User login
Acute limb ischemia (ALI) is an emergent medical condition that is characterized by a precipitous decrease in limb perfusion that threatens the viability of the affected limb, and symptoms that have been present for 14 days or less.1-5 The incidence of lower extremity ALI in the US is nine to 16 cases per 100,000 persons per year, and one to three cases per 100,000 persons per year for upper extremity ALI.3 Symptoms suggestive of this condition classically include pain in the effected limb at rest, loss of sensation, impaired motor function, and cyanosis/pallor. Irreparable damage can occur in as quickly as 4 to 6 hours with complete arterial occlusion. A majority of ALI is caused by thrombosis, with the remainder of cases caused by embolism, 85% and 15% respectively.1-6 If not addressed promptly, this condition carries a high degree of morbidity (ie, limb loss) and mortality. Immediate initiation of anticoagulation therapy and vascular surgery consultation are mainstays of ED management. Time-to-treatment is a predictor of success and is an area where the emergency physician (EP) can make a significant difference in patient outcomes.7 Although vascular interruption via traumatic mechanism is not addressed in this review, it should remain a consideration in relation to the historical features of each case.
Presentation
Traditionally, symptoms of ALI can be remembered as the “six Ps”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia. Note that ALI represents a spectrum of disease, and the presenting features depend heavily on the level and degree of obstruction. Pain, however, is most frequently the first presenting symptom of patients with ALI.1-6 Pain will occur in muscle groups distal to the occlusion. Obstruction of the aortoiliac region might produce pain in the buttocks, thigh, and hip, whereas occlusion of the femoral artery may produce pain in the calf. Single-level disease will often produce claudication, but multilevel disease may present as a nonhealing ulcer or with focal gangrene. The differential diagnosis of ALI includes direct arterial injury, vasospasm, compartment syndrome, chronic peripheral artery disease, stroke, spinal cord injury, vasculitis, muscular trauma, and radiculopathy.3,5
Etiology
As stated previously, ALI is a result of either thrombotic or embolic phenomena that either partially or completely occludes a vessel such that adequate perfusion is no longer achieved. Consequently, there is a decrease in the metabolism of the tissue supplied in those territories, which can rapidly progress to necrosis. Thrombosis is the most common cause of ALI, accounting for approximately 85%.2,3Typically, thrombosis presents in the setting of pre-existing peripheral artery disease (PAD).8 As PAD worsens, damage to the arterial endothelium triggers platelet activation, and accumulation in a manner conceptually similar to thrombosis in myocardial infarction.9 Hypercoagulable states increase the risk of thrombi development.10 Symptoms of thrombosis are often more insidious in onset when compared to embolism, and signs/symptoms of prior claudication are almost always present. Consequently, there is a greater chance that collateral circulation has developed over time.2,6,11,12
By contrast, ALI caused by embolism is more acute in onset, and is often more emergent due to complete occlusion without chance of collateral vessel development.1,3,6,12 See Table 1 for differences in risk factors and presentation between thrombotic and embolic occlusion.
Historical Features
Information should be obtained systematically in all presentations and should include time of symptom onset, duration, severity, and location; however, special attention should be paid to the evolution of symptoms over time. One historical pearl that should raise suspicion of embolism vs thrombus is a patient history with a specific time of symptom onset. Specific questioning about history of claudication, including description of typical symptoms, is critical. A full list of other medical comorbidities should be obtained. This may reveal risk factors for thrombotic and embolic disease.1,3-5 This also may alter available treatment options.7,8,12,13 At presentation, the EP should obtain a list of the patient’s current medications, and special attention should be given to prothrombotic medications and medications that may interact with anticoagulants.
Physical Examination
On physical examination, the EP should pay particular attention to both the external appearance and temperature of the skin of the patient’s affected extremity. The presence or absence of peripheral pulses is an important feature when assessing for ALI; however, the presence of peripheral pulses does not exclude ALI from the differential.
Precise evaluation of sensation is important to attempt to quantify the extent of injury, localize a possible obstruction, and differentiate ALI from other possible causes of sensation deficits. Though not referenced in the six Ps, the patient’s limb may also appear mottled or marbled. Embolic phenomena occur at sites of vascular bifurcation. In a patient with aortic embolism, femoral pulses will be absent with bilateral mottling and paralysis of the lower extremities. Iliac artery embolism will produce the same symptoms, except unilateral. Femoral artery embolism will yield coolness and mottling distal to the inguinal ligament. Similarly, popliteal artery embolism will produce those same findings, except distal to the knee with preserved femoral pulses.
Diagnosis and Evaluation
After the history and physical examination are completed, further diagnostic methodologies should be considered, including laboratory evaluation and additional imaging studies.
