space neck infection can also result from laceration of the floor of the mouth, mandibular fracture, tumor, lymphadenitis, sialadenitis, patient injection of intravenous (IV) drugs, systemic infection, hematogenous spread of infection, and foreign body ingestion.4,6 In adults, branchial sinuses, thyroglossal duct cysts, tuberculosis, and malignancies can masquerade as infections or can present with secondary infection.7-9 In children, acute tonsillitis and pharyngitis remain the most commonly identified inciting conditions.9
Larawin et al10 reported the incidence of infection in children and adults in a retrospective study of 103 cases occurring at a teaching hospital. In this study, Ludwig’s angina was seen in 37% of cases, submandibular space infection in 27%, masticator space infection in 13%, parapharyngeal abscess in 11%, parotid space abscess 6%, retropharyngeal abscess in 5%, and the prevertebral in 1%.10 The predominant site of neck abscesses in children was in the retropharyngeal or parapharyngeal spaces, followed by the anterior or posterior triangle.11,12 While most deep neck infections will involve a single anatomic space, two or more spaces were involved in nearly 30% of patients in one retrospective study.6
Presentation
In adults, the most common presenting complaint in deep space neck infection is odynophagia, affecting 83.9% of patients. Other symptoms include dysphagia (71%), fever (67.7%), neck pain (54.8%), swelling (45.2%), trismus (38.7%), and respiratory distress (9.7%).10 Children and geriatric patients (age 65 years or older) tend to have a more subtle presentation. Pediatric patients are seldom able to verbalize their symptoms and their history commonly includes recent upper respiratory infection with fever, neck mass or swelling, and difficulty eating and drinking.11
As increased disease severity is linked to lack of preventive dental and primary care and delayed presentation, patients with severe infections tend to be of low socioeconomic status.7,13,14 Immunosuppression and the use of multiple medications have also been correlated with increased rates of infection.2 Deep space infections in diabetic patients, who have greater susceptibility to infection and are likely to be older at diagnosis, tend to be more severe and result in more serious complications, prolonged hospital stays, and higher mortality.1 Additionally, the bacterial flora may be unusual in diabetic patients, making culture and sensitivity more important for directed antimicrobial therapy.8 Interestingly, in one review, a seasonal distribution of cases was found with the highest incidence in autumn (46.2%) and far fewer cases in winter (15.6%).6
Pathophysiology and Microbiology
Deep space neck infections often begin as cellulitis adjacent to the primary source of infection and may progress to abscess formation. Abscess may also directly arise from perforation of the lymph node capsule. Fascial layers initially confine both cellulitis and abscess. Further spread tends to involve adjacent or communicating compartments.15,16
In the pre-antibiotic era, the most common organism associated with deep space neck infection was Staphylococcus aureus. Now, due to drug resistance and microbial flora change, these infections are most commonly associated with aerobic streptococcal species and nonstreptococcal anaerobes.4Streptococcus viridans is the predominant organism in adult neck infection (43.7%), with Klebsiella pneumoniae slightly more prevalent in diabetic patients (56.1%).8 The standard of care is to presume polymicrobial infection and to provide empiric coverage for both aerobic and anaerobic infection. Any condition that reduces the blood supply to an affected area (eg, trauma, foreign body, malignancy, surgery, edema, shock, vascular disease) creates a hypoxic environment—ideal for anaerobic infection.2
Anatomy
For successful management of patients with deep space neck infections, the provider must have a working knowledge of neck anatomy, common etiologies, typical presentations, and potential complications.3 Several classification systems are currently used to describe specific deep neck spaces. Essentially, there are three major clinically important spaces between the deep cervical fascia: the parapharyngeal, submental and submandibular, and retropharyngeal spaces.
