User login
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
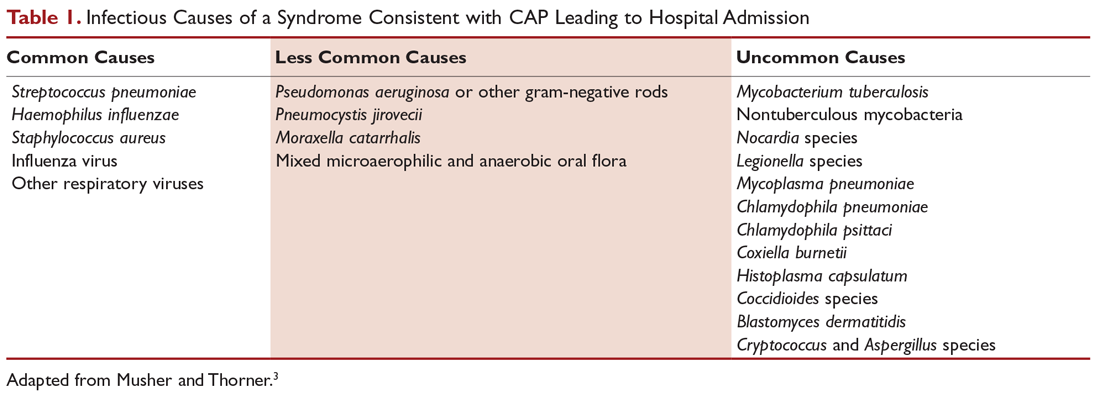
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
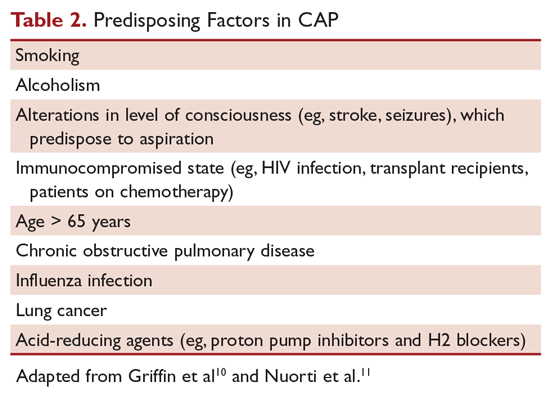
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11

Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.

Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11

Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.

Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.