User login
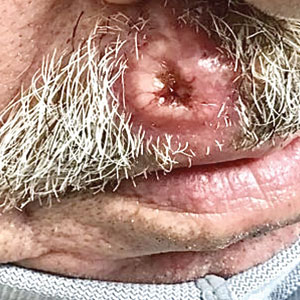
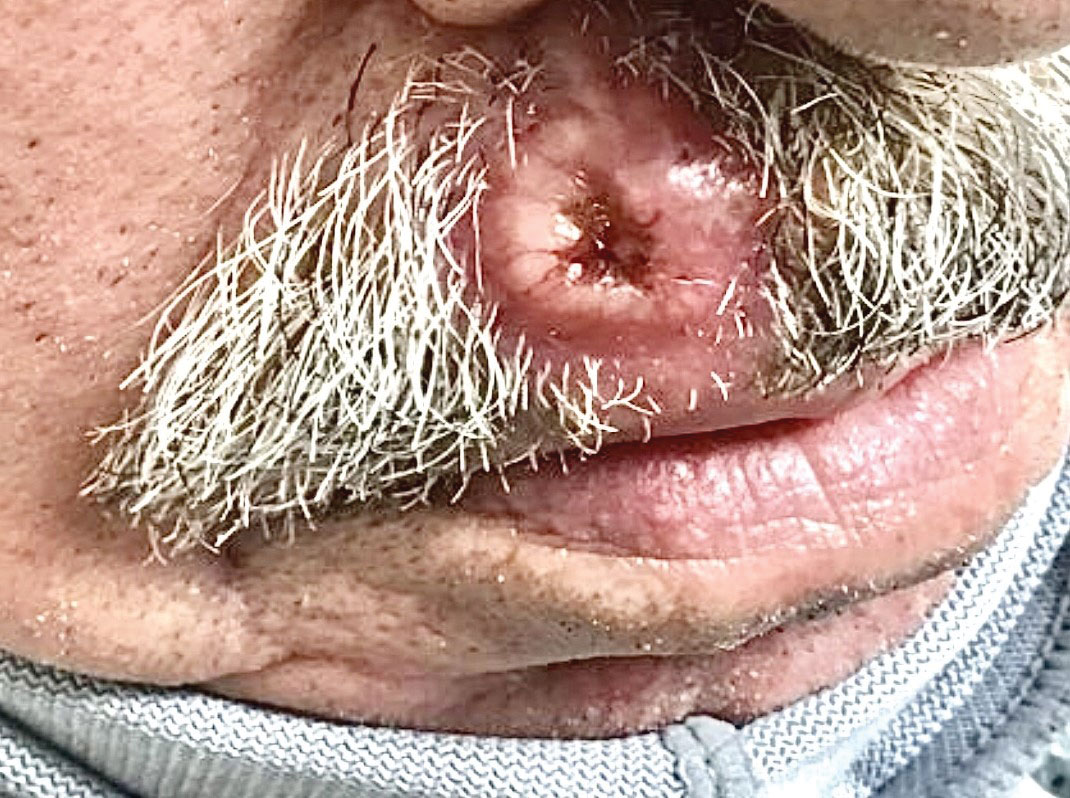
Ulcerated Nodule on the Lip
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
A 79-year-old man with a medical history of type 2 diabetes mellitus, hypothyroidism, and atrial fibrillation presented with an enlarging lesion on the right side of the upper cutaneous lip of 5 weeks’ duration. He had no personal history of skin cancer or other malignancy and was up to date on all routine cancer screenings. He reported associated lip and oral cavity tenderness, weakness, and a 13.6-kg (30-lb) unintentional weight loss over the last 6 months. He had used over-the-counter bacitracin ointment on the lesion without relief. A full-body skin examination revealed a firm, mobile, flesh-colored, nondraining nodule in the right axillary vault.
Granulomatous Pigmented Purpuric Dermatosis
Pigmented purpuric dermatoses (PPDs) are a spectrum of chronic disorders that present as speckled brown to purpuric lesions and orange-brown discoloration of the skin.1 Eruptions generally occur in middle-aged to elderly patients and commonly follow a chronic waxing and waning course.2 Lesions usually are found in a localized distribution on the legs. Histologically, PPD presents with perivascular infiltrates of lymphocytes and macrophages centered around the superficial small blood vessels with narrowing of the lumina. Extravasation of red blood cells and hemosiderin deposition are commonly seen in the absence of vasculitis.