The parapharyngeal (or lateral pharyngeal), located in the upper neck, extends superior to the hyoid bone. The parotid gland and mandible (lateral) are located in this space and are bound by the pretracheal fascia of the visceral compartment and the superficial fascia (which appears as an inverted cone). The submental and submandibular triangles contain the second space, located between the mucosa of the floor of the mouth and superficial layer of the deep fascia. Lastly, the retropharyngeal space running from the base of the skull to the posterior mediastinumis is bordered posteriorly by the prevertebral fascia and anteriorly by the posterior portion of the pretracheal fascia.4,15,16
A knowledge of cervical fascia anatomy is critical to understanding the likely source, predicting the extent of the progression of infection as well as aiding in the choice of medical versus surgical treatment.4 From a purely anatomic standpoint, deep space neck infections follow the path of least resistance, penetrating the nearest and thinnest cortical bone and tracking along the fascial planes in the neck and face.8 The tough connective tissue of the deep cervical fascia in the neck, which is divided into superficial, middle, and deep layers, prevents the egress of pus toward the skin. As a result, infections will alternatively descend toward the mediastinum, ascend to the lateral pharynx and masticator spaces, or expand to the point of airway obstruction. (See the Figure for a sagittal illustration of the fascial spaces.)
Ludwig’s Angina
While the aim of this article is for comprehensive knowledge of deep space neck infections, it is worth turning attention to one condition that has high name recognition and is representative of the discussion. Ludwig’s angina was first described in 1836 by German physician Wilhelm Frederick von Ludwig as a rapidly and frequently fatal progressive gangrenous cellulitis and edema of the soft tissues of the neck and floor of the mouth, which he considered a “morbid entity.”17
Although the term “Ludwig’s” is often loosely applied to deep space neck infections, it should be limited to those infections which are bilateral and involve the submandibular space (including both the sublingual and submylohyoid spaces). Prior to the advent of antibiotics, swelling frequently led to respiratory obstruction and death; thus, the term angina was added to the description (angina coming from the word angere, meaning “to strangle.”4,18,19
Diagnosis
Physical Examination
When evaluating a patient with a suspected deep space neck infection, an orderly evaluation should be conducted following the basic principles of a thorough physical examination, including visualization, palpation, and percussion.4 Careful examination alone may obviate the need for imaging prior to consultation and result in decreased time to surgical intervention.
Vital Signs. Patients with deep space neck infections are often quite ill, some with associated shock. As is the case with all presentations to the ED, the physical examination should always begin by documenting the patient’s blood pressure, pulse, temperature, peripheral perfusion by assessment of capillary refill time, skin temperature, degree of moisture/dryness, and oxygen saturation.
General Observations. Note the overall level of patient comfort and position. A seated patient leaning forward in the sniffing position is an ominous sign, and placing such a patient supine may lead to complete airway collapse.
Mouth. Visually assess the uvula, tonsils, and posterior pharynx for symmetry, color, edema, and presence of exudate. Poor oral intake is the norm for these patients. Comment on whether the oral mucosa is moist or dry, and assess for sublingual edema, tongue elevation, and pooling of oral secretions. Closely inspect gums and teeth for gingival disease, dental caries, fractures, and purulent drainage, with extra consideration for lower molars as a frequent source. Palpate to assess fluctuance, induration, or draining sinuses; crepitus may signal involvement of a gas-producing organism. Percussion of teeth may elicit pain from the involved nerve root. Trismus and limited mouth opening should give one pause in attempting orotracheal intubation, as this finding is associated with a difficult airway.
Neck. Examine the skin for swelling, erythema, ecchymosis, pustules, or “pointing” infection. Palpate the anterior and posterior triangles. Hesitation with range of motion may indicate retro-involvement. Auscultate for bruits and note the appearance of unilateral jugular venous distension; jugular vein thrombosis is associated with infections in these spaces. Palpate the trachea to assess whether the position is midline or deviated. Consider additional causes for the patient’s clinical condition such as branchial cyst, thyroid carcinoma, and aneurysm.
Chest. Observe the patient’s respiratory rate, use of accessory muscles, presence of retractions, and overall work of breathing. Auscultate, as descending infections leading to mediastinitis have been associated with pleural effusion, empyema, and pneumonia.
