User login
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
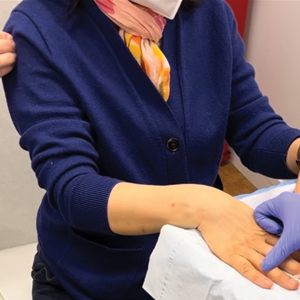
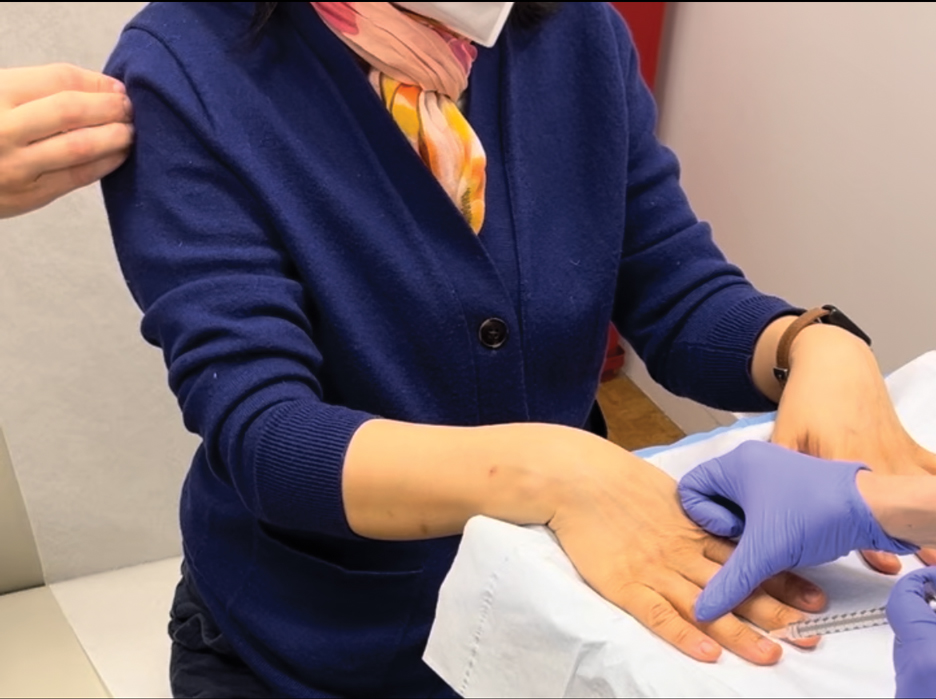
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.
This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).
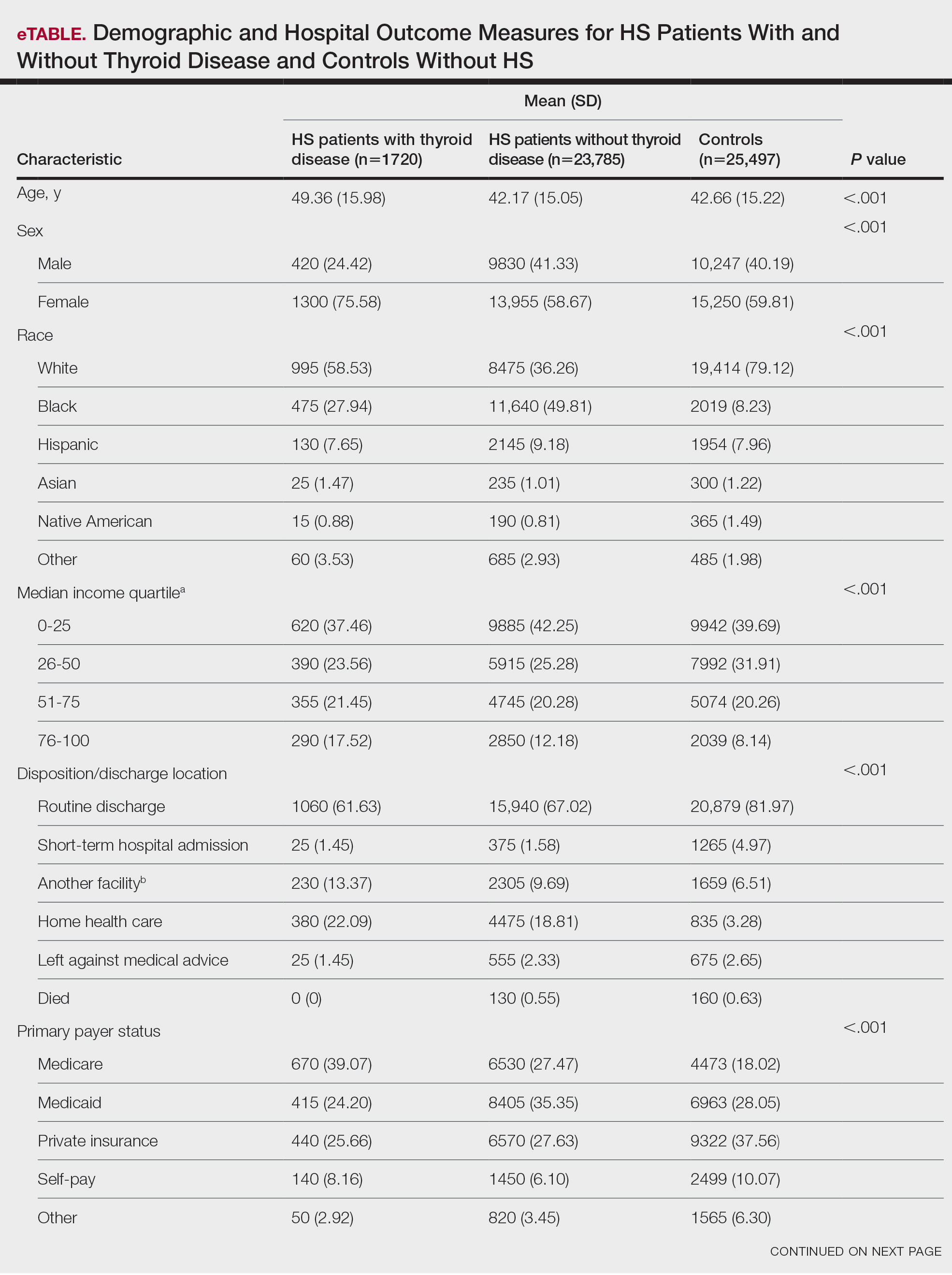