The etiology of PPD is unknown; however, important cofactors include venous hypertension, exercise and gravitational dependency, capillary fragility, focal infections, and chemical ingestions.1 Drugs are the most important provoking factors, including acetaminophen, aspirin, adalin, carbromal, chlordiazepoxide, glipizide, glybuzole, hydralazine, meprobamate, dipyridamole, reserpine, thiamine, and interferon-alfa, as well as medroxyprogesterone acetate injection. Other phenomena include contact allergy and alcohol ingestion.1
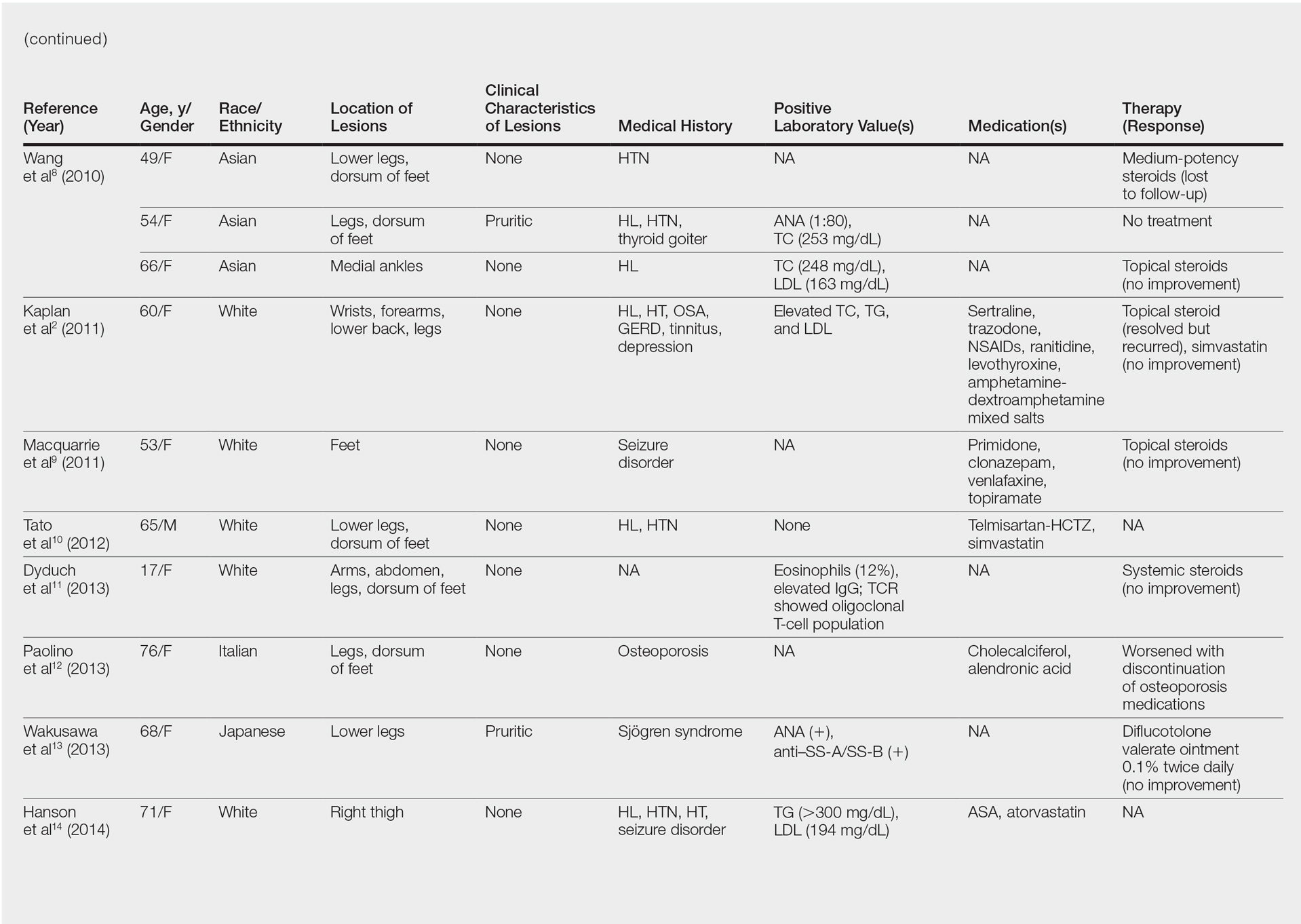
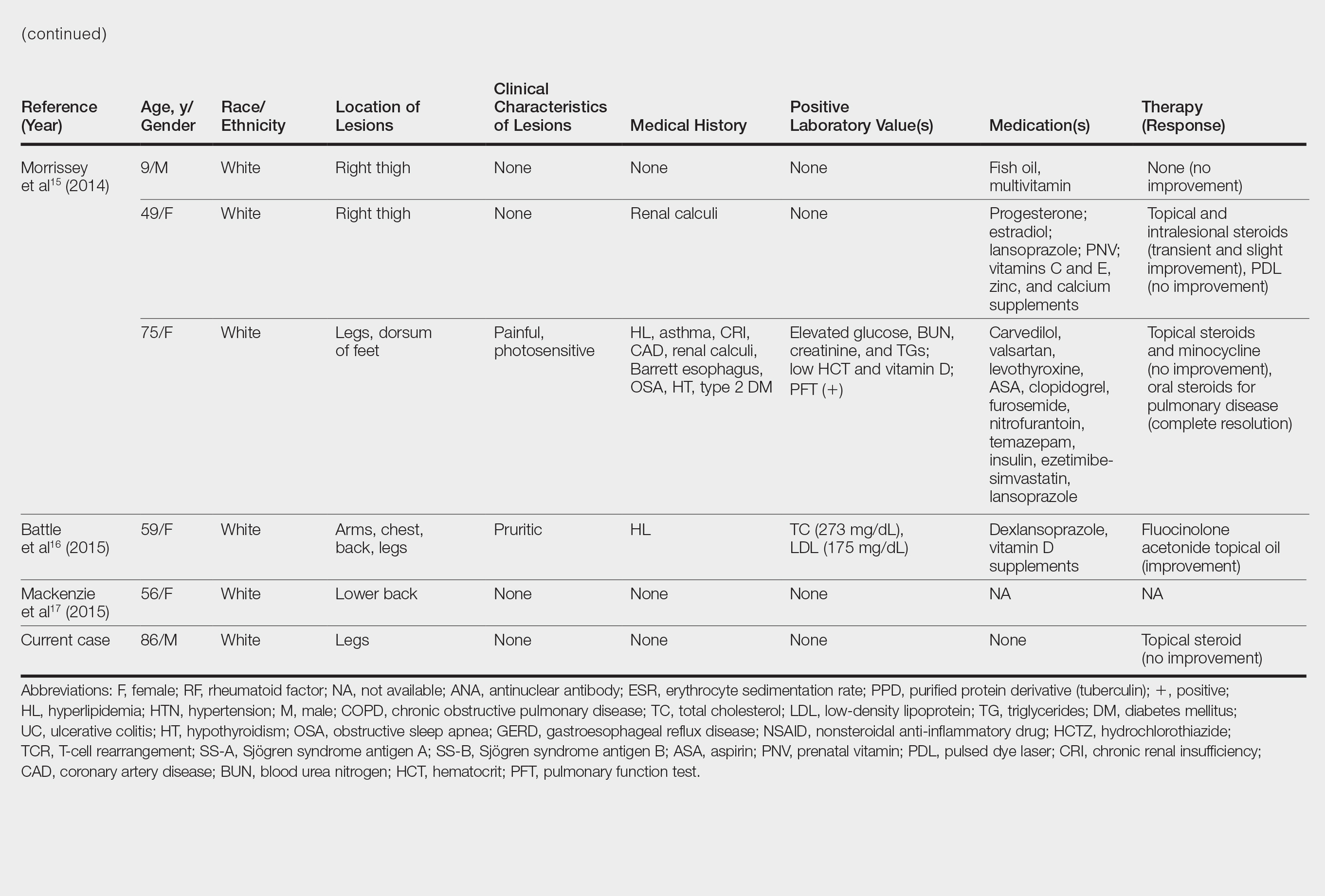
Although the diagnosis often is made clinically, many forms of PPD exist. The 4 main forms include Schaumberg disease, purpura annularis telangiectaticum of Majocchi, pigmented purpuric lichenoid dermatitis of Gougerot and Blum, and eczematoidlike purpura of Doucas and Kapetanakis. Less common variants include itching purpura of Lowenthal, lichen purpuricus, lichen aureus, granulomatous pigmented purpura, transitory pigmented purpuric dermatosis, and linear pigmented purpura.1Granulomatous PPD (GPPD) is a rare histologic variant of PPD. Clinically, it is indistinguishable from other forms of PPD but reveals itself histologically with granulomatous infiltrates superimposed on classic PPD. We report a case of GPPD and provide a thorough literature review focusing on epidemiology, clinical symptoms, and treatment.2-17 The eTable summarizes all reported cases of GPPD.