Laboratory Studies
As in the case of most infections, no single laboratory test provides definitive diagnosis of deep space neck infection. Consensus recommendations advise obtaining a complete blood count, basic metabolic panel, blood cultures, and coagulation studies, while considering measures of inflammatory markers.
A study by Bakir et al6 found that 56% of patients with diagnosed deep space neck infection had white blood cell counts higher than 10,000 cells/mm3. Although the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are typically elevated, specificity of both is lacking. In a review by Boscolo-Rizzo, et al20 of patients with deep space neck infections, 100% had a “pathologic” elevation in the ESR, with an average value of 48.3 mm/h. In this study, CRP was also consistently elevated.20
In patients with diabetes, glycemic control is important in the treatment of infection. Impaired neutrophil bactericidal function is associated with poor blood glucose control and neutrophil bactericidal function will likely improve as blood glucose control improves.1 Although blood cultures are typically obtained in patients with critical illness, the value of cultures has been questioned. Some reports indicate that culture and sensitivity data do not lead to change in antibiotic selection or treatment. Therefore, the data clearly are not comprehensive enough for a formal recommendation.8,9
Imaging
The literature supports radiologic evaluation to identify the location, extension, and characteristics of deep space neck infections.6 Although computed tomography (CT) is by far the modality of choice and the one most widely used for diagnosing these infections, ultrasonography has gained acceptance as the sole imaging procedure. While CT is less expensive than magnetic resonance imaging (MRI) and is more readily available, quickly acquired, and useful to localize abscesses in the head and neck, as well as other structural abnormalities,4 it is not as effective as ultrasound in differentiating abscesses from cellulitis.
A combination of clinical evaluation and CT findings led to 89% accuracy in identifying drainable collections of pus, with high sensitivity (95%) and specificity (80%).4,16 Yet even with contrast enhancement, the false-positive rate of CT in evaluating surgical drainage remains above 10%. In patients with deep space abscess, enhanced-contrast CT results were useful for determining which patients might avoid surgical drainage. Nearly half of patients who met defined criteria responded well to medical therapy alone and had rapid improvement.21
Ultrasound can be performed at the bedside, which may improve time to diagnosis, initiation of medical management, and admission.22 Furthermore, ultrasound can be used to direct an aspirating needle, and involves no radiation. This modality is particularly useful in pediatric patients and in peritonsillar-upper parapharyngeal infections to avoid incision and drainage. While ultrasound is more accurate than CT in differentiating drainable abscesses, it can rarely discern deeper neck spaces.16
Soft tissue definition is better visualized with MRI than CT and avoids radiation exposure to the patient. However, MRI requires patient participation and often has a longer acquisition time. In a patient with a potential airway obstruction, the use of MRI in the acute setting is limited. Once the patient is stabilized and the airway is secured, MRI may be advantageous for those with a high suspicion of neurovascular involvement.4,16
Complications
Even in the modern antibiotic era, life-threatening complications, namely, airway compromise, jugular vein thrombosis, mediastinal involvement, pneumonia, septic shock, and intracranial extensions may develop due to delays in diagnosis and treatment.7,10 Rare reports of cavernous sinus thrombosis have even been described.23 Abscesses left untreated can rupture spontaneously into the pharynx, leading to aspiration.15 In one study of 20 patients with descending necrotizing mediastinitis (DNM) as a severe complication of odontogenic infections, 30.4% of patients died as a result of septic shock and multiorgan failure.24 Descending necrotizing mediastinitis is associated with chest pain, dyspnea, fever, and significant toxemia. When DNM is suspected, CT imaging should be continued inferiorly into the chest, and a thoracic surgeon should be consulted.
