User login
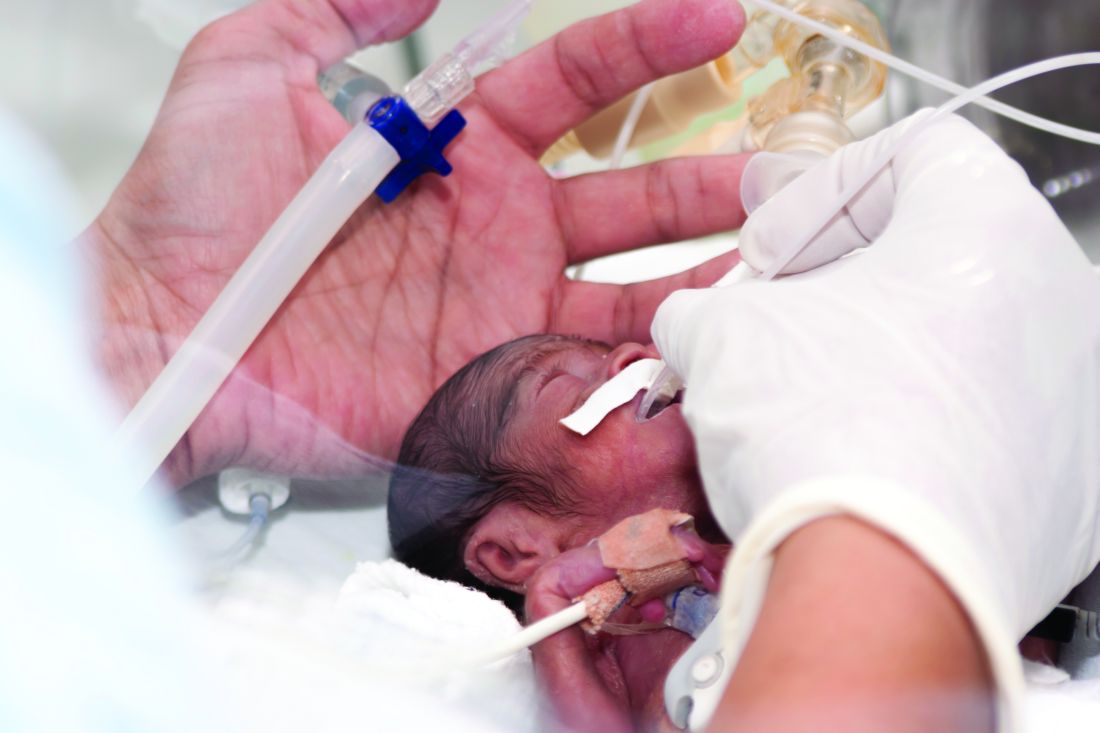
Antenatal corticosteroids may significantly decrease neonatal mortality and morbidity even when they are given just hours before preterm delivery, according to a report published online May 15 in JAMA Pediatrics.
In women at risk of preterm delivery, antenatal corticosteroids given between 1 and 7 days before birth reduce infant mortality by an estimated 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%. But until now their effect when given less than 24 hours before preterm birth has been described as “suboptimal,” “partial,” or “incomplete,” said Mikael Norman, MD, PhD, of Karolinska Institutet in Stockholm, Sweden, and his associates.
“Our findings challenge current thinking about the optimal timing” of antenatal corticosteroids and encourage “a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury,” the investigators said.
To further examine this issue, they assessed the effects of antenatal corticosteroids when given at different intervals before preterm birth, using data from a prospective cohort study of perinatal intensive care. That study involved 10,329 very preterm births throughout 11 countries in Europe during a 1-year period.
For their analysis, Dr. Norman and his associates focused on 4,594 singleton births at 24-31 weeks’ gestation. They classified the timing of antenatal corticosteroids into four categories: no injections (662 infants, or 14.4% of the study population), first injection at less than 24 hours before birth (1,111 infants, or 24.2%), first injection at the recommended 1-7 days before birth (1,871 infants, or 40.7%), and first injection more than 7 days before birth (950 infants, or 20.7%).
Receiving antenatal corticosteroids at any interval before preterm birth was associated with lower infant mortality, a lower rate of severe neonatal morbidity, and a lower rate of severe neonatal brain injury, compared with not receiving any antenatal corticosteroids. The largest reduction in risk (more than 50%) occurred at the recommended interval of 1-7 days before birth. However, receiving the treatment less than 24 hours before birth also significantly reduced these risks (JAMA Pediatr. 2017 May 15. doi: 10.1001/jamapediatrics.2017.0602).
Using their findings on treatment intervals and effectiveness, the investigators created a simulation model for the 661 infants in this cohort who did not receive any antenatal corticosteroids. Their model predicted that if these infants had received treatment at least 3 hours before delivery, overall mortality would have decreased by 26%. If they had received treatment 3-5 hours before delivery, mortality would have decreased by 37%, and if they had received treatment at 6-12 hours before delivery it would have decreased by 51%.
At the other end of the timing spectrum, infant mortality increased 40% when corticosteroids were given more than 7 days before delivery, compared with when they were given within the recommended 1-7 days. This represents a substantial number of infants – approximately 20% of the study cohort.