Imaging Studies
Doppler ultrasound examination to assess for pulses on the affected limb should be performed immediately. Bedside continuous-wave Doppler may be used to differentiate arterial vs venous signal.6,14-16 An ankle brachial index may be calculated through either direct auscultation of pulses or Doppler signal. A ratio of less than 0.9 is suggestive of ischemia, while a ratio of less than 0.5 is considered critical ischemia.2
Laboratory Evaluation
Laboratory evaluation of hematocrit, coagulation studies, renal function, electrolyte levels, lactate, and creatinine kinase should be completed.1,5 An electrocardiogram and bedside/formal echocardiogram may be considered if embolism is suspected.1,6,17
Imaging
Angiography is currently the gold standard imaging modality to diagnose ALI.16 However, other imaging modalities exist that expose the patient to less radiation and are generally preferred by patients.18 Duplex ultrasonography is the least invasive imaging modality, with a reported sensitivity of 88% and specificity of 96% for the detection of greater than 50% stenosis.6,19 Contrast-enhanced magnetic resonance angiography (MRA) of the lower extremity is also relatively noninvasive, but is more limited in its application. It carries a reported sensitivity of 95% and a specificity of 97% for detection of stenosis of greater than 50%.6,19 An MRA without contrast may be considered for patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and who are not on dialysis, but the diagnostic efficacy is reduced.16 Finally, the sensitivity and specificity of computed tomography angiography (CTA) to detect aortoiliac stenosis of greater than 50% is 96% and 98% respectively.6,18,19 Other studies demonstrated similar results for other arterial locations in the lower extremities.18,19 A principal advantage of CTA is that direct visualization of calcifications, clips, stents, and prior bypasses is possible without the limitations of MRA.16 The American College of Radiology (ACR) lists ultrasound duplex Doppler and noncontrast MRA of the lower extremity as “may be appropriate.”16 In contrast, the ACR classifies MRA with contrast, CTA with contrast, and angiography of the lower extremity as “usually appropriate.”16 Selecting the optimal imaging study for a patient may require involvement of a facility’s radiology department and/or a vascular surgeon.14,15
Management in the ED
Even before the definitive diagnosis of ALI is made, steps can be taken to minimize and/or slow progression of injury. Placing the affected limb in a dependent position and providing intravenous (IV) fluid hydration will maximize perfusion.1,3,5 Even with consideration of the diagnostic modalities previously described, ALI is often considered a clinical diagnosis. Since time is critical, early consultation with vascular surgery services is imperative if ALI is suspected.
Intravenous Fluid Therapy
Suspected ALI should be treated in the ED with an IV heparin bolus, followed by constant IV infusion. Heparin prevents proximal and distal propagation of the thrombus.1,3,5,6,15,17,20 Additionally, it helps maintain the microcirculation surrounding the affected area.6 A heparin bolus dose of 100 U/kg followed by a continuous infusion of heparin 1,000 U/h is the recommended standard.1,3,5,6,15 A goal partial thromboplastin time of 60 to 100 seconds, or an international normalized ratio of 2 to 3 is desirable.8,21
Staging: The Rutherford Classification System
Once immediate therapies are started, the stage of PAD should be determined using the Rutherford classification system in collaboration with vascular surgery services; this will further guide treatment and disposition.
Category I. In category I ALI, the affected limb is considered viable and not immediately threatened.
Category IIa. The limb is considered to be marginally threatened and salvageable.
Category IIb. An ALI classified as a category IIb is the most urgent type in which the limb is immediately threatened.
Category III. The limb is classified as having irreversible damage.2,3,17,22
(See Table 2 for a more detailed summary of the Rutherford classification system.)
Treatment
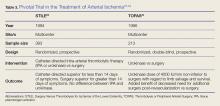
Definitive treatment decisions should be made in consultation with vascular surgery services. The results of three clinical trials developed the foundation for management of ALI: the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, Surgery Versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, and Rutherford trial.22-24 Table 3 provides a summary of the treatment techniques utilized, which include mechanical recanalization with percutaneous aspiration thrombectomy, percutaneous mechanical thrombectomy,6,12,25-28 pharmacological recanalization via catheter-directed thrombolysis,6,8,12,20,23-28 and/or surgical management via thrombectomy or embolectomy.6,11,23,24,27
Complications
The complications of treating ALI can be stratified into three subsets: pharmacologic, mechanical, and reperfusion injury-related. Medical treatment with heparin is not without risk. Intracranial hemorrhage, major bleeding at other sites, and compartment syndrome secondary to bleeding can all occur. Treatment may also cause distal embolization and lead to mechanical occlusion of another site. Finally, reperfusion injury predisposes the patient to a host of new problems. Reperfusion injury occurs when fresh blood enters a previously ischemic zone. When this occurs, oxygen free radicals, inflammatory mediators, and catabolism byproducts mix with the blood. This process can damage surrounding epithelial cells and lead to increased interstitial permeability. As a result, compartment syndrome may also develop via this mechanism.1,3,5,6 The anterior compartment of the lower extremity is most at risk. As such, assessment of peroneal nerve activity via dorsiflexion of the foot and sensation testing is important after the initiation of treatment.6 Systemic distribution of these harmful byproducts can also cause arrhythmias, renal failure, and systemic acadisos.1,3,5,6 It is important for physicians and staff to monitor the patient closely for the development of these complications and be prepared to intervene.
Opportunities for Improvement
In a condition where minutes matter, expedient action is critical. Time-to-recognition, imaging, consultation, and intervention all are potential sources of delay. Two recent studies have investigated the treatment timeline of ALI. The first study showed that the greatest source of delay is time from symptom onset to presentation in the ED; an average of 11.35 hours. The second largest source of delay was time from recognition of ALI to imaging, with an average delay of 4.75 hours. The average ED evaluation time was 40 minutes, and the average total time to intervention was 10.2 hours. While time-to-symptom presentation may represent a failure of public health, time-to-imaging was identified as an area of unacceptable delay by the authors.29 A second study examined the effect of pre-hospital care on the time-to-treatment. Persons who were transported via emergency medical services (EMS) arrived to the hospital at a median time of 5 hours after symptom onset, were seen by a physician at a median time of 51 minutes, and had revascularization at a median time of 23 hours. Those not transported by EMS arrived to the hospital at a median time of 48 hours after symptom onset, were seen by a physician at a median time of 80 minutes, and had revascularization at a median time of 93 hours. As a secondary goal, the study examined the effect of heparinization in the ED and showed that treatment with heparin was associated with a favorable outcome.30 While the total treatment times in each study vary widely, they share several important commonalities and reinforce expedient management as an important goal.
