User login
Approximately 15% to 20% of inpatients develop significant adverse events, which are often preceded by a change in the patient's condition.[1, 2] Many hospitals utilize medical emergency teams (METs) to deliver prompt care for deteriorating patients. METs have an emerging role in end‐of‐life care; up to 35% of patients who die in the hospital with a do not resuscitate (DNR) order have a MET activation during the admission.[3] Thus, METs are well positioned to discuss preferences for cardiopulmonary resuscitation in a population at high risk for cardiac arrest.[4]
MET activations involve a change in code status in approximately 3% to 10% of cases.[5, 6, 7, 8] However, previous studies primarily involved hospitals in Australia, making the results difficult to generalize to other countries.[3, 6, 7] There may be important cultural differences in patient and clinician attitudes toward end‐of‐life care among different countries.[9] In addition, little is known about METs and hospital resources utilized in patients with MET‐implemented changes in code status. Anecdotal evidence suggests that MET activations addressing code status are more time consuming.[3] Conversely, by identifying patients unlikely to benefit from restorative care, METs may facilitate end‐of‐life comfort care, thereby reducing unplanned intensive care unit (ICU) admissions and hospital length of stay. Finally, preliminary evidence suggests that METs may improve the quality of end‐of‐life care.[10] However, the use of end‐of‐life resources, including inpatient palliative care consultation and hospice care following MET activation has not been well studied.
The purpose of our study was to examine the role of a US MET in end‐of‐life care. First, we assessed the proportion of MET calls that resulted in a new DNR order in a US hospital and compared this to previous reports in other countries. We also examined MET and hospital resource utilization in MET activations involving changes in code status by evaluating the duration of MET activations, the need for telemetry or ICU transfer, and hospital length of stay following MET activation. Finally, we explored the quality of end‐of‐life care in patients with MET‐implemented DNR orders by assessing the utilization of inpatient palliative care consultation and hospice care compared to patients with a preexisting DNR order.
MATERIALS AND METHODS
We conducted this study at Lahey Clinic, a 350‐bed academic, tertiary care center. The institutional review board approved this study and waived the need for informed consent. We performed a retrospective review of a prospectively collected MET registry. We included consecutive, adult (>18 years old) inpatient MET activations and excluded nonhospitalized patients. Data were recorded in an intranet registry at the time of the event by the MET nurse, including preexisting code status (full code or DNR), any change to the patient's code status (full code to DNR or DNR to full code) during the MET event, date of the event, disposition after the event (no transfer, transferred to ICU, or transfer to telemetry), and a description of MET interventions. The primary reason for the event was also recorded, including cardiovascular (systolic blood pressure <90 mm Hg, pulse <40 or >130 bpm), respiratory (respiratory rate <10 or >24 breaths per minute, need for noninvasive positive pressure ventilation, oxygen saturation <90%), neurologic (loss of consciousness, change in mental status, seizure, or suspected stroke), or clinical deterioration causing staff to become worried. MET activation occurs at our institution by utilizing a text paging system that records the date and time of the call, along with the patient's location. The event start time was recorded from the paging system. We considered the event stop time to be the time at which the patient is transferred to a different level of care (ie, ICU) or the MET members leave the bedside and transfer care back to the primary service. MET nurses recorded the duration of MET activation in the intranet database immediately following the activation. Data were collected from the medical record, including age, gender, race, medical insurance, religion, admission source (home, assisted living, rehabilitation, or other hospital), admission team (internal medicine or surgery), admission date and time, discharge or death date and time, disposition at discharge (home, rehabilitation, death with or without a DNR order, or hospice care), and admission diagnosis. We categorized patients discharged to a hospice bridge program as being discharged with hospice care. We also recorded whether or not code status was discussed at the time of admission. Our hospital policy requires that an advanced directives order (either full code or DNR) must be placed on all patients at the time of admission. However, we only considered a code status discussion to have occurred if there was explicit documentation of a code status discussion in the medical record at the time of hospital admission. Data from the time of hospital admission were collected to calculate a Charlson Comorbidity Index, a well‐validated predictor of mortality that uses a weighted sum of 17 medical conditions, with scores ranging from 0 to 37.[11] Higher scores indicate a greater burden of illness. We also recorded whether or not the inpatient palliative care service was consulted following MET activation by examining the medical record for a consult note. The inpatient palliative care service at our institution was implemented in 2005 and receives over 700 new inpatient consults per year. The most common reason for consultation is to assist the primary service with discussing goals of care and code status.
Our MET was established in 2005 and responds to approximately 30 to 40 events per 1000 admissions. There are 4 members on our MET, including a critical care nurse, a nursing supervisor, a respiratory therapist, and a team leader who, depending on a predetermined schedule, is either an attending hospital medicine physician, an attending critical care physician, a critical care training physician, or a critical care physician assistant.
The data were transferred from the intranet registry to an Excel (Microsoft Corp., Redmond, WA) spreadsheet for statistical analysis. The primary outcome was the proportion of activations resulting in a MET‐implemented DNR order. Secondary outcomes included the duration of MET activation, need for transfer to telemetry or the ICU, hospital length of stay following MET activation (time from the end of the MET activation to hospital discharge or death), and the frequency with which inpatient palliative care consultation and outpatient hospice care were utilized. For repeat MET activations in a single patient, we considered each MET activation as a separate event as the code status could potentially change more than once. We used SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC) statistical analysis software for data analysis. The 2 method was used for categorical variables, and either a 2‐sample t test or Wilcoxon rank sum test was utilized for continuous variables. A P value 0.05 was considered significant.
RESULTS
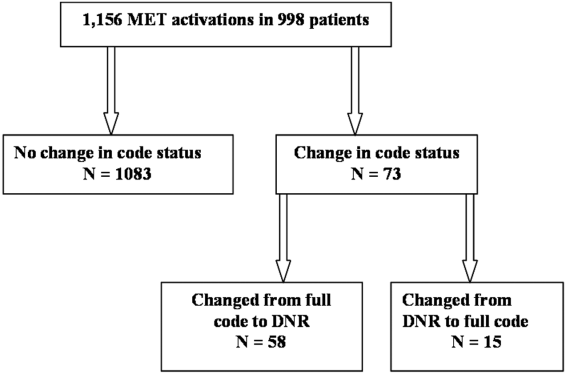
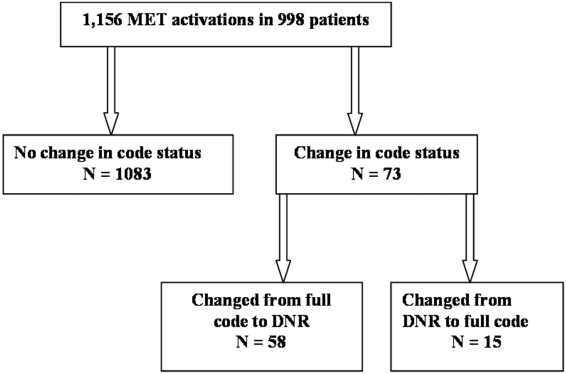
We observed 1156 MET activations in 998 patients. The mean age was 67 years and 57% (565/998) were male (Table 1). The mean Charlson Comorbidity Index was 5.4. Most patients were admitted from home (76%, 760/998), to a medical service (72%, 720/998), and to a teaching service (73%, 732/998). Sepsis (11%, 109/998) and trauma (11%, 105/998) were the most common admission diagnoses. A cardiovascular abnormality was the most common (35%, 399/1156) reason for activation. A code status discussion was documented on admission in 44% (440/998) of all patients.
| Variable | Total, n=998 | No Change in Code Status During MET Activation, n=926 | MET‐Implemented Change in Code Status, n=72a | P Valueb | DNR Prior to MET Activation, n=100 | MET‐Implemented DNR Order, n=58 | P Valuec |
|---|---|---|---|---|---|---|---|
| |||||||
| Gender, male | 565 (56.6) | 527 (56.9) | 38 (52.8) | 0.50 | 52 (52.0) | 34 (58.6) | 0.42 |
| Age, yr, meanSTD | 6717 | 6717 | 7115 | 0.08 | 8111 | 7016 | <0.0001 |
| Race | |||||||
| Caucasian | 927 (92.9) | 859 (92.8) | 68(94.4) | 0.59 | 99 (99) | 54 (93.1) | 0.04 |
| Minorityd | 71 (7.1) | 67 (7.2) | 4(5.6) | 1 (1.0) | 4 (6.9) | ||
| Insurance | |||||||
| Medicare | 694 (69.5) | 635 (68.6) | 59 (81.9) | 0.02 | 89 (89.0) | 45 (77.6) | 0.05 |
| Private | 244 (24.5) | 235 (25.4) | 9 (12.5) | 0.01 | 10 (10.0) | 9 (15.5) | 0.30 |
| Medicaid | 41 (4.1) | 39 (4.2) | 2 (2.8) | 0.55 | 1 (1.0) | 2 (3.5) | 0.27 |
| None | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 0 (0) | 1 (1.7) | 0.19 |
| Religion | |||||||
| Christian | 748 (75.0) | 691 (74.6) | 57 (79.2) | 0.39 | 82 (82.0) | 46 (79.3) | 0.68 |
| None specified | 226 (22.7) | 213 (23.0) | 13 (18.1) | 0.33 | 14 (14.0) | 10 (17.2) | 0.58 |
| Other religions | 24 (2.4) | 22 (2.4) | 2 (2.7) | 0.83 | 4 (4.0) | 2 (3.5) | 0.86 |
| Admission diagnosis | |||||||
| Sepsis | 109 (10.9) | 100 (10.8) | 9 (12.5) | 0.66 | 11 (11.0) | 8 (13.8) | 0.60 |
| Trauma/fall | 105 (10.5) | 100 (10.8) | 5 (6.9) | 0.30 | 6 (6.0) | 5 (8.6) | 0.53 |
| Malignancy related | 79 (7.9) | 73 (7.9) | 6 (8.3) | 0.89 | 7 (7.0) | 4 (6.9) | 0.98 |
| Stroke | 47 (4.7) | 43 (4.6) | 4 (5.6) | 0.73 | 6 (6.0) | 4 (6.9) | 0.82 |
| Pneumonia | 46 (4.6) | 41 (4.4) | 5 (6.9) | 0.33 | 11 (11.0) | 3 (5.2) | 0.21 |
| Altered mental status | 43 (4.3) | 40 (4.3) | 3 (4.2) | 0.95 | 5 (5.0) | 3 (5.2) | 0.96 |