Predictors of complications include patients older than age 65 years (OR, 6.12; 95% CI, 1.63-22.89), diabetes mellitus (OR, 9.0; 95% CI, 2.08-38.95), other comorbidities (OR, 5.44; 95% CI, 1.72-17.17), multiple space involvement (OR, 10.80; 95% CI, 2.59-44.97), and anterior visceral space involvement (larynx, thyroid, trachea, cervical esophagus) (OR, 26.80; 95% CI, 5.95-120.76).20 The size, shape, and dimensions of the anterior visceral space plays a key role in determining airway obstruction as well as the spread of infection to the anterior mediastinum.20
Treatment and Management
Deep neck infections are treated with airway control, IV antibiotics, and, if necessary, surgical drainage.20 Although surgical drainage has been the mainstay of treatment for decades, newer studies suggest that patients who have abscesses less than 3.0 cm, are in stable clinical condition, and lack “danger” space involvement, may do well with antimicrobial therapy alone. Patients tolerating this approach tend to have shorter hospital stays with decreases in overall cost of treatment. However, prompt consultation for diagnosed or presumed deep space neck infection is key. In one study, more than 90% of the patients required surgical intervention. Without rapid medical/surgical intervention, patients tend to progress to critical status.3 Thus, the general consensus is for admission to a tertiary care center under the supervision of the head and neck surgical team.
Antibiotic Therapy
Due to the predictable makeup of the majority of polymicrobial neck infections, most patients can be treated empirically with antibiotic regimens that include clindamycin and β lactams alone or in combination with metronidazole. In odontogenic infections, nearly 20% of isolated species were penicillin resistant5; only 4% were resistant to clindamycin.8 Empiric antibiotic coverage must consider aerobic and anaerobic pathogens that synthesize β lactamase.3 Second- or third-generation cephalosporins such as cefoxitin or ceftriaxone, also are effective. Alternatively, a penicillin and β-lactamase inhibitor combination such as ampicillin-sulbactam provides appropriate coverage of predicted flora. De-escalation of antibiotic regimen is typical following return of culture and sensitivity testing.
Other Medical Therapy
Additional medical management should include initiation of IV fluid resuscitation. Until patients have demonstrated stability with positive clinical trajectory, the possibility of impending airway compromise should be considered and patients should be placed on a “nothing by mouth” status.
The debate is ongoing over use of corticosteroids, with controversy stemming from the anti-inflammatory and immunosuppressant effects of this intervention. Consensus recommendations are for dexamethasone 10 mg as an initial dose with 4 mg every 6 hours for 48 hours to aid in decreasing edema and chemical decompression with a goal of airway protection and improved antibiotic tissue penetration.24
Airway Management
Airway compromise can progress rapidly and is the most immediate, life-threatening complications encountered in the management of deep space neck infections. Direct compression of the airway may result from either displacement of the tongue posteriorly or secondary to laryngeal edema. Patients should be presumed to have a difficult airway and present challenges even when in a relatively controlled operating-room environment. In several studies, up to 75% of patients with Ludwig’s angina required tracheostomy. In a study reviewing 20 attempts at oral intubation, 11 were unsuccessful with failed airways requiring emergent tracheostomy. In one of the few prospective studies, airway management was accomplished by endotracheal intubation in over 90% of patients aided by a near equal number of fiberoptic and laryngoscopic approaches.5
There are multiple options for airway management, including close clinical observation, endotracheal intubation (fiberoptic or direct), and surgery. There is a lack of data in the literature to help determine the best method.25 A decision to observe the airway, perform intubation, or obtain tracheostomy must be made on an individual basis, considering the advantages and disadvantages of each.4,20
Observation. If initial evaluation reveals no impending airway compromise, close observation of the airway in an ICU setting is appropriate. The main complications of observation without mechanical intervention are unrecognized impending airway loss, risk of over sedation and loss of airway, or extension of infection and edema leading to asphyxiation. The benefit is that there is no mechanical intervention, which carries inherent risks.
Direct Endotracheal Intubation. The advantages of endotracheal intubation include the speed at which airway control can be achieved, and that it is a nonsurgical procedure. Disadvantages include the potential for failed intubation, inability to bypass upper airway obstruction, requirement for mechanical ventilation, and subglottic stenosis.