Case Report
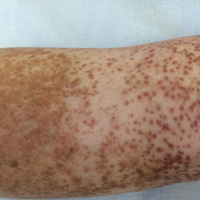
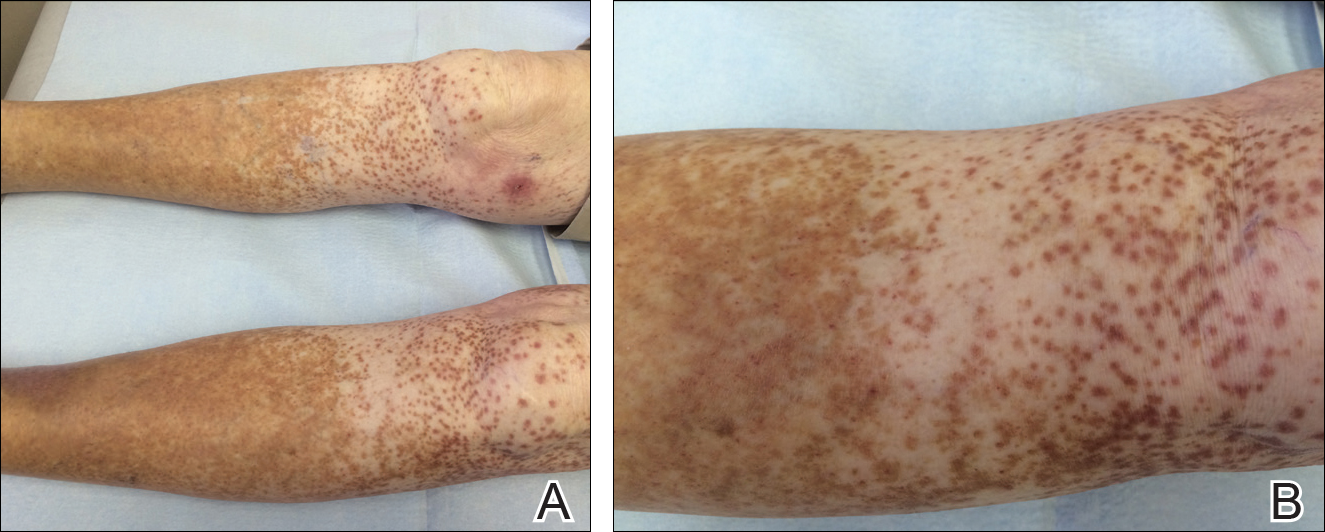
An 86-year-old white man with no remarkable medical history presented with an asymptomatic eruption over the bilateral shins extending up both thighs of 6 years’ duration (Figure 1). It began as a 15-cm patch on the right medial thigh that rapidly spread over 1 year to involve the majority of the legs. Physical examination revealed scattered 1- to 2-mm brown macules coalescing into patches on both legs. The patches increased in density distally and extended from the bilateral thighs to the ankles. Edema of the legs was absent, and lesions were nonblanchable and without scale or induration. The differential diagnoses included stasis dermatitis, vasculitis, and PPD. All laboratory values were within reference range, including complete blood cell count, comprehensive metabolic panel, urine analysis, and lipid profile.
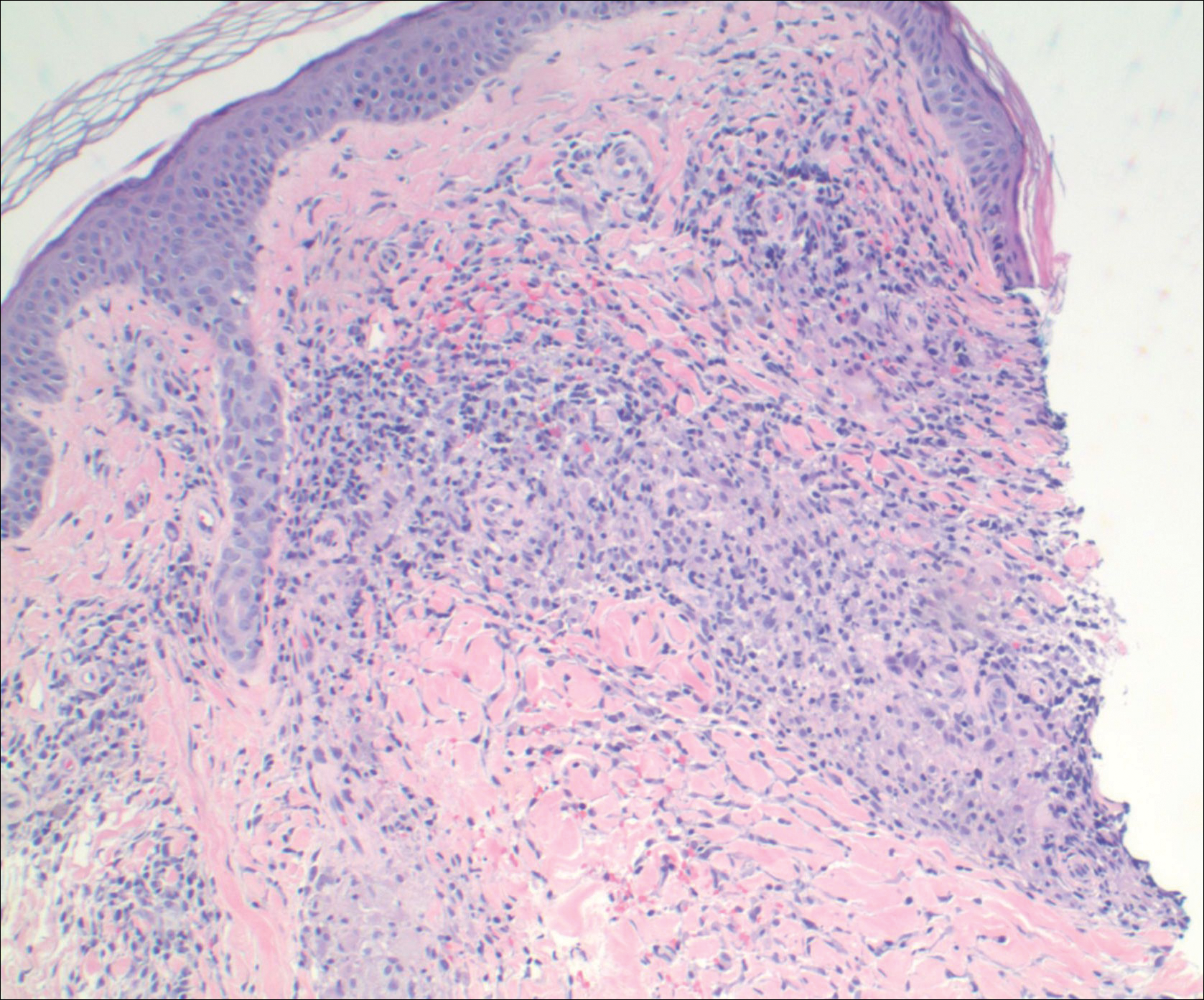
A punch biopsy from the distal right thigh revealed a superficial to mid dermal perivascular lymphocyte-predominant infiltrate with associated siderophages and a focal granulomatous infiltrate comprised of histiocytes (Figure 2). Periodic acid–Schiff, acid-fast bacilli, and Fite stains were negative for microorganisms. No eosinophils or leukocytoclasia were seen. The patient applied betamethasone dipropionate cream 0.05% twice daily for several weeks without improvement. Because the lesions were asymptomatic, he discontinued the topical medication.