The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Antenatal corticosteroids may significantly decrease neonatal mortality and morbidity even when they are given just hours before preterm delivery, according to a report published online May 15 in JAMA Pediatrics.
In women at risk of preterm delivery, antenatal corticosteroids given between 1 and 7 days before birth reduce infant mortality by an estimated 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%. But until now their effect when given less than 24 hours before preterm birth has been described as “suboptimal,” “partial,” or “incomplete,” said Mikael Norman, MD, PhD, of Karolinska Institutet in Stockholm, Sweden, and his associates.
“Our findings challenge current thinking about the optimal timing” of antenatal corticosteroids and encourage “a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury,” the investigators said.
To further examine this issue, they assessed the effects of antenatal corticosteroids when given at different intervals before preterm birth, using data from a prospective cohort study of perinatal intensive care. That study involved 10,329 very preterm births throughout 11 countries in Europe during a 1-year period.
For their analysis, Dr. Norman and his associates focused on 4,594 singleton births at 24-31 weeks’ gestation. They classified the timing of antenatal corticosteroids into four categories: no injections (662 infants, or 14.4% of the study population), first injection at less than 24 hours before birth (1,111 infants, or 24.2%), first injection at the recommended 1-7 days before birth (1,871 infants, or 40.7%), and first injection more than 7 days before birth (950 infants, or 20.7%).
Receiving antenatal corticosteroids at any interval before preterm birth was associated with lower infant mortality, a lower rate of severe neonatal morbidity, and a lower rate of severe neonatal brain injury, compared with not receiving any antenatal corticosteroids. The largest reduction in risk (more than 50%) occurred at the recommended interval of 1-7 days before birth. However, receiving the treatment less than 24 hours before birth also significantly reduced these risks (JAMA Pediatr. 2017 May 15. doi: 10.1001/jamapediatrics.2017.0602).
Using their findings on treatment intervals and effectiveness, the investigators created a simulation model for the 661 infants in this cohort who did not receive any antenatal corticosteroids. Their model predicted that if these infants had received treatment at least 3 hours before delivery, overall mortality would have decreased by 26%. If they had received treatment 3-5 hours before delivery, mortality would have decreased by 37%, and if they had received treatment at 6-12 hours before delivery it would have decreased by 51%.
At the other end of the timing spectrum, infant mortality increased 40% when corticosteroids were given more than 7 days before delivery, compared with when they were given within the recommended 1-7 days. This represents a substantial number of infants – approximately 20% of the study cohort.
The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Antenatal corticosteroids may significantly decrease neonatal mortality and morbidity even when they are given just hours before preterm delivery, according to a report published online May 15 in JAMA Pediatrics.
In women at risk of preterm delivery, antenatal corticosteroids given between 1 and 7 days before birth reduce infant mortality by an estimated 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%. But until now their effect when given less than 24 hours before preterm birth has been described as “suboptimal,” “partial,” or “incomplete,” said Mikael Norman, MD, PhD, of Karolinska Institutet in Stockholm, Sweden, and his associates.
“Our findings challenge current thinking about the optimal timing” of antenatal corticosteroids and encourage “a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury,” the investigators said.
To further examine this issue, they assessed the effects of antenatal corticosteroids when given at different intervals before preterm birth, using data from a prospective cohort study of perinatal intensive care. That study involved 10,329 very preterm births throughout 11 countries in Europe during a 1-year period.
For their analysis, Dr. Norman and his associates focused on 4,594 singleton births at 24-31 weeks’ gestation. They classified the timing of antenatal corticosteroids into four categories: no injections (662 infants, or 14.4% of the study population), first injection at less than 24 hours before birth (1,111 infants, or 24.2%), first injection at the recommended 1-7 days before birth (1,871 infants, or 40.7%), and first injection more than 7 days before birth (950 infants, or 20.7%).
Receiving antenatal corticosteroids at any interval before preterm birth was associated with lower infant mortality, a lower rate of severe neonatal morbidity, and a lower rate of severe neonatal brain injury, compared with not receiving any antenatal corticosteroids. The largest reduction in risk (more than 50%) occurred at the recommended interval of 1-7 days before birth. However, receiving the treatment less than 24 hours before birth also significantly reduced these risks (JAMA Pediatr. 2017 May 15. doi: 10.1001/jamapediatrics.2017.0602).
Using their findings on treatment intervals and effectiveness, the investigators created a simulation model for the 661 infants in this cohort who did not receive any antenatal corticosteroids. Their model predicted that if these infants had received treatment at least 3 hours before delivery, overall mortality would have decreased by 26%. If they had received treatment 3-5 hours before delivery, mortality would have decreased by 37%, and if they had received treatment at 6-12 hours before delivery it would have decreased by 51%.
At the other end of the timing spectrum, infant mortality increased 40% when corticosteroids were given more than 7 days before delivery, compared with when they were given within the recommended 1-7 days. This represents a substantial number of infants – approximately 20% of the study cohort.
The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Key clinical point:
Major finding: A simulation model predicted that if untreated infants had received antenatal corticosteroids 6-12 hours before delivery, overall mortality would have decreased by 51%.
Data source: A secondary analysis of data from a population-based cohort study of perinatal intensive care across Europe, involving 4,594 preterm singleton births.
Disclosures: The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.