Tracheostomy. This surgical intervention allows for the bypass of upper airway obstruction. It is a very secure airway, there is less need for sedation and mechanical ventilation, and it allows for earlier transfer out of the critical care unit.25 Tracheostomy carries inherent risks such as pneumothorax, bleeding, subglottic stenosis, fistula formation, and unsightly scarring. Training and comfort with airway management procedures, as well as available hospital resources such as anesthesiology, fiberoptic equipment, and critical care resources, also have an impact.
Conventional endotracheal intubation is often difficult in patients with deep space neck infections, and direct laryngoscopy may precipitate acute airway collapse. Blind nasal intubation carries a considerable risk of damage to the swollen and fragile pharyngeal mucosa, with bleeding, abscess perforation, and complete upper airway obstruction as possible outcomes. Tracheostomy while the patient is awake remains the gold standard in airway management for deep space neck infections, but achieving adequate anesthesia can be problematic. Patients are frequently ill, panic stricken, and hypoxic.2,26
Fiberoptic Intubation. In the awake patient, fiberoptic intubation overcomes some of the problems associated with tracheostomy. The procedure requires the patient to sit in front of the operator. The head-up position prevents the tongue and pharynx from collapsing backwards and obstructing the view. However, this technique is not without risk. A good understanding of the equipment, proficiency in its use, and knowledge on how to best preserve airway patency is mandatory. Anecdotal surveys suggest that many anesthetists and emergency care providers do not feel confident using fiberoptic scopes due to lack of training and infrequent chances for exposure.2 This is a key skill that the ED must learn and practice to maintain.
Conclusion
Despite the significant decrease in the incidence of deep space neck infections, these infections still do occur as a spectrum of pathology, with many patients initially presenting to the ED. Since these infections often lack outward visual findings on physical examination, bedside ultrasound and consultation with an otolaryngologist should be obtained as soon as an abscess is suspected. Timely diagnosis and intervention are essential to prevent life-threatening complications such as airway compromise, jugular vein thrombosis, mediastinal involvement, pneumonia, septic shock, and intracranial extensions. In all cases of deep space neck infection, the EP should anticipate an early critical course.
Dr Leigh is a clinical associate in the Mayo Clinic department of emergency medicine and Mayo Clinic Health System Hospitals, Rochester, Minnesota. Dr Bellew is a second-year emergency medicine resident at the Mayo Clinic, Rochester, Minnesota. Dr Wangsgard is a simulation fellow at the Mayo Clinic department of emergency medicine, Rochester, Minnesota. Dr Cabrera is an assistant professor in the department of emergency medicine at the Mayo Clinic, Rochester, Minnesota.
- Chen MK, Wen YS, Chang CC, Lee HS, Huang MT, Hsiao HC. Deep neck infections in diabetic patients. Am J Otolaryngol. 2000;21(3):169-173.
- Karkos PD, Leong SC, Beer H, Apostolidou MT, Panarese A. Challenging airways in deep neck space infections. Am J Otolaryngol. 2007;28(6):415-418.
- Daramola OO, Flanagan CE, Maisel RH, Odland RM. Diagnosis and treatment of deep neck space abscesses. Otolaryngol Head Neck Surg. 2009;141(1):123-130.
- Osborn TM, Assael LA, Bell RB. Deep space neck infection: principles of surgical management. Oral Maxillofac Surg Clin North Am. 2008;20(3):353-365.
- Flynn TR, Shanti RM, Levi MH, Adamo AK, Kraut RA, Trieger N. Severe odontogenic infections, part 1: prospective report. J Oral Maxillofac Surg. 2006;64(7):1093-1103.
- Bakir S, Tanriverdi MH, Gün R, et al. Deep neck space infections: a retrospective review of 173 cases. Am J Otolaryngol. 2012;33(1):56-63.
- Lee JK, Kim HD, Lim SC. Predisposing factors of complicated deep neck infection: an analysis of 158 cases. Yonsei Med J. 200728;48(1):55-62.
- Caccamese JF Jr, Coletti DP. Deep neck infections: clinical considerations in aggressive disease. Oral Maxillofac Surg Clin North Am. 2008;20(3):367-380.
