User login
Choosing Wisely in Pediatric Medicine
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
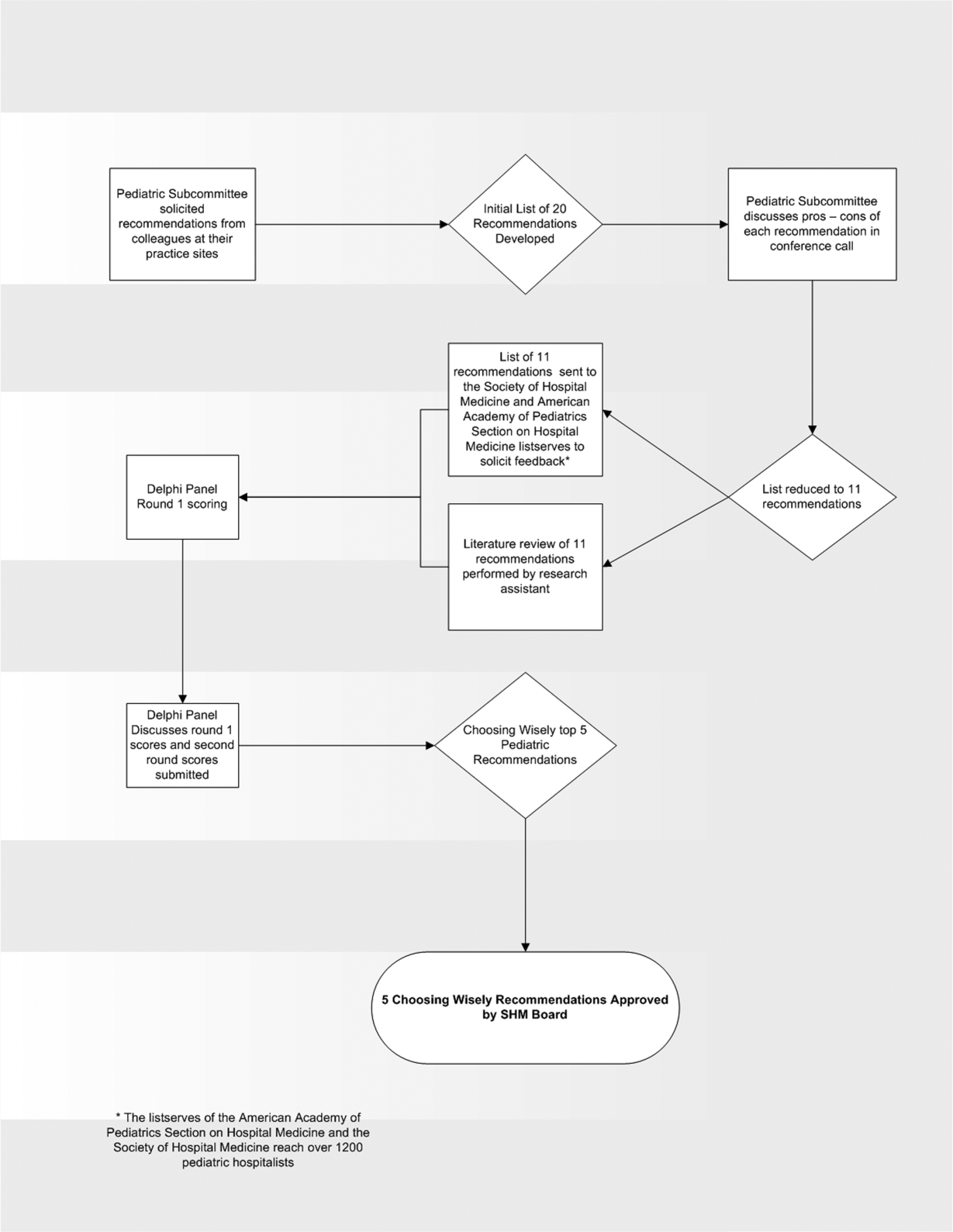
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.
Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
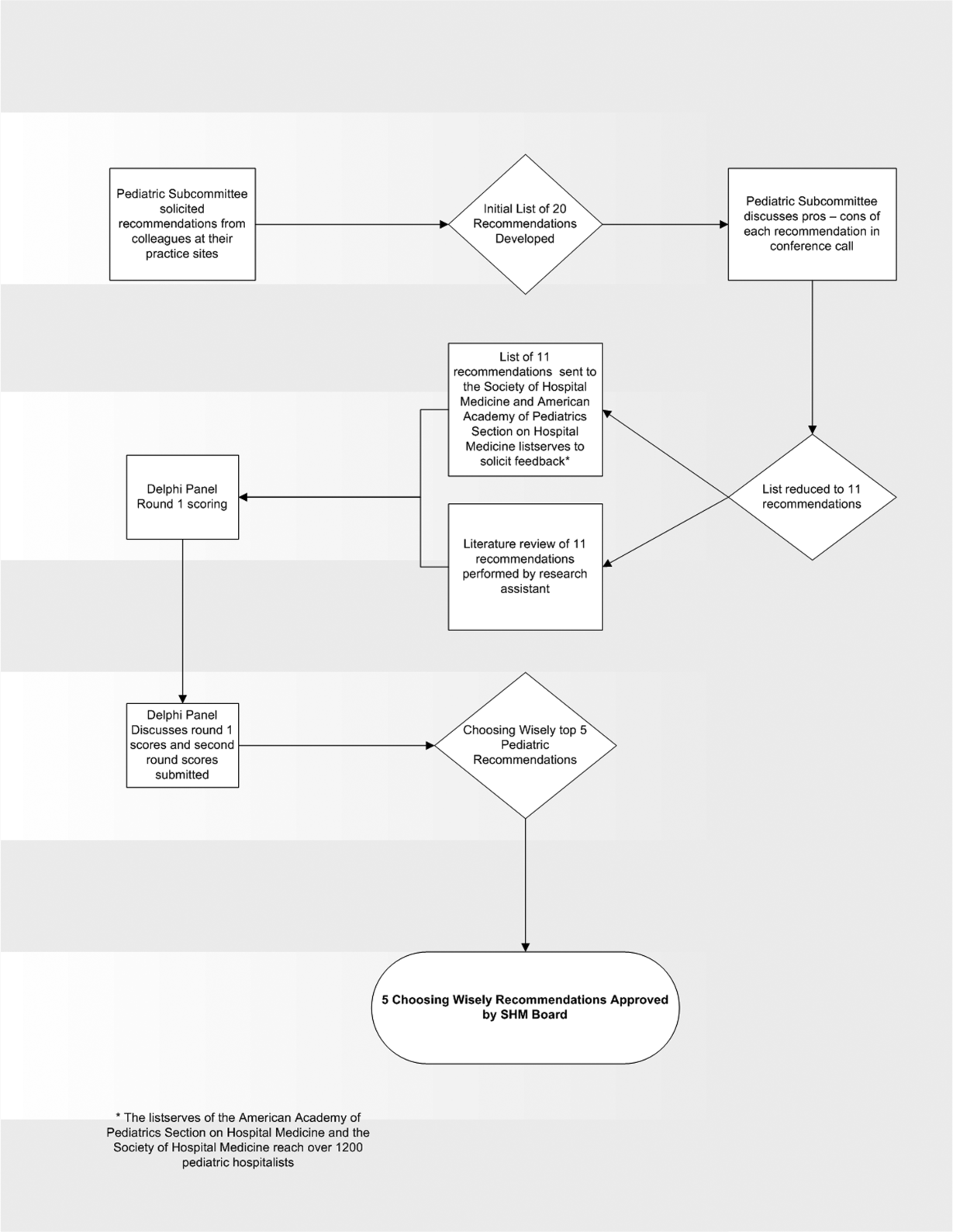
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.

Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.

Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
Copyright © 2013 Society of Hospital Medicine
Choosing Wisely in Hospital Medicine
The overuse of medical tests and treatments is a growing concern. A recent survey revealed that 2 in 5 primary care physicians perceive that patients in their own practice are receiving too much care.[1] Twenty‐eight percent of the physicians indicated they provide more care than they should. When queried about reasons for the aggressiveness of care, responses included fear of malpractice litigation, adherence to clinical performance measures that require following protocols, and inadequacy of time spent with patients. Overutilization of healthcare resources is a complex issue promulgated not only by the factors cited by the physicians but also a culture in the United States habituated to believe more care is better care.[2, 3, 4] In 2010, $2.6 trillion was spent on healthcare, an increase of $1.3 trillion between 2000 and 2010.5 As much as 30% of healthcare spending may be wasted.[6] Because physicians influence approximately 80% of healthcare expenditures, including ordering tests and treatments, it is imperative that physicians take a leadership role in reversing this trend.[7]
In response to this need, several physician‐led projects have emerged.[8, 9, 10] One such initiative is the American Board of Internal Medicine Foundation's (ABIM‐F's) Choosing Wisely campaign.[11] The ABIM‐F contacted a variety of specialty societies and asked each to identify the 5 top tests or treatments relevant to their specialty that may frequently be overused. Phase 1 of the Choosing Wisely campaign was launched in April 2012 with 9 specialty societies participating. The second phase was unveiled in February 2013 and comprised of 16 additional groups including the Society of Hospital Medicine (SHM). The SHM represents 35,000 hospitalists in the United States whose primary focus is the general medical care of hospitalized patients. This is especially important because almost one‐third of total US healthcare expenditures are on hospital care,[12] and hospitalists care for an increasing number of hospitalized patients.[13] In this article, we describe the used to derive the adult hospital medicine Choosing processes Wisely list, review the tests and treatments that the SHM's Choosing Wisely Subcommittee chose, and discuss potential next steps in implementation of the adult hospital medicine recommendations.