| Myocardial infarct | 42 (4.2) | 40 (4.3) | 2 (2.8) | 0.53 | 1 (1.0) | 1 (1.7) | 0.69 |
| Respiratory failure | 40 (4.0) | 36 (3.9) | 4 (5.6) | 0.49 | 2 (2.0) | 3 (5.2) | 0.27 |
| Arrhythmia | 37 (3.7) | 33 (3.6) | 4 (5.6) | 0.39 | 2 (2.0) | 3 (5.2) | 0.27 |
| Heart failure | 35 (3.5) | 33 (3.6) | 2 (2.8) | 0.72 | 10 (10.0) | 2 (3.5) | 0.13 |
| Other | 415 (41.6) | 387 (41.8) | 28 (38.9) | 0.63 | 39 (39.0) | 22 (37.9) | 0.89 |
| Admission type | |||||||
| Medical | 720 (72.1) | 662 (71.5) | 58 (80.6) | 0.10 | 91 (91.0) | 46 (79.3) | 0.04 |
| Surgical | 278 (27.9) | 264 (28.5) | 14 (19.4) | 9 (9.0) | 12 (20.7) | ||
| Admission source | |||||||
| Home | 760 (76.2) | 706 (76.2) | 54 (75.0) | 0.81 | 60 (60.0) | 44 (75.9) | 0.04 |
| Assisted Living | 29 (2.9) | 28(3.0) | 1 (1.4) | 0.43 | 9 (9.0) | 1 (1.7) | 0.07 |
| Nursing Home | 69 (6.9) | 65(7.0) | 4 (5.6) | 0.64 | 19 (19.0) | 1 (1.7) | <0.01 |
| Outside hospital | 139 (13.9) | 126(13.6) | 13 (18.1) | 0.29 | 12 (12.0) | 12 (20.7) | 0.14 |
| Other | 1 (0.1) | 0(0) | 1 (0.1) | 0.78 | 0 (0) | 0 | |
| Teaching service | 732 (73.4) | 678 (73.2) | 54 (75.0) | 0.74 | 84 (84.0) | 41 (70.7) | 0.05 |
| Code status discussed on admission | 440(44.1) | 397 (42.9) | 43 (59.7) | 0.01 | 70 (70.0) | 32 (55.2) | 0.06 |
| CCI, meanSTDe | 5.43.0 | 5.43.0 | 5.83.0 | 0.21 | 7.72.4 | 5.73.0 | <0.001 |
| MI | 226 (22.7) | 210 (22.7) | 16 (22.2) | 0.93 | 36 (36.0) | 13 (22.4) | 0.08 |
| Heart failure | 138 (13.8) | 127 (13.7) | 11 (15.3) | 0.71 | 28 (28.0) | 8 (13.8) | 0.04 |
| PVD | 90 (9.0) | 85 (9.2) | 5 (6.9) | 0.52 | 14 (14.0) | 4(6.9) | 0.18 |
| Stroke | 131 (13.1) | 121 (13.1) | 10 (13.9) | 0.84 | 30 (30.0) | 9 (15.5) | 0.04 |
| Dementia | 58 (5.8) | 51(5.5) | 7 (9.7) | 0.14 | 19 (19.0) | 5 (8.6) | 0.08 |
| COPD | 173 (17.3) | 161 (17.4) | 12 (16.7) | 0.88 | 23 (23.0) | 8 (13.8) | 0.16 |
| CTD | 58 (5.8) | 56 (6.1) | 2 (2.8) | 0.25 | 6 (6.0) | 2 (3.5) | 0.48 |
| Peptic ulcer disease | 26 (2.6) | 25 (2.7) | 1 (1.4) | 0.50 | 2 (2.0) | 1 (1.7) | 0.90 |
| Mild liver disease | 33 (3.3) | 32 (3.5) | 1 (1.4) | 0.34 | 1 (1.0) | 1 (1.7) | 0.69 |
| DM | 213 (21.3) | 194 (21.0) | 19 (16.4) | 0.28 | 19 (19.0) | 15 (25.9) | 0.31 |
| Hemiplegia | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 2 (2.0) | 1 (1.7) | 0.90 |
| Renal disease | 131 (13.1) | 119 (12.9) | 12 (16.7) | 0.36 | 21 (21.0) | 9 (15.5) | 0.40 |
| DM+organ damage | 68 (6.8) | 64 (6.9) | 4 (5.6) | 0.66 | 7 (7.0) | 3 (5.2) | 0.65 |
| Any tumor | 188 (18.8) | 173 (18.7) | 15 (20.8) | 0.65 | 25 (25.0) | 14 (24.1) | 0.90 |
| Lymphoma | 21 (2.1) | 20 (2.2) | 1 (1.4) | 0.66 | 2 (2.0) | 1 (1.7) | 0.90 |
| Leukemia | 20 (2.0) | 18 (1.9) | 2 (2.8) | 0.63 | 1 (1.0) | 0 (0.0) | 0.45 |
| Moderate/severe liver disease | 45 (4.5) | 39 (4.2) | 6 (8.3) | 0.10 | 0 (0.0) | 6 (10.3) | 0.001 |
| Metastatic tumor | 61 (6.1) | 51 (5.5) | 10 (13.9) | 0.004 | 10 (10.0) | 8 (13.8) | 0.47 |
| AIDS | 4 (0.4) | 3 (0.3) | 1 (1.4) | 0.17 | 0 (0.0) | 1 (1.7) | 0.19 |
MET activation resulted in a DNR order in 5% (58/1156) of cases (Figure 1). In activations involving a change in code status, 21% (15/73) were changed from DNR to full code. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order where younger (70 vs 81 years, P<0.0001), more commonly admitted from home (60% vs 44%, P=0.04), less frequently from a nursing home (1% vs 9%, P<0.01), and had a lower Charlson index (5.7 vs 7.7, P<0.001) (Table 1). Moderate to severe liver disease was more common in patients with a MET‐implemented DNR order (10% vs 0%, P=0.001). Admission diagnoses were similar between patients with a preexisting DNR and a MET‐implemented DNR order (Table 1).
The median time spent on activations with a change in code status was significantly longer than activations without a change (66 vs 60 minutes, P=0.05). The rates of telemetry (6% vs 3%, P=0.24) and ICU transfer (40% vs 41%, P=0.8) were similar between patients with a change in code status and patients without a change (Table 2). Patients with a MET‐implemented DNR order were more frequently transferred to the ICU than patients with a preexisting DNR order (36% vs 17%, P<0.01). The median hospital length of stay following MET activation was shorter in patients with a change in code status compared to patients with no change (3 vs 5 days, P<0.0001).
| Variable | Total, N=1,156 | No Change in Code Status During MET Activation, n=1,083 | MET‐Implemented Change in Code Status, n=73a | PValuec | DNR Prior to MET Activation, n=115 | MET‐Implemented DNR Order, n=58 | P Valued |
|---|---|---|---|---|---|---|---|
| |||||||
| Reason for callb | |||||||
| Cardiovascular | 399 (34.5) | 379 (35.0) | 20 (27.4) | 0.19 | 39 (33.9) | 19 (32.8) | 0.88 |
| Respiratory | 319 (27.6) | 295 (27.2) | 2 (32.9) | 0.30 | 40 (34.8) | 18 (31.0) | 0.62 |
| Neurologic | 215 (18.6) | 196 (18.1) | 19 (26.0) | 0.09 | 21 (18.3) | 15 (25.9) | 0.25 |
| Other | 323 (27.9) | 303 (28.0) | 20 (27.4) | 0.92 | 22 (19.1) | 15 (25.9) | 0.31 |
| MET resources, call duration, min, median (IQR) | 60 (4090) | 60 (4090) | 66 (43100) | 0.05 | 50 (3075) | 67 (50100) | <0.001 |
| Hospital resources | |||||||
| Tele transfer | 68 (5.9) | 663 (6.1) | 2 (2.7) | 0.24 | 3 (2.6) | 2(3.5) | 0.76 |
| ICU transfer | 459 (39.7) | 429 (39.6) | 30 (41.1) | 0.8 | 19 (16.5) | 21 (36.2) | <0.01 |
| LOS after MET activation, d, median (IQR) | 5.2 (0.2510.7) | 5 (5.411.0) | 2.8 (0.66.7) | <0.0001 | 3.8 (1.56.5) | 2.9 (0.56.5) | 0.06 |
| End‐of‐life care | n=191e | n=157 | n=34 | n=41 | n=26 | ||
| Palliative care | 31 (16.2) | 27 (17.2) | 4 (11.8) | 0.44 | 8 (19.5) | 3 (11.5) | 0.39 |
| CMO orders | 159 (83.3) | 127 (80.9) | 32 (94.1) | 0.06 | 33 (80.5) | 25 (96.2) | 0.07 |
| Died full code | 10 (5.2) | 10 (6.4) | 0 (0) | 0.13 | 2 (4.9) | 0 (0) | 0.25 |
| Died DNR | 155 (81.2) | 123 (78.3) | 32 (94.1) | 0.03 | 27 (65.9) | 25 (96.2) | 0.004 |
| Hospice | 26 (13.6) | 24 (15.3) | 2 (5.9) | 0.15 | 12 (29.3) | 1 (3.9) | 0.01 |
The inpatient mortality was 17% (165/998). Most patients who died had the focus of care changed to comfort measures only (88%, 146/165). When examining the group of patients who died in the hospital with comfort care, we found that 58% (92/159) were transferred to the ICU following the MET call, 5% (8/159) were changed to comfort care during the MET call, and 18% (29/159) had a palliative care consult. We also observed that 16% (25/159) patients who died with comfort care were made DNR during MET activation. The inpatient mortality was significantly higher in patients with a change in code status compared to patients with no change in code status (44% vs 14%, 133/926, P<0.0001). Patients with a MET‐implemented DNR order had a higher inpatient mortality than patients with a preexisting DNR (43% vs 27%, P=0.04). Twenty‐five patients with a MET‐implemented DNR order died in the hospital. When examining a subgroup of patients who required end‐of‐life care (died or discharged from the hospital with hospice), we found patients with a MET‐implemented DNR order were less likely to be discharged with hospice care than patients with a preexisting DNR (4% vs 29%, P=0.01). There was no difference in the use of inpatient palliative care consultation at the end of life in patients with a preexisting DNR versus MET‐implemented DNR order (20% vs 12%, P=0.39). Patients with a MET‐implemented DNR order also had a significantly shorter median time from implementation of comfort care orders to death or discharge with hospice compared to patients with a preexisting DNR order (7 hours, interquartile range [IQR], 416 hours vs 22 hours, IQR 939 hours).
DISCUSSION
We observed a MET‐implemented DNR order in 5% of activations. Little is known about the role of METs in end‐of‐life discussions in the United States, and past experience has primarily come from Australian hospitals. Important differences in end‐of‐life care exist among different countries, particularly with regard to placing limitations on treatment.[9] Our observed rate is similar to the 3% to 10% rate of MET‐implemented DNR orders in previous reports worldwide.[3, 5, 8, 12, 13] Recent data from the United States suggest that METs initiate a DNR order in 28% of cases.[14] However, most DNR orders in that study were placed in the ICU days to weeks after MET activation, likely accounting for the high DNR rate. Our data add to the growing body of evidence that METs play an important role in end‐of‐life discussions among different countries throughout the world, including the United States.
To our knowledge, no prior study has evaluated the impact of code status discussions on MET resource utilization. Our MET spent 6 minutes longer on activations involving a change in code status when compared to activations with no changes made to code status. Presumably, some of this time was spent discussing goals of care. In our opinion, the additional time spent on these activations was invaluable, particularly when considering MET‐initiated end‐of‐life discussions may have prevented several unwanted resuscitations (25 patients died with a MET‐implemented DNR order). Interestingly, less than half of patients had code status discussions at the time of hospital admission. This finding suggests that clinicians could be more vigilant about discussing preferences for resuscitation at the time of admission in patients at risk for clinical deterioration. We suspect that in some cases code status discussions may have occurred between the patient and the primary service later in the patient's hospitalization, which were not captured in our study.
