User login
Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Pin the Pinworm
An 84‐year‐old female patient with hypertension, osteoarthritis, hypothyroidism, and remote breast cancer was admitted with complaints of generalized abdominal pain of 2 months' duration. Pain was described as noncolicky in nature and was associated with diarrhea. She reported 78 daily episodes of watery, non‐foul‐smelling diarrhea. She denied any nausea, vomiting, fever, joint pains, oral ulcers, eye redness, stool incontinence, melena, hematochezia, or weight loss. There was no history of recent travel, antibiotic use, or exposure to sick contacts. She had no risk factors for HIV infection or other sexually transmitted infections. Her social history was significant for dining out on a regular basis and living in an assisted living facility. However, she denied any relationship between her abdominal symptoms and any particular food intake or with bowel movements. She denied any anal pruritis but reported seeing white squiggly things on tissue paper after bowel movements. She denied use of over‐the‐counter laxatives or herbal supplements. None of her prescription medications had diarrhea as a major side effect. Her social history was unremarkable for smoking, alcohol use, or illicit drug use. There were no prior abdominal surgeries. The patient's physical exam showed normal vitals on presentation and was unremarkable except for vague, generalized abdominal tenderness with no involuntary guarding or rebound pain. Her initial laboratory evaluation showed normal complete blood counts with no eosinophilia and normal serum electrolytes and liver and thyroid panel. Acute‐phase reactants, erythrocyte sedimentation rate, and C‐reactive protein were not elevated. Stool evaluation was unremarkable for Clostridium difficile toxin, fat droplets, leukocytes, erythrocytes, ova, parasites, or any bacterial growth on cultures. Computed tomography scans of the abdomen and pelvis were nonrevealing. Her colonoscopic examination 1 year prior was significant only for diverticulosis.1, 2
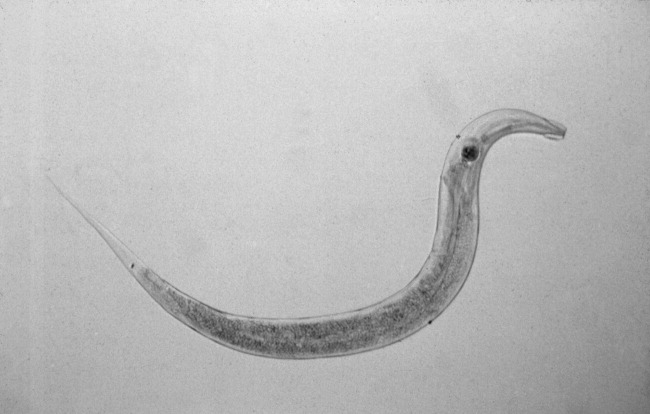
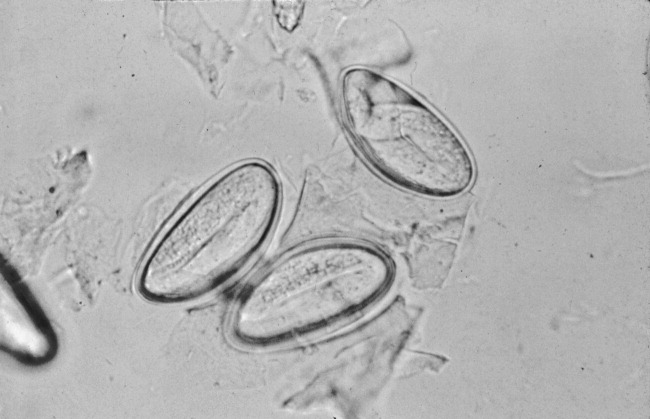
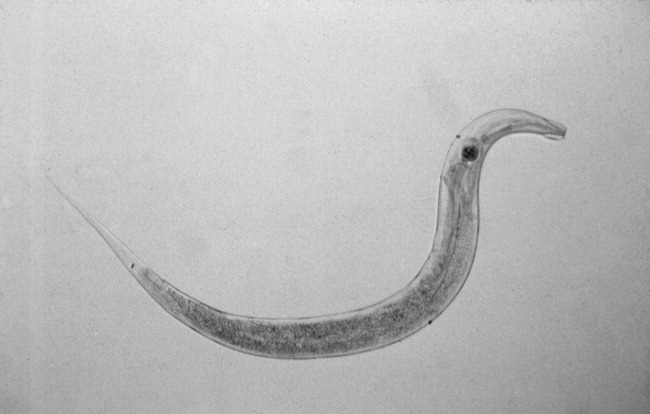
The patient was treated with loperamide as an outpatient with no relief. She was then admitted to the hospital for further diagnostic workup. Hospital workup included a Scotch tape test, which showed adult pinworms. She was treated with a single dose of 400 mg of albendazole with complete resolution of her symptoms within 2 days. No further workup was done. Patient was discharged with advice to contact her primary care doctor for reevaluation if symptoms recurred. However, the patient remained symptom free 1 year after discharge.