Outcomes
Advances in the treatment of this condition began in the 1970s, with an explosion of new techniques and data in the past several years. Still, ALI is a high morbidity and high mortality condition. Rates of limb loss are approximately 30% for all cases, and it is a fatal condition for as many as one-in-five. As discussed earlier, the patients who are most likely to experience ALI typically have several medical comorbidities. Perhaps unsurprisingly, ALI portends a poor prognosis even if the limb is salvaged. Approximately 15% to 20% of patients with ALI will die within 1 year of diagnosis.3,4,6,12
Conclusion
Acute limb ischemia is a rare condition, but a true medical emergency. Prompt recognition of the signs and symptoms of ALI are critical for optimizing patient outcomes. A high index of clinical suspicion in patients with risk factors may lead to early diagnosis. Early vascular surgery consultation and early IV heparin treatment are important aspects of care. Prompt imaging and staging guide further management. Special care should be paid to possible post-treatment complications and to the investigation of contributing factors. The EP has a great opportunity to positively impact care with expeditious management. Early diagnosis, targeted history and physical examination, prompt treatment, collaboration with specialists, and prevention and treatment of life-threatening complications are all hallmarks of emergency medicine.
1. Braun R, Lin M. Acute limb ischemia: a case report and literature review. J Emerg Med. 2015;49(6):1011-1017. doi:10.1016/j.jemermed.2015.03.008.
2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ. 2000;320(7237):764-767.
3. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198-2206. doi:10.1056/NEJMcp1006054.
4. Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interv Cardiol Clin. 2014;3(4):557-572.
5. Santistevan JR. Acute limb ischemia: an emergency medicine approach. Emerg Med Clin North Am. 2017;35(4):889-909. doi:10.1016/j.emc.2017.07.006.
6. Acar RD, Sahin M, Kirma C. One of the most urgent vascular circumstances: acute limb ischemia. SAGE Open Med. 2013;1:2050312113516110. doi:10.1177/2050312113516110.
7. Abou-Zamzam AM Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458-463.
8. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465-1508.
9. Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46(3):485-490.
10. Deitcher SR, Carman TL, Sheikh MA, Gomes M. Hypercoagulable syndromes: evaluation and management strategies for acute limb ischemia. Semin Vasc Surg. 2001;14(2):74-85.
11. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
12. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988-995.
13. Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;65(1):108-118. doi:10.1016/j.jvs.2016.06.105.
14. Aboyans V, Björck M, Brodmann M, et al; ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017. doi:10.1093/eurheartj/ehx499. [Epub ahead of print]
15. Authors/Task Force Members, Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017. doi:10.1016/j.ejvs.2017.07.018. [Epub ahead of print]
16. Expert Panel on Vascular Imaging: Weiss CR, Azene EM, Majdalany BS, et al. ACR Appropriateness Criteria® sudden onset of cold, painful leg. J Am Coll Radiol. 2017;14(5S):S307-S313. doi:10.1016/j.jacr.2017.02.015.
17. Dieter RS, Dieter RA Jr, Dieter RA 3rd, Nanjundappa A. Critical Limb Ischemia: Acute and Chronic. New York, NY: Springer; 2016.
18. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415-424.
19. Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
20. Giannini D, Balbarini A. Thrombolytic therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):249-258.
21. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S. doi:10.1378/chest.08-0658.
22. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
23. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23(1):64-73; discussion 74-75.
24. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220(3):251-266; discussion 266-268.
25. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53(2):340-346. doi:10.1016/j.jvs.2010.08.064.
26. Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15.
27. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61(1):147-154. doi:10.1016/j.jvs.2014.06.109.
28. Zeller T, Tepe G. Treatment of acute limb ischemia with focus on endovascular techniques. Vasa. 2009;38(2):123-133. doi: 10.1024/0301-1526.38.2.123.
29. Normahani P, Standfield NJ, Jaffer U. Sources of delay in the acute limb ischemia patient pathway. Ann Vasc Surg. 2017;38:279-285. doi:10.1016/j.avsg.2016.05.118.
30. Langenskiöld M, Smidfelt K, Karlsson A, Bohm C, Herlitz J, Nordanstig J. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54(2):235-240. doi:10.1016/j.ejvs.2017.04.010.
Acute limb ischemia (ALI) is an emergent medical condition that is characterized by a precipitous decrease in limb perfusion that threatens the viability of the affected limb, and symptoms that have been present for 14 days or less.1-5 The incidence of lower extremity ALI in the US is nine to 16 cases per 100,000 persons per year, and one to three cases per 100,000 persons per year for upper extremity ALI.3 Symptoms suggestive of this condition classically include pain in the effected limb at rest, loss of sensation, impaired motor function, and cyanosis/pallor. Irreparable damage can occur in as quickly as 4 to 6 hours with complete arterial occlusion. A majority of ALI is caused by thrombosis, with the remainder of cases caused by embolism, 85% and 15% respectively.1-6 If not addressed promptly, this condition carries a high degree of morbidity (ie, limb loss) and mortality. Immediate initiation of anticoagulation therapy and vascular surgery consultation are mainstays of ED management. Time-to-treatment is a predictor of success and is an area where the emergency physician (EP) can make a significant difference in patient outcomes.7 Although vascular interruption via traumatic mechanism is not addressed in this review, it should remain a consideration in relation to the historical features of each case.
