User login
What's your diagnosis? - September 2018
Endovascular walled-off pancreatic necrosis complicating a pancreatic duct-portal vein fistula
Pancreatic fistula occurs primarily as a result of abdominal trauma, pancreatic surgery, or disruption of the pancreatic duct. In the vast majority of the cases, the latter is encountered in the context of chronic pancreatitis, and results in chronic pancreatic or peripancreatic fluid or necrotic collections. Rarely, such ductal disruption leads to a direct communication between the ruptured duct and the portal vein lumen. Such pancreas-portal venous fistulas are extremely rare, with less than 20 cases reported in published literature.1 The location of the fistula is within the head of the pancreas in most cases, and it is associated with intrapancreatic necrotic collection in close proximity to the portal vein, as in the present case. The intravascular flow of pancreatic enzymes leads to local and progressively extensive portal vein thrombosis. Importantly, most portal vein thromboses in the context of acute or chronic pancreatitis do not result from pancreas-portal venous fistula, and are explained by local vascular compression by the inflammatory pancreatic head, and acquired coagulation abnormalities owing to the pancreatitis. In case of fistula, the resulting high blood level of the pancreatic enzymes may lead to a range of clinical presentations, from vague abdominal pain to disseminated fat necrosis. Painful erythematous lesions on the lower extremities and arthritis have also been described. The present case is an exceptional complication of pancreatic duct-portal vein fistula, with endovascular organization of walled-off pancreatic necrosis.2 The direct visualization of the fistula is difficult, endoscopic retrograde or, more frequently, magnetic resonance cholangiopancreatography being the most useful technique.3
Ultrasound imaging can be useful by showing the heterogeneous yet hypoechoic content of the portal venous system. Percutaneous transhepatic puncture has also been described, and is performed, as in our case, to obtain fluid sample and to perform evacuation of fluid/drainage if necessary. Percutaneous puncture may also provide precise extension of the portal venous invasion. The management of patients with pancreatic-portal vein fistula is poorly codified and relies on individual clinical and imaging analysis. Early surgical intervention has been described in patients with disseminated fat necrosis to limit morbidity and prevent mortality. Later in the evolution of the disease, surgery can be performed if the fistula remains active to alleviate the patient's symptoms and prevent future complications. Finally, conservative treatment can be proposed in selected patients with dried up fistula, as in the present report.
References
1. Brown A., Malden E., Kugelmas M., et al. Diagnosis of pancreatic duct-portal vein fistula; A case report and review of the literature. J Radiol Case Rep. 2014;8:31-8.
2. Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
3. Yoon S.E., Lee Y.H., Yoon K.H., et al. Spontaneous pancreatic pseudocyst-portal vein fistula presenting with pancreatic ascites: Strength of MR cholangiopancreatography. Br J Radiol. 2008;81:e13-6.
Endovascular walled-off pancreatic necrosis complicating a pancreatic duct-portal vein fistula
Pancreatic fistula occurs primarily as a result of abdominal trauma, pancreatic surgery, or disruption of the pancreatic duct. In the vast majority of the cases, the latter is encountered in the context of chronic pancreatitis, and results in chronic pancreatic or peripancreatic fluid or necrotic collections. Rarely, such ductal disruption leads to a direct communication between the ruptured duct and the portal vein lumen. Such pancreas-portal venous fistulas are extremely rare, with less than 20 cases reported in published literature.1 The location of the fistula is within the head of the pancreas in most cases, and it is associated with intrapancreatic necrotic collection in close proximity to the portal vein, as in the present case. The intravascular flow of pancreatic enzymes leads to local and progressively extensive portal vein thrombosis. Importantly, most portal vein thromboses in the context of acute or chronic pancreatitis do not result from pancreas-portal venous fistula, and are explained by local vascular compression by the inflammatory pancreatic head, and acquired coagulation abnormalities owing to the pancreatitis. In case of fistula, the resulting high blood level of the pancreatic enzymes may lead to a range of clinical presentations, from vague abdominal pain to disseminated fat necrosis. Painful erythematous lesions on the lower extremities and arthritis have also been described. The present case is an exceptional complication of pancreatic duct-portal vein fistula, with endovascular organization of walled-off pancreatic necrosis.2 The direct visualization of the fistula is difficult, endoscopic retrograde or, more frequently, magnetic resonance cholangiopancreatography being the most useful technique.3
Ultrasound imaging can be useful by showing the heterogeneous yet hypoechoic content of the portal venous system. Percutaneous transhepatic puncture has also been described, and is performed, as in our case, to obtain fluid sample and to perform evacuation of fluid/drainage if necessary. Percutaneous puncture may also provide precise extension of the portal venous invasion. The management of patients with pancreatic-portal vein fistula is poorly codified and relies on individual clinical and imaging analysis. Early surgical intervention has been described in patients with disseminated fat necrosis to limit morbidity and prevent mortality. Later in the evolution of the disease, surgery can be performed if the fistula remains active to alleviate the patient's symptoms and prevent future complications. Finally, conservative treatment can be proposed in selected patients with dried up fistula, as in the present report.
References
1. Brown A., Malden E., Kugelmas M., et al. Diagnosis of pancreatic duct-portal vein fistula; A case report and review of the literature. J Radiol Case Rep. 2014;8:31-8.
2. Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
3. Yoon S.E., Lee Y.H., Yoon K.H., et al. Spontaneous pancreatic pseudocyst-portal vein fistula presenting with pancreatic ascites: Strength of MR cholangiopancreatography. Br J Radiol. 2008;81:e13-6.
Endovascular walled-off pancreatic necrosis complicating a pancreatic duct-portal vein fistula
Pancreatic fistula occurs primarily as a result of abdominal trauma, pancreatic surgery, or disruption of the pancreatic duct. In the vast majority of the cases, the latter is encountered in the context of chronic pancreatitis, and results in chronic pancreatic or peripancreatic fluid or necrotic collections. Rarely, such ductal disruption leads to a direct communication between the ruptured duct and the portal vein lumen. Such pancreas-portal venous fistulas are extremely rare, with less than 20 cases reported in published literature.1 The location of the fistula is within the head of the pancreas in most cases, and it is associated with intrapancreatic necrotic collection in close proximity to the portal vein, as in the present case. The intravascular flow of pancreatic enzymes leads to local and progressively extensive portal vein thrombosis. Importantly, most portal vein thromboses in the context of acute or chronic pancreatitis do not result from pancreas-portal venous fistula, and are explained by local vascular compression by the inflammatory pancreatic head, and acquired coagulation abnormalities owing to the pancreatitis. In case of fistula, the resulting high blood level of the pancreatic enzymes may lead to a range of clinical presentations, from vague abdominal pain to disseminated fat necrosis. Painful erythematous lesions on the lower extremities and arthritis have also been described. The present case is an exceptional complication of pancreatic duct-portal vein fistula, with endovascular organization of walled-off pancreatic necrosis.2 The direct visualization of the fistula is difficult, endoscopic retrograde or, more frequently, magnetic resonance cholangiopancreatography being the most useful technique.3
Ultrasound imaging can be useful by showing the heterogeneous yet hypoechoic content of the portal venous system. Percutaneous transhepatic puncture has also been described, and is performed, as in our case, to obtain fluid sample and to perform evacuation of fluid/drainage if necessary. Percutaneous puncture may also provide precise extension of the portal venous invasion. The management of patients with pancreatic-portal vein fistula is poorly codified and relies on individual clinical and imaging analysis. Early surgical intervention has been described in patients with disseminated fat necrosis to limit morbidity and prevent mortality. Later in the evolution of the disease, surgery can be performed if the fistula remains active to alleviate the patient's symptoms and prevent future complications. Finally, conservative treatment can be proposed in selected patients with dried up fistula, as in the present report.
References
1. Brown A., Malden E., Kugelmas M., et al. Diagnosis of pancreatic duct-portal vein fistula; A case report and review of the literature. J Radiol Case Rep. 2014;8:31-8.
2. Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
3. Yoon S.E., Lee Y.H., Yoon K.H., et al. Spontaneous pancreatic pseudocyst-portal vein fistula presenting with pancreatic ascites: Strength of MR cholangiopancreatography. Br J Radiol. 2008;81:e13-6.
A 51-year-old man with a history of chronic pancreatitis presented with fatigue, weight loss, and right abdominal pain. He reported excessive alcohol consumption (> 200 g of alcohol per day during the past 35 years), active tobacco smoking (70 pack-years), and diabetes mellitus treated by insulin therapy. He had suffered from recurrent epigastric pain, left unexplored, for several weeks. Abdominal examination revealed no anomaly. Laboratory test results showed serum lipase 146 U/L (normal, < 78), alkaline phosphatase 477 U/L (normal, < 130), gamma-glutamyl transpeptidase 503 U/L (normal, < 55), albumin 23 g/L (normal, 40-49), and prealbumin 0.11 g/L (normal, 0.22-0.39).
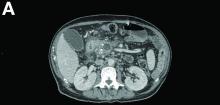
Contrast-enhanced computed tomography scanning showed features of chronic pancreatitis including pancreatic atrophy, parenchymal calcifications, marked peripancreatic fat stranding, and cephalic hypoattenuating well-delineated collection (Figure A).
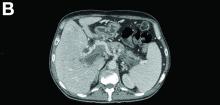
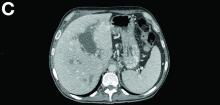
There was a chronic obstruction of the portal vein that was surrounded by numerous tortuous venous channels (Figure B). The entire intrahepatic portal branches were occluded, the right and left portal branches showed marked dilatation with a lumen filled with fluid-like material (mean density, 18 Hounsfield units), and no contrast uptake (Figure C).
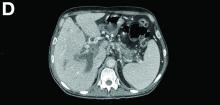
Portal vein walls were thickened and showed contrast enhancement (Figure D). The superior mesenteric vein was also thrombosed, whereas the splenic vein remained patent. Bile ducts were unnoticeable.
Ultrasound-guided transhepatic puncture of the left portal branch was performed and allowed for the aspiration of a brown fluid. Analysis showed lipase 89,990 UI/dL and amylase 43,125 UI/dL. The patient was treated by parenteral nutrition, anticoagulation therapy, and somatostatin analogues. The patient is doing well at the 6-month follow-up.