DISCUSSION
Enterobius vermicularis is a parasite that infects 2040 million people annually in the United States and about 200 million people worldwide. Equal infection rates are seen in all races, socioeconomic classes, and cultures.1 It is more prevalent among those in crowded living conditions. Humans are the primary natural host for the parasite, although it has been documented in cockroaches and primates. Transmission occurs via the feco‐oral route or via airborne eggs that are dislodged from contaminated clothing or bed linen. Its life cycle begins with parasite eggs hatching in the duodenum, usually within 6 hours of ingestion. They mature into adults in as little as 2 weeks and have a life span of approximately 2 months. Enterobius vermicularis normally inhabits distal small bowel including the terminal ileum, cecum, and vermiform appendix, as well as the proximal ascending colon. After copulation, an adult female will migrate to the perineum, often at night, and lay an average of 10,00015,000 eggs. These eggs mature in about 6 hours and are then transmitted to a new host by the feco‐oral route. The worms live mainly in the intestinal lumen and do not invade tissue. Hence, pinworm infections, unlike many other parasitic infections, are rarely associated with serum eosinophilia or elevated serum IgE levels.
E. vermicularis is generally considered to be an innocuous parasite. Perianal pruritis, especially during the nighttime, is the most common symptom. Patients may develop secondary bacterial infection of the irritated anal skin. Rarely, E. vermicularis infection may result in a life‐threatening illness. A literature review showed pinworm infection to be an infrequent cause of eosinophilic enterocolitis, appendicitis, intestinal obstruction, intestinal perforation, hepatic infection, urinary tract infection, sialoadenitis, salpingitis, enterocolitis, eosinophilic ileocolitis, vulvovaginitis, pelvic inflammatory disease, conditions mimicking inflammatory bowel diseases, perianal abscesses, and perianal granulomas. In a retrospective review of 180 colonoscopies done on patients with rectal bleeding or suspected inflammatory bowel disease, E. vermicularis was identified macroscopically in 31 cases (17.2%). Data collected on 23 of these cases showed that symptoms were present for a mean of 17 months; the symptoms with the highest frequency were abdominal pain (73%), rectal bleeding (62%), chronic diarrhea (50%), and weight loss (42%). None of these patients experienced perianal pruritis or developed inflammatory bowel disease during the follow‐up period of up to 5 years, although 21 patients demonstrated histopathological evidence of nonspecific colitis.6
The gold standard for diagnosing E. vermicularis infection is by visualizing the worms directly or by examination of the parasitic eggs under a microscope. The Scotch tape test is a simple, inexpensive, and quick way for confirming the infection. It is performed by doubling clear cellophane Scotch tape onto a wooden stick so that the sticky side points outward and pressing it against the perianal skin. The kidney‐bean‐shaped eggs (50 25 m) will stick to the tape and can then be directly visualized under a microscope. Pinworms are most active during the night, and eggs are deposited around the perianal region and are best recovered before defecation, early in the morning. The sensitivity of this test is 90% if done on 3 consecutive mornings and goes up to 99% when performed on 5 consecutive mornings.2, 3 Female adult worms are pin‐shaped, about 813 mm long, and white in color. They may be seen by direct visualization in the perianal region or more invasively by an anoscopic or colonoscopic examination. However, endoscopic examination may sometimes give false‐negative results as the worms are small, (ie, only a few millimeters in length) and may be missed if the endoscopist is not actively looking for them.