Presentation
Traditionally, symptoms of ALI can be remembered as the “six Ps”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia. Note that ALI represents a spectrum of disease, and the presenting features depend heavily on the level and degree of obstruction. Pain, however, is most frequently the first presenting symptom of patients with ALI.1-6 Pain will occur in muscle groups distal to the occlusion. Obstruction of the aortoiliac region might produce pain in the buttocks, thigh, and hip, whereas occlusion of the femoral artery may produce pain in the calf. Single-level disease will often produce claudication, but multilevel disease may present as a nonhealing ulcer or with focal gangrene. The differential diagnosis of ALI includes direct arterial injury, vasospasm, compartment syndrome, chronic peripheral artery disease, stroke, spinal cord injury, vasculitis, muscular trauma, and radiculopathy.3,5
Etiology
As stated previously, ALI is a result of either thrombotic or embolic phenomena that either partially or completely occludes a vessel such that adequate perfusion is no longer achieved. Consequently, there is a decrease in the metabolism of the tissue supplied in those territories, which can rapidly progress to necrosis. Thrombosis is the most common cause of ALI, accounting for approximately 85%.2,3Typically, thrombosis presents in the setting of pre-existing peripheral artery disease (PAD).8 As PAD worsens, damage to the arterial endothelium triggers platelet activation, and accumulation in a manner conceptually similar to thrombosis in myocardial infarction.9 Hypercoagulable states increase the risk of thrombi development.10 Symptoms of thrombosis are often more insidious in onset when compared to embolism, and signs/symptoms of prior claudication are almost always present. Consequently, there is a greater chance that collateral circulation has developed over time.2,6,11,12
By contrast, ALI caused by embolism is more acute in onset, and is often more emergent due to complete occlusion without chance of collateral vessel development.1,3,6,12 See Table 1 for differences in risk factors and presentation between thrombotic and embolic occlusion.
Historical Features
Information should be obtained systematically in all presentations and should include time of symptom onset, duration, severity, and location; however, special attention should be paid to the evolution of symptoms over time. One historical pearl that should raise suspicion of embolism vs thrombus is a patient history with a specific time of symptom onset. Specific questioning about history of claudication, including description of typical symptoms, is critical. A full list of other medical comorbidities should be obtained. This may reveal risk factors for thrombotic and embolic disease.1,3-5 This also may alter available treatment options.7,8,12,13 At presentation, the EP should obtain a list of the patient’s current medications, and special attention should be given to prothrombotic medications and medications that may interact with anticoagulants.
Physical Examination
On physical examination, the EP should pay particular attention to both the external appearance and temperature of the skin of the patient’s affected extremity. The presence or absence of peripheral pulses is an important feature when assessing for ALI; however, the presence of peripheral pulses does not exclude ALI from the differential.
Precise evaluation of sensation is important to attempt to quantify the extent of injury, localize a possible obstruction, and differentiate ALI from other possible causes of sensation deficits. Though not referenced in the six Ps, the patient’s limb may also appear mottled or marbled. Embolic phenomena occur at sites of vascular bifurcation. In a patient with aortic embolism, femoral pulses will be absent with bilateral mottling and paralysis of the lower extremities. Iliac artery embolism will produce the same symptoms, except unilateral. Femoral artery embolism will yield coolness and mottling distal to the inguinal ligament. Similarly, popliteal artery embolism will produce those same findings, except distal to the knee with preserved femoral pulses.
Diagnosis and Evaluation
After the history and physical examination are completed, further diagnostic methodologies should be considered, including laboratory evaluation and additional imaging studies.
Imaging Studies
Doppler ultrasound examination to assess for pulses on the affected limb should be performed immediately. Bedside continuous-wave Doppler may be used to differentiate arterial vs venous signal.6,14-16 An ankle brachial index may be calculated through either direct auscultation of pulses or Doppler signal. A ratio of less than 0.9 is suggestive of ischemia, while a ratio of less than 0.5 is considered critical ischemia.2
Laboratory Evaluation
Laboratory evaluation of hematocrit, coagulation studies, renal function, electrolyte levels, lactate, and creatinine kinase should be completed.1,5 An electrocardiogram and bedside/formal echocardiogram may be considered if embolism is suspected.1,6,17
Imaging
Angiography is currently the gold standard imaging modality to diagnose ALI.16 However, other imaging modalities exist that expose the patient to less radiation and are generally preferred by patients.18 Duplex ultrasonography is the least invasive imaging modality, with a reported sensitivity of 88% and specificity of 96% for the detection of greater than 50% stenosis.6,19 Contrast-enhanced magnetic resonance angiography (MRA) of the lower extremity is also relatively noninvasive, but is more limited in its application. It carries a reported sensitivity of 95% and a specificity of 97% for detection of stenosis of greater than 50%.6,19 An MRA without contrast may be considered for patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and who are not on dialysis, but the diagnostic efficacy is reduced.16 Finally, the sensitivity and specificity of computed tomography angiography (CTA) to detect aortoiliac stenosis of greater than 50% is 96% and 98% respectively.6,18,19 Other studies demonstrated similar results for other arterial locations in the lower extremities.18,19 A principal advantage of CTA is that direct visualization of calcifications, clips, stents, and prior bypasses is possible without the limitations of MRA.16 The American College of Radiology (ACR) lists ultrasound duplex Doppler and noncontrast MRA of the lower extremity as “may be appropriate.”16 In contrast, the ACR classifies MRA with contrast, CTA with contrast, and angiography of the lower extremity as “usually appropriate.”16 Selecting the optimal imaging study for a patient may require involvement of a facility’s radiology department and/or a vascular surgeon.14,15
Management in the ED
Even before the definitive diagnosis of ALI is made, steps can be taken to minimize and/or slow progression of injury. Placing the affected limb in a dependent position and providing intravenous (IV) fluid hydration will maximize perfusion.1,3,5 Even with consideration of the diagnostic modalities previously described, ALI is often considered a clinical diagnosis. Since time is critical, early consultation with vascular surgery services is imperative if ALI is suspected.
Intravenous Fluid Therapy
Suspected ALI should be treated in the ED with an IV heparin bolus, followed by constant IV infusion. Heparin prevents proximal and distal propagation of the thrombus.1,3,5,6,15,17,20 Additionally, it helps maintain the microcirculation surrounding the affected area.6 A heparin bolus dose of 100 U/kg followed by a continuous infusion of heparin 1,000 U/h is the recommended standard.1,3,5,6,15 A goal partial thromboplastin time of 60 to 100 seconds, or an international normalized ratio of 2 to 3 is desirable.8,21
Staging: The Rutherford Classification System
Once immediate therapies are started, the stage of PAD should be determined using the Rutherford classification system in collaboration with vascular surgery services; this will further guide treatment and disposition.