Comment
Pathogenesis/Etiology of GPPD
Granulomatous PPD is a rare histological variant of PPD, which was first reported in 1996 by Saito and Matsuoka.3 Originally, GPPD was mainly thought to affect individuals in the Far East and be associated with the hepatitis C virus, antinuclear antibodies, or rheumatoid factor.3 Since its initial description, GPPD continues to predominantly be seen on the distal legs. According to a PubMed search of articles indexed for MEDLINE and the Michigan State University library database using the terms granulomatous pigmented purpuric dermatosis and pigmented purpuric dermatosis, 26 known cases including the current case (Asian, n=13; white, n=13) have been reported. The mean age of onset was 54.5 years and the female to male ratio was 2.5 to 1.
Currently, the etiology of GPPD is unknown; however, 13 reported cases have been associated with hyperlipidemia,2,4,5,7,8,10,14-16 which has led to the speculation that they may be related. Previous investigators have postulated that the granulomatous infiltrate is a response to lipid deposition in the endothelial cells or that the elevated lipid levels launch an incompetent helper T cell (TH1) response, leading to granuloma formation.5,7,8 Currently, hyperlipidemia is present in 50% of patients and appears to be trending downward as more cases present in the literature.
Medications have been implicated in the pathogenesis of PPD and may have a possible role in the development of the granulomatous variant.9 One case report noted preceding medication changes, alluding to the possibility of aminosalicylates being the culprit.6
Another case described GPPD appearing after an upper respiratory tract infection.11 Comorbidities are not uncommon in patients presenting with GPPD. Although the majority of cases are single reports, they include systemic derangements such as hepatitis C,3,5 Sjögren syndrome,13 hypertension,2,4,5,8,10,14,15 seizure disorder,9,14 ulcerative colitis,6 diabetes mellitus,5,15 meningioma,3 renal calculi,15 thrombocytopenia,5 chronic obstructive pulmonary disease,4 thyroid goiter,8 obstructive sleep apnea,2,15 osteoporosis,12 asthma,15 gastroesophageal reflux disease/Barrett esophagus,2,15 hypothyroidism,2,14,15 and hyperuricemia.5
Clinical Presentation
Clinically, GPPD commonly presents as asymptomatic petechiae and bronze discoloration of the lower legs. The clinical presentation can vary from a solitary lesion to a localized eruption typically on the lower legs or rarely a widespread eruption. A review of the literature revealed 5 cases presenting on the upper arms2,5,11,16 and 4 on the trunk.2,11,16,17 Four patients presented with pruritus3,8,13,16 and 1 described pain and photosensitive lesions.15 No other clinical signs of hyperlipidemia were described (eg, xanthomas). The duration of the disease has a wide spectrum, ranging from 3 weeks to 20 years.4,16
Histopathology
With the increasing trend toward dermatoscopic evaluation, 2 reviews evaluated dermatoscopic features of GPPD. These reports described scattered, round to oval, red dots, globules, and patches with a diffuse red-brown or coppery background of pigmentation.14,17
The granulomatous variant of PPD is characterized histopathologically by ill-defined, nonnecrotizing granulomas admixed with a lymphocytic infiltrate. Commonly, erythrocyte extravasation and hemosiderin are seen with granulomas superimposed on classic changes of PPD.15 Vasculitis features including endothelial swelling, fibrinoid necrosis, and leukocytoclasia are absent. Rarely, eosinophils are seen.6 Mild epidermal spongiosis and exocytosis of lymphocytes may be seen in all variants of PPD, except lichen aureus.1 This exocytosis was observed focally in one case of GPPD.4 Although loosely formed granulomas in the papillary dermis are characteristic, 7 cases have had a concomitant lichenoid infiltrate.2,9-11,15,16
Kaplan et al2 reported granulomatous and nongranulomatous PPD occurring together in different areas of the body. A new granulomatous variant was proposed in a 2015 report that revealed 2 patients with granulomatous infiltrates in the mid to deep dermis rather than the classic superficial dermis.15 One case of GPPD was suspicious for progression into mycosis fungoides (MF) and described a lichenoid infiltrate with mild atypical and small lymphocytes migrating into the epidermis.11 Follow-up biopsy lacked epidermotropism and quantitative representation of T-cell subsets. The diagnosis of early-phase MF was based on the progressive clinical course rather than immunohistologic and molecular findings.11 One other case exhibited minimal epidermotropism.15
Management of GPPD should require a lipid profile with other tests to assess cardiovascular risk.10 A thorough medication review and a punch rather than a shave biopsy should be performed, especially because granulomatous infiltrates have been found in the mid to deep dermis.15 With the lack of rebiopsies documented, follow-up and rebiopsy has been suggested if there is suspicion of MF; however, we favor rebiopsy at a later time to help reveal the course of this disease and rule out progression into MF.
Therapy
Thus far, therapy has mostly been with oral and topical steroids. Five case reports noted improvement,2,3,6,15,16 2 with oral and 3 with topical steroids. However, therapy has been discouraging, with clinical improvement being transient in most treatment-responsive patients. One case spontaneously resolved.3 Ten cases did not document therapy or follow-up.4,5,7,10,14,17 Only 1 case reported follow-up after treatment with simvastatin; unfortunately, the patient had no improvement.2 Our case revealed no improvement with topical steroids.