Surprisingly, when examining the use of hospital resources, we found no difference in the rate of unplanned ICU transfer in patients with a change in code status. In fact, we observed a higher rate of ICU transfer in patients with a MET‐implemented DNR order compared to those with a preexisting DNR order (36% vs 17%). These results were at odds with our hypothesis of a lower rate of ICU transfer in patients with MET‐implemented limitations in care. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order were younger, more commonly admitted from home, and had a lower Charlson index. Despite evidence of a lower burden of chronic illness and younger age, patients with a MET‐implemented DNR order had higher inpatient mortality than patients with a preexisting DNR order (43% vs 27%), suggesting an acute and rapidly progressive disease process. These observations may have compelled the MET to advocate for aggressive ICU‐level care in patients with a MET‐implemented DNR order. Another possible explanation for the relatively high rate of ICU transfer is that the MET is, in part, led by ICU staff. Thus, our MET may have made the decision to transfer the patient to the ICU and then subsequently initiated end‐of‐life discussions only after taking ownership of the patient. Furthermore, almost 20% of MET‐implemented changes to code status involved reversing status from DNR to full code. These data suggest that METs are not merely serving as a resource to review code status, but rather providing intensive treatment for acutely ill patients and simultaneously initiating end‐of‐life discussions in a population with a high inpatient mortality rate. The practice pattern observed in our study of transferring patients to the ICU for a trial of intensive therapy at the end of life is consistent with the overall trend in the United States for increased inpatient treatment intensity at the end of life.[14, 15, 16]
Our data suggest that the increased use of ICU resources in patients with a MET‐implemented DNR may be balanced by a shorter hospital length of stay following MET activation. In a multicenter observational study, Jones et al. found hospital length of stay to be similar in patients with and without a MET‐implemented limitation of medical therapy.[3] The authors did not examine length of stay specifically in patients with a DNR order, but rather examined patients with any limitation in medical therapy, including not for ICU admission. We suspect that the shorter length of stay following MET activation in our study was related to the fact that patients with a change in code status had a significantly higher inpatient mortality.
We observed several interesting findings with regard to end‐of‐life care following MET‐implemented DNR orders. First, the inpatient mortality in this population was remarkably high at 43%, compared to 27% in patients with a preexisting DNR order. Interestingly, there was no difference in the rate of palliative care consultation between the 2 groups despite the fact that all 25 patients who died following a MET‐implemented DNR order did so with a comfort measures only order. We also found that patients with a preexisting DNR also had a higher rate of discharge with hospice compared to patients with a MET‐implemented DNR order (29% vs 4%). Thus, our data suggest that inpatient palliative care consultation and hospice services are not resources that are routinely utilized in patients with MET‐initiated DNR orders. It may be the case that the acuity and severity of illness or patient preferences may have precluded the possibility of discharging some patients in our study home with hospice care or implementing comfort care earlier in the hospital course. Patients with MET‐implemented DNR orders were younger, had fewer comorbidities, and died sooner after comfort care orders were written. The overall rate of comfort care provided to patients who died was high at 88%. We have an inpatient comfort measures only order set at our hospital, which may account for the large proportion of patients receiving comfort care at the end of life. In addition, this order set may also help to improve the quality of end‐of‐life care and thus limit the need for palliative care consultation to some extent. However, we found that patients with a MET‐implemented DNR order had a shorter time from comfort care orders to death than patients with a preexisting DNR order. This finding suggests that patients with MET‐implemented DNR orders may have had comfort care implemented relatively late in the course of illness and had less‐than‐optimal end‐of‐life care. Vazquez et al. reported improved quality of end‐of‐life care after implementation of a MET.[10] However, an inpatient palliative care service was not available in that study, and it is not clear whether or not a comfort care order set was available. Evidence suggests that utilization of palliative care resources improves end‐of‐life care in the ICU.[17, 18, 19] We found that more than half of patients who died with comfort care in the hospital did so after being transferred to the ICU for a trial of aggressive care, suggesting that this population may have benefited from more involvement of our palliative care service. In summary, our data on end‐of‐life care following MET activation suggest that the METs are able to take advantage of an opportunity to identify patients who would not want resuscitation efforts because of personal preferences or futility of treatments. However, our surrogate measures of the quality of end‐of‐life care suggest that patients with MET‐implemented DNR orders may benefit from coordinated care with inpatient palliative care services, timelier implementation of comfort care orders, and possibly increased referrals for hospice care to help improve the quality of end‐of‐life care in this population.
Our study is subject to a number of limitations. This was a single‐center study, making the results difficult to generalize. The retrospective nature of our study makes it subject to the limitations inherent in this study design, including bias and confounding. The duration of MET activation was difficult to accurately and objectively measure and is subject to reporting bias. The event stop time, in particular, was subjectively measured by the MET nurse and is difficult to accurately assess, because MET members occasionally leave the bedside and return to reevaluate the response to therapy. We tried to account for this by clearly defining the MET stop time as the point at which MET members leave the bedside and transfer care back to the primary service or physically transfer the patient to a higher level of care. It also bears mentioning that the nurses performing data entry were not aware of the study hypothesis at the time of data entry. Despite including over 1100 MET calls in our analysis, the number of patients with a MET‐implemented DNR order was relatively small, which may have limited our ability to detect differences among subgroups during our analysis. We also did not document the clinical circumstances surrounding the MET‐implemented DNR order. Although we hypothesized that these patients had a higher mortality due to a higher acuity of illness, we were unable to support this hypothesis with the data available in our retrospective study. We did not record which providers were involved in code status discussions and the exact amount time spent on these discussions, making it difficult to accurately quantify the MET resources utilized on the calls. Our MET works closely with the patient's primary service, and it is possible that some of the changes to code status were implemented by the primary service and not MET providers. The patient's primary service may have a preexisting relationship with the patient and would be in a better position to discuss goals of care than MET providers who have had no prior relationship with the patient. However, even in this case, the clinical deterioration prompting MET activation was likely the event that triggered end‐of‐life discussions. Prospective studies would be helpful not only to identify the individuals involved in code status discussions during MET activations, but also to objectively measure the time spent on such discussions. Finally, our study population consisted primarily of Caucasian patients. Preferences for end‐of‐life care may differ among socioeconomic and ethnic groups, thus limiting the generalizability of our study findings.[20, 21]
In conclusion, we found the rate of MET‐implemented DNR orders in the United States to be similar to that of previous reports in other countries. MET events involving a change in code status are associated with increased utilization of MET and ICU resources, but a shorter hospital length of stay. Despite a high inpatient mortality rate, patients with a MET‐implemented DNR had a relatively low utilization of end‐of‐life resources, including palliative care and home hospice services. Coordinated care between METs and palliative care may help to improve of end‐of‐life care in patients with a change in code status following MET activation.
Acknowledgements
The authors acknowledge the hard work and dedication provided by Elizabeth Spellman during the data collection process.
Disclosures: This work was performed at Lahey Hospital and Medical Center. The authors report no conflicts of interest.
- , , , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645.
- , , , et al. Antecedents to hospital deaths. Intern Med J. 2001;31(6):343–348.
- , , , et al. The role of the medical emergency team in end‐of‐life care: a multicenter, prospective, observational study. Crit Care Med. 2012;40(1):98–103.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow‐up study. Resuscitation. 2010;81(1):31–35.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , et al. The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79(3):391–397.
- . Cultural differences in end‐of‐life care. Crit Care Med. 2001;29(2 Suppl):N52–N55.
- , , , et al. Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9(2):151–156.
- , , , et al. A retrospective cohort study of the effect of medical emergency teams on documentation of advance care directives. Crit Care Resusc. 2011;13(3):167–174.
- , , , et al. The medical emergency team a call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and healthcare transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39(2):363–375.
- , , , et al. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients. Crit Care Med. 2007;35(6):1530–1535.
- , . Impact of a proactive approach to improve end‐of‐life care in a medical ICU. Chest. 2003;123(1):266–271.
- , , , et al. In their own words: patients and families define high‐quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818.
- , , , et al. The influence of race/ethnicity and socioeconomic status on end‐of‐life care in the ICU. Chest. 2011;139(5):1025–1033.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137.
Approximately 15% to 20% of inpatients develop significant adverse events, which are often preceded by a change in the patient's condition.[1, 2] Many hospitals utilize medical emergency teams (METs) to deliver prompt care for deteriorating patients. METs have an emerging role in end‐of‐life care; up to 35% of patients who die in the hospital with a do not resuscitate (DNR) order have a MET activation during the admission.[3] Thus, METs are well positioned to discuss preferences for cardiopulmonary resuscitation in a population at high risk for cardiac arrest.[4]
MET activations involve a change in code status in approximately 3% to 10% of cases.[5, 6, 7, 8] However, previous studies primarily involved hospitals in Australia, making the results difficult to generalize to other countries.[3, 6, 7] There may be important cultural differences in patient and clinician attitudes toward end‐of‐life care among different countries.[9] In addition, little is known about METs and hospital resources utilized in patients with MET‐implemented changes in code status. Anecdotal evidence suggests that MET activations addressing code status are more time consuming.[3] Conversely, by identifying patients unlikely to benefit from restorative care, METs may facilitate end‐of‐life comfort care, thereby reducing unplanned intensive care unit (ICU) admissions and hospital length of stay. Finally, preliminary evidence suggests that METs may improve the quality of end‐of‐life care.[10] However, the use of end‐of‐life resources, including inpatient palliative care consultation and hospice care following MET activation has not been well studied.
The purpose of our study was to examine the role of a US MET in end‐of‐life care. First, we assessed the proportion of MET calls that resulted in a new DNR order in a US hospital and compared this to previous reports in other countries. We also examined MET and hospital resource utilization in MET activations involving changes in code status by evaluating the duration of MET activations, the need for telemetry or ICU transfer, and hospital length of stay following MET activation. Finally, we explored the quality of end‐of‐life care in patients with MET‐implemented DNR orders by assessing the utilization of inpatient palliative care consultation and hospice care compared to patients with a preexisting DNR order.