Category I. In category I ALI, the affected limb is considered viable and not immediately threatened.
Category IIa. The limb is considered to be marginally threatened and salvageable.
Category IIb. An ALI classified as a category IIb is the most urgent type in which the limb is immediately threatened.
Category III. The limb is classified as having irreversible damage.2,3,17,22
(See Table 2 for a more detailed summary of the Rutherford classification system.)
Treatment
Definitive treatment decisions should be made in consultation with vascular surgery services. The results of three clinical trials developed the foundation for management of ALI: the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, Surgery Versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, and Rutherford trial.22-24 Table 3 provides a summary of the treatment techniques utilized, which include mechanical recanalization with percutaneous aspiration thrombectomy, percutaneous mechanical thrombectomy,6,12,25-28 pharmacological recanalization via catheter-directed thrombolysis,6,8,12,20,23-28 and/or surgical management via thrombectomy or embolectomy.6,11,23,24,27
Complications
The complications of treating ALI can be stratified into three subsets: pharmacologic, mechanical, and reperfusion injury-related. Medical treatment with heparin is not without risk. Intracranial hemorrhage, major bleeding at other sites, and compartment syndrome secondary to bleeding can all occur. Treatment may also cause distal embolization and lead to mechanical occlusion of another site. Finally, reperfusion injury predisposes the patient to a host of new problems. Reperfusion injury occurs when fresh blood enters a previously ischemic zone. When this occurs, oxygen free radicals, inflammatory mediators, and catabolism byproducts mix with the blood. This process can damage surrounding epithelial cells and lead to increased interstitial permeability. As a result, compartment syndrome may also develop via this mechanism.1,3,5,6 The anterior compartment of the lower extremity is most at risk. As such, assessment of peroneal nerve activity via dorsiflexion of the foot and sensation testing is important after the initiation of treatment.6 Systemic distribution of these harmful byproducts can also cause arrhythmias, renal failure, and systemic acadisos.1,3,5,6 It is important for physicians and staff to monitor the patient closely for the development of these complications and be prepared to intervene.
Opportunities for Improvement
In a condition where minutes matter, expedient action is critical. Time-to-recognition, imaging, consultation, and intervention all are potential sources of delay. Two recent studies have investigated the treatment timeline of ALI. The first study showed that the greatest source of delay is time from symptom onset to presentation in the ED; an average of 11.35 hours. The second largest source of delay was time from recognition of ALI to imaging, with an average delay of 4.75 hours. The average ED evaluation time was 40 minutes, and the average total time to intervention was 10.2 hours. While time-to-symptom presentation may represent a failure of public health, time-to-imaging was identified as an area of unacceptable delay by the authors.29 A second study examined the effect of pre-hospital care on the time-to-treatment. Persons who were transported via emergency medical services (EMS) arrived to the hospital at a median time of 5 hours after symptom onset, were seen by a physician at a median time of 51 minutes, and had revascularization at a median time of 23 hours. Those not transported by EMS arrived to the hospital at a median time of 48 hours after symptom onset, were seen by a physician at a median time of 80 minutes, and had revascularization at a median time of 93 hours. As a secondary goal, the study examined the effect of heparinization in the ED and showed that treatment with heparin was associated with a favorable outcome.30 While the total treatment times in each study vary widely, they share several important commonalities and reinforce expedient management as an important goal.
Outcomes
Advances in the treatment of this condition began in the 1970s, with an explosion of new techniques and data in the past several years. Still, ALI is a high morbidity and high mortality condition. Rates of limb loss are approximately 30% for all cases, and it is a fatal condition for as many as one-in-five. As discussed earlier, the patients who are most likely to experience ALI typically have several medical comorbidities. Perhaps unsurprisingly, ALI portends a poor prognosis even if the limb is salvaged. Approximately 15% to 20% of patients with ALI will die within 1 year of diagnosis.3,4,6,12
Conclusion
Acute limb ischemia is a rare condition, but a true medical emergency. Prompt recognition of the signs and symptoms of ALI are critical for optimizing patient outcomes. A high index of clinical suspicion in patients with risk factors may lead to early diagnosis. Early vascular surgery consultation and early IV heparin treatment are important aspects of care. Prompt imaging and staging guide further management. Special care should be paid to possible post-treatment complications and to the investigation of contributing factors. The EP has a great opportunity to positively impact care with expeditious management. Early diagnosis, targeted history and physical examination, prompt treatment, collaboration with specialists, and prevention and treatment of life-threatening complications are all hallmarks of emergency medicine.
Acute limb ischemia (ALI) is an emergent medical condition that is characterized by a precipitous decrease in limb perfusion that threatens the viability of the affected limb, and symptoms that have been present for 14 days or less.1-5 The incidence of lower extremity ALI in the US is nine to 16 cases per 100,000 persons per year, and one to three cases per 100,000 persons per year for upper extremity ALI.3 Symptoms suggestive of this condition classically include pain in the effected limb at rest, loss of sensation, impaired motor function, and cyanosis/pallor. Irreparable damage can occur in as quickly as 4 to 6 hours with complete arterial occlusion. A majority of ALI is caused by thrombosis, with the remainder of cases caused by embolism, 85% and 15% respectively.1-6 If not addressed promptly, this condition carries a high degree of morbidity (ie, limb loss) and mortality. Immediate initiation of anticoagulation therapy and vascular surgery consultation are mainstays of ED management. Time-to-treatment is a predictor of success and is an area where the emergency physician (EP) can make a significant difference in patient outcomes.7 Although vascular interruption via traumatic mechanism is not addressed in this review, it should remain a consideration in relation to the historical features of each case.