- Ridder GJ, Technau-Ihling K, Sander A, Boedeker CC. Spectrum and management of deep neck space infections: an 8-year experience of 234 cases. Otolaryngol Head Neck Surg. 2005;133(5):709-714.
- Larawin V, Naipao J, Dubey SP. Head and neck space infections. Otolaryngol Head Neck Surg. 2006;135(6):889-893.
- Songu M, Demiray U, Adibelli ZH, Adibelli H.Bilateral deep neck space infection in the paediatric age group: a case report and review of the literature. Acta Otorhinolaryngol Ital. 2010;31(3):190-193.
- Coticchia JM, Getnick GS, Yun RD, Arnold JE. Age-, site-, and time-specific differences in pediatric deep neck abscesses. Arch Otolaryngol Head Neck Surg. 2004;130(2):201-207.
- Agarwal AK, Ashwani Sethi, Deepika Sethi, Sumit Mrig, Shamit Chopra. Role of socioeconomic factors in deep neck abscess: A prospective study of 120 patients. Br J Oral Maxillofac Surg. 2007;45(7):553-555.
- Meher R, Jain A, Sabharwal A, Gupta B, Singh I, Agarwal AK. Deep neck abscess: a prospective study of 54 cases. J Laryngol Otol. 2005;119(4):299-302.
- Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62(12):1545-1550.
- Maroldi R, Farina D, Ravanelli M, Lombardi D, Nicolai P. Emergency imaging assessment of deep neck space infections. Semin Ultrasound CT MR. 2012;33(5):432-442.
- Thomas TT. Ludwig’s augina. An anatomical, clinical, and statistical study. Ann Surg. 1908;47(2): 161-183.
- Moreland LW, Corey J, McKenzie R. Ludwig’s Angina: Report of a Case and Review of the Literature. Arch Intern Med. 1988;148(2)461-466.
- Wang LF, Kuo WR, Tsai SM, Huang KJ. Characterizations of life-threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol. 2003;24(2)111-117.
- Boscolo-Rizzo P, Da Mosto MC. Submandibular space infection: a potentially lethal infection. Int J Infect Dis. 2009;13(3):327-333.
- Boscolo-Rizzo P, Marchiori C, Zanetti F, Vaglia A, Da Mosto MC. Conservative management of deep neck abscesses in adults: the importance of CECT findings. Otolaryngol Head Neck Surg. 2006;135(6):894-899.
- Gaspari RJ. Bedside ultrasound of the soft tissue of the face: a case of early Ludwig’s angina. J Emerg Med. 2006;31(3):287-291.
- Feldman DP, Picerno NA, Porubsky ES. Cavernous sinus thrombosis complicating odontogenic parapharyngeal space neck abscess: a case report and discussion. Otolaryngol Head Neck Surg. 2000;123(6):744-745.
- Roccia F, Pecorari GC, Oliaro A, et al. Ten years of descending necrotizing mediastinitis: management of 23 cases. J Oral Maxillofac Surg. 2007;65(9):1716-1724.
- Potter JK, Herford AS, Ellis E 3rd. Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002;60(4):349-354.
- Neff SP, Merry AF, Anderson B. Airway management in Ludwig’s angina. Anesth Intensive Care. 1999;27(6):659-661.
- Chen MK, Wen YS, Chang CC, Lee HS, Huang MT, Hsiao HC. Deep neck infections in diabetic patients. Am J Otolaryngol. 2000;21(3):169-173.
- Karkos PD, Leong SC, Beer H, Apostolidou MT, Panarese A. Challenging airways in deep neck space infections. Am J Otolaryngol. 2007;28(6):415-418.
- Daramola OO, Flanagan CE, Maisel RH, Odland RM. Diagnosis and treatment of deep neck space abscesses. Otolaryngol Head Neck Surg. 2009;141(1):123-130.
- Osborn TM, Assael LA, Bell RB. Deep space neck infection: principles of surgical management. Oral Maxillofac Surg Clin North Am. 2008;20(3):353-365.