Conclusion
The exact pathogenesis of GPPD is unknown. The initial impression that GPPD was a disease in Far East Asians and patients with hyperlipidemia is becoming less clear. Based on the current literature including the addition of our case, the prevalence appears to be equal among white individuals and Asians, possibly due to increased awareness of this condition and documentation in the literature. Correlation with systemic disorders such as hyperlipidemia and hypertensive medications needs further review. Eight cases reported a medical history of hypertension.4,5,8,10,14 With antihypertensive medications being a potential culprit of PPD, this etiology should not be overlooked. A punch biopsy should be performed, especially because granulomatous infiltrates may be lurking in the mid to deep dermis.15 Granulomatous PPD has a chronic course with a disappointing response to therapy but appears to be benign in nature.12 A rebiopsy is recommended if MF is suspected. Evaluation of GPPD following therapy for hyperlipidemia is not well documented and should be pursued. Clinicians and pathologists should be aware of the suspected associations and consider this variant when dermal granulomatous infiltrates are present with a background of PPD.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Kaplan J, Burgin S, Sepehr A. Granulomatous pigmented purpura: report of a case and review of the literature. J Cutan Pathol. 2011;38:984-989.
- Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
- Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
- Lin WL, Kuo TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia. Clin Exp Dermatol. 2007;32:513-515.
- Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
- Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidaemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
- Wang J, Wu Y, Hsiao P, et al. Granulomatous pigmented purpuric dermatoses: report of three cases and review of the literature. Dermatologica Sinica. 2010;28:77-81.
- Macquarrie EK, Pasternak S, Torok M, et al. Persistent pigmented purpuric dermatitis: granulomatous variant. J Cutan Pathol. 2011;38:979-983.
- Tato BP, Marinero Escobedo S, Pérez González YC, et al. Granulomatous variant of pigmented purpuric dermatosis. Am J Dermatopathol. 2012;34:746-748.
- Dyduch G, Zuber Z, Turowska-Heydel D, et al. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157-159; answer 160.
- Paolino S, Cinotti E, Merlo V, et al. Progressive petechial and pigmented macules and papules on the lower extremities. Am J Dermatopathol. 2013;35:370, 388.
- Wakusawa C, Fujimura T, Haga T, et al. Granulomatous pigmented purpuric dermatitis associated with primary Sjögren’s syndrome. Acta Derm Venereol. 2013;93:95-96.
- Hanson C, Fischer R, Fraga G, et al. Granulomatous pigmented purpuric dermatosis: an unusual variant associated with hyperlipidemia. Dermatol Online J. 2014;21. pii:13030/qt0tp272d1.
- Morrissey K, Rosenbach M, DeHoratius D, et al. Granulomatous changes associated with pigmented purpuric dermatosis. Cutis. 2014;94:197-202.
- Battle LR, Shalin SC, Gao L. Granulomatous pigmented purpuric dermatosis [published online December 18, 2014]. Clin Exp Dermatol. 2015;40:387-390.
- Mackenzie AI, Biswas A. Granulomatous pigmented purpuric dermatosis: report of a case with atypical clinical presentation including dermoscopic findings. Am J Dermatopathol. 2015;37:311-314.
Pigmented purpuric dermatoses (PPDs) are a spectrum of chronic disorders that present as speckled brown to purpuric lesions and orange-brown discoloration of the skin.1 Eruptions generally occur in middle-aged to elderly patients and commonly follow a chronic waxing and waning course.2 Lesions usually are found in a localized distribution on the legs. Histologically, PPD presents with perivascular infiltrates of lymphocytes and macrophages centered around the superficial small blood vessels with narrowing of the lumina. Extravasation of red blood cells and hemosiderin deposition are commonly seen in the absence of vasculitis.
The etiology of PPD is unknown; however, important cofactors include venous hypertension, exercise and gravitational dependency, capillary fragility, focal infections, and chemical ingestions.1 Drugs are the most important provoking factors, including acetaminophen, aspirin, adalin, carbromal, chlordiazepoxide, glipizide, glybuzole, hydralazine, meprobamate, dipyridamole, reserpine, thiamine, and interferon-alfa, as well as medroxyprogesterone acetate injection. Other phenomena include contact allergy and alcohol ingestion.1
Although the diagnosis often is made clinically, many forms of PPD exist. The 4 main forms include Schaumberg disease, purpura annularis telangiectaticum of Majocchi, pigmented purpuric lichenoid dermatitis of Gougerot and Blum, and eczematoidlike purpura of Doucas and Kapetanakis. Less common variants include itching purpura of Lowenthal, lichen purpuricus, lichen aureus, granulomatous pigmented purpura, transitory pigmented purpuric dermatosis, and linear pigmented purpura.1Granulomatous PPD (GPPD) is a rare histologic variant of PPD. Clinically, it is indistinguishable from other forms of PPD but reveals itself histologically with granulomatous infiltrates superimposed on classic PPD. We report a case of GPPD and provide a thorough literature review focusing on epidemiology, clinical symptoms, and treatment.2-17 The eTable summarizes all reported cases of GPPD.



Case Report
An 86-year-old white man with no remarkable medical history presented with an asymptomatic eruption over the bilateral shins extending up both thighs of 6 years’ duration (Figure 1). It began as a 15-cm patch on the right medial thigh that rapidly spread over 1 year to involve the majority of the legs. Physical examination revealed scattered 1- to 2-mm brown macules coalescing into patches on both legs. The patches increased in density distally and extended from the bilateral thighs to the ankles. Edema of the legs was absent, and lesions were nonblanchable and without scale or induration. The differential diagnoses included stasis dermatitis, vasculitis, and PPD. All laboratory values were within reference range, including complete blood cell count, comprehensive metabolic panel, urine analysis, and lipid profile.

A punch biopsy from the distal right thigh revealed a superficial to mid dermal perivascular lymphocyte-predominant infiltrate with associated siderophages and a focal granulomatous infiltrate comprised of histiocytes (Figure 2). Periodic acid–Schiff, acid-fast bacilli, and Fite stains were negative for microorganisms. No eosinophils or leukocytoclasia were seen. The patient applied betamethasone dipropionate cream 0.05% twice daily for several weeks without improvement. Because the lesions were asymptomatic, he discontinued the topical medication.

Comment
Pathogenesis/Etiology of GPPD
Granulomatous PPD is a rare histological variant of PPD, which was first reported in 1996 by Saito and Matsuoka.3 Originally, GPPD was mainly thought to affect individuals in the Far East and be associated with the hepatitis C virus, antinuclear antibodies, or rheumatoid factor.3 Since its initial description, GPPD continues to predominantly be seen on the distal legs. According to a PubMed search of articles indexed for MEDLINE and the Michigan State University library database using the terms granulomatous pigmented purpuric dermatosis and pigmented purpuric dermatosis, 26 known cases including the current case (Asian, n=13; white, n=13) have been reported. The mean age of onset was 54.5 years and the female to male ratio was 2.5 to 1.