Presentation
Traditionally, symptoms of ALI can be remembered as the “six Ps”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia. Note that ALI represents a spectrum of disease, and the presenting features depend heavily on the level and degree of obstruction. Pain, however, is most frequently the first presenting symptom of patients with ALI.1-6 Pain will occur in muscle groups distal to the occlusion. Obstruction of the aortoiliac region might produce pain in the buttocks, thigh, and hip, whereas occlusion of the femoral artery may produce pain in the calf. Single-level disease will often produce claudication, but multilevel disease may present as a nonhealing ulcer or with focal gangrene. The differential diagnosis of ALI includes direct arterial injury, vasospasm, compartment syndrome, chronic peripheral artery disease, stroke, spinal cord injury, vasculitis, muscular trauma, and radiculopathy.3,5
Etiology
As stated previously, ALI is a result of either thrombotic or embolic phenomena that either partially or completely occludes a vessel such that adequate perfusion is no longer achieved. Consequently, there is a decrease in the metabolism of the tissue supplied in those territories, which can rapidly progress to necrosis. Thrombosis is the most common cause of ALI, accounting for approximately 85%.2,3Typically, thrombosis presents in the setting of pre-existing peripheral artery disease (PAD).8 As PAD worsens, damage to the arterial endothelium triggers platelet activation, and accumulation in a manner conceptually similar to thrombosis in myocardial infarction.9 Hypercoagulable states increase the risk of thrombi development.10 Symptoms of thrombosis are often more insidious in onset when compared to embolism, and signs/symptoms of prior claudication are almost always present. Consequently, there is a greater chance that collateral circulation has developed over time.2,6,11,12
By contrast, ALI caused by embolism is more acute in onset, and is often more emergent due to complete occlusion without chance of collateral vessel development.1,3,6,12 See Table 1 for differences in risk factors and presentation between thrombotic and embolic occlusion.
Historical Features
Information should be obtained systematically in all presentations and should include time of symptom onset, duration, severity, and location; however, special attention should be paid to the evolution of symptoms over time. One historical pearl that should raise suspicion of embolism vs thrombus is a patient history with a specific time of symptom onset. Specific questioning about history of claudication, including description of typical symptoms, is critical. A full list of other medical comorbidities should be obtained. This may reveal risk factors for thrombotic and embolic disease.1,3-5 This also may alter available treatment options.7,8,12,13 At presentation, the EP should obtain a list of the patient’s current medications, and special attention should be given to prothrombotic medications and medications that may interact with anticoagulants.
Physical Examination
On physical examination, the EP should pay particular attention to both the external appearance and temperature of the skin of the patient’s affected extremity. The presence or absence of peripheral pulses is an important feature when assessing for ALI; however, the presence of peripheral pulses does not exclude ALI from the differential.
Precise evaluation of sensation is important to attempt to quantify the extent of injury, localize a possible obstruction, and differentiate ALI from other possible causes of sensation deficits. Though not referenced in the six Ps, the patient’s limb may also appear mottled or marbled. Embolic phenomena occur at sites of vascular bifurcation. In a patient with aortic embolism, femoral pulses will be absent with bilateral mottling and paralysis of the lower extremities. Iliac artery embolism will produce the same symptoms, except unilateral. Femoral artery embolism will yield coolness and mottling distal to the inguinal ligament. Similarly, popliteal artery embolism will produce those same findings, except distal to the knee with preserved femoral pulses.
Diagnosis and Evaluation
After the history and physical examination are completed, further diagnostic methodologies should be considered, including laboratory evaluation and additional imaging studies.
Imaging Studies
Doppler ultrasound examination to assess for pulses on the affected limb should be performed immediately. Bedside continuous-wave Doppler may be used to differentiate arterial vs venous signal.6,14-16 An ankle brachial index may be calculated through either direct auscultation of pulses or Doppler signal. A ratio of less than 0.9 is suggestive of ischemia, while a ratio of less than 0.5 is considered critical ischemia.2
Laboratory Evaluation
Laboratory evaluation of hematocrit, coagulation studies, renal function, electrolyte levels, lactate, and creatinine kinase should be completed.1,5 An electrocardiogram and bedside/formal echocardiogram may be considered if embolism is suspected.1,6,17
Imaging
Angiography is currently the gold standard imaging modality to diagnose ALI.16 However, other imaging modalities exist that expose the patient to less radiation and are generally preferred by patients.18 Duplex ultrasonography is the least invasive imaging modality, with a reported sensitivity of 88% and specificity of 96% for the detection of greater than 50% stenosis.6,19 Contrast-enhanced magnetic resonance angiography (MRA) of the lower extremity is also relatively noninvasive, but is more limited in its application. It carries a reported sensitivity of 95% and a specificity of 97% for detection of stenosis of greater than 50%.6,19 An MRA without contrast may be considered for patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and who are not on dialysis, but the diagnostic efficacy is reduced.16 Finally, the sensitivity and specificity of computed tomography angiography (CTA) to detect aortoiliac stenosis of greater than 50% is 96% and 98% respectively.6,18,19 Other studies demonstrated similar results for other arterial locations in the lower extremities.18,19 A principal advantage of CTA is that direct visualization of calcifications, clips, stents, and prior bypasses is possible without the limitations of MRA.16 The American College of Radiology (ACR) lists ultrasound duplex Doppler and noncontrast MRA of the lower extremity as “may be appropriate.”16 In contrast, the ACR classifies MRA with contrast, CTA with contrast, and angiography of the lower extremity as “usually appropriate.”16 Selecting the optimal imaging study for a patient may require involvement of a facility’s radiology department and/or a vascular surgeon.14,15
Management in the ED
Even before the definitive diagnosis of ALI is made, steps can be taken to minimize and/or slow progression of injury. Placing the affected limb in a dependent position and providing intravenous (IV) fluid hydration will maximize perfusion.1,3,5 Even with consideration of the diagnostic modalities previously described, ALI is often considered a clinical diagnosis. Since time is critical, early consultation with vascular surgery services is imperative if ALI is suspected.
