User login
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
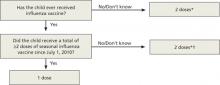
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
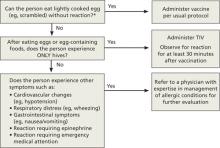
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.