Currently, the etiology of GPPD is unknown; however, 13 reported cases have been associated with hyperlipidemia,2,4,5,7,8,10,14-16 which has led to the speculation that they may be related. Previous investigators have postulated that the granulomatous infiltrate is a response to lipid deposition in the endothelial cells or that the elevated lipid levels launch an incompetent helper T cell (TH1) response, leading to granuloma formation.5,7,8 Currently, hyperlipidemia is present in 50% of patients and appears to be trending downward as more cases present in the literature.
Medications have been implicated in the pathogenesis of PPD and may have a possible role in the development of the granulomatous variant.9 One case report noted preceding medication changes, alluding to the possibility of aminosalicylates being the culprit.6
Another case described GPPD appearing after an upper respiratory tract infection.11 Comorbidities are not uncommon in patients presenting with GPPD. Although the majority of cases are single reports, they include systemic derangements such as hepatitis C,3,5 Sjögren syndrome,13 hypertension,2,4,5,8,10,14,15 seizure disorder,9,14 ulcerative colitis,6 diabetes mellitus,5,15 meningioma,3 renal calculi,15 thrombocytopenia,5 chronic obstructive pulmonary disease,4 thyroid goiter,8 obstructive sleep apnea,2,15 osteoporosis,12 asthma,15 gastroesophageal reflux disease/Barrett esophagus,2,15 hypothyroidism,2,14,15 and hyperuricemia.5
Clinical Presentation
Clinically, GPPD commonly presents as asymptomatic petechiae and bronze discoloration of the lower legs. The clinical presentation can vary from a solitary lesion to a localized eruption typically on the lower legs or rarely a widespread eruption. A review of the literature revealed 5 cases presenting on the upper arms2,5,11,16 and 4 on the trunk.2,11,16,17 Four patients presented with pruritus3,8,13,16 and 1 described pain and photosensitive lesions.15 No other clinical signs of hyperlipidemia were described (eg, xanthomas). The duration of the disease has a wide spectrum, ranging from 3 weeks to 20 years.4,16
Histopathology
With the increasing trend toward dermatoscopic evaluation, 2 reviews evaluated dermatoscopic features of GPPD. These reports described scattered, round to oval, red dots, globules, and patches with a diffuse red-brown or coppery background of pigmentation.14,17
The granulomatous variant of PPD is characterized histopathologically by ill-defined, nonnecrotizing granulomas admixed with a lymphocytic infiltrate. Commonly, erythrocyte extravasation and hemosiderin are seen with granulomas superimposed on classic changes of PPD.15 Vasculitis features including endothelial swelling, fibrinoid necrosis, and leukocytoclasia are absent. Rarely, eosinophils are seen.6 Mild epidermal spongiosis and exocytosis of lymphocytes may be seen in all variants of PPD, except lichen aureus.1 This exocytosis was observed focally in one case of GPPD.4 Although loosely formed granulomas in the papillary dermis are characteristic, 7 cases have had a concomitant lichenoid infiltrate.2,9-11,15,16
Kaplan et al2 reported granulomatous and nongranulomatous PPD occurring together in different areas of the body. A new granulomatous variant was proposed in a 2015 report that revealed 2 patients with granulomatous infiltrates in the mid to deep dermis rather than the classic superficial dermis.15 One case of GPPD was suspicious for progression into mycosis fungoides (MF) and described a lichenoid infiltrate with mild atypical and small lymphocytes migrating into the epidermis.11 Follow-up biopsy lacked epidermotropism and quantitative representation of T-cell subsets. The diagnosis of early-phase MF was based on the progressive clinical course rather than immunohistologic and molecular findings.11 One other case exhibited minimal epidermotropism.15
Management of GPPD should require a lipid profile with other tests to assess cardiovascular risk.10 A thorough medication review and a punch rather than a shave biopsy should be performed, especially because granulomatous infiltrates have been found in the mid to deep dermis.15 With the lack of rebiopsies documented, follow-up and rebiopsy has been suggested if there is suspicion of MF; however, we favor rebiopsy at a later time to help reveal the course of this disease and rule out progression into MF.
Therapy
Thus far, therapy has mostly been with oral and topical steroids. Five case reports noted improvement,2,3,6,15,16 2 with oral and 3 with topical steroids. However, therapy has been discouraging, with clinical improvement being transient in most treatment-responsive patients. One case spontaneously resolved.3 Ten cases did not document therapy or follow-up.4,5,7,10,14,17 Only 1 case reported follow-up after treatment with simvastatin; unfortunately, the patient had no improvement.2 Our case revealed no improvement with topical steroids.
Conclusion
The exact pathogenesis of GPPD is unknown. The initial impression that GPPD was a disease in Far East Asians and patients with hyperlipidemia is becoming less clear. Based on the current literature including the addition of our case, the prevalence appears to be equal among white individuals and Asians, possibly due to increased awareness of this condition and documentation in the literature. Correlation with systemic disorders such as hyperlipidemia and hypertensive medications needs further review. Eight cases reported a medical history of hypertension.4,5,8,10,14 With antihypertensive medications being a potential culprit of PPD, this etiology should not be overlooked. A punch biopsy should be performed, especially because granulomatous infiltrates may be lurking in the mid to deep dermis.15 Granulomatous PPD has a chronic course with a disappointing response to therapy but appears to be benign in nature.12 A rebiopsy is recommended if MF is suspected. Evaluation of GPPD following therapy for hyperlipidemia is not well documented and should be pursued. Clinicians and pathologists should be aware of the suspected associations and consider this variant when dermal granulomatous infiltrates are present with a background of PPD.
Pigmented purpuric dermatoses (PPDs) are a spectrum of chronic disorders that present as speckled brown to purpuric lesions and orange-brown discoloration of the skin.1 Eruptions generally occur in middle-aged to elderly patients and commonly follow a chronic waxing and waning course.2 Lesions usually are found in a localized distribution on the legs. Histologically, PPD presents with perivascular infiltrates of lymphocytes and macrophages centered around the superficial small blood vessels with narrowing of the lumina. Extravasation of red blood cells and hemosiderin deposition are commonly seen in the absence of vasculitis.
