User login
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
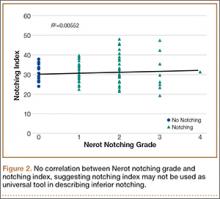
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
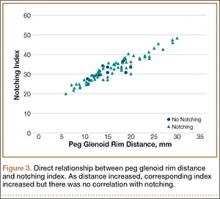
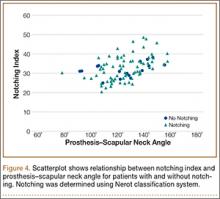
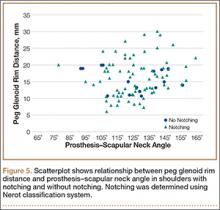
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.