A single oral dose of benzimidazoles (100 mg of mebendazole or 400 mg of albendazole) results in a cure of rate of 95% and 100%, respectively. Despite the high initial cure rates, reinfection remains common; hence, a second dose 12 weeks after the initial treatment is often given to prevent it.4, 5 Pyrantel pamoate and piperazine are alternate treatments. However, they have lower efficacy and are more toxic than benzimidazoles.
Close contacts such as household members are often concurrently infected, and treatment of the remaining household members or of the group institution is also indicated. All bedding and clothes should be laundered. Personal hygiene such as fingernail clipping, frequent hand washing, and bathing should also be encouraged.
Although the pinworm's entire life cycle is in the human intestinal tract, gastrointestinal symptoms have seldom been reported. However, this may be because of underreporting. Given the increasing number of patients living in institutionalized environments such as nursing homes and assisted living, it is important to consider the possibility of E. vermicularis infection early on in a diagnostic workup of patients presenting with symptoms of colitis, even when not accompanied by anal pruritis. In a patient presenting with symptoms of inflammatory bowel disease with histopathological evaluation of nonspecific colitis should prompt clinicians to consider E. vermicularis infection.6 On the other hand, in patients who fail to respond to antiparasitic therapy or those who present with weight loss, change in bowel habits, or melena, colonscopic examination is warranted. Considering pinworm infection early during evaluation of nonspecific abdominal complaints may avoid an unnecessary and expensive diagnostic workup.
KEY POINTS
-
Recognize early on that Enterobius vermicularis infection is an important differential diagnosis for patients presenting with symptoms of colitis, thus avoiding unnecessary, expensive, and potentially harmful invasive testing.
-
Recognize that a simple and inexpensive Scotch tape test and/or direct visualization is an easy and effective way of confirming diagnosis and that stool examination may be unhelpful.
-
Recognize that reinfection may be prevented using a second dose of the antiparasitic drug.
- .The pinworm, Enterobius vermicularis.Prim Care.1991;18:13–24.
- ,,,,.Prevalence of intestinal parasites in three socioeconomically‐different regions of Sivas, Turkey.J Health Popul Nutr.2005;23:184–191.
- ,,,.Pinworm infection.Gastrointest Endosc.2001;53:210.
- ,,.Mebendazole (R 17635) in enterobiasis. A clinical trial in mental retardates.Chemotherapy.1975;21:255–260.
- ,,, et al.Field trials on the efficacy of albendazole composite against intestinal nematodiasis.Chung Kuo Chi Sheng Chung Hsueh Yu Chi Sheng Chung Ping Tsa Chih.1998;16:1–5.
- ,,.Enterobius vermicularis and colitis in children.J Pediatr Gastroenterol Nutr.2006;43:610–612.
An 84‐year‐old female patient with hypertension, osteoarthritis, hypothyroidism, and remote breast cancer was admitted with complaints of generalized abdominal pain of 2 months' duration. Pain was described as noncolicky in nature and was associated with diarrhea. She reported 78 daily episodes of watery, non‐foul‐smelling diarrhea. She denied any nausea, vomiting, fever, joint pains, oral ulcers, eye redness, stool incontinence, melena, hematochezia, or weight loss. There was no history of recent travel, antibiotic use, or exposure to sick contacts. She had no risk factors for HIV infection or other sexually transmitted infections. Her social history was significant for dining out on a regular basis and living in an assisted living facility. However, she denied any relationship between her abdominal symptoms and any particular food intake or with bowel movements. She denied any anal pruritis but reported seeing white squiggly things on tissue paper after bowel movements. She denied use of over‐the‐counter laxatives or herbal supplements. None of her prescription medications had diarrhea as a major side effect. Her social history was unremarkable for smoking, alcohol use, or illicit drug use. There were no prior abdominal surgeries. The patient's physical exam showed normal vitals on presentation and was unremarkable except for vague, generalized abdominal tenderness with no involuntary guarding or rebound pain. Her initial laboratory evaluation showed normal complete blood counts with no eosinophilia and normal serum electrolytes and liver and thyroid panel. Acute‐phase reactants, erythrocyte sedimentation rate, and C‐reactive protein were not elevated. Stool evaluation was unremarkable for Clostridium difficile toxin, fat droplets, leukocytes, erythrocytes, ova, parasites, or any bacterial growth on cultures. Computed tomography scans of the abdomen and pelvis were nonrevealing. Her colonoscopic examination 1 year prior was significant only for diverticulosis.1, 2


The patient was treated with loperamide as an outpatient with no relief. She was then admitted to the hospital for further diagnostic workup. Hospital workup included a Scotch tape test, which showed adult pinworms. She was treated with a single dose of 400 mg of albendazole with complete resolution of her symptoms within 2 days. No further workup was done. Patient was discharged with advice to contact her primary care doctor for reevaluation if symptoms recurred. However, the patient remained symptom free 1 year after discharge.