The etiology of PPD is unknown; however, important cofactors include venous hypertension, exercise and gravitational dependency, capillary fragility, focal infections, and chemical ingestions.1 Drugs are the most important provoking factors, including acetaminophen, aspirin, adalin, carbromal, chlordiazepoxide, glipizide, glybuzole, hydralazine, meprobamate, dipyridamole, reserpine, thiamine, and interferon-alfa, as well as medroxyprogesterone acetate injection. Other phenomena include contact allergy and alcohol ingestion.1
Although the diagnosis often is made clinically, many forms of PPD exist. The 4 main forms include Schaumberg disease, purpura annularis telangiectaticum of Majocchi, pigmented purpuric lichenoid dermatitis of Gougerot and Blum, and eczematoidlike purpura of Doucas and Kapetanakis. Less common variants include itching purpura of Lowenthal, lichen purpuricus, lichen aureus, granulomatous pigmented purpura, transitory pigmented purpuric dermatosis, and linear pigmented purpura.1Granulomatous PPD (GPPD) is a rare histologic variant of PPD. Clinically, it is indistinguishable from other forms of PPD but reveals itself histologically with granulomatous infiltrates superimposed on classic PPD. We report a case of GPPD and provide a thorough literature review focusing on epidemiology, clinical symptoms, and treatment.2-17 The eTable summarizes all reported cases of GPPD.



Case Report
An 86-year-old white man with no remarkable medical history presented with an asymptomatic eruption over the bilateral shins extending up both thighs of 6 years’ duration (Figure 1). It began as a 15-cm patch on the right medial thigh that rapidly spread over 1 year to involve the majority of the legs. Physical examination revealed scattered 1- to 2-mm brown macules coalescing into patches on both legs. The patches increased in density distally and extended from the bilateral thighs to the ankles. Edema of the legs was absent, and lesions were nonblanchable and without scale or induration. The differential diagnoses included stasis dermatitis, vasculitis, and PPD. All laboratory values were within reference range, including complete blood cell count, comprehensive metabolic panel, urine analysis, and lipid profile.

A punch biopsy from the distal right thigh revealed a superficial to mid dermal perivascular lymphocyte-predominant infiltrate with associated siderophages and a focal granulomatous infiltrate comprised of histiocytes (Figure 2). Periodic acid–Schiff, acid-fast bacilli, and Fite stains were negative for microorganisms. No eosinophils or leukocytoclasia were seen. The patient applied betamethasone dipropionate cream 0.05% twice daily for several weeks without improvement. Because the lesions were asymptomatic, he discontinued the topical medication.

Comment
Pathogenesis/Etiology of GPPD
Granulomatous PPD is a rare histological variant of PPD, which was first reported in 1996 by Saito and Matsuoka.3 Originally, GPPD was mainly thought to affect individuals in the Far East and be associated with the hepatitis C virus, antinuclear antibodies, or rheumatoid factor.3 Since its initial description, GPPD continues to predominantly be seen on the distal legs. According to a PubMed search of articles indexed for MEDLINE and the Michigan State University library database using the terms granulomatous pigmented purpuric dermatosis and pigmented purpuric dermatosis, 26 known cases including the current case (Asian, n=13; white, n=13) have been reported. The mean age of onset was 54.5 years and the female to male ratio was 2.5 to 1.
Currently, the etiology of GPPD is unknown; however, 13 reported cases have been associated with hyperlipidemia,2,4,5,7,8,10,14-16 which has led to the speculation that they may be related. Previous investigators have postulated that the granulomatous infiltrate is a response to lipid deposition in the endothelial cells or that the elevated lipid levels launch an incompetent helper T cell (TH1) response, leading to granuloma formation.5,7,8 Currently, hyperlipidemia is present in 50% of patients and appears to be trending downward as more cases present in the literature.
Medications have been implicated in the pathogenesis of PPD and may have a possible role in the development of the granulomatous variant.9 One case report noted preceding medication changes, alluding to the possibility of aminosalicylates being the culprit.6
Another case described GPPD appearing after an upper respiratory tract infection.11 Comorbidities are not uncommon in patients presenting with GPPD. Although the majority of cases are single reports, they include systemic derangements such as hepatitis C,3,5 Sjögren syndrome,13 hypertension,2,4,5,8,10,14,15 seizure disorder,9,14 ulcerative colitis,6 diabetes mellitus,5,15 meningioma,3 renal calculi,15 thrombocytopenia,5 chronic obstructive pulmonary disease,4 thyroid goiter,8 obstructive sleep apnea,2,15 osteoporosis,12 asthma,15 gastroesophageal reflux disease/Barrett esophagus,2,15 hypothyroidism,2,14,15 and hyperuricemia.5
Clinical Presentation
Clinically, GPPD commonly presents as asymptomatic petechiae and bronze discoloration of the lower legs. The clinical presentation can vary from a solitary lesion to a localized eruption typically on the lower legs or rarely a widespread eruption. A review of the literature revealed 5 cases presenting on the upper arms2,5,11,16 and 4 on the trunk.2,11,16,17 Four patients presented with pruritus3,8,13,16 and 1 described pain and photosensitive lesions.15 No other clinical signs of hyperlipidemia were described (eg, xanthomas). The duration of the disease has a wide spectrum, ranging from 3 weeks to 20 years.4,16
Histopathology
With the increasing trend toward dermatoscopic evaluation, 2 reviews evaluated dermatoscopic features of GPPD. These reports described scattered, round to oval, red dots, globules, and patches with a diffuse red-brown or coppery background of pigmentation.14,17
The granulomatous variant of PPD is characterized histopathologically by ill-defined, nonnecrotizing granulomas admixed with a lymphocytic infiltrate. Commonly, erythrocyte extravasation and hemosiderin are seen with granulomas superimposed on classic changes of PPD.15 Vasculitis features including endothelial swelling, fibrinoid necrosis, and leukocytoclasia are absent. Rarely, eosinophils are seen.6 Mild epidermal spongiosis and exocytosis of lymphocytes may be seen in all variants of PPD, except lichen aureus.1 This exocytosis was observed focally in one case of GPPD.4 Although loosely formed granulomas in the papillary dermis are characteristic, 7 cases have had a concomitant lichenoid infiltrate.2,9-11,15,16
Kaplan et al2 reported granulomatous and nongranulomatous PPD occurring together in different areas of the body. A new granulomatous variant was proposed in a 2015 report that revealed 2 patients with granulomatous infiltrates in the mid to deep dermis rather than the classic superficial dermis.15 One case of GPPD was suspicious for progression into mycosis fungoides (MF) and described a lichenoid infiltrate with mild atypical and small lymphocytes migrating into the epidermis.11 Follow-up biopsy lacked epidermotropism and quantitative representation of T-cell subsets. The diagnosis of early-phase MF was based on the progressive clinical course rather than immunohistologic and molecular findings.11 One other case exhibited minimal epidermotropism.15
Management of GPPD should require a lipid profile with other tests to assess cardiovascular risk.10 A thorough medication review and a punch rather than a shave biopsy should be performed, especially because granulomatous infiltrates have been found in the mid to deep dermis.15 With the lack of rebiopsies documented, follow-up and rebiopsy has been suggested if there is suspicion of MF; however, we favor rebiopsy at a later time to help reveal the course of this disease and rule out progression into MF.