METHODOLOGY
Upon invitation to participate in the Choosing Wisely campaign, SHM's Hospital Quality and Patient Safety (HQPS) Committee formally convened the Choosing Wisely Subcommittee. The subcommittee identified and executed a methodology (see Supporting Figure 1 and Supporting Table 1 in the online version of this article) to create the list of 5 tests and treatments that the SHM submitted to the ABIM‐F. All subcommittee members participated fully in the voting and refinement process. The Choosing Wisely Subcommittee worked closely with the SHM's Pediatrics Choosing Wisely Subcommittee to develop both adult and pediatric lists.
Convening the Choosing Wisely Subcommittee
The HQPS Committee convened a subcommittee consisting of 9 members. The subcommittee represented a diverse group of hospitalists reflecting different institution types, geographic regions, and experience. All Choosing Wisely Subcommittee members signed conflict of interest statements and reported no conflict related to the conclusions, implications, or opinions stated. The subcommittee did not consult other external stakeholders in the development of recommendations.
Identification and Refinement of Potential Wasteful Practices
To generate an initial list of potential recommendations, members of all of the SHM committees were surveyed and asked to submit 5 tests and treatments that are inappropriately used or overused. SHM staff removed duplicates and categorized submissions by topic, highlighting overlapping recommendations. Tests and treatments that are used infrequently and items included in phase 1 society lists were also excluded. Subcommittee members then ranked the resultant list using a 5‐point Likert scale. All SHM members were then given the opportunity to rank their agreement with the tests and treatments on the list, as refined at the time based upon their own experience and consideration of the following criteria: tests and procedures within the control and purview of hospital medicine, the frequency with which the tests or procedures occur, and the significance of associated costs. This was accomplished via electronic survey.
Establishing an Evidence Base
SHM staff conducted a literature review of the list of tests and treatments that was further refined by the SHM membership's ranking using a standard template. Two reviewers (W.N. and J.G.) conducted an independent literature review of the remaining tests and treatments using PubMed, MEDLINE, and Cochrane Library. The reviewers also conducted generic Internet searches. The literature review included all literature published through 2012 as well as nonEnglish language publications. The reviewers included clinical research guidelines and primary and secondary research studies. Studies included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, reviewed the harm associated with the administration of a test or treatment, and explored the cost associated with the test or treatment as well as the overall strength of evidence. Additionally, the reference lists included in articles were reviewed to identify supplementary literature sources. The reviewers read and analyzed the articles identified in the initial search for relevant subject matter and summarized the findings in a table.
Delphi Panels
A Delphi scoring process was utilized to complete list refinement.[14] Subcommittee members anonymously voted via email for the strength of the test and treatment recommendation based upon specific criteria. To assist with this process, they received a copy of the completed literature review and an evidence summary of the literature. The following categories were used to guide the scoring: validity/evidence base to support, feasibility of implementation, frequency of occurrence, cost of occurrence, yield/emmpact, harm, and potential to improve. Results were aggregated and shared with the Choosing Wisely Subcommittee. The subcommittee conferred a final time, editing the recommendations for clarification and improved wording. A second anonymous vote was then conducted for the remaining tests and treatments through a revised scoring spreadsheet. The penultimate list was presented to the SHM's Board. Upon the Board's approval, the final list was submitted to the ABIM‐F.
RESULTS
The results of each stage of the list development process are shown in the online supporting information (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). The initial survey of SHM committee members garnered in excess of 150 tests and treatments from approximately 40 SHM committee members. The subsequent list refinement by SHM staff narrowed this list to 65 items, which were then further reduced to 15 items after ranking by members of the subcommittee (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). Voting by members of the general SHM membership further reduced the list to 11 tests and treatments.
The final list of 5 tests and treatments submitted to the ABIM‐F were:
- Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
- Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal (GI) complications.
- Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
- Do not order continuous telemetry monitoring outside of the intensive care unit (ICU) without using a protocol that governs continuation.
- Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability (Table 1).
|
| Test/Treatment Recommendations |
| Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis).[21, 50] |
| Do not prescribe GI prophylaxis to medical inpatients without clear‐cut indication or high risk for GI complication.[24] |
| Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms.[29, 51] |
| Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes.[35, 43, 52, 53] |
| Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.[44, 54, 55] |
RECOMMENDATIONS
Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
Despite guidelines identifying appropriate indications for the placement of urinary catheters, urinary tract infections due to catheter use remain the most frequent type of infection in acute care settings. Nearly 1 in every 5 patients in the hospital receives an indwelling catheter, and up to half are placed inappropriately.[15] Twenty‐six percent of patients who have indwelling catheters for 2 to 10 days will develop bacteriuria; subsequently, 24% of those patients will develop a catheter‐associated urinary tract infection (CAUTI).[15] More than 13,000 deaths due to CAUTI occur annually.[16] In addition to urinary tract infections and their complications, additional adverse outcomes related to indwelling catheters include formation of encrustations and restrictions to flow, prolonged hospital stay, and exposure to multidrug resistant organisms due to increased use of antibiotics. Evidence suggests that infections due to catheters are frequently preventable.[17, 18]
The economic burden associated with indwelling catheter complications is also substantial. Each episode of symptomatic urinary tract infection adds $676 in incremental costs, and catheter‐related bacteremia costs at least $2836.15 According to Scott, nearly 450,000 CAUTIs were estimated to have occurred in 2007, resulting in direct medical costs of between $340 to $370 million.[19]
Several organizations simultaneously released guidelines to provide a roadmap for appropriate catheter use and prevention of CAUTIs.[20, 21] Despite explicit guidelines, the Centers for Disease Control and Prevention recently reported that there was no improvement in CAUTIs between 2010 and 2011.[22] Implementing these strategies for CAUTI reduction include establishing a multidisciplinary team that applies a clear protocol, with daily reminders about catheters and stop orders for catheter discontinuation.
Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
Stress ulcer prophylaxis in the hospital with proton pump inhibitors (PPIs) or histamine‐2 antagonists are common. As many as 71% of patients admitted to the hospital receive some form of prophylaxis without appropriate indication.[23] Guidelines exist for appropriate use; however, therapy is commonly used in the inpatient setting for indications not investigated or supported by the literature.[24]
Inappropriate prescribing practices have been associated with multiple adverse events, including drug interactions, hospital‐acquired infections, and increased costs of care. Although consensus among physicians regarding whether GI prophylaxis causes harm is lacking, studies demonstrate a strong correlation between use of PPIs and common adverse events such as pneumonia and Clostridium difficile infection.[25, 26] For instance, inpatients receiving PPIs were 3.6 times more likely to develop C. difficile‐associated diarrhea than inpatients not exposed to PPIs.[27]
The American Society of Health‐System Pharmacists Therapeutic Guidelines on Stress Ulcer Prophylaxis provide guidance regarding the optimal indication for administration of acid‐suppression medication for patients in the hospital setting. The clinical guidelines specify that stress ulcer prophylaxis is not recommended for adult patients in non‐ICU settings. The recommendations are applicable to general medical and surgical patients with fewer than 2 risk factors for clinically important bleeding. Indications for use of stress ulcer prophylaxis in the ICU include coagulopathy and mechanical ventilation.[24]
Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
Anemia is a frequent comorbid condition in hospitalized patients. Correcting anemia by means of allogeneic blood transfusions with the goal of maximizing oxygen delivery is common practice in many hospitals. Varied threshold levels of hemoglobin and hematocrit are used, which is unsupported by evidence.[28, 29]
Acute anemia with normovolemic hemodilution has been proven safe in patients with coronary artery disease, heart valve disease, and the elderly. A restrictive transfusion approach with hemoglobin cutoff of 7 g/dL, as opposed to higher thresholds, has shown improved outcomes (lower mortality and lower rate of rebleeding) in adult and pediatric critical care as well as surgical patients.[30] Large studies in patients with acute myocardial infarction demonstrated that restrictive transfusional strategies are associated with decreased in‐hospital mortality, rate of reinfarction, and worsening heart failure, as well as 30‐day mortality.[31] A randomized trial in patients with active GI bleeding showed that a restrictive strategy of hemoglobin threshold of 7 g/dL was associated with improved outcomes (less mortality, less rate of rebleeding), compared with a strategy to transfuse patients with hemoglobin less than 9 g/dL.[32] In addition, increased awareness of the high cost of blood ($700$900 per unit) associated with the blood banking process as well as risk of potential infectious and noninfectious adverse reactions (eg, human immunodeficiency virus, hepatitis C virus, transfusion‐related lung injury, transfusion‐related circulatory overload) must be considered in the risk/benefit equation.[28]
Based on current available evidence, the American Association of Blood Banks recommends adhering to a restrictive transfusion strategy (7 g/dL) in hospitalized stable patients, and this threshold is raised to 8 g/dL in patients with preexisting cardiovascular disease or with active symptoms.[28] This should be combined with techniques such as preoperative anemia optimization by hematinics replacement (eg, iron, vitamin B12, folate, erythropoietin), intraoperative strategies (eg, antifibrinolytics, hypotension, normovolemic hemodilution, etc.), and postoperative strategies (eg, intraoperative cell salvage). These strategies have been shown to result in parsimonious red blood cell utilization as well as in substantial healthcare cost savings.[33]
Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
Telemetry use in the hospital is common and clearly has a role for patients with certain cardiac conditions and those at risk for cardiac events. Telemetry is resource intensive, requiring dedicated multidisciplinary staff with specialized training. Many hospitals lack the ability to maintain and staff telemetry beds.[34] Physicians may overestimate the role of telemetry in guiding patient management.[35] One study concluded that only 12.6% of patients on a non‐ICU cardiac telemetry unit required telemetric monitoring, and only 7% received modified management as a result of telemetry findings.[36]
Inappropriate utilization of telemetry can be linked to increased length of stay or boarding in the emergency department, reduced hospital throughput, increased ambulance diversion, and increased operational costs.[37] In addition, the use of telemetry can lead to a false sense of security and alarm fatigue.[38] Telemetry artifacts may result in unnecessary testing and procedures for patients.[39] Furthermore, to accommodate the need for telemetry, frequent room changes may occur that may lead to decreased patient satisfaction. Low‐risk chest pain patients (hemodynamically stable with negative biomarkers, no electrocardiogram changes, and no indication for invasive procedure) do not require telemetry monitoring, because it rarely affects direct care of these patients.[36, 40] A 2009 study concluded that telemetry monitoring does not affect the care or the outcome of low‐risk patients.[41] Patients with other diagnoses, such as chronic obstructive pulmonary disease exacerbation or hemodynamically stable pulmonary embolism, and those requiring blood transfusions, are often placed in monitored beds without evidence that this will impact their care.[37]
The American Heart Association has published guidelines on the use of cardiac telemetry.[35] Patients are risk stratified into 3 categories, with class III patients being those who are low risk and do not require telemetry. Seventy percent of patients with the top 10 diagnoses that were admitted from the emergency department may clinically warrant telemetry.[37] Implementing a systematic evidence‐based approach to telemetry use can decrease unnecessary telemetry days,[42] reduce costs, and avoid unnecessary testing for rhythm artifacts.[39, 43]
Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability.