Intravenous Fluid Therapy
Suspected ALI should be treated in the ED with an IV heparin bolus, followed by constant IV infusion. Heparin prevents proximal and distal propagation of the thrombus.1,3,5,6,15,17,20 Additionally, it helps maintain the microcirculation surrounding the affected area.6 A heparin bolus dose of 100 U/kg followed by a continuous infusion of heparin 1,000 U/h is the recommended standard.1,3,5,6,15 A goal partial thromboplastin time of 60 to 100 seconds, or an international normalized ratio of 2 to 3 is desirable.8,21
Staging: The Rutherford Classification System
Once immediate therapies are started, the stage of PAD should be determined using the Rutherford classification system in collaboration with vascular surgery services; this will further guide treatment and disposition.
Category I. In category I ALI, the affected limb is considered viable and not immediately threatened.
Category IIa. The limb is considered to be marginally threatened and salvageable.
Category IIb. An ALI classified as a category IIb is the most urgent type in which the limb is immediately threatened.
Category III. The limb is classified as having irreversible damage.2,3,17,22
(See Table 2 for a more detailed summary of the Rutherford classification system.)
Treatment
Definitive treatment decisions should be made in consultation with vascular surgery services. The results of three clinical trials developed the foundation for management of ALI: the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, Surgery Versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, and Rutherford trial.22-24 Table 3 provides a summary of the treatment techniques utilized, which include mechanical recanalization with percutaneous aspiration thrombectomy, percutaneous mechanical thrombectomy,6,12,25-28 pharmacological recanalization via catheter-directed thrombolysis,6,8,12,20,23-28 and/or surgical management via thrombectomy or embolectomy.6,11,23,24,27
Complications
The complications of treating ALI can be stratified into three subsets: pharmacologic, mechanical, and reperfusion injury-related. Medical treatment with heparin is not without risk. Intracranial hemorrhage, major bleeding at other sites, and compartment syndrome secondary to bleeding can all occur. Treatment may also cause distal embolization and lead to mechanical occlusion of another site. Finally, reperfusion injury predisposes the patient to a host of new problems. Reperfusion injury occurs when fresh blood enters a previously ischemic zone. When this occurs, oxygen free radicals, inflammatory mediators, and catabolism byproducts mix with the blood. This process can damage surrounding epithelial cells and lead to increased interstitial permeability. As a result, compartment syndrome may also develop via this mechanism.1,3,5,6 The anterior compartment of the lower extremity is most at risk. As such, assessment of peroneal nerve activity via dorsiflexion of the foot and sensation testing is important after the initiation of treatment.6 Systemic distribution of these harmful byproducts can also cause arrhythmias, renal failure, and systemic acadisos.1,3,5,6 It is important for physicians and staff to monitor the patient closely for the development of these complications and be prepared to intervene.
Opportunities for Improvement
In a condition where minutes matter, expedient action is critical. Time-to-recognition, imaging, consultation, and intervention all are potential sources of delay. Two recent studies have investigated the treatment timeline of ALI. The first study showed that the greatest source of delay is time from symptom onset to presentation in the ED; an average of 11.35 hours. The second largest source of delay was time from recognition of ALI to imaging, with an average delay of 4.75 hours. The average ED evaluation time was 40 minutes, and the average total time to intervention was 10.2 hours. While time-to-symptom presentation may represent a failure of public health, time-to-imaging was identified as an area of unacceptable delay by the authors.29 A second study examined the effect of pre-hospital care on the time-to-treatment. Persons who were transported via emergency medical services (EMS) arrived to the hospital at a median time of 5 hours after symptom onset, were seen by a physician at a median time of 51 minutes, and had revascularization at a median time of 23 hours. Those not transported by EMS arrived to the hospital at a median time of 48 hours after symptom onset, were seen by a physician at a median time of 80 minutes, and had revascularization at a median time of 93 hours. As a secondary goal, the study examined the effect of heparinization in the ED and showed that treatment with heparin was associated with a favorable outcome.30 While the total treatment times in each study vary widely, they share several important commonalities and reinforce expedient management as an important goal.
Outcomes
Advances in the treatment of this condition began in the 1970s, with an explosion of new techniques and data in the past several years. Still, ALI is a high morbidity and high mortality condition. Rates of limb loss are approximately 30% for all cases, and it is a fatal condition for as many as one-in-five. As discussed earlier, the patients who are most likely to experience ALI typically have several medical comorbidities. Perhaps unsurprisingly, ALI portends a poor prognosis even if the limb is salvaged. Approximately 15% to 20% of patients with ALI will die within 1 year of diagnosis.3,4,6,12
Conclusion
Acute limb ischemia is a rare condition, but a true medical emergency. Prompt recognition of the signs and symptoms of ALI are critical for optimizing patient outcomes. A high index of clinical suspicion in patients with risk factors may lead to early diagnosis. Early vascular surgery consultation and early IV heparin treatment are important aspects of care. Prompt imaging and staging guide further management. Special care should be paid to possible post-treatment complications and to the investigation of contributing factors. The EP has a great opportunity to positively impact care with expeditious management. Early diagnosis, targeted history and physical examination, prompt treatment, collaboration with specialists, and prevention and treatment of life-threatening complications are all hallmarks of emergency medicine.
1. Braun R, Lin M. Acute limb ischemia: a case report and literature review. J Emerg Med. 2015;49(6):1011-1017. doi:10.1016/j.jemermed.2015.03.008.
2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ. 2000;320(7237):764-767.
3. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198-2206. doi:10.1056/NEJMcp1006054.
4. Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interv Cardiol Clin. 2014;3(4):557-572.
5. Santistevan JR. Acute limb ischemia: an emergency medicine approach. Emerg Med Clin North Am. 2017;35(4):889-909. doi:10.1016/j.emc.2017.07.006.
6. Acar RD, Sahin M, Kirma C. One of the most urgent vascular circumstances: acute limb ischemia. SAGE Open Med. 2013;1:2050312113516110. doi:10.1177/2050312113516110.
7. Abou-Zamzam AM Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458-463.
8. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465-1508.
9. Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46(3):485-490.
10. Deitcher SR, Carman TL, Sheikh MA, Gomes M. Hypercoagulable syndromes: evaluation and management strategies for acute limb ischemia. Semin Vasc Surg. 2001;14(2):74-85.
11. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
12. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988-995.
13. Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;65(1):108-118. doi:10.1016/j.jvs.2016.06.105.
14. Aboyans V, Björck M, Brodmann M, et al; ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017. doi:10.1093/eurheartj/ehx499. [Epub ahead of print]
15. Authors/Task Force Members, Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017. doi:10.1016/j.ejvs.2017.07.018. [Epub ahead of print]
16. Expert Panel on Vascular Imaging: Weiss CR, Azene EM, Majdalany BS, et al. ACR Appropriateness Criteria® sudden onset of cold, painful leg. J Am Coll Radiol. 2017;14(5S):S307-S313. doi:10.1016/j.jacr.2017.02.015.
17. Dieter RS, Dieter RA Jr, Dieter RA 3rd, Nanjundappa A. Critical Limb Ischemia: Acute and Chronic. New York, NY: Springer; 2016.
18. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415-424.
19. Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
20. Giannini D, Balbarini A. Thrombolytic therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):249-258.
21. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S. doi:10.1378/chest.08-0658.
22. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
23. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23(1):64-73; discussion 74-75.
24. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220(3):251-266; discussion 266-268.
25. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53(2):340-346. doi:10.1016/j.jvs.2010.08.064.
26. Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15.
27. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61(1):147-154. doi:10.1016/j.jvs.2014.06.109.
28. Zeller T, Tepe G. Treatment of acute limb ischemia with focus on endovascular techniques. Vasa. 2009;38(2):123-133. doi: 10.1024/0301-1526.38.2.123.
29. Normahani P, Standfield NJ, Jaffer U. Sources of delay in the acute limb ischemia patient pathway. Ann Vasc Surg. 2017;38:279-285. doi:10.1016/j.avsg.2016.05.118.
30. Langenskiöld M, Smidfelt K, Karlsson A, Bohm C, Herlitz J, Nordanstig J. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54(2):235-240. doi:10.1016/j.ejvs.2017.04.010.
1. Braun R, Lin M. Acute limb ischemia: a case report and literature review. J Emerg Med. 2015;49(6):1011-1017. doi:10.1016/j.jemermed.2015.03.008.
2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ. 2000;320(7237):764-767.
3. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198-2206. doi:10.1056/NEJMcp1006054.
4. Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interv Cardiol Clin. 2014;3(4):557-572.
5. Santistevan JR. Acute limb ischemia: an emergency medicine approach. Emerg Med Clin North Am. 2017;35(4):889-909. doi:10.1016/j.emc.2017.07.006.
6. Acar RD, Sahin M, Kirma C. One of the most urgent vascular circumstances: acute limb ischemia. SAGE Open Med. 2013;1:2050312113516110. doi:10.1177/2050312113516110.
7. Abou-Zamzam AM Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458-463.
8. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465-1508.
9. Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46(3):485-490.
10. Deitcher SR, Carman TL, Sheikh MA, Gomes M. Hypercoagulable syndromes: evaluation and management strategies for acute limb ischemia. Semin Vasc Surg. 2001;14(2):74-85.
11. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
12. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988-995.
13. Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;65(1):108-118. doi:10.1016/j.jvs.2016.06.105.
14. Aboyans V, Björck M, Brodmann M, et al; ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017. doi:10.1093/eurheartj/ehx499. [Epub ahead of print]
15. Authors/Task Force Members, Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017. doi:10.1016/j.ejvs.2017.07.018. [Epub ahead of print]
16. Expert Panel on Vascular Imaging: Weiss CR, Azene EM, Majdalany BS, et al. ACR Appropriateness Criteria® sudden onset of cold, painful leg. J Am Coll Radiol. 2017;14(5S):S307-S313. doi:10.1016/j.jacr.2017.02.015.
17. Dieter RS, Dieter RA Jr, Dieter RA 3rd, Nanjundappa A. Critical Limb Ischemia: Acute and Chronic. New York, NY: Springer; 2016.
18. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415-424.
19. Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
20. Giannini D, Balbarini A. Thrombolytic therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):249-258.
21. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S. doi:10.1378/chest.08-0658.
22. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
23. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23(1):64-73; discussion 74-75.
24. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220(3):251-266; discussion 266-268.
25. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53(2):340-346. doi:10.1016/j.jvs.2010.08.064.
26. Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15.
27. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61(1):147-154. doi:10.1016/j.jvs.2014.06.109.
28. Zeller T, Tepe G. Treatment of acute limb ischemia with focus on endovascular techniques. Vasa. 2009;38(2):123-133. doi: 10.1024/0301-1526.38.2.123.
29. Normahani P, Standfield NJ, Jaffer U. Sources of delay in the acute limb ischemia patient pathway. Ann Vasc Surg. 2017;38:279-285. doi:10.1016/j.avsg.2016.05.118.
30. Langenskiöld M, Smidfelt K, Karlsson A, Bohm C, Herlitz J, Nordanstig J. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54(2):235-240. doi:10.1016/j.ejvs.2017.04.010.