Therapy
Thus far, therapy has mostly been with oral and topical steroids. Five case reports noted improvement,2,3,6,15,16 2 with oral and 3 with topical steroids. However, therapy has been discouraging, with clinical improvement being transient in most treatment-responsive patients. One case spontaneously resolved.3 Ten cases did not document therapy or follow-up.4,5,7,10,14,17 Only 1 case reported follow-up after treatment with simvastatin; unfortunately, the patient had no improvement.2 Our case revealed no improvement with topical steroids.
Conclusion
The exact pathogenesis of GPPD is unknown. The initial impression that GPPD was a disease in Far East Asians and patients with hyperlipidemia is becoming less clear. Based on the current literature including the addition of our case, the prevalence appears to be equal among white individuals and Asians, possibly due to increased awareness of this condition and documentation in the literature. Correlation with systemic disorders such as hyperlipidemia and hypertensive medications needs further review. Eight cases reported a medical history of hypertension.4,5,8,10,14 With antihypertensive medications being a potential culprit of PPD, this etiology should not be overlooked. A punch biopsy should be performed, especially because granulomatous infiltrates may be lurking in the mid to deep dermis.15 Granulomatous PPD has a chronic course with a disappointing response to therapy but appears to be benign in nature.12 A rebiopsy is recommended if MF is suspected. Evaluation of GPPD following therapy for hyperlipidemia is not well documented and should be pursued. Clinicians and pathologists should be aware of the suspected associations and consider this variant when dermal granulomatous infiltrates are present with a background of PPD.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Kaplan J, Burgin S, Sepehr A. Granulomatous pigmented purpura: report of a case and review of the literature. J Cutan Pathol. 2011;38:984-989.
- Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
- Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
- Lin WL, Kuo TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia. Clin Exp Dermatol. 2007;32:513-515.
- Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
- Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidaemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
- Wang J, Wu Y, Hsiao P, et al. Granulomatous pigmented purpuric dermatoses: report of three cases and review of the literature. Dermatologica Sinica. 2010;28:77-81.
- Macquarrie EK, Pasternak S, Torok M, et al. Persistent pigmented purpuric dermatitis: granulomatous variant. J Cutan Pathol. 2011;38:979-983.
- Tato BP, Marinero Escobedo S, Pérez González YC, et al. Granulomatous variant of pigmented purpuric dermatosis. Am J Dermatopathol. 2012;34:746-748.
- Dyduch G, Zuber Z, Turowska-Heydel D, et al. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157-159; answer 160.
- Paolino S, Cinotti E, Merlo V, et al. Progressive petechial and pigmented macules and papules on the lower extremities. Am J Dermatopathol. 2013;35:370, 388.
- Wakusawa C, Fujimura T, Haga T, et al. Granulomatous pigmented purpuric dermatitis associated with primary Sjögren’s syndrome. Acta Derm Venereol. 2013;93:95-96.
- Hanson C, Fischer R, Fraga G, et al. Granulomatous pigmented purpuric dermatosis: an unusual variant associated with hyperlipidemia. Dermatol Online J. 2014;21. pii:13030/qt0tp272d1.
- Morrissey K, Rosenbach M, DeHoratius D, et al. Granulomatous changes associated with pigmented purpuric dermatosis. Cutis. 2014;94:197-202.
- Battle LR, Shalin SC, Gao L. Granulomatous pigmented purpuric dermatosis [published online December 18, 2014]. Clin Exp Dermatol. 2015;40:387-390.
- Mackenzie AI, Biswas A. Granulomatous pigmented purpuric dermatosis: report of a case with atypical clinical presentation including dermoscopic findings. Am J Dermatopathol. 2015;37:311-314.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Kaplan J, Burgin S, Sepehr A. Granulomatous pigmented purpura: report of a case and review of the literature. J Cutan Pathol. 2011;38:984-989.
- Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
- Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
- Lin WL, Kuo TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia. Clin Exp Dermatol. 2007;32:513-515.
- Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
- Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidaemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
- Wang J, Wu Y, Hsiao P, et al. Granulomatous pigmented purpuric dermatoses: report of three cases and review of the literature. Dermatologica Sinica. 2010;28:77-81.
- Macquarrie EK, Pasternak S, Torok M, et al. Persistent pigmented purpuric dermatitis: granulomatous variant. J Cutan Pathol. 2011;38:979-983.
- Tato BP, Marinero Escobedo S, Pérez González YC, et al. Granulomatous variant of pigmented purpuric dermatosis. Am J Dermatopathol. 2012;34:746-748.
- Dyduch G, Zuber Z, Turowska-Heydel D, et al. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157-159; answer 160.
- Paolino S, Cinotti E, Merlo V, et al. Progressive petechial and pigmented macules and papules on the lower extremities. Am J Dermatopathol. 2013;35:370, 388.
- Wakusawa C, Fujimura T, Haga T, et al. Granulomatous pigmented purpuric dermatitis associated with primary Sjögren’s syndrome. Acta Derm Venereol. 2013;93:95-96.
- Hanson C, Fischer R, Fraga G, et al. Granulomatous pigmented purpuric dermatosis: an unusual variant associated with hyperlipidemia. Dermatol Online J. 2014;21. pii:13030/qt0tp272d1.
- Morrissey K, Rosenbach M, DeHoratius D, et al. Granulomatous changes associated with pigmented purpuric dermatosis. Cutis. 2014;94:197-202.
- Battle LR, Shalin SC, Gao L. Granulomatous pigmented purpuric dermatosis [published online December 18, 2014]. Clin Exp Dermatol. 2015;40:387-390.
- Mackenzie AI, Biswas A. Granulomatous pigmented purpuric dermatosis: report of a case with atypical clinical presentation including dermoscopic findings. Am J Dermatopathol. 2015;37:311-314.
Practice Points
- Granulomatous pigmented purpuric dermatosis is not only seen in Far East Asians and patients with hyperlipidemia.
- Suspected pigmented purpuric dermatoses should be managed with a punch biopsy to exclude the granulomatous variant.