MATERIALS AND METHODS
We conducted this study at Lahey Clinic, a 350‐bed academic, tertiary care center. The institutional review board approved this study and waived the need for informed consent. We performed a retrospective review of a prospectively collected MET registry. We included consecutive, adult (>18 years old) inpatient MET activations and excluded nonhospitalized patients. Data were recorded in an intranet registry at the time of the event by the MET nurse, including preexisting code status (full code or DNR), any change to the patient's code status (full code to DNR or DNR to full code) during the MET event, date of the event, disposition after the event (no transfer, transferred to ICU, or transfer to telemetry), and a description of MET interventions. The primary reason for the event was also recorded, including cardiovascular (systolic blood pressure <90 mm Hg, pulse <40 or >130 bpm), respiratory (respiratory rate <10 or >24 breaths per minute, need for noninvasive positive pressure ventilation, oxygen saturation <90%), neurologic (loss of consciousness, change in mental status, seizure, or suspected stroke), or clinical deterioration causing staff to become worried. MET activation occurs at our institution by utilizing a text paging system that records the date and time of the call, along with the patient's location. The event start time was recorded from the paging system. We considered the event stop time to be the time at which the patient is transferred to a different level of care (ie, ICU) or the MET members leave the bedside and transfer care back to the primary service. MET nurses recorded the duration of MET activation in the intranet database immediately following the activation. Data were collected from the medical record, including age, gender, race, medical insurance, religion, admission source (home, assisted living, rehabilitation, or other hospital), admission team (internal medicine or surgery), admission date and time, discharge or death date and time, disposition at discharge (home, rehabilitation, death with or without a DNR order, or hospice care), and admission diagnosis. We categorized patients discharged to a hospice bridge program as being discharged with hospice care. We also recorded whether or not code status was discussed at the time of admission. Our hospital policy requires that an advanced directives order (either full code or DNR) must be placed on all patients at the time of admission. However, we only considered a code status discussion to have occurred if there was explicit documentation of a code status discussion in the medical record at the time of hospital admission. Data from the time of hospital admission were collected to calculate a Charlson Comorbidity Index, a well‐validated predictor of mortality that uses a weighted sum of 17 medical conditions, with scores ranging from 0 to 37.[11] Higher scores indicate a greater burden of illness. We also recorded whether or not the inpatient palliative care service was consulted following MET activation by examining the medical record for a consult note. The inpatient palliative care service at our institution was implemented in 2005 and receives over 700 new inpatient consults per year. The most common reason for consultation is to assist the primary service with discussing goals of care and code status.
Our MET was established in 2005 and responds to approximately 30 to 40 events per 1000 admissions. There are 4 members on our MET, including a critical care nurse, a nursing supervisor, a respiratory therapist, and a team leader who, depending on a predetermined schedule, is either an attending hospital medicine physician, an attending critical care physician, a critical care training physician, or a critical care physician assistant.
The data were transferred from the intranet registry to an Excel (Microsoft Corp., Redmond, WA) spreadsheet for statistical analysis. The primary outcome was the proportion of activations resulting in a MET‐implemented DNR order. Secondary outcomes included the duration of MET activation, need for transfer to telemetry or the ICU, hospital length of stay following MET activation (time from the end of the MET activation to hospital discharge or death), and the frequency with which inpatient palliative care consultation and outpatient hospice care were utilized. For repeat MET activations in a single patient, we considered each MET activation as a separate event as the code status could potentially change more than once. We used SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC) statistical analysis software for data analysis. The 2 method was used for categorical variables, and either a 2‐sample t test or Wilcoxon rank sum test was utilized for continuous variables. A P value 0.05 was considered significant.
RESULTS
We observed 1156 MET activations in 998 patients. The mean age was 67 years and 57% (565/998) were male (Table 1). The mean Charlson Comorbidity Index was 5.4. Most patients were admitted from home (76%, 760/998), to a medical service (72%, 720/998), and to a teaching service (73%, 732/998). Sepsis (11%, 109/998) and trauma (11%, 105/998) were the most common admission diagnoses. A cardiovascular abnormality was the most common (35%, 399/1156) reason for activation. A code status discussion was documented on admission in 44% (440/998) of all patients.
| Variable | Total, n=998 | No Change in Code Status During MET Activation, n=926 | MET‐Implemented Change in Code Status, n=72a | P Valueb | DNR Prior to MET Activation, n=100 | MET‐Implemented DNR Order, n=58 | P Valuec |
|---|---|---|---|---|---|---|---|
| |||||||
| Gender, male | 565 (56.6) | 527 (56.9) | 38 (52.8) | 0.50 | 52 (52.0) | 34 (58.6) | 0.42 |
| Age, yr, meanSTD | 6717 | 6717 | 7115 | 0.08 | 8111 | 7016 | <0.0001 |
| Race | |||||||
| Caucasian | 927 (92.9) | 859 (92.8) | 68(94.4) | 0.59 | 99 (99) | 54 (93.1) | 0.04 |
| Minorityd | 71 (7.1) | 67 (7.2) | 4(5.6) | 1 (1.0) | 4 (6.9) | ||
| Insurance | |||||||
| Medicare | 694 (69.5) | 635 (68.6) | 59 (81.9) | 0.02 | 89 (89.0) | 45 (77.6) | 0.05 |
| Private | 244 (24.5) | 235 (25.4) | 9 (12.5) | 0.01 | 10 (10.0) | 9 (15.5) | 0.30 |
| Medicaid | 41 (4.1) | 39 (4.2) | 2 (2.8) | 0.55 | 1 (1.0) | 2 (3.5) | 0.27 |
| None | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 0 (0) | 1 (1.7) | 0.19 |
| Religion | |||||||
| Christian | 748 (75.0) | 691 (74.6) | 57 (79.2) | 0.39 | 82 (82.0) | 46 (79.3) | 0.68 |
| None specified | 226 (22.7) | 213 (23.0) | 13 (18.1) | 0.33 | 14 (14.0) | 10 (17.2) | 0.58 |
| Other religions | 24 (2.4) | 22 (2.4) | 2 (2.7) | 0.83 | 4 (4.0) | 2 (3.5) | 0.86 |
| Admission diagnosis | |||||||
| Sepsis | 109 (10.9) | 100 (10.8) | 9 (12.5) | 0.66 | 11 (11.0) | 8 (13.8) | 0.60 |
| Trauma/fall | 105 (10.5) | 100 (10.8) | 5 (6.9) | 0.30 | 6 (6.0) | 5 (8.6) | 0.53 |
| Malignancy related | 79 (7.9) | 73 (7.9) | 6 (8.3) | 0.89 | 7 (7.0) | 4 (6.9) | 0.98 |
| Stroke | 47 (4.7) | 43 (4.6) | 4 (5.6) | 0.73 | 6 (6.0) | 4 (6.9) | 0.82 |
| Pneumonia | 46 (4.6) | 41 (4.4) | 5 (6.9) | 0.33 | 11 (11.0) | 3 (5.2) | 0.21 |
| Altered mental status | 43 (4.3) | 40 (4.3) | 3 (4.2) | 0.95 | 5 (5.0) | 3 (5.2) | 0.96 |
| Myocardial infarct | 42 (4.2) | 40 (4.3) | 2 (2.8) | 0.53 | 1 (1.0) | 1 (1.7) | 0.69 |
| Respiratory failure | 40 (4.0) | 36 (3.9) | 4 (5.6) | 0.49 | 2 (2.0) | 3 (5.2) | 0.27 |
| Arrhythmia | 37 (3.7) | 33 (3.6) | 4 (5.6) | 0.39 | 2 (2.0) | 3 (5.2) | 0.27 |
| Heart failure | 35 (3.5) | 33 (3.6) | 2 (2.8) | 0.72 | 10 (10.0) | 2 (3.5) | 0.13 |
| Other | 415 (41.6) | 387 (41.8) | 28 (38.9) | 0.63 | 39 (39.0) | 22 (37.9) | 0.89 |
| Admission type | |||||||
| Medical | 720 (72.1) | 662 (71.5) | 58 (80.6) | 0.10 | 91 (91.0) | 46 (79.3) | 0.04 |
| Surgical | 278 (27.9) | 264 (28.5) | 14 (19.4) | 9 (9.0) | 12 (20.7) | ||
| Admission source | |||||||
| Home | 760 (76.2) | 706 (76.2) | 54 (75.0) | 0.81 | 60 (60.0) | 44 (75.9) | 0.04 |
| Assisted Living | 29 (2.9) | 28(3.0) | 1 (1.4) | 0.43 | 9 (9.0) | 1 (1.7) | 0.07 |
| Nursing Home | 69 (6.9) | 65(7.0) | 4 (5.6) | 0.64 | 19 (19.0) | 1 (1.7) | <0.01 |
| Outside hospital | 139 (13.9) | 126(13.6) | 13 (18.1) | 0.29 | 12 (12.0) | 12 (20.7) | 0.14 |
| Other | 1 (0.1) | 0(0) | 1 (0.1) | 0.78 | 0 (0) | 0 | |
| Teaching service | 732 (73.4) | 678 (73.2) | 54 (75.0) | 0.74 | 84 (84.0) | 41 (70.7) | 0.05 |
| Code status discussed on admission | 440(44.1) | 397 (42.9) | 43 (59.7) | 0.01 | 70 (70.0) | 32 (55.2) | 0.06 |
| CCI, meanSTDe | 5.43.0 | 5.43.0 | 5.83.0 | 0.21 | 7.72.4 | 5.73.0 | <0.001 |
| MI | 226 (22.7) | 210 (22.7) | 16 (22.2) | 0.93 | 36 (36.0) | 13 (22.4) | 0.08 |
| Heart failure | 138 (13.8) | 127 (13.7) | 11 (15.3) | 0.71 | 28 (28.0) | 8 (13.8) | 0.04 |
| PVD | 90 (9.0) | 85 (9.2) | 5 (6.9) | 0.52 | 14 (14.0) | 4(6.9) | 0.18 |
| Stroke | 131 (13.1) | 121 (13.1) | 10 (13.9) | 0.84 | 30 (30.0) | 9 (15.5) | 0.04 |
| Dementia | 58 (5.8) | 51(5.5) | 7 (9.7) | 0.14 | 19 (19.0) | 5 (8.6) | 0.08 |
| COPD | 173 (17.3) | 161 (17.4) | 12 (16.7) | 0.88 | 23 (23.0) | 8 (13.8) | 0.16 |
| CTD | 58 (5.8) | 56 (6.1) | 2 (2.8) | 0.25 | 6 (6.0) | 2 (3.5) | 0.48 |
| Peptic ulcer disease | 26 (2.6) | 25 (2.7) | 1 (1.4) | 0.50 | 2 (2.0) | 1 (1.7) | 0.90 |
| Mild liver disease | 33 (3.3) | 32 (3.5) | 1 (1.4) | 0.34 | 1 (1.0) | 1 (1.7) | 0.69 |
| DM | 213 (21.3) | 194 (21.0) | 19 (16.4) | 0.28 | 19 (19.0) | 15 (25.9) | 0.31 |
| Hemiplegia | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 2 (2.0) | 1 (1.7) | 0.90 |
| Renal disease | 131 (13.1) | 119 (12.9) | 12 (16.7) | 0.36 | 21 (21.0) | 9 (15.5) | 0.40 |
| DM+organ damage | 68 (6.8) | 64 (6.9) | 4 (5.6) | 0.66 | 7 (7.0) | 3 (5.2) | 0.65 |
| Any tumor | 188 (18.8) | 173 (18.7) | 15 (20.8) | 0.65 | 25 (25.0) | 14 (24.1) | 0.90 |
| Lymphoma | 21 (2.1) | 20 (2.2) | 1 (1.4) | 0.66 | 2 (2.0) | 1 (1.7) | 0.90 |
| Leukemia | 20 (2.0) | 18 (1.9) | 2 (2.8) | 0.63 | 1 (1.0) | 0 (0.0) | 0.45 |
| Moderate/severe liver disease | 45 (4.5) | 39 (4.2) | 6 (8.3) | 0.10 | 0 (0.0) | 6 (10.3) | 0.001 |
| Metastatic tumor | 61 (6.1) | 51 (5.5) | 10 (13.9) | 0.004 | 10 (10.0) | 8 (13.8) | 0.47 |
| AIDS | 4 (0.4) | 3 (0.3) | 1 (1.4) | 0.17 | 0 (0.0) | 1 (1.7) | 0.19 |
MET activation resulted in a DNR order in 5% (58/1156) of cases (Figure 1). In activations involving a change in code status, 21% (15/73) were changed from DNR to full code. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order where younger (70 vs 81 years, P<0.0001), more commonly admitted from home (60% vs 44%, P=0.04), less frequently from a nursing home (1% vs 9%, P<0.01), and had a lower Charlson index (5.7 vs 7.7, P<0.001) (Table 1). Moderate to severe liver disease was more common in patients with a MET‐implemented DNR order (10% vs 0%, P=0.001). Admission diagnoses were similar between patients with a preexisting DNR and a MET‐implemented DNR order (Table 1).