- Flynn TR, Shanti RM, Levi MH, Adamo AK, Kraut RA, Trieger N. Severe odontogenic infections, part 1: prospective report. J Oral Maxillofac Surg. 2006;64(7):1093-1103.
- Bakir S, Tanriverdi MH, Gün R, et al. Deep neck space infections: a retrospective review of 173 cases. Am J Otolaryngol. 2012;33(1):56-63.
- Lee JK, Kim HD, Lim SC. Predisposing factors of complicated deep neck infection: an analysis of 158 cases. Yonsei Med J. 200728;48(1):55-62.
- Caccamese JF Jr, Coletti DP. Deep neck infections: clinical considerations in aggressive disease. Oral Maxillofac Surg Clin North Am. 2008;20(3):367-380.
- Ridder GJ, Technau-Ihling K, Sander A, Boedeker CC. Spectrum and management of deep neck space infections: an 8-year experience of 234 cases. Otolaryngol Head Neck Surg. 2005;133(5):709-714.
- Larawin V, Naipao J, Dubey SP. Head and neck space infections. Otolaryngol Head Neck Surg. 2006;135(6):889-893.
- Songu M, Demiray U, Adibelli ZH, Adibelli H.Bilateral deep neck space infection in the paediatric age group: a case report and review of the literature. Acta Otorhinolaryngol Ital. 2010;31(3):190-193.
- Coticchia JM, Getnick GS, Yun RD, Arnold JE. Age-, site-, and time-specific differences in pediatric deep neck abscesses. Arch Otolaryngol Head Neck Surg. 2004;130(2):201-207.
- Agarwal AK, Ashwani Sethi, Deepika Sethi, Sumit Mrig, Shamit Chopra. Role of socioeconomic factors in deep neck abscess: A prospective study of 120 patients. Br J Oral Maxillofac Surg. 2007;45(7):553-555.
- Meher R, Jain A, Sabharwal A, Gupta B, Singh I, Agarwal AK. Deep neck abscess: a prospective study of 54 cases. J Laryngol Otol. 2005;119(4):299-302.
- Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62(12):1545-1550.
- Maroldi R, Farina D, Ravanelli M, Lombardi D, Nicolai P. Emergency imaging assessment of deep neck space infections. Semin Ultrasound CT MR. 2012;33(5):432-442.
- Thomas TT. Ludwig’s augina. An anatomical, clinical, and statistical study. Ann Surg. 1908;47(2): 161-183.
- Moreland LW, Corey J, McKenzie R. Ludwig’s Angina: Report of a Case and Review of the Literature. Arch Intern Med. 1988;148(2)461-466.
- Wang LF, Kuo WR, Tsai SM, Huang KJ. Characterizations of life-threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol. 2003;24(2)111-117.
- Boscolo-Rizzo P, Da Mosto MC. Submandibular space infection: a potentially lethal infection. Int J Infect Dis. 2009;13(3):327-333.
- Boscolo-Rizzo P, Marchiori C, Zanetti F, Vaglia A, Da Mosto MC. Conservative management of deep neck abscesses in adults: the importance of CECT findings. Otolaryngol Head Neck Surg. 2006;135(6):894-899.
- Gaspari RJ. Bedside ultrasound of the soft tissue of the face: a case of early Ludwig’s angina. J Emerg Med. 2006;31(3):287-291.
- Feldman DP, Picerno NA, Porubsky ES. Cavernous sinus thrombosis complicating odontogenic parapharyngeal space neck abscess: a case report and discussion. Otolaryngol Head Neck Surg. 2000;123(6):744-745.
- Roccia F, Pecorari GC, Oliaro A, et al. Ten years of descending necrotizing mediastinitis: management of 23 cases. J Oral Maxillofac Surg. 2007;65(9):1716-1724.
- Potter JK, Herford AS, Ellis E 3rd. Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002;60(4):349-354.
- Neff SP, Merry AF, Anderson B. Airway management in Ludwig’s angina. Anesth Intensive Care. 1999;27(6):659-661.