Although unnecessary laboratory testing is widely perceived as ineffective and wasteful, no national guideline or consensus statement exists regarding the utility or timing of repetitive laboratory testing. Multiple studies showed no difference in readmission rates, transfers to ICUs, lengths of stay, rates of adverse events, or mortality when the frequency of laboratory testing was reduced. Charges for daily laboratory testing were estimated to be $150/patient/day.[44] In a study at a university‐associated teaching hospital, an intervention to reduce the frequency of laboratory testing was associated with a total decrease of nearly 98,000 tests over a 3‐year period.[45] The cost savings in this study was estimated to be almost $2 million over the same time period. A second study at a teaching hospital, involving a computerized physician order entry (CPOE)‐based intervention, showed a reduction of almost 72,000 tests over a 1‐year period, which reduced the total number of inpatient phlebotomies by approximately 21%.[46]
The cost of routine, daily laboratory testing for a given patient or health system is not insignificant. When healthcare providers are made aware of the cost of daily laboratory testing, this might reduce the number of laboratory tests ordered and result in significant savings for a health system, as well as improve the patient experience.[44]
Developing guidelines or strategies to reduce repetitive laboratory testing in the face of clinical or laboratory stability would likely produce significant cost savings for both the individual patient as well as the health system, and could possibly would likely improve the hospital experience for many patients. Widespread adoption of CPOE by the US healthcare system has the potential to facilitate decision support that can change laboratory ordering practices.
DISCUSSION
Eliminating waste in healthcare is a priority for physicians,[6, 7, 8, 9, 10] and the ABIM‐F's Choosing Wisely campaign is a key component of this effort.[11] The SHM chose 5 tests and treatments relevant to the specialty of hospital medicine that occur at a high frequency, have significant cost and affect to patients, and that can feasibly be impacted. Given that a high percentage of healthcare costs occur in the hospital[5] and hospitalists care for an increasing number of these patients,[13] successful implementation of the SHM's adult hospital medicine Choosing Wisely list has great potential to decrease waste in the hospital, reduce harm, and improve patient outcomes.
The methodology chosen to develop the adult Choosing Wisely recommendations was intended to be both pragmatic and evidence based. A broad range of opinions was solicited, including from the SHM's general membership. The final refinement included a literature review and a Delphi process.
Review of cost and utilization data to determine the scope of the problem was used for decision‐making by subcommittee members to formulate the SHM's recommendations. For some recommendations, there were significant data, whereas for others, this information was sparse. As has been noted, we were unable to identify the total number of patients in the United States who receive telemetry on an annual basis, and thus were unable to make an estimate about the total population that would be impacted by improved utilization. However, several studies do indicate inappropriate use in significant patient populations and widespread use of the resource. Similarly, we were able to identify the costs associated with a CBC, but were unable to calculate the total number of CBCs administered annually. In the absence of these data, subcommittee members utilized other criteria, including frequency of test or treatment, patient harm or benefit, and utility for making treatment/management decisions.
In general, the tests and treatments contained in the adult hospital medicine Choosing Wisely list are not requested by patients. As such, physicians' choices play a greater role, potentially magnifying the impact hospitalists could make. Overuse of medical tests is multifactorial, and culture plays a significant role in the United States.[2, 3, 4] Although each of the tests and treatments identified by the SHM is within the purview of hospitalists, ensuring that guidelines are reliably followed will require interdisciplinary process changes. Ample opportunity exists to partner with nurses (urinary catheters and telemetry), pharmacists (stress ulcer prophylaxis), blood banks, and laboratories (transfusions and lab testing), as well as other healthcare providers and physicians in multiple specialties.
Successful implementation of each guideline will require improvement of systems within hospitals to drive reliability.[47] Provider education, training programs, protocols and reminders may prove to be significant catalysts in overcoming misinformation or no information about specific guidelines. More importantly, interdisciplinary teams will need to assess the current practice patterns within their hospitals prior to implementing solutions that standardize and automate the ordering processes for these tests and treatments.[48] Additionally, the culture within individual patient care units will need to be modified.[49] The challenge of changing the behavior of multiple stakeholders and hardwiring systems changes represent significant potential barriers to success.
There are several potential concerns with the recommendations. Concepts such as high risk and clinical stability exist in several of the recommendations. In most cases, specific guidelines exist that explicitly define the appropriate use of the test or treatment. Where they do not, implementers will need to define the operational definitions, such as the number of normal CBCs that define stability. Although the recommendations are based on the best evidence available, consensus still plays a role. As has been noted, the risk of malpractice litigation influences physicians' decisions.[1] Although evidence‐based recommendations such as these help shape the standard of care and mitigate risk, they may not completely eliminate this concern. Providers should always weigh the risks and benefits of any test or treatment. Finally, the approach taken to establish the list was both pragmatic and evidence based. Published evidence was not reviewed until the list was honed to 11. When the evidence was reviewed, the strength of the evidence was judged in a subjective manner by members of the committee as part of the Delphi panel voting.
CONCLUSION
As healthcare providers enter an era of more cost conscious decision‐making about provision of care based upon necessity, hospitalists have an excellent opportunity to impact overutilization. The 5 recommendations comprising the adult hospital medicine Choosing Wisely list offer an explicit starting point. The SHM hopes to lead this process during the coming months and years and to offer additional recommendations, providing a foundation for hospitalists to decrease unnecessary tests and treatments and improve healthcare value.
Acknowledgments
The authors thank the additional members of the Choosing Wisely subcommittee of the SHM's Healthcare Quality and Patient Safety Committee: Krishna Das, MD; Shelley Taylor, MD; Kevin O'Leary, MD; and Nasim Afsarmanesh, MD. The authors also thank SHM staff who were involved in all facets of the recommendation development process, particularly Brendon Shank, who provided significant input into the survey and dissemination process.
Disclosure
Nothing to report.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- , , , et al. Evidence that consumers are skeptical about evidence‐based health care. Health Aff (Millwood). 2010;29:1400–1406.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Too much treatment? Aggressive medical care can lead to more pain, with no gain. Consum Rep. 2008;73:40–44.
- Centers for Medicare and Medicaid Services. Historical national health expenditure data. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed February 12, 2013.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- . Change the microenvironment: delivery system reform essential to controlling costs. Available at: http://www.commonwealthfund.org/Publications/Commentaries/2009/Apr/Change‐the‐Microenvironment.aspx. Accessed February 12, 2013.
- Costs of care. Available at: http://www.costsofcare.org. Accessed February 12, 2013.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750.
- , , , ; Clinical Guidelines Committee of the American College of Physicians. High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- ABIM Foundation. U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM‐Foundation‐News/2012/Choosing‐Wisely.aspx. Accessed February 12, 2013.
- , . Addressing requests by patients for nonbeneficial interventions. JAMA. 2012;307:149–150.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102–1112.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28:68–75.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- , , , et al. A compendium of strategies to prevent healthcare‐associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1): S12–S21.
- , , , et al. Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Available at: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed February 12, 2013.
- , , , , . Healthcare Infection Control Practices Advisory Committee, guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–326.
- , , , et al. Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
- , , , et al. 2011 National and State Healthcare‐Associated Infection Standardized Infection Ratio Report. Available at: http://www.cdc.gov/hai/pdfs/SIR/SIR‐Report_02_07_2013.pdf. Accessed February 13, 2013.
- , . Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64:1396–1400.
- . ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–379.
- , , , , , . Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292:1955–1960.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301:2120–2128.
- , , , . Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile‐associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- , , , et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
- , , , . Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818.
- , , , , . Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Intern Med 2013;173:132–139.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , . Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med. 1993;94:509–514.
- , , , , , . The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97:1483–1487.
- , , , et al. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Circulation. 1999;100:886–893.
- , , , et al. Role of telemetry monitoring in the non‐intensive care unit. Am J Cardiol. 1995;76:960–965.
- , . When do patients need admission to a telemetry bed? J Emerg Med. 2007;33:53–60.
- , . Electrocardiographic monitoring in the hospitalized patient: a diagnostic intervention of uncertain clinical impact. Am J Emerg Med. 2008;26:1047–1055.
- , , , , . Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341:1270–1274.
- , , , . Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in‐hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci. 2005;60:605–606.
- , , , , . Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8:125–126.
- Agency for Healthcare Research and Quality. Winawer N. Redesign of telemetry unit admission and transfer criteria leads to improved patient flow and reduced emergency department waiting times. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2239. Accessed February 12, 2013.
- , , , et al. Is telemetry monitoring necessary in low‐risk suspected acute chest pain syndromes? Chest. 2002;122:517–523.
- , . Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146:524–527.
- , , , et al. A cost‐effective method for reducing the volume of laboratory tests in a university‐associated teaching hospital. Mt Sinai J Med. 2006;73:787–794.
- , , , et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200–206.
- . Making noncatastrophic health care processes reliable: learning to walk before running in creating high‐reliability organizations. Health Serv Res. 2006;41:1677–1689.
- , , , et al. What have we learned about interventions to reduce medical errors? Annu Rev Public Health. 2010;31:479–497.
- , . Safe Patients, Smart Hospitals: How One Doctor's Checklist Can Help Us Change Health Care From The Inside Out. New York, NY: Hudson Street Press; 2010.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.
- Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703.
- , , , , , . Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76:368–372.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653.
- , , , , . Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524.
The overuse of medical tests and treatments is a growing concern. A recent survey revealed that 2 in 5 primary care physicians perceive that patients in their own practice are receiving too much care.[1] Twenty‐eight percent of the physicians indicated they provide more care than they should. When queried about reasons for the aggressiveness of care, responses included fear of malpractice litigation, adherence to clinical performance measures that require following protocols, and inadequacy of time spent with patients. Overutilization of healthcare resources is a complex issue promulgated not only by the factors cited by the physicians but also a culture in the United States habituated to believe more care is better care.[2, 3, 4] In 2010, $2.6 trillion was spent on healthcare, an increase of $1.3 trillion between 2000 and 2010.5 As much as 30% of healthcare spending may be wasted.[6] Because physicians influence approximately 80% of healthcare expenditures, including ordering tests and treatments, it is imperative that physicians take a leadership role in reversing this trend.[7]
In response to this need, several physician‐led projects have emerged.[8, 9, 10] One such initiative is the American Board of Internal Medicine Foundation's (ABIM‐F's) Choosing Wisely campaign.[11] The ABIM‐F contacted a variety of specialty societies and asked each to identify the 5 top tests or treatments relevant to their specialty that may frequently be overused. Phase 1 of the Choosing Wisely campaign was launched in April 2012 with 9 specialty societies participating. The second phase was unveiled in February 2013 and comprised of 16 additional groups including the Society of Hospital Medicine (SHM). The SHM represents 35,000 hospitalists in the United States whose primary focus is the general medical care of hospitalized patients. This is especially important because almost one‐third of total US healthcare expenditures are on hospital care,[12] and hospitalists care for an increasing number of hospitalized patients.[13] In this article, we describe the used to derive the adult hospital medicine Choosing processes Wisely list, review the tests and treatments that the SHM's Choosing Wisely Subcommittee chose, and discuss potential next steps in implementation of the adult hospital medicine recommendations.
METHODOLOGY
Upon invitation to participate in the Choosing Wisely campaign, SHM's Hospital Quality and Patient Safety (HQPS) Committee formally convened the Choosing Wisely Subcommittee. The subcommittee identified and executed a methodology (see Supporting Figure 1 and Supporting Table 1 in the online version of this article) to create the list of 5 tests and treatments that the SHM submitted to the ABIM‐F. All subcommittee members participated fully in the voting and refinement process. The Choosing Wisely Subcommittee worked closely with the SHM's Pediatrics Choosing Wisely Subcommittee to develop both adult and pediatric lists.
Convening the Choosing Wisely Subcommittee
The HQPS Committee convened a subcommittee consisting of 9 members. The subcommittee represented a diverse group of hospitalists reflecting different institution types, geographic regions, and experience. All Choosing Wisely Subcommittee members signed conflict of interest statements and reported no conflict related to the conclusions, implications, or opinions stated. The subcommittee did not consult other external stakeholders in the development of recommendations.
Identification and Refinement of Potential Wasteful Practices
To generate an initial list of potential recommendations, members of all of the SHM committees were surveyed and asked to submit 5 tests and treatments that are inappropriately used or overused. SHM staff removed duplicates and categorized submissions by topic, highlighting overlapping recommendations. Tests and treatments that are used infrequently and items included in phase 1 society lists were also excluded. Subcommittee members then ranked the resultant list using a 5‐point Likert scale. All SHM members were then given the opportunity to rank their agreement with the tests and treatments on the list, as refined at the time based upon their own experience and consideration of the following criteria: tests and procedures within the control and purview of hospital medicine, the frequency with which the tests or procedures occur, and the significance of associated costs. This was accomplished via electronic survey.
Establishing an Evidence Base
SHM staff conducted a literature review of the list of tests and treatments that was further refined by the SHM membership's ranking using a standard template. Two reviewers (W.N. and J.G.) conducted an independent literature review of the remaining tests and treatments using PubMed, MEDLINE, and Cochrane Library. The reviewers also conducted generic Internet searches. The literature review included all literature published through 2012 as well as nonEnglish language publications. The reviewers included clinical research guidelines and primary and secondary research studies. Studies included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, reviewed the harm associated with the administration of a test or treatment, and explored the cost associated with the test or treatment as well as the overall strength of evidence. Additionally, the reference lists included in articles were reviewed to identify supplementary literature sources. The reviewers read and analyzed the articles identified in the initial search for relevant subject matter and summarized the findings in a table.
Delphi Panels
A Delphi scoring process was utilized to complete list refinement.[14] Subcommittee members anonymously voted via email for the strength of the test and treatment recommendation based upon specific criteria. To assist with this process, they received a copy of the completed literature review and an evidence summary of the literature. The following categories were used to guide the scoring: validity/evidence base to support, feasibility of implementation, frequency of occurrence, cost of occurrence, yield/emmpact, harm, and potential to improve. Results were aggregated and shared with the Choosing Wisely Subcommittee. The subcommittee conferred a final time, editing the recommendations for clarification and improved wording. A second anonymous vote was then conducted for the remaining tests and treatments through a revised scoring spreadsheet. The penultimate list was presented to the SHM's Board. Upon the Board's approval, the final list was submitted to the ABIM‐F.
RESULTS
The results of each stage of the list development process are shown in the online supporting information (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). The initial survey of SHM committee members garnered in excess of 150 tests and treatments from approximately 40 SHM committee members. The subsequent list refinement by SHM staff narrowed this list to 65 items, which were then further reduced to 15 items after ranking by members of the subcommittee (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). Voting by members of the general SHM membership further reduced the list to 11 tests and treatments.
The final list of 5 tests and treatments submitted to the ABIM‐F were:
- Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
- Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal (GI) complications.
- Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
- Do not order continuous telemetry monitoring outside of the intensive care unit (ICU) without using a protocol that governs continuation.
- Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability (Table 1).
|
| Test/Treatment Recommendations |
| Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis).[21, 50] |
| Do not prescribe GI prophylaxis to medical inpatients without clear‐cut indication or high risk for GI complication.[24] |
| Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms.[29, 51] |
| Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes.[35, 43, 52, 53] |
| Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.[44, 54, 55] |
RECOMMENDATIONS
Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
Despite guidelines identifying appropriate indications for the placement of urinary catheters, urinary tract infections due to catheter use remain the most frequent type of infection in acute care settings. Nearly 1 in every 5 patients in the hospital receives an indwelling catheter, and up to half are placed inappropriately.[15] Twenty‐six percent of patients who have indwelling catheters for 2 to 10 days will develop bacteriuria; subsequently, 24% of those patients will develop a catheter‐associated urinary tract infection (CAUTI).[15] More than 13,000 deaths due to CAUTI occur annually.[16] In addition to urinary tract infections and their complications, additional adverse outcomes related to indwelling catheters include formation of encrustations and restrictions to flow, prolonged hospital stay, and exposure to multidrug resistant organisms due to increased use of antibiotics. Evidence suggests that infections due to catheters are frequently preventable.[17, 18]
The economic burden associated with indwelling catheter complications is also substantial. Each episode of symptomatic urinary tract infection adds $676 in incremental costs, and catheter‐related bacteremia costs at least $2836.15 According to Scott, nearly 450,000 CAUTIs were estimated to have occurred in 2007, resulting in direct medical costs of between $340 to $370 million.[19]
Several organizations simultaneously released guidelines to provide a roadmap for appropriate catheter use and prevention of CAUTIs.[20, 21] Despite explicit guidelines, the Centers for Disease Control and Prevention recently reported that there was no improvement in CAUTIs between 2010 and 2011.[22] Implementing these strategies for CAUTI reduction include establishing a multidisciplinary team that applies a clear protocol, with daily reminders about catheters and stop orders for catheter discontinuation.
Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
Stress ulcer prophylaxis in the hospital with proton pump inhibitors (PPIs) or histamine‐2 antagonists are common. As many as 71% of patients admitted to the hospital receive some form of prophylaxis without appropriate indication.[23] Guidelines exist for appropriate use; however, therapy is commonly used in the inpatient setting for indications not investigated or supported by the literature.[24]
Inappropriate prescribing practices have been associated with multiple adverse events, including drug interactions, hospital‐acquired infections, and increased costs of care. Although consensus among physicians regarding whether GI prophylaxis causes harm is lacking, studies demonstrate a strong correlation between use of PPIs and common adverse events such as pneumonia and Clostridium difficile infection.[25, 26] For instance, inpatients receiving PPIs were 3.6 times more likely to develop C. difficile‐associated diarrhea than inpatients not exposed to PPIs.[27]
The American Society of Health‐System Pharmacists Therapeutic Guidelines on Stress Ulcer Prophylaxis provide guidance regarding the optimal indication for administration of acid‐suppression medication for patients in the hospital setting. The clinical guidelines specify that stress ulcer prophylaxis is not recommended for adult patients in non‐ICU settings. The recommendations are applicable to general medical and surgical patients with fewer than 2 risk factors for clinically important bleeding. Indications for use of stress ulcer prophylaxis in the ICU include coagulopathy and mechanical ventilation.[24]
Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
Anemia is a frequent comorbid condition in hospitalized patients. Correcting anemia by means of allogeneic blood transfusions with the goal of maximizing oxygen delivery is common practice in many hospitals. Varied threshold levels of hemoglobin and hematocrit are used, which is unsupported by evidence.[28, 29]
Acute anemia with normovolemic hemodilution has been proven safe in patients with coronary artery disease, heart valve disease, and the elderly. A restrictive transfusion approach with hemoglobin cutoff of 7 g/dL, as opposed to higher thresholds, has shown improved outcomes (lower mortality and lower rate of rebleeding) in adult and pediatric critical care as well as surgical patients.[30] Large studies in patients with acute myocardial infarction demonstrated that restrictive transfusional strategies are associated with decreased in‐hospital mortality, rate of reinfarction, and worsening heart failure, as well as 30‐day mortality.[31] A randomized trial in patients with active GI bleeding showed that a restrictive strategy of hemoglobin threshold of 7 g/dL was associated with improved outcomes (less mortality, less rate of rebleeding), compared with a strategy to transfuse patients with hemoglobin less than 9 g/dL.[32] In addition, increased awareness of the high cost of blood ($700$900 per unit) associated with the blood banking process as well as risk of potential infectious and noninfectious adverse reactions (eg, human immunodeficiency virus, hepatitis C virus, transfusion‐related lung injury, transfusion‐related circulatory overload) must be considered in the risk/benefit equation.[28]
Based on current available evidence, the American Association of Blood Banks recommends adhering to a restrictive transfusion strategy (7 g/dL) in hospitalized stable patients, and this threshold is raised to 8 g/dL in patients with preexisting cardiovascular disease or with active symptoms.[28] This should be combined with techniques such as preoperative anemia optimization by hematinics replacement (eg, iron, vitamin B12, folate, erythropoietin), intraoperative strategies (eg, antifibrinolytics, hypotension, normovolemic hemodilution, etc.), and postoperative strategies (eg, intraoperative cell salvage). These strategies have been shown to result in parsimonious red blood cell utilization as well as in substantial healthcare cost savings.[33]
Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
Telemetry use in the hospital is common and clearly has a role for patients with certain cardiac conditions and those at risk for cardiac events. Telemetry is resource intensive, requiring dedicated multidisciplinary staff with specialized training. Many hospitals lack the ability to maintain and staff telemetry beds.[34] Physicians may overestimate the role of telemetry in guiding patient management.[35] One study concluded that only 12.6% of patients on a non‐ICU cardiac telemetry unit required telemetric monitoring, and only 7% received modified management as a result of telemetry findings.[36]
Inappropriate utilization of telemetry can be linked to increased length of stay or boarding in the emergency department, reduced hospital throughput, increased ambulance diversion, and increased operational costs.[37] In addition, the use of telemetry can lead to a false sense of security and alarm fatigue.[38] Telemetry artifacts may result in unnecessary testing and procedures for patients.[39] Furthermore, to accommodate the need for telemetry, frequent room changes may occur that may lead to decreased patient satisfaction. Low‐risk chest pain patients (hemodynamically stable with negative biomarkers, no electrocardiogram changes, and no indication for invasive procedure) do not require telemetry monitoring, because it rarely affects direct care of these patients.[36, 40] A 2009 study concluded that telemetry monitoring does not affect the care or the outcome of low‐risk patients.[41] Patients with other diagnoses, such as chronic obstructive pulmonary disease exacerbation or hemodynamically stable pulmonary embolism, and those requiring blood transfusions, are often placed in monitored beds without evidence that this will impact their care.[37]
The American Heart Association has published guidelines on the use of cardiac telemetry.[35] Patients are risk stratified into 3 categories, with class III patients being those who are low risk and do not require telemetry. Seventy percent of patients with the top 10 diagnoses that were admitted from the emergency department may clinically warrant telemetry.[37] Implementing a systematic evidence‐based approach to telemetry use can decrease unnecessary telemetry days,[42] reduce costs, and avoid unnecessary testing for rhythm artifacts.[39, 43]
Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability.
Although unnecessary laboratory testing is widely perceived as ineffective and wasteful, no national guideline or consensus statement exists regarding the utility or timing of repetitive laboratory testing. Multiple studies showed no difference in readmission rates, transfers to ICUs, lengths of stay, rates of adverse events, or mortality when the frequency of laboratory testing was reduced. Charges for daily laboratory testing were estimated to be $150/patient/day.[44] In a study at a university‐associated teaching hospital, an intervention to reduce the frequency of laboratory testing was associated with a total decrease of nearly 98,000 tests over a 3‐year period.[45] The cost savings in this study was estimated to be almost $2 million over the same time period. A second study at a teaching hospital, involving a computerized physician order entry (CPOE)‐based intervention, showed a reduction of almost 72,000 tests over a 1‐year period, which reduced the total number of inpatient phlebotomies by approximately 21%.[46]
The cost of routine, daily laboratory testing for a given patient or health system is not insignificant. When healthcare providers are made aware of the cost of daily laboratory testing, this might reduce the number of laboratory tests ordered and result in significant savings for a health system, as well as improve the patient experience.[44]
Developing guidelines or strategies to reduce repetitive laboratory testing in the face of clinical or laboratory stability would likely produce significant cost savings for both the individual patient as well as the health system, and could possibly would likely improve the hospital experience for many patients. Widespread adoption of CPOE by the US healthcare system has the potential to facilitate decision support that can change laboratory ordering practices.
DISCUSSION
Eliminating waste in healthcare is a priority for physicians,[6, 7, 8, 9, 10] and the ABIM‐F's Choosing Wisely campaign is a key component of this effort.[11] The SHM chose 5 tests and treatments relevant to the specialty of hospital medicine that occur at a high frequency, have significant cost and affect to patients, and that can feasibly be impacted. Given that a high percentage of healthcare costs occur in the hospital[5] and hospitalists care for an increasing number of these patients,[13] successful implementation of the SHM's adult hospital medicine Choosing Wisely list has great potential to decrease waste in the hospital, reduce harm, and improve patient outcomes.
The methodology chosen to develop the adult Choosing Wisely recommendations was intended to be both pragmatic and evidence based. A broad range of opinions was solicited, including from the SHM's general membership. The final refinement included a literature review and a Delphi process.
Review of cost and utilization data to determine the scope of the problem was used for decision‐making by subcommittee members to formulate the SHM's recommendations. For some recommendations, there were significant data, whereas for others, this information was sparse. As has been noted, we were unable to identify the total number of patients in the United States who receive telemetry on an annual basis, and thus were unable to make an estimate about the total population that would be impacted by improved utilization. However, several studies do indicate inappropriate use in significant patient populations and widespread use of the resource. Similarly, we were able to identify the costs associated with a CBC, but were unable to calculate the total number of CBCs administered annually. In the absence of these data, subcommittee members utilized other criteria, including frequency of test or treatment, patient harm or benefit, and utility for making treatment/management decisions.
In general, the tests and treatments contained in the adult hospital medicine Choosing Wisely list are not requested by patients. As such, physicians' choices play a greater role, potentially magnifying the impact hospitalists could make. Overuse of medical tests is multifactorial, and culture plays a significant role in the United States.[2, 3, 4] Although each of the tests and treatments identified by the SHM is within the purview of hospitalists, ensuring that guidelines are reliably followed will require interdisciplinary process changes. Ample opportunity exists to partner with nurses (urinary catheters and telemetry), pharmacists (stress ulcer prophylaxis), blood banks, and laboratories (transfusions and lab testing), as well as other healthcare providers and physicians in multiple specialties.