The median time spent on activations with a change in code status was significantly longer than activations without a change (66 vs 60 minutes, P=0.05). The rates of telemetry (6% vs 3%, P=0.24) and ICU transfer (40% vs 41%, P=0.8) were similar between patients with a change in code status and patients without a change (Table 2). Patients with a MET‐implemented DNR order were more frequently transferred to the ICU than patients with a preexisting DNR order (36% vs 17%, P<0.01). The median hospital length of stay following MET activation was shorter in patients with a change in code status compared to patients with no change (3 vs 5 days, P<0.0001).
| Variable | Total, N=1,156 | No Change in Code Status During MET Activation, n=1,083 | MET‐Implemented Change in Code Status, n=73a | PValuec | DNR Prior to MET Activation, n=115 | MET‐Implemented DNR Order, n=58 | P Valued |
|---|---|---|---|---|---|---|---|
| |||||||
| Reason for callb | |||||||
| Cardiovascular | 399 (34.5) | 379 (35.0) | 20 (27.4) | 0.19 | 39 (33.9) | 19 (32.8) | 0.88 |
| Respiratory | 319 (27.6) | 295 (27.2) | 2 (32.9) | 0.30 | 40 (34.8) | 18 (31.0) | 0.62 |
| Neurologic | 215 (18.6) | 196 (18.1) | 19 (26.0) | 0.09 | 21 (18.3) | 15 (25.9) | 0.25 |
| Other | 323 (27.9) | 303 (28.0) | 20 (27.4) | 0.92 | 22 (19.1) | 15 (25.9) | 0.31 |
| MET resources, call duration, min, median (IQR) | 60 (4090) | 60 (4090) | 66 (43100) | 0.05 | 50 (3075) | 67 (50100) | <0.001 |
| Hospital resources | |||||||
| Tele transfer | 68 (5.9) | 663 (6.1) | 2 (2.7) | 0.24 | 3 (2.6) | 2(3.5) | 0.76 |
| ICU transfer | 459 (39.7) | 429 (39.6) | 30 (41.1) | 0.8 | 19 (16.5) | 21 (36.2) | <0.01 |
| LOS after MET activation, d, median (IQR) | 5.2 (0.2510.7) | 5 (5.411.0) | 2.8 (0.66.7) | <0.0001 | 3.8 (1.56.5) | 2.9 (0.56.5) | 0.06 |
| End‐of‐life care | n=191e | n=157 | n=34 | n=41 | n=26 | ||
| Palliative care | 31 (16.2) | 27 (17.2) | 4 (11.8) | 0.44 | 8 (19.5) | 3 (11.5) | 0.39 |
| CMO orders | 159 (83.3) | 127 (80.9) | 32 (94.1) | 0.06 | 33 (80.5) | 25 (96.2) | 0.07 |
| Died full code | 10 (5.2) | 10 (6.4) | 0 (0) | 0.13 | 2 (4.9) | 0 (0) | 0.25 |
| Died DNR | 155 (81.2) | 123 (78.3) | 32 (94.1) | 0.03 | 27 (65.9) | 25 (96.2) | 0.004 |
| Hospice | 26 (13.6) | 24 (15.3) | 2 (5.9) | 0.15 | 12 (29.3) | 1 (3.9) | 0.01 |
The inpatient mortality was 17% (165/998). Most patients who died had the focus of care changed to comfort measures only (88%, 146/165). When examining the group of patients who died in the hospital with comfort care, we found that 58% (92/159) were transferred to the ICU following the MET call, 5% (8/159) were changed to comfort care during the MET call, and 18% (29/159) had a palliative care consult. We also observed that 16% (25/159) patients who died with comfort care were made DNR during MET activation. The inpatient mortality was significantly higher in patients with a change in code status compared to patients with no change in code status (44% vs 14%, 133/926, P<0.0001). Patients with a MET‐implemented DNR order had a higher inpatient mortality than patients with a preexisting DNR (43% vs 27%, P=0.04). Twenty‐five patients with a MET‐implemented DNR order died in the hospital. When examining a subgroup of patients who required end‐of‐life care (died or discharged from the hospital with hospice), we found patients with a MET‐implemented DNR order were less likely to be discharged with hospice care than patients with a preexisting DNR (4% vs 29%, P=0.01). There was no difference in the use of inpatient palliative care consultation at the end of life in patients with a preexisting DNR versus MET‐implemented DNR order (20% vs 12%, P=0.39). Patients with a MET‐implemented DNR order also had a significantly shorter median time from implementation of comfort care orders to death or discharge with hospice compared to patients with a preexisting DNR order (7 hours, interquartile range [IQR], 416 hours vs 22 hours, IQR 939 hours).
DISCUSSION
We observed a MET‐implemented DNR order in 5% of activations. Little is known about the role of METs in end‐of‐life discussions in the United States, and past experience has primarily come from Australian hospitals. Important differences in end‐of‐life care exist among different countries, particularly with regard to placing limitations on treatment.[9] Our observed rate is similar to the 3% to 10% rate of MET‐implemented DNR orders in previous reports worldwide.[3, 5, 8, 12, 13] Recent data from the United States suggest that METs initiate a DNR order in 28% of cases.[14] However, most DNR orders in that study were placed in the ICU days to weeks after MET activation, likely accounting for the high DNR rate. Our data add to the growing body of evidence that METs play an important role in end‐of‐life discussions among different countries throughout the world, including the United States.
To our knowledge, no prior study has evaluated the impact of code status discussions on MET resource utilization. Our MET spent 6 minutes longer on activations involving a change in code status when compared to activations with no changes made to code status. Presumably, some of this time was spent discussing goals of care. In our opinion, the additional time spent on these activations was invaluable, particularly when considering MET‐initiated end‐of‐life discussions may have prevented several unwanted resuscitations (25 patients died with a MET‐implemented DNR order). Interestingly, less than half of patients had code status discussions at the time of hospital admission. This finding suggests that clinicians could be more vigilant about discussing preferences for resuscitation at the time of admission in patients at risk for clinical deterioration. We suspect that in some cases code status discussions may have occurred between the patient and the primary service later in the patient's hospitalization, which were not captured in our study.
Surprisingly, when examining the use of hospital resources, we found no difference in the rate of unplanned ICU transfer in patients with a change in code status. In fact, we observed a higher rate of ICU transfer in patients with a MET‐implemented DNR order compared to those with a preexisting DNR order (36% vs 17%). These results were at odds with our hypothesis of a lower rate of ICU transfer in patients with MET‐implemented limitations in care. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order were younger, more commonly admitted from home, and had a lower Charlson index. Despite evidence of a lower burden of chronic illness and younger age, patients with a MET‐implemented DNR order had higher inpatient mortality than patients with a preexisting DNR order (43% vs 27%), suggesting an acute and rapidly progressive disease process. These observations may have compelled the MET to advocate for aggressive ICU‐level care in patients with a MET‐implemented DNR order. Another possible explanation for the relatively high rate of ICU transfer is that the MET is, in part, led by ICU staff. Thus, our MET may have made the decision to transfer the patient to the ICU and then subsequently initiated end‐of‐life discussions only after taking ownership of the patient. Furthermore, almost 20% of MET‐implemented changes to code status involved reversing status from DNR to full code. These data suggest that METs are not merely serving as a resource to review code status, but rather providing intensive treatment for acutely ill patients and simultaneously initiating end‐of‐life discussions in a population with a high inpatient mortality rate. The practice pattern observed in our study of transferring patients to the ICU for a trial of intensive therapy at the end of life is consistent with the overall trend in the United States for increased inpatient treatment intensity at the end of life.[14, 15, 16]
Our data suggest that the increased use of ICU resources in patients with a MET‐implemented DNR may be balanced by a shorter hospital length of stay following MET activation. In a multicenter observational study, Jones et al. found hospital length of stay to be similar in patients with and without a MET‐implemented limitation of medical therapy.[3] The authors did not examine length of stay specifically in patients with a DNR order, but rather examined patients with any limitation in medical therapy, including not for ICU admission. We suspect that the shorter length of stay following MET activation in our study was related to the fact that patients with a change in code status had a significantly higher inpatient mortality.
We observed several interesting findings with regard to end‐of‐life care following MET‐implemented DNR orders. First, the inpatient mortality in this population was remarkably high at 43%, compared to 27% in patients with a preexisting DNR order. Interestingly, there was no difference in the rate of palliative care consultation between the 2 groups despite the fact that all 25 patients who died following a MET‐implemented DNR order did so with a comfort measures only order. We also found that patients with a preexisting DNR also had a higher rate of discharge with hospice compared to patients with a MET‐implemented DNR order (29% vs 4%). Thus, our data suggest that inpatient palliative care consultation and hospice services are not resources that are routinely utilized in patients with MET‐initiated DNR orders. It may be the case that the acuity and severity of illness or patient preferences may have precluded the possibility of discharging some patients in our study home with hospice care or implementing comfort care earlier in the hospital course. Patients with MET‐implemented DNR orders were younger, had fewer comorbidities, and died sooner after comfort care orders were written. The overall rate of comfort care provided to patients who died was high at 88%. We have an inpatient comfort measures only order set at our hospital, which may account for the large proportion of patients receiving comfort care at the end of life. In addition, this order set may also help to improve the quality of end‐of‐life care and thus limit the need for palliative care consultation to some extent. However, we found that patients with a MET‐implemented DNR order had a shorter time from comfort care orders to death than patients with a preexisting DNR order. This finding suggests that patients with MET‐implemented DNR orders may have had comfort care implemented relatively late in the course of illness and had less‐than‐optimal end‐of‐life care. Vazquez et al. reported improved quality of end‐of‐life care after implementation of a MET.[10] However, an inpatient palliative care service was not available in that study, and it is not clear whether or not a comfort care order set was available. Evidence suggests that utilization of palliative care resources improves end‐of‐life care in the ICU.[17, 18, 19] We found that more than half of patients who died with comfort care in the hospital did so after being transferred to the ICU for a trial of aggressive care, suggesting that this population may have benefited from more involvement of our palliative care service. In summary, our data on end‐of‐life care following MET activation suggest that the METs are able to take advantage of an opportunity to identify patients who would not want resuscitation efforts because of personal preferences or futility of treatments. However, our surrogate measures of the quality of end‐of‐life care suggest that patients with MET‐implemented DNR orders may benefit from coordinated care with inpatient palliative care services, timelier implementation of comfort care orders, and possibly increased referrals for hospice care to help improve the quality of end‐of‐life care in this population.