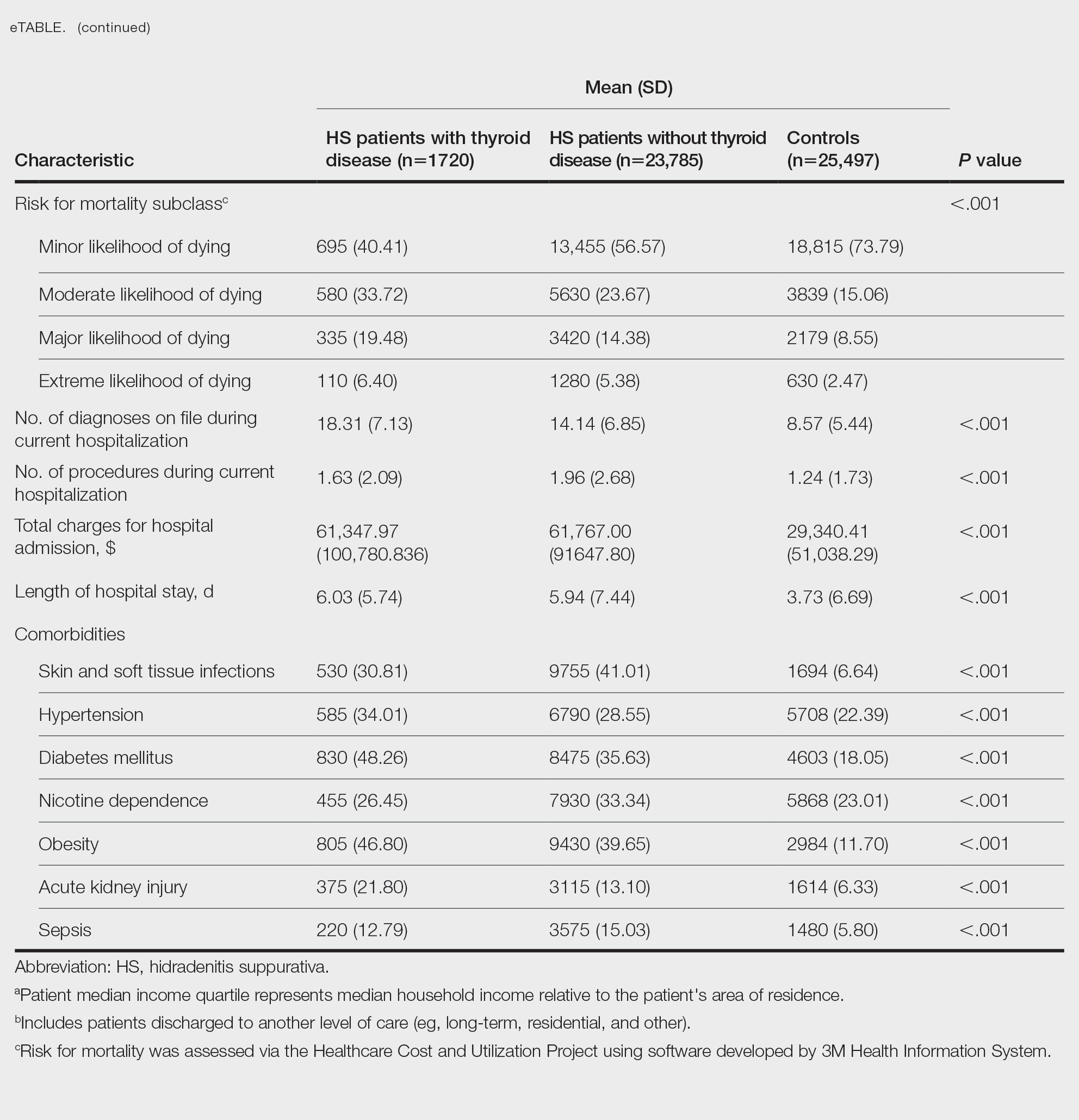
On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
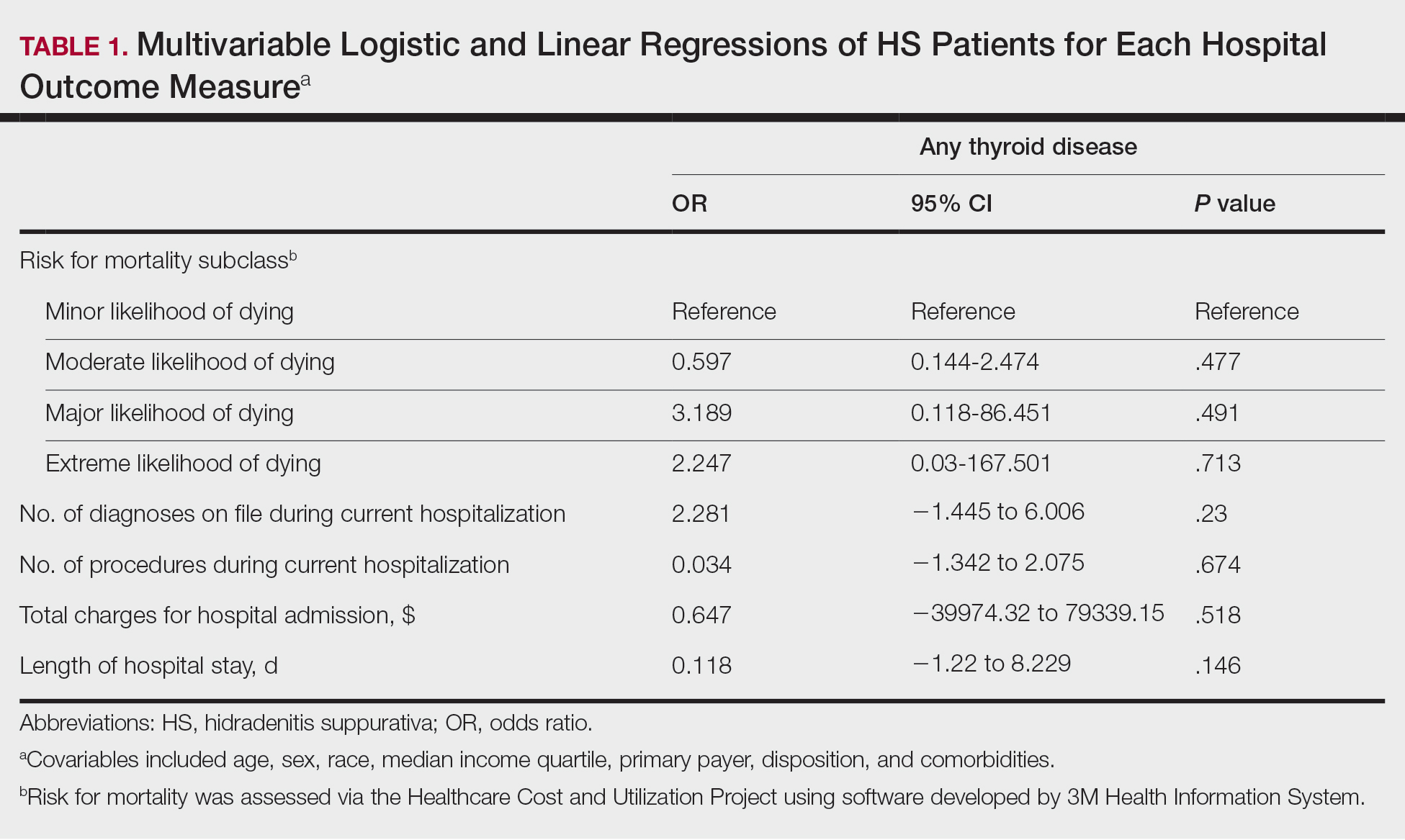
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).

We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).


On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).


We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).


On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).


We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
PRACTICE
- Hidradenitis suppurativa (HS) is associated with autoimmune and endocrine conditions, but the association between HS and thyroid disorders is poorly characterized.