Successful implementation of each guideline will require improvement of systems within hospitals to drive reliability.[47] Provider education, training programs, protocols and reminders may prove to be significant catalysts in overcoming misinformation or no information about specific guidelines. More importantly, interdisciplinary teams will need to assess the current practice patterns within their hospitals prior to implementing solutions that standardize and automate the ordering processes for these tests and treatments.[48] Additionally, the culture within individual patient care units will need to be modified.[49] The challenge of changing the behavior of multiple stakeholders and hardwiring systems changes represent significant potential barriers to success.
There are several potential concerns with the recommendations. Concepts such as high risk and clinical stability exist in several of the recommendations. In most cases, specific guidelines exist that explicitly define the appropriate use of the test or treatment. Where they do not, implementers will need to define the operational definitions, such as the number of normal CBCs that define stability. Although the recommendations are based on the best evidence available, consensus still plays a role. As has been noted, the risk of malpractice litigation influences physicians' decisions.[1] Although evidence‐based recommendations such as these help shape the standard of care and mitigate risk, they may not completely eliminate this concern. Providers should always weigh the risks and benefits of any test or treatment. Finally, the approach taken to establish the list was both pragmatic and evidence based. Published evidence was not reviewed until the list was honed to 11. When the evidence was reviewed, the strength of the evidence was judged in a subjective manner by members of the committee as part of the Delphi panel voting.
CONCLUSION
As healthcare providers enter an era of more cost conscious decision‐making about provision of care based upon necessity, hospitalists have an excellent opportunity to impact overutilization. The 5 recommendations comprising the adult hospital medicine Choosing Wisely list offer an explicit starting point. The SHM hopes to lead this process during the coming months and years and to offer additional recommendations, providing a foundation for hospitalists to decrease unnecessary tests and treatments and improve healthcare value.
Acknowledgments
The authors thank the additional members of the Choosing Wisely subcommittee of the SHM's Healthcare Quality and Patient Safety Committee: Krishna Das, MD; Shelley Taylor, MD; Kevin O'Leary, MD; and Nasim Afsarmanesh, MD. The authors also thank SHM staff who were involved in all facets of the recommendation development process, particularly Brendon Shank, who provided significant input into the survey and dissemination process.
Disclosure
Nothing to report.
The overuse of medical tests and treatments is a growing concern. A recent survey revealed that 2 in 5 primary care physicians perceive that patients in their own practice are receiving too much care.[1] Twenty‐eight percent of the physicians indicated they provide more care than they should. When queried about reasons for the aggressiveness of care, responses included fear of malpractice litigation, adherence to clinical performance measures that require following protocols, and inadequacy of time spent with patients. Overutilization of healthcare resources is a complex issue promulgated not only by the factors cited by the physicians but also a culture in the United States habituated to believe more care is better care.[2, 3, 4] In 2010, $2.6 trillion was spent on healthcare, an increase of $1.3 trillion between 2000 and 2010.5 As much as 30% of healthcare spending may be wasted.[6] Because physicians influence approximately 80% of healthcare expenditures, including ordering tests and treatments, it is imperative that physicians take a leadership role in reversing this trend.[7]
In response to this need, several physician‐led projects have emerged.[8, 9, 10] One such initiative is the American Board of Internal Medicine Foundation's (ABIM‐F's) Choosing Wisely campaign.[11] The ABIM‐F contacted a variety of specialty societies and asked each to identify the 5 top tests or treatments relevant to their specialty that may frequently be overused. Phase 1 of the Choosing Wisely campaign was launched in April 2012 with 9 specialty societies participating. The second phase was unveiled in February 2013 and comprised of 16 additional groups including the Society of Hospital Medicine (SHM). The SHM represents 35,000 hospitalists in the United States whose primary focus is the general medical care of hospitalized patients. This is especially important because almost one‐third of total US healthcare expenditures are on hospital care,[12] and hospitalists care for an increasing number of hospitalized patients.[13] In this article, we describe the used to derive the adult hospital medicine Choosing processes Wisely list, review the tests and treatments that the SHM's Choosing Wisely Subcommittee chose, and discuss potential next steps in implementation of the adult hospital medicine recommendations.
METHODOLOGY
Upon invitation to participate in the Choosing Wisely campaign, SHM's Hospital Quality and Patient Safety (HQPS) Committee formally convened the Choosing Wisely Subcommittee. The subcommittee identified and executed a methodology (see Supporting Figure 1 and Supporting Table 1 in the online version of this article) to create the list of 5 tests and treatments that the SHM submitted to the ABIM‐F. All subcommittee members participated fully in the voting and refinement process. The Choosing Wisely Subcommittee worked closely with the SHM's Pediatrics Choosing Wisely Subcommittee to develop both adult and pediatric lists.
Convening the Choosing Wisely Subcommittee
The HQPS Committee convened a subcommittee consisting of 9 members. The subcommittee represented a diverse group of hospitalists reflecting different institution types, geographic regions, and experience. All Choosing Wisely Subcommittee members signed conflict of interest statements and reported no conflict related to the conclusions, implications, or opinions stated. The subcommittee did not consult other external stakeholders in the development of recommendations.
Identification and Refinement of Potential Wasteful Practices
To generate an initial list of potential recommendations, members of all of the SHM committees were surveyed and asked to submit 5 tests and treatments that are inappropriately used or overused. SHM staff removed duplicates and categorized submissions by topic, highlighting overlapping recommendations. Tests and treatments that are used infrequently and items included in phase 1 society lists were also excluded. Subcommittee members then ranked the resultant list using a 5‐point Likert scale. All SHM members were then given the opportunity to rank their agreement with the tests and treatments on the list, as refined at the time based upon their own experience and consideration of the following criteria: tests and procedures within the control and purview of hospital medicine, the frequency with which the tests or procedures occur, and the significance of associated costs. This was accomplished via electronic survey.
Establishing an Evidence Base
SHM staff conducted a literature review of the list of tests and treatments that was further refined by the SHM membership's ranking using a standard template. Two reviewers (W.N. and J.G.) conducted an independent literature review of the remaining tests and treatments using PubMed, MEDLINE, and Cochrane Library. The reviewers also conducted generic Internet searches. The literature review included all literature published through 2012 as well as nonEnglish language publications. The reviewers included clinical research guidelines and primary and secondary research studies. Studies included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, reviewed the harm associated with the administration of a test or treatment, and explored the cost associated with the test or treatment as well as the overall strength of evidence. Additionally, the reference lists included in articles were reviewed to identify supplementary literature sources. The reviewers read and analyzed the articles identified in the initial search for relevant subject matter and summarized the findings in a table.
Delphi Panels
A Delphi scoring process was utilized to complete list refinement.[14] Subcommittee members anonymously voted via email for the strength of the test and treatment recommendation based upon specific criteria. To assist with this process, they received a copy of the completed literature review and an evidence summary of the literature. The following categories were used to guide the scoring: validity/evidence base to support, feasibility of implementation, frequency of occurrence, cost of occurrence, yield/emmpact, harm, and potential to improve. Results were aggregated and shared with the Choosing Wisely Subcommittee. The subcommittee conferred a final time, editing the recommendations for clarification and improved wording. A second anonymous vote was then conducted for the remaining tests and treatments through a revised scoring spreadsheet. The penultimate list was presented to the SHM's Board. Upon the Board's approval, the final list was submitted to the ABIM‐F.
RESULTS
The results of each stage of the list development process are shown in the online supporting information (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). The initial survey of SHM committee members garnered in excess of 150 tests and treatments from approximately 40 SHM committee members. The subsequent list refinement by SHM staff narrowed this list to 65 items, which were then further reduced to 15 items after ranking by members of the subcommittee (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). Voting by members of the general SHM membership further reduced the list to 11 tests and treatments.
The final list of 5 tests and treatments submitted to the ABIM‐F were:
- Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
- Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal (GI) complications.
- Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
- Do not order continuous telemetry monitoring outside of the intensive care unit (ICU) without using a protocol that governs continuation.
- Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability (Table 1).
|
| Test/Treatment Recommendations |
| Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis).[21, 50] |
| Do not prescribe GI prophylaxis to medical inpatients without clear‐cut indication or high risk for GI complication.[24] |
| Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms.[29, 51] |
| Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes.[35, 43, 52, 53] |
| Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.[44, 54, 55] |
RECOMMENDATIONS
Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
Despite guidelines identifying appropriate indications for the placement of urinary catheters, urinary tract infections due to catheter use remain the most frequent type of infection in acute care settings. Nearly 1 in every 5 patients in the hospital receives an indwelling catheter, and up to half are placed inappropriately.[15] Twenty‐six percent of patients who have indwelling catheters for 2 to 10 days will develop bacteriuria; subsequently, 24% of those patients will develop a catheter‐associated urinary tract infection (CAUTI).[15] More than 13,000 deaths due to CAUTI occur annually.[16] In addition to urinary tract infections and their complications, additional adverse outcomes related to indwelling catheters include formation of encrustations and restrictions to flow, prolonged hospital stay, and exposure to multidrug resistant organisms due to increased use of antibiotics. Evidence suggests that infections due to catheters are frequently preventable.[17, 18]
The economic burden associated with indwelling catheter complications is also substantial. Each episode of symptomatic urinary tract infection adds $676 in incremental costs, and catheter‐related bacteremia costs at least $2836.15 According to Scott, nearly 450,000 CAUTIs were estimated to have occurred in 2007, resulting in direct medical costs of between $340 to $370 million.[19]
Several organizations simultaneously released guidelines to provide a roadmap for appropriate catheter use and prevention of CAUTIs.[20, 21] Despite explicit guidelines, the Centers for Disease Control and Prevention recently reported that there was no improvement in CAUTIs between 2010 and 2011.[22] Implementing these strategies for CAUTI reduction include establishing a multidisciplinary team that applies a clear protocol, with daily reminders about catheters and stop orders for catheter discontinuation.
Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
Stress ulcer prophylaxis in the hospital with proton pump inhibitors (PPIs) or histamine‐2 antagonists are common. As many as 71% of patients admitted to the hospital receive some form of prophylaxis without appropriate indication.[23] Guidelines exist for appropriate use; however, therapy is commonly used in the inpatient setting for indications not investigated or supported by the literature.[24]
Inappropriate prescribing practices have been associated with multiple adverse events, including drug interactions, hospital‐acquired infections, and increased costs of care. Although consensus among physicians regarding whether GI prophylaxis causes harm is lacking, studies demonstrate a strong correlation between use of PPIs and common adverse events such as pneumonia and Clostridium difficile infection.[25, 26] For instance, inpatients receiving PPIs were 3.6 times more likely to develop C. difficile‐associated diarrhea than inpatients not exposed to PPIs.[27]
The American Society of Health‐System Pharmacists Therapeutic Guidelines on Stress Ulcer Prophylaxis provide guidance regarding the optimal indication for administration of acid‐suppression medication for patients in the hospital setting. The clinical guidelines specify that stress ulcer prophylaxis is not recommended for adult patients in non‐ICU settings. The recommendations are applicable to general medical and surgical patients with fewer than 2 risk factors for clinically important bleeding. Indications for use of stress ulcer prophylaxis in the ICU include coagulopathy and mechanical ventilation.[24]
Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
Anemia is a frequent comorbid condition in hospitalized patients. Correcting anemia by means of allogeneic blood transfusions with the goal of maximizing oxygen delivery is common practice in many hospitals. Varied threshold levels of hemoglobin and hematocrit are used, which is unsupported by evidence.[28, 29]
Acute anemia with normovolemic hemodilution has been proven safe in patients with coronary artery disease, heart valve disease, and the elderly. A restrictive transfusion approach with hemoglobin cutoff of 7 g/dL, as opposed to higher thresholds, has shown improved outcomes (lower mortality and lower rate of rebleeding) in adult and pediatric critical care as well as surgical patients.[30] Large studies in patients with acute myocardial infarction demonstrated that restrictive transfusional strategies are associated with decreased in‐hospital mortality, rate of reinfarction, and worsening heart failure, as well as 30‐day mortality.[31] A randomized trial in patients with active GI bleeding showed that a restrictive strategy of hemoglobin threshold of 7 g/dL was associated with improved outcomes (less mortality, less rate of rebleeding), compared with a strategy to transfuse patients with hemoglobin less than 9 g/dL.[32] In addition, increased awareness of the high cost of blood ($700$900 per unit) associated with the blood banking process as well as risk of potential infectious and noninfectious adverse reactions (eg, human immunodeficiency virus, hepatitis C virus, transfusion‐related lung injury, transfusion‐related circulatory overload) must be considered in the risk/benefit equation.[28]
Based on current available evidence, the American Association of Blood Banks recommends adhering to a restrictive transfusion strategy (7 g/dL) in hospitalized stable patients, and this threshold is raised to 8 g/dL in patients with preexisting cardiovascular disease or with active symptoms.[28] This should be combined with techniques such as preoperative anemia optimization by hematinics replacement (eg, iron, vitamin B12, folate, erythropoietin), intraoperative strategies (eg, antifibrinolytics, hypotension, normovolemic hemodilution, etc.), and postoperative strategies (eg, intraoperative cell salvage). These strategies have been shown to result in parsimonious red blood cell utilization as well as in substantial healthcare cost savings.[33]
Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
Telemetry use in the hospital is common and clearly has a role for patients with certain cardiac conditions and those at risk for cardiac events. Telemetry is resource intensive, requiring dedicated multidisciplinary staff with specialized training. Many hospitals lack the ability to maintain and staff telemetry beds.[34] Physicians may overestimate the role of telemetry in guiding patient management.[35] One study concluded that only 12.6% of patients on a non‐ICU cardiac telemetry unit required telemetric monitoring, and only 7% received modified management as a result of telemetry findings.[36]
Inappropriate utilization of telemetry can be linked to increased length of stay or boarding in the emergency department, reduced hospital throughput, increased ambulance diversion, and increased operational costs.[37] In addition, the use of telemetry can lead to a false sense of security and alarm fatigue.[38] Telemetry artifacts may result in unnecessary testing and procedures for patients.[39] Furthermore, to accommodate the need for telemetry, frequent room changes may occur that may lead to decreased patient satisfaction. Low‐risk chest pain patients (hemodynamically stable with negative biomarkers, no electrocardiogram changes, and no indication for invasive procedure) do not require telemetry monitoring, because it rarely affects direct care of these patients.[36, 40] A 2009 study concluded that telemetry monitoring does not affect the care or the outcome of low‐risk patients.[41] Patients with other diagnoses, such as chronic obstructive pulmonary disease exacerbation or hemodynamically stable pulmonary embolism, and those requiring blood transfusions, are often placed in monitored beds without evidence that this will impact their care.[37]
The American Heart Association has published guidelines on the use of cardiac telemetry.[35] Patients are risk stratified into 3 categories, with class III patients being those who are low risk and do not require telemetry. Seventy percent of patients with the top 10 diagnoses that were admitted from the emergency department may clinically warrant telemetry.[37] Implementing a systematic evidence‐based approach to telemetry use can decrease unnecessary telemetry days,[42] reduce costs, and avoid unnecessary testing for rhythm artifacts.[39, 43]
Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability.
Although unnecessary laboratory testing is widely perceived as ineffective and wasteful, no national guideline or consensus statement exists regarding the utility or timing of repetitive laboratory testing. Multiple studies showed no difference in readmission rates, transfers to ICUs, lengths of stay, rates of adverse events, or mortality when the frequency of laboratory testing was reduced. Charges for daily laboratory testing were estimated to be $150/patient/day.[44] In a study at a university‐associated teaching hospital, an intervention to reduce the frequency of laboratory testing was associated with a total decrease of nearly 98,000 tests over a 3‐year period.[45] The cost savings in this study was estimated to be almost $2 million over the same time period. A second study at a teaching hospital, involving a computerized physician order entry (CPOE)‐based intervention, showed a reduction of almost 72,000 tests over a 1‐year period, which reduced the total number of inpatient phlebotomies by approximately 21%.[46]
The cost of routine, daily laboratory testing for a given patient or health system is not insignificant. When healthcare providers are made aware of the cost of daily laboratory testing, this might reduce the number of laboratory tests ordered and result in significant savings for a health system, as well as improve the patient experience.[44]
Developing guidelines or strategies to reduce repetitive laboratory testing in the face of clinical or laboratory stability would likely produce significant cost savings for both the individual patient as well as the health system, and could possibly would likely improve the hospital experience for many patients. Widespread adoption of CPOE by the US healthcare system has the potential to facilitate decision support that can change laboratory ordering practices.
DISCUSSION
Eliminating waste in healthcare is a priority for physicians,[6, 7, 8, 9, 10] and the ABIM‐F's Choosing Wisely campaign is a key component of this effort.[11] The SHM chose 5 tests and treatments relevant to the specialty of hospital medicine that occur at a high frequency, have significant cost and affect to patients, and that can feasibly be impacted. Given that a high percentage of healthcare costs occur in the hospital[5] and hospitalists care for an increasing number of these patients,[13] successful implementation of the SHM's adult hospital medicine Choosing Wisely list has great potential to decrease waste in the hospital, reduce harm, and improve patient outcomes.
The methodology chosen to develop the adult Choosing Wisely recommendations was intended to be both pragmatic and evidence based. A broad range of opinions was solicited, including from the SHM's general membership. The final refinement included a literature review and a Delphi process.
Review of cost and utilization data to determine the scope of the problem was used for decision‐making by subcommittee members to formulate the SHM's recommendations. For some recommendations, there were significant data, whereas for others, this information was sparse. As has been noted, we were unable to identify the total number of patients in the United States who receive telemetry on an annual basis, and thus were unable to make an estimate about the total population that would be impacted by improved utilization. However, several studies do indicate inappropriate use in significant patient populations and widespread use of the resource. Similarly, we were able to identify the costs associated with a CBC, but were unable to calculate the total number of CBCs administered annually. In the absence of these data, subcommittee members utilized other criteria, including frequency of test or treatment, patient harm or benefit, and utility for making treatment/management decisions.
In general, the tests and treatments contained in the adult hospital medicine Choosing Wisely list are not requested by patients. As such, physicians' choices play a greater role, potentially magnifying the impact hospitalists could make. Overuse of medical tests is multifactorial, and culture plays a significant role in the United States.[2, 3, 4] Although each of the tests and treatments identified by the SHM is within the purview of hospitalists, ensuring that guidelines are reliably followed will require interdisciplinary process changes. Ample opportunity exists to partner with nurses (urinary catheters and telemetry), pharmacists (stress ulcer prophylaxis), blood banks, and laboratories (transfusions and lab testing), as well as other healthcare providers and physicians in multiple specialties.