Our study is subject to a number of limitations. This was a single‐center study, making the results difficult to generalize. The retrospective nature of our study makes it subject to the limitations inherent in this study design, including bias and confounding. The duration of MET activation was difficult to accurately and objectively measure and is subject to reporting bias. The event stop time, in particular, was subjectively measured by the MET nurse and is difficult to accurately assess, because MET members occasionally leave the bedside and return to reevaluate the response to therapy. We tried to account for this by clearly defining the MET stop time as the point at which MET members leave the bedside and transfer care back to the primary service or physically transfer the patient to a higher level of care. It also bears mentioning that the nurses performing data entry were not aware of the study hypothesis at the time of data entry. Despite including over 1100 MET calls in our analysis, the number of patients with a MET‐implemented DNR order was relatively small, which may have limited our ability to detect differences among subgroups during our analysis. We also did not document the clinical circumstances surrounding the MET‐implemented DNR order. Although we hypothesized that these patients had a higher mortality due to a higher acuity of illness, we were unable to support this hypothesis with the data available in our retrospective study. We did not record which providers were involved in code status discussions and the exact amount time spent on these discussions, making it difficult to accurately quantify the MET resources utilized on the calls. Our MET works closely with the patient's primary service, and it is possible that some of the changes to code status were implemented by the primary service and not MET providers. The patient's primary service may have a preexisting relationship with the patient and would be in a better position to discuss goals of care than MET providers who have had no prior relationship with the patient. However, even in this case, the clinical deterioration prompting MET activation was likely the event that triggered end‐of‐life discussions. Prospective studies would be helpful not only to identify the individuals involved in code status discussions during MET activations, but also to objectively measure the time spent on such discussions. Finally, our study population consisted primarily of Caucasian patients. Preferences for end‐of‐life care may differ among socioeconomic and ethnic groups, thus limiting the generalizability of our study findings.[20, 21]
In conclusion, we found the rate of MET‐implemented DNR orders in the United States to be similar to that of previous reports in other countries. MET events involving a change in code status are associated with increased utilization of MET and ICU resources, but a shorter hospital length of stay. Despite a high inpatient mortality rate, patients with a MET‐implemented DNR had a relatively low utilization of end‐of‐life resources, including palliative care and home hospice services. Coordinated care between METs and palliative care may help to improve of end‐of‐life care in patients with a change in code status following MET activation.
Acknowledgements
The authors acknowledge the hard work and dedication provided by Elizabeth Spellman during the data collection process.
Disclosures: This work was performed at Lahey Hospital and Medical Center. The authors report no conflicts of interest.
Approximately 15% to 20% of inpatients develop significant adverse events, which are often preceded by a change in the patient's condition.[1, 2] Many hospitals utilize medical emergency teams (METs) to deliver prompt care for deteriorating patients. METs have an emerging role in end‐of‐life care; up to 35% of patients who die in the hospital with a do not resuscitate (DNR) order have a MET activation during the admission.[3] Thus, METs are well positioned to discuss preferences for cardiopulmonary resuscitation in a population at high risk for cardiac arrest.[4]
MET activations involve a change in code status in approximately 3% to 10% of cases.[5, 6, 7, 8] However, previous studies primarily involved hospitals in Australia, making the results difficult to generalize to other countries.[3, 6, 7] There may be important cultural differences in patient and clinician attitudes toward end‐of‐life care among different countries.[9] In addition, little is known about METs and hospital resources utilized in patients with MET‐implemented changes in code status. Anecdotal evidence suggests that MET activations addressing code status are more time consuming.[3] Conversely, by identifying patients unlikely to benefit from restorative care, METs may facilitate end‐of‐life comfort care, thereby reducing unplanned intensive care unit (ICU) admissions and hospital length of stay. Finally, preliminary evidence suggests that METs may improve the quality of end‐of‐life care.[10] However, the use of end‐of‐life resources, including inpatient palliative care consultation and hospice care following MET activation has not been well studied.
The purpose of our study was to examine the role of a US MET in end‐of‐life care. First, we assessed the proportion of MET calls that resulted in a new DNR order in a US hospital and compared this to previous reports in other countries. We also examined MET and hospital resource utilization in MET activations involving changes in code status by evaluating the duration of MET activations, the need for telemetry or ICU transfer, and hospital length of stay following MET activation. Finally, we explored the quality of end‐of‐life care in patients with MET‐implemented DNR orders by assessing the utilization of inpatient palliative care consultation and hospice care compared to patients with a preexisting DNR order.
MATERIALS AND METHODS
We conducted this study at Lahey Clinic, a 350‐bed academic, tertiary care center. The institutional review board approved this study and waived the need for informed consent. We performed a retrospective review of a prospectively collected MET registry. We included consecutive, adult (>18 years old) inpatient MET activations and excluded nonhospitalized patients. Data were recorded in an intranet registry at the time of the event by the MET nurse, including preexisting code status (full code or DNR), any change to the patient's code status (full code to DNR or DNR to full code) during the MET event, date of the event, disposition after the event (no transfer, transferred to ICU, or transfer to telemetry), and a description of MET interventions. The primary reason for the event was also recorded, including cardiovascular (systolic blood pressure <90 mm Hg, pulse <40 or >130 bpm), respiratory (respiratory rate <10 or >24 breaths per minute, need for noninvasive positive pressure ventilation, oxygen saturation <90%), neurologic (loss of consciousness, change in mental status, seizure, or suspected stroke), or clinical deterioration causing staff to become worried. MET activation occurs at our institution by utilizing a text paging system that records the date and time of the call, along with the patient's location. The event start time was recorded from the paging system. We considered the event stop time to be the time at which the patient is transferred to a different level of care (ie, ICU) or the MET members leave the bedside and transfer care back to the primary service. MET nurses recorded the duration of MET activation in the intranet database immediately following the activation. Data were collected from the medical record, including age, gender, race, medical insurance, religion, admission source (home, assisted living, rehabilitation, or other hospital), admission team (internal medicine or surgery), admission date and time, discharge or death date and time, disposition at discharge (home, rehabilitation, death with or without a DNR order, or hospice care), and admission diagnosis. We categorized patients discharged to a hospice bridge program as being discharged with hospice care. We also recorded whether or not code status was discussed at the time of admission. Our hospital policy requires that an advanced directives order (either full code or DNR) must be placed on all patients at the time of admission. However, we only considered a code status discussion to have occurred if there was explicit documentation of a code status discussion in the medical record at the time of hospital admission. Data from the time of hospital admission were collected to calculate a Charlson Comorbidity Index, a well‐validated predictor of mortality that uses a weighted sum of 17 medical conditions, with scores ranging from 0 to 37.[11] Higher scores indicate a greater burden of illness. We also recorded whether or not the inpatient palliative care service was consulted following MET activation by examining the medical record for a consult note. The inpatient palliative care service at our institution was implemented in 2005 and receives over 700 new inpatient consults per year. The most common reason for consultation is to assist the primary service with discussing goals of care and code status.
Our MET was established in 2005 and responds to approximately 30 to 40 events per 1000 admissions. There are 4 members on our MET, including a critical care nurse, a nursing supervisor, a respiratory therapist, and a team leader who, depending on a predetermined schedule, is either an attending hospital medicine physician, an attending critical care physician, a critical care training physician, or a critical care physician assistant.
The data were transferred from the intranet registry to an Excel (Microsoft Corp., Redmond, WA) spreadsheet for statistical analysis. The primary outcome was the proportion of activations resulting in a MET‐implemented DNR order. Secondary outcomes included the duration of MET activation, need for transfer to telemetry or the ICU, hospital length of stay following MET activation (time from the end of the MET activation to hospital discharge or death), and the frequency with which inpatient palliative care consultation and outpatient hospice care were utilized. For repeat MET activations in a single patient, we considered each MET activation as a separate event as the code status could potentially change more than once. We used SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC) statistical analysis software for data analysis. The 2 method was used for categorical variables, and either a 2‐sample t test or Wilcoxon rank sum test was utilized for continuous variables. A P value 0.05 was considered significant.
RESULTS
We observed 1156 MET activations in 998 patients. The mean age was 67 years and 57% (565/998) were male (Table 1). The mean Charlson Comorbidity Index was 5.4. Most patients were admitted from home (76%, 760/998), to a medical service (72%, 720/998), and to a teaching service (73%, 732/998). Sepsis (11%, 109/998) and trauma (11%, 105/998) were the most common admission diagnoses. A cardiovascular abnormality was the most common (35%, 399/1156) reason for activation. A code status discussion was documented on admission in 44% (440/998) of all patients.
| Variable | Total, n=998 | No Change in Code Status During MET Activation, n=926 | MET‐Implemented Change in Code Status, n=72a | P Valueb | DNR Prior to MET Activation, n=100 | MET‐Implemented DNR Order, n=58 | P Valuec |
|---|---|---|---|---|---|---|---|
| |||||||
| Gender, male | 565 (56.6) | 527 (56.9) | 38 (52.8) | 0.50 | 52 (52.0) | 34 (58.6) | 0.42 |
| Age, yr, meanSTD | 6717 | 6717 | 7115 | 0.08 | 8111 | 7016 | <0.0001 |
| Race | |||||||
| Caucasian | 927 (92.9) | 859 (92.8) | 68(94.4) | 0.59 | 99 (99) | 54 (93.1) | 0.04 |
| Minorityd | 71 (7.1) | 67 (7.2) | 4(5.6) | 1 (1.0) | 4 (6.9) | ||
| Insurance | |||||||
| Medicare | 694 (69.5) | 635 (68.6) | 59 (81.9) | 0.02 | 89 (89.0) | 45 (77.6) | 0.05 |
| Private | 244 (24.5) | 235 (25.4) | 9 (12.5) | 0.01 | 10 (10.0) | 9 (15.5) | 0.30 |
| Medicaid | 41 (4.1) | 39 (4.2) | 2 (2.8) | 0.55 | 1 (1.0) | 2 (3.5) | 0.27 |
| None | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 0 (0) | 1 (1.7) | 0.19 |
| Religion | |||||||
| Christian | 748 (75.0) | 691 (74.6) | 57 (79.2) | 0.39 | 82 (82.0) | 46 (79.3) | 0.68 |
| None specified | 226 (22.7) | 213 (23.0) | 13 (18.1) | 0.33 | 14 (14.0) | 10 (17.2) | 0.58 |
| Other religions | 24 (2.4) | 22 (2.4) | 2 (2.7) | 0.83 | 4 (4.0) | 2 (3.5) | 0.86 |
| Admission diagnosis | |||||||
| Sepsis | 109 (10.9) | 100 (10.8) | 9 (12.5) | 0.66 | 11 (11.0) | 8 (13.8) | 0.60 |
| Trauma/fall | 105 (10.5) | 100 (10.8) | 5 (6.9) | 0.30 | 6 (6.0) | 5 (8.6) | 0.53 |
| Malignancy related | 79 (7.9) | 73 (7.9) | 6 (8.3) | 0.89 | 7 (7.0) | 4 (6.9) | 0.98 |
| Stroke | 47 (4.7) | 43 (4.6) | 4 (5.6) | 0.73 | 6 (6.0) | 4 (6.9) | 0.82 |
| Pneumonia | 46 (4.6) | 41 (4.4) | 5 (6.9) | 0.33 | 11 (11.0) | 3 (5.2) | 0.21 |
| Altered mental status | 43 (4.3) | 40 (4.3) | 3 (4.2) | 0.95 | 5 (5.0) | 3 (5.2) | 0.96 |
| Myocardial infarct | 42 (4.2) | 40 (4.3) | 2 (2.8) | 0.53 | 1 (1.0) | 1 (1.7) | 0.69 |
| Respiratory failure | 40 (4.0) | 36 (3.9) | 4 (5.6) | 0.49 | 2 (2.0) | 3 (5.2) | 0.27 |
| Arrhythmia | 37 (3.7) | 33 (3.6) | 4 (5.6) | 0.39 | 2 (2.0) | 3 (5.2) | 0.27 |
| Heart failure | 35 (3.5) | 33 (3.6) | 2 (2.8) | 0.72 | 10 (10.0) | 2 (3.5) | 0.13 |
| Other | 415 (41.6) | 387 (41.8) | 28 (38.9) | 0.63 | 39 (39.0) | 22 (37.9) | 0.89 |
| Admission type | |||||||
| Medical | 720 (72.1) | 662 (71.5) | 58 (80.6) | 0.10 | 91 (91.0) | 46 (79.3) | 0.04 |
| Surgical | 278 (27.9) | 264 (28.5) | 14 (19.4) | 9 (9.0) | 12 (20.7) | ||
| Admission source | |||||||
| Home | 760 (76.2) | 706 (76.2) | 54 (75.0) | 0.81 | 60 (60.0) | 44 (75.9) | 0.04 |
| Assisted Living | 29 (2.9) | 28(3.0) | 1 (1.4) | 0.43 | 9 (9.0) | 1 (1.7) | 0.07 |
| Nursing Home | 69 (6.9) | 65(7.0) | 4 (5.6) | 0.64 | 19 (19.0) | 1 (1.7) | <0.01 |
| Outside hospital | 139 (13.9) | 126(13.6) | 13 (18.1) | 0.29 | 12 (12.0) | 12 (20.7) | 0.14 |
| Other | 1 (0.1) | 0(0) | 1 (0.1) | 0.78 | 0 (0) | 0 | |
| Teaching service | 732 (73.4) | 678 (73.2) | 54 (75.0) | 0.74 | 84 (84.0) | 41 (70.7) | 0.05 |
| Code status discussed on admission | 440(44.1) | 397 (42.9) | 43 (59.7) | 0.01 | 70 (70.0) | 32 (55.2) | 0.06 |
| CCI, meanSTDe | 5.43.0 | 5.43.0 | 5.83.0 | 0.21 | 7.72.4 | 5.73.0 | <0.001 |
| MI | 226 (22.7) | 210 (22.7) | 16 (22.2) | 0.93 | 36 (36.0) | 13 (22.4) | 0.08 |
| Heart failure | 138 (13.8) | 127 (13.7) | 11 (15.3) | 0.71 | 28 (28.0) | 8 (13.8) | 0.04 |
| PVD | 90 (9.0) | 85 (9.2) | 5 (6.9) | 0.52 | 14 (14.0) | 4(6.9) | 0.18 |
| Stroke | 131 (13.1) | 121 (13.1) | 10 (13.9) | 0.84 | 30 (30.0) | 9 (15.5) | 0.04 |
| Dementia | 58 (5.8) | 51(5.5) | 7 (9.7) | 0.14 | 19 (19.0) | 5 (8.6) | 0.08 |
| COPD | 173 (17.3) | 161 (17.4) | 12 (16.7) | 0.88 | 23 (23.0) | 8 (13.8) | 0.16 |
| CTD | 58 (5.8) | 56 (6.1) | 2 (2.8) | 0.25 | 6 (6.0) | 2 (3.5) | 0.48 |
| Peptic ulcer disease | 26 (2.6) | 25 (2.7) | 1 (1.4) | 0.50 | 2 (2.0) | 1 (1.7) | 0.90 |
| Mild liver disease | 33 (3.3) | 32 (3.5) | 1 (1.4) | 0.34 | 1 (1.0) | 1 (1.7) | 0.69 |
| DM | 213 (21.3) | 194 (21.0) | 19 (16.4) | 0.28 | 19 (19.0) | 15 (25.9) | 0.31 |
| Hemiplegia | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 2 (2.0) | 1 (1.7) | 0.90 |
| Renal disease | 131 (13.1) | 119 (12.9) | 12 (16.7) | 0.36 | 21 (21.0) | 9 (15.5) | 0.40 |
| DM+organ damage | 68 (6.8) | 64 (6.9) | 4 (5.6) | 0.66 | 7 (7.0) | 3 (5.2) | 0.65 |
| Any tumor | 188 (18.8) | 173 (18.7) | 15 (20.8) | 0.65 | 25 (25.0) | 14 (24.1) | 0.90 |
| Lymphoma | 21 (2.1) | 20 (2.2) | 1 (1.4) | 0.66 | 2 (2.0) | 1 (1.7) | 0.90 |
| Leukemia | 20 (2.0) | 18 (1.9) | 2 (2.8) | 0.63 | 1 (1.0) | 0 (0.0) | 0.45 |
| Moderate/severe liver disease | 45 (4.5) | 39 (4.2) | 6 (8.3) | 0.10 | 0 (0.0) | 6 (10.3) | 0.001 |
| Metastatic tumor | 61 (6.1) | 51 (5.5) | 10 (13.9) | 0.004 | 10 (10.0) | 8 (13.8) | 0.47 |
| AIDS | 4 (0.4) | 3 (0.3) | 1 (1.4) | 0.17 | 0 (0.0) | 1 (1.7) | 0.19 |
MET activation resulted in a DNR order in 5% (58/1156) of cases (Figure 1). In activations involving a change in code status, 21% (15/73) were changed from DNR to full code. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order where younger (70 vs 81 years, P<0.0001), more commonly admitted from home (60% vs 44%, P=0.04), less frequently from a nursing home (1% vs 9%, P<0.01), and had a lower Charlson index (5.7 vs 7.7, P<0.001) (Table 1). Moderate to severe liver disease was more common in patients with a MET‐implemented DNR order (10% vs 0%, P=0.001). Admission diagnoses were similar between patients with a preexisting DNR and a MET‐implemented DNR order (Table 1).

The median time spent on activations with a change in code status was significantly longer than activations without a change (66 vs 60 minutes, P=0.05). The rates of telemetry (6% vs 3%, P=0.24) and ICU transfer (40% vs 41%, P=0.8) were similar between patients with a change in code status and patients without a change (Table 2). Patients with a MET‐implemented DNR order were more frequently transferred to the ICU than patients with a preexisting DNR order (36% vs 17%, P<0.01). The median hospital length of stay following MET activation was shorter in patients with a change in code status compared to patients with no change (3 vs 5 days, P<0.0001).
| Variable | Total, N=1,156 | No Change in Code Status During MET Activation, n=1,083 | MET‐Implemented Change in Code Status, n=73a | PValuec | DNR Prior to MET Activation, n=115 | MET‐Implemented DNR Order, n=58 | P Valued |
|---|---|---|---|---|---|---|---|
| |||||||
| Reason for callb | |||||||
| Cardiovascular | 399 (34.5) | 379 (35.0) | 20 (27.4) | 0.19 | 39 (33.9) | 19 (32.8) | 0.88 |
| Respiratory | 319 (27.6) | 295 (27.2) | 2 (32.9) | 0.30 | 40 (34.8) | 18 (31.0) | 0.62 |
| Neurologic | 215 (18.6) | 196 (18.1) | 19 (26.0) | 0.09 | 21 (18.3) | 15 (25.9) | 0.25 |
| Other | 323 (27.9) | 303 (28.0) | 20 (27.4) | 0.92 | 22 (19.1) | 15 (25.9) | 0.31 |
| MET resources, call duration, min, median (IQR) | 60 (4090) | 60 (4090) | 66 (43100) | 0.05 | 50 (3075) | 67 (50100) | <0.001 |
| Hospital resources | |||||||
| Tele transfer | 68 (5.9) | 663 (6.1) | 2 (2.7) | 0.24 | 3 (2.6) | 2(3.5) | 0.76 |
| ICU transfer | 459 (39.7) | 429 (39.6) | 30 (41.1) | 0.8 | 19 (16.5) | 21 (36.2) | <0.01 |
| LOS after MET activation, d, median (IQR) | 5.2 (0.2510.7) | 5 (5.411.0) | 2.8 (0.66.7) | <0.0001 | 3.8 (1.56.5) | 2.9 (0.56.5) | 0.06 |
| End‐of‐life care | n=191e | n=157 | n=34 | n=41 | n=26 | ||
| Palliative care | 31 (16.2) | 27 (17.2) | 4 (11.8) | 0.44 | 8 (19.5) | 3 (11.5) | 0.39 |
| CMO orders | 159 (83.3) | 127 (80.9) | 32 (94.1) | 0.06 | 33 (80.5) | 25 (96.2) | 0.07 |
| Died full code | 10 (5.2) | 10 (6.4) | 0 (0) | 0.13 | 2 (4.9) | 0 (0) | 0.25 |
| Died DNR | 155 (81.2) | 123 (78.3) | 32 (94.1) | 0.03 | 27 (65.9) | 25 (96.2) | 0.004 |
| Hospice | 26 (13.6) | 24 (15.3) | 2 (5.9) | 0.15 | 12 (29.3) | 1 (3.9) | 0.01 |
The inpatient mortality was 17% (165/998). Most patients who died had the focus of care changed to comfort measures only (88%, 146/165). When examining the group of patients who died in the hospital with comfort care, we found that 58% (92/159) were transferred to the ICU following the MET call, 5% (8/159) were changed to comfort care during the MET call, and 18% (29/159) had a palliative care consult. We also observed that 16% (25/159) patients who died with comfort care were made DNR during MET activation. The inpatient mortality was significantly higher in patients with a change in code status compared to patients with no change in code status (44% vs 14%, 133/926, P<0.0001). Patients with a MET‐implemented DNR order had a higher inpatient mortality than patients with a preexisting DNR (43% vs 27%, P=0.04). Twenty‐five patients with a MET‐implemented DNR order died in the hospital. When examining a subgroup of patients who required end‐of‐life care (died or discharged from the hospital with hospice), we found patients with a MET‐implemented DNR order were less likely to be discharged with hospice care than patients with a preexisting DNR (4% vs 29%, P=0.01). There was no difference in the use of inpatient palliative care consultation at the end of life in patients with a preexisting DNR versus MET‐implemented DNR order (20% vs 12%, P=0.39). Patients with a MET‐implemented DNR order also had a significantly shorter median time from implementation of comfort care orders to death or discharge with hospice compared to patients with a preexisting DNR order (7 hours, interquartile range [IQR], 416 hours vs 22 hours, IQR 939 hours).