Successful implementation of each guideline will require improvement of systems within hospitals to drive reliability.[47] Provider education, training programs, protocols and reminders may prove to be significant catalysts in overcoming misinformation or no information about specific guidelines. More importantly, interdisciplinary teams will need to assess the current practice patterns within their hospitals prior to implementing solutions that standardize and automate the ordering processes for these tests and treatments.[48] Additionally, the culture within individual patient care units will need to be modified.[49] The challenge of changing the behavior of multiple stakeholders and hardwiring systems changes represent significant potential barriers to success.
There are several potential concerns with the recommendations. Concepts such as high risk and clinical stability exist in several of the recommendations. In most cases, specific guidelines exist that explicitly define the appropriate use of the test or treatment. Where they do not, implementers will need to define the operational definitions, such as the number of normal CBCs that define stability. Although the recommendations are based on the best evidence available, consensus still plays a role. As has been noted, the risk of malpractice litigation influences physicians' decisions.[1] Although evidence‐based recommendations such as these help shape the standard of care and mitigate risk, they may not completely eliminate this concern. Providers should always weigh the risks and benefits of any test or treatment. Finally, the approach taken to establish the list was both pragmatic and evidence based. Published evidence was not reviewed until the list was honed to 11. When the evidence was reviewed, the strength of the evidence was judged in a subjective manner by members of the committee as part of the Delphi panel voting.
CONCLUSION
As healthcare providers enter an era of more cost conscious decision‐making about provision of care based upon necessity, hospitalists have an excellent opportunity to impact overutilization. The 5 recommendations comprising the adult hospital medicine Choosing Wisely list offer an explicit starting point. The SHM hopes to lead this process during the coming months and years and to offer additional recommendations, providing a foundation for hospitalists to decrease unnecessary tests and treatments and improve healthcare value.
Acknowledgments
The authors thank the additional members of the Choosing Wisely subcommittee of the SHM's Healthcare Quality and Patient Safety Committee: Krishna Das, MD; Shelley Taylor, MD; Kevin O'Leary, MD; and Nasim Afsarmanesh, MD. The authors also thank SHM staff who were involved in all facets of the recommendation development process, particularly Brendon Shank, who provided significant input into the survey and dissemination process.
Disclosure
Nothing to report.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- , , , et al. Evidence that consumers are skeptical about evidence‐based health care. Health Aff (Millwood). 2010;29:1400–1406.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Too much treatment? Aggressive medical care can lead to more pain, with no gain. Consum Rep. 2008;73:40–44.
- Centers for Medicare and Medicaid Services. Historical national health expenditure data. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed February 12, 2013.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- . Change the microenvironment: delivery system reform essential to controlling costs. Available at: http://www.commonwealthfund.org/Publications/Commentaries/2009/Apr/Change‐the‐Microenvironment.aspx. Accessed February 12, 2013.
- Costs of care. Available at: http://www.costsofcare.org. Accessed February 12, 2013.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750.
- , , , ; Clinical Guidelines Committee of the American College of Physicians. High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- ABIM Foundation. U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM‐Foundation‐News/2012/Choosing‐Wisely.aspx. Accessed February 12, 2013.
- , . Addressing requests by patients for nonbeneficial interventions. JAMA. 2012;307:149–150.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102–1112.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28:68–75.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- , , , et al. A compendium of strategies to prevent healthcare‐associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1): S12–S21.
- , , , et al. Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Available at: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed February 12, 2013.
- , , , , . Healthcare Infection Control Practices Advisory Committee, guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–326.
- , , , et al. Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
- , , , et al. 2011 National and State Healthcare‐Associated Infection Standardized Infection Ratio Report. Available at: http://www.cdc.gov/hai/pdfs/SIR/SIR‐Report_02_07_2013.pdf. Accessed February 13, 2013.
- , . Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64:1396–1400.
- . ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–379.
- , , , , , . Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292:1955–1960.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301:2120–2128.
- , , , . Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile‐associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- , , , et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
- , , , . Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818.
- , , , , . Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Intern Med 2013;173:132–139.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , . Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med. 1993;94:509–514.
- , , , , , . The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97:1483–1487.
- , , , et al. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Circulation. 1999;100:886–893.
- , , , et al. Role of telemetry monitoring in the non‐intensive care unit. Am J Cardiol. 1995;76:960–965.
- , . When do patients need admission to a telemetry bed? J Emerg Med. 2007;33:53–60.
- , . Electrocardiographic monitoring in the hospitalized patient: a diagnostic intervention of uncertain clinical impact. Am J Emerg Med. 2008;26:1047–1055.
- , , , , . Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341:1270–1274.
- , , , . Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in‐hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci. 2005;60:605–606.
- , , , , . Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8:125–126.
- Agency for Healthcare Research and Quality. Winawer N. Redesign of telemetry unit admission and transfer criteria leads to improved patient flow and reduced emergency department waiting times. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2239. Accessed February 12, 2013.
- , , , et al. Is telemetry monitoring necessary in low‐risk suspected acute chest pain syndromes? Chest. 2002;122:517–523.
- , . Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146:524–527.
- , , , et al. A cost‐effective method for reducing the volume of laboratory tests in a university‐associated teaching hospital. Mt Sinai J Med. 2006;73:787–794.
- , , , et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200–206.
- . Making noncatastrophic health care processes reliable: learning to walk before running in creating high‐reliability organizations. Health Serv Res. 2006;41:1677–1689.
- , , , et al. What have we learned about interventions to reduce medical errors? Annu Rev Public Health. 2010;31:479–497.
- , . Safe Patients, Smart Hospitals: How One Doctor's Checklist Can Help Us Change Health Care From The Inside Out. New York, NY: Hudson Street Press; 2010.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.
- Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703.
- , , , , , . Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76:368–372.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653.
- , , , , . Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- , , , et al. Evidence that consumers are skeptical about evidence‐based health care. Health Aff (Millwood). 2010;29:1400–1406.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Too much treatment? Aggressive medical care can lead to more pain, with no gain. Consum Rep. 2008;73:40–44.
- Centers for Medicare and Medicaid Services. Historical national health expenditure data. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed February 12, 2013.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- . Change the microenvironment: delivery system reform essential to controlling costs. Available at: http://www.commonwealthfund.org/Publications/Commentaries/2009/Apr/Change‐the‐Microenvironment.aspx. Accessed February 12, 2013.
- Costs of care. Available at: http://www.costsofcare.org. Accessed February 12, 2013.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750.
- , , , ; Clinical Guidelines Committee of the American College of Physicians. High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- ABIM Foundation. U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM‐Foundation‐News/2012/Choosing‐Wisely.aspx. Accessed February 12, 2013.
- , . Addressing requests by patients for nonbeneficial interventions. JAMA. 2012;307:149–150.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102–1112.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28:68–75.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- , , , et al. A compendium of strategies to prevent healthcare‐associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1): S12–S21.
- , , , et al. Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Available at: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed February 12, 2013.
- , , , , . Healthcare Infection Control Practices Advisory Committee, guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–326.
- , , , et al. Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
- , , , et al. 2011 National and State Healthcare‐Associated Infection Standardized Infection Ratio Report. Available at: http://www.cdc.gov/hai/pdfs/SIR/SIR‐Report_02_07_2013.pdf. Accessed February 13, 2013.
- , . Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64:1396–1400.
- . ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–379.
- , , , , , . Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292:1955–1960.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301:2120–2128.
- , , , . Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile‐associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- , , , et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
- , , , . Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818.
- , , , , . Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Intern Med 2013;173:132–139.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , . Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med. 1993;94:509–514.
- , , , , , . The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97:1483–1487.
- , , , et al. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Circulation. 1999;100:886–893.
- , , , et al. Role of telemetry monitoring in the non‐intensive care unit. Am J Cardiol. 1995;76:960–965.
- , . When do patients need admission to a telemetry bed? J Emerg Med. 2007;33:53–60.
- , . Electrocardiographic monitoring in the hospitalized patient: a diagnostic intervention of uncertain clinical impact. Am J Emerg Med. 2008;26:1047–1055.
- , , , , . Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341:1270–1274.
- , , , . Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in‐hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci. 2005;60:605–606.
- , , , , . Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8:125–126.
- Agency for Healthcare Research and Quality. Winawer N. Redesign of telemetry unit admission and transfer criteria leads to improved patient flow and reduced emergency department waiting times. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2239. Accessed February 12, 2013.
- , , , et al. Is telemetry monitoring necessary in low‐risk suspected acute chest pain syndromes? Chest. 2002;122:517–523.
- , . Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146:524–527.
- , , , et al. A cost‐effective method for reducing the volume of laboratory tests in a university‐associated teaching hospital. Mt Sinai J Med. 2006;73:787–794.
- , , , et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200–206.
- . Making noncatastrophic health care processes reliable: learning to walk before running in creating high‐reliability organizations. Health Serv Res. 2006;41:1677–1689.
- , , , et al. What have we learned about interventions to reduce medical errors? Annu Rev Public Health. 2010;31:479–497.
- , . Safe Patients, Smart Hospitals: How One Doctor's Checklist Can Help Us Change Health Care From The Inside Out. New York, NY: Hudson Street Press; 2010.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.
- Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703.
- , , , , , . Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76:368–372.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653.
- , , , , . Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524.
Copyright © 2013 Society of Hospital Medicine