DISCUSSION
We observed a MET‐implemented DNR order in 5% of activations. Little is known about the role of METs in end‐of‐life discussions in the United States, and past experience has primarily come from Australian hospitals. Important differences in end‐of‐life care exist among different countries, particularly with regard to placing limitations on treatment.[9] Our observed rate is similar to the 3% to 10% rate of MET‐implemented DNR orders in previous reports worldwide.[3, 5, 8, 12, 13] Recent data from the United States suggest that METs initiate a DNR order in 28% of cases.[14] However, most DNR orders in that study were placed in the ICU days to weeks after MET activation, likely accounting for the high DNR rate. Our data add to the growing body of evidence that METs play an important role in end‐of‐life discussions among different countries throughout the world, including the United States.
To our knowledge, no prior study has evaluated the impact of code status discussions on MET resource utilization. Our MET spent 6 minutes longer on activations involving a change in code status when compared to activations with no changes made to code status. Presumably, some of this time was spent discussing goals of care. In our opinion, the additional time spent on these activations was invaluable, particularly when considering MET‐initiated end‐of‐life discussions may have prevented several unwanted resuscitations (25 patients died with a MET‐implemented DNR order). Interestingly, less than half of patients had code status discussions at the time of hospital admission. This finding suggests that clinicians could be more vigilant about discussing preferences for resuscitation at the time of admission in patients at risk for clinical deterioration. We suspect that in some cases code status discussions may have occurred between the patient and the primary service later in the patient's hospitalization, which were not captured in our study.
Surprisingly, when examining the use of hospital resources, we found no difference in the rate of unplanned ICU transfer in patients with a change in code status. In fact, we observed a higher rate of ICU transfer in patients with a MET‐implemented DNR order compared to those with a preexisting DNR order (36% vs 17%). These results were at odds with our hypothesis of a lower rate of ICU transfer in patients with MET‐implemented limitations in care. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order were younger, more commonly admitted from home, and had a lower Charlson index. Despite evidence of a lower burden of chronic illness and younger age, patients with a MET‐implemented DNR order had higher inpatient mortality than patients with a preexisting DNR order (43% vs 27%), suggesting an acute and rapidly progressive disease process. These observations may have compelled the MET to advocate for aggressive ICU‐level care in patients with a MET‐implemented DNR order. Another possible explanation for the relatively high rate of ICU transfer is that the MET is, in part, led by ICU staff. Thus, our MET may have made the decision to transfer the patient to the ICU and then subsequently initiated end‐of‐life discussions only after taking ownership of the patient. Furthermore, almost 20% of MET‐implemented changes to code status involved reversing status from DNR to full code. These data suggest that METs are not merely serving as a resource to review code status, but rather providing intensive treatment for acutely ill patients and simultaneously initiating end‐of‐life discussions in a population with a high inpatient mortality rate. The practice pattern observed in our study of transferring patients to the ICU for a trial of intensive therapy at the end of life is consistent with the overall trend in the United States for increased inpatient treatment intensity at the end of life.[14, 15, 16]
Our data suggest that the increased use of ICU resources in patients with a MET‐implemented DNR may be balanced by a shorter hospital length of stay following MET activation. In a multicenter observational study, Jones et al. found hospital length of stay to be similar in patients with and without a MET‐implemented limitation of medical therapy.[3] The authors did not examine length of stay specifically in patients with a DNR order, but rather examined patients with any limitation in medical therapy, including not for ICU admission. We suspect that the shorter length of stay following MET activation in our study was related to the fact that patients with a change in code status had a significantly higher inpatient mortality.
We observed several interesting findings with regard to end‐of‐life care following MET‐implemented DNR orders. First, the inpatient mortality in this population was remarkably high at 43%, compared to 27% in patients with a preexisting DNR order. Interestingly, there was no difference in the rate of palliative care consultation between the 2 groups despite the fact that all 25 patients who died following a MET‐implemented DNR order did so with a comfort measures only order. We also found that patients with a preexisting DNR also had a higher rate of discharge with hospice compared to patients with a MET‐implemented DNR order (29% vs 4%). Thus, our data suggest that inpatient palliative care consultation and hospice services are not resources that are routinely utilized in patients with MET‐initiated DNR orders. It may be the case that the acuity and severity of illness or patient preferences may have precluded the possibility of discharging some patients in our study home with hospice care or implementing comfort care earlier in the hospital course. Patients with MET‐implemented DNR orders were younger, had fewer comorbidities, and died sooner after comfort care orders were written. The overall rate of comfort care provided to patients who died was high at 88%. We have an inpatient comfort measures only order set at our hospital, which may account for the large proportion of patients receiving comfort care at the end of life. In addition, this order set may also help to improve the quality of end‐of‐life care and thus limit the need for palliative care consultation to some extent. However, we found that patients with a MET‐implemented DNR order had a shorter time from comfort care orders to death than patients with a preexisting DNR order. This finding suggests that patients with MET‐implemented DNR orders may have had comfort care implemented relatively late in the course of illness and had less‐than‐optimal end‐of‐life care. Vazquez et al. reported improved quality of end‐of‐life care after implementation of a MET.[10] However, an inpatient palliative care service was not available in that study, and it is not clear whether or not a comfort care order set was available. Evidence suggests that utilization of palliative care resources improves end‐of‐life care in the ICU.[17, 18, 19] We found that more than half of patients who died with comfort care in the hospital did so after being transferred to the ICU for a trial of aggressive care, suggesting that this population may have benefited from more involvement of our palliative care service. In summary, our data on end‐of‐life care following MET activation suggest that the METs are able to take advantage of an opportunity to identify patients who would not want resuscitation efforts because of personal preferences or futility of treatments. However, our surrogate measures of the quality of end‐of‐life care suggest that patients with MET‐implemented DNR orders may benefit from coordinated care with inpatient palliative care services, timelier implementation of comfort care orders, and possibly increased referrals for hospice care to help improve the quality of end‐of‐life care in this population.
Our study is subject to a number of limitations. This was a single‐center study, making the results difficult to generalize. The retrospective nature of our study makes it subject to the limitations inherent in this study design, including bias and confounding. The duration of MET activation was difficult to accurately and objectively measure and is subject to reporting bias. The event stop time, in particular, was subjectively measured by the MET nurse and is difficult to accurately assess, because MET members occasionally leave the bedside and return to reevaluate the response to therapy. We tried to account for this by clearly defining the MET stop time as the point at which MET members leave the bedside and transfer care back to the primary service or physically transfer the patient to a higher level of care. It also bears mentioning that the nurses performing data entry were not aware of the study hypothesis at the time of data entry. Despite including over 1100 MET calls in our analysis, the number of patients with a MET‐implemented DNR order was relatively small, which may have limited our ability to detect differences among subgroups during our analysis. We also did not document the clinical circumstances surrounding the MET‐implemented DNR order. Although we hypothesized that these patients had a higher mortality due to a higher acuity of illness, we were unable to support this hypothesis with the data available in our retrospective study. We did not record which providers were involved in code status discussions and the exact amount time spent on these discussions, making it difficult to accurately quantify the MET resources utilized on the calls. Our MET works closely with the patient's primary service, and it is possible that some of the changes to code status were implemented by the primary service and not MET providers. The patient's primary service may have a preexisting relationship with the patient and would be in a better position to discuss goals of care than MET providers who have had no prior relationship with the patient. However, even in this case, the clinical deterioration prompting MET activation was likely the event that triggered end‐of‐life discussions. Prospective studies would be helpful not only to identify the individuals involved in code status discussions during MET activations, but also to objectively measure the time spent on such discussions. Finally, our study population consisted primarily of Caucasian patients. Preferences for end‐of‐life care may differ among socioeconomic and ethnic groups, thus limiting the generalizability of our study findings.[20, 21]
In conclusion, we found the rate of MET‐implemented DNR orders in the United States to be similar to that of previous reports in other countries. MET events involving a change in code status are associated with increased utilization of MET and ICU resources, but a shorter hospital length of stay. Despite a high inpatient mortality rate, patients with a MET‐implemented DNR had a relatively low utilization of end‐of‐life resources, including palliative care and home hospice services. Coordinated care between METs and palliative care may help to improve of end‐of‐life care in patients with a change in code status following MET activation.
Acknowledgements
The authors acknowledge the hard work and dedication provided by Elizabeth Spellman during the data collection process.
Disclosures: This work was performed at Lahey Hospital and Medical Center. The authors report no conflicts of interest.
- , , , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645.
- , , , et al. Antecedents to hospital deaths. Intern Med J. 2001;31(6):343–348.
- , , , et al. The role of the medical emergency team in end‐of‐life care: a multicenter, prospective, observational study. Crit Care Med. 2012;40(1):98–103.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow‐up study. Resuscitation. 2010;81(1):31–35.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , et al. The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79(3):391–397.
- . Cultural differences in end‐of‐life care. Crit Care Med. 2001;29(2 Suppl):N52–N55.
- , , , et al. Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9(2):151–156.
- , , , et al. A retrospective cohort study of the effect of medical emergency teams on documentation of advance care directives. Crit Care Resusc. 2011;13(3):167–174.
- , , , et al. The medical emergency team a call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and healthcare transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39(2):363–375.
- , , , et al. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients. Crit Care Med. 2007;35(6):1530–1535.
- , . Impact of a proactive approach to improve end‐of‐life care in a medical ICU. Chest. 2003;123(1):266–271.
- , , , et al. In their own words: patients and families define high‐quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818.
- , , , et al. The influence of race/ethnicity and socioeconomic status on end‐of‐life care in the ICU. Chest. 2011;139(5):1025–1033.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137.
- , , , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645.
- , , , et al. Antecedents to hospital deaths. Intern Med J. 2001;31(6):343–348.
- , , , et al. The role of the medical emergency team in end‐of‐life care: a multicenter, prospective, observational study. Crit Care Med. 2012;40(1):98–103.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow‐up study. Resuscitation. 2010;81(1):31–35.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , et al. The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79(3):391–397.
- . Cultural differences in end‐of‐life care. Crit Care Med. 2001;29(2 Suppl):N52–N55.
- , , , et al. Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9(2):151–156.
- , , , et al. A retrospective cohort study of the effect of medical emergency teams on documentation of advance care directives. Crit Care Resusc. 2011;13(3):167–174.
- , , , et al. The medical emergency team a call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and healthcare transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39(2):363–375.
- , , , et al. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients. Crit Care Med. 2007;35(6):1530–1535.
- , . Impact of a proactive approach to improve end‐of‐life care in a medical ICU. Chest. 2003;123(1):266–271.
- , , , et al. In their own words: patients and families define high‐quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818.
- , , , et al. The influence of race/ethnicity and socioeconomic status on end‐of‐life care in the ICU. Chest. 2011;139(5):1025–1033.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137.
© 2014 Society of Hospital Medicine