DISCUSSION
Enterobius vermicularis is a parasite that infects 2040 million people annually in the United States and about 200 million people worldwide. Equal infection rates are seen in all races, socioeconomic classes, and cultures.1 It is more prevalent among those in crowded living conditions. Humans are the primary natural host for the parasite, although it has been documented in cockroaches and primates. Transmission occurs via the feco‐oral route or via airborne eggs that are dislodged from contaminated clothing or bed linen. Its life cycle begins with parasite eggs hatching in the duodenum, usually within 6 hours of ingestion. They mature into adults in as little as 2 weeks and have a life span of approximately 2 months. Enterobius vermicularis normally inhabits distal small bowel including the terminal ileum, cecum, and vermiform appendix, as well as the proximal ascending colon. After copulation, an adult female will migrate to the perineum, often at night, and lay an average of 10,00015,000 eggs. These eggs mature in about 6 hours and are then transmitted to a new host by the feco‐oral route. The worms live mainly in the intestinal lumen and do not invade tissue. Hence, pinworm infections, unlike many other parasitic infections, are rarely associated with serum eosinophilia or elevated serum IgE levels.
E. vermicularis is generally considered to be an innocuous parasite. Perianal pruritis, especially during the nighttime, is the most common symptom. Patients may develop secondary bacterial infection of the irritated anal skin. Rarely, E. vermicularis infection may result in a life‐threatening illness. A literature review showed pinworm infection to be an infrequent cause of eosinophilic enterocolitis, appendicitis, intestinal obstruction, intestinal perforation, hepatic infection, urinary tract infection, sialoadenitis, salpingitis, enterocolitis, eosinophilic ileocolitis, vulvovaginitis, pelvic inflammatory disease, conditions mimicking inflammatory bowel diseases, perianal abscesses, and perianal granulomas. In a retrospective review of 180 colonoscopies done on patients with rectal bleeding or suspected inflammatory bowel disease, E. vermicularis was identified macroscopically in 31 cases (17.2%). Data collected on 23 of these cases showed that symptoms were present for a mean of 17 months; the symptoms with the highest frequency were abdominal pain (73%), rectal bleeding (62%), chronic diarrhea (50%), and weight loss (42%). None of these patients experienced perianal pruritis or developed inflammatory bowel disease during the follow‐up period of up to 5 years, although 21 patients demonstrated histopathological evidence of nonspecific colitis.6
The gold standard for diagnosing E. vermicularis infection is by visualizing the worms directly or by examination of the parasitic eggs under a microscope. The Scotch tape test is a simple, inexpensive, and quick way for confirming the infection. It is performed by doubling clear cellophane Scotch tape onto a wooden stick so that the sticky side points outward and pressing it against the perianal skin. The kidney‐bean‐shaped eggs (50 25 m) will stick to the tape and can then be directly visualized under a microscope. Pinworms are most active during the night, and eggs are deposited around the perianal region and are best recovered before defecation, early in the morning. The sensitivity of this test is 90% if done on 3 consecutive mornings and goes up to 99% when performed on 5 consecutive mornings.2, 3 Female adult worms are pin‐shaped, about 813 mm long, and white in color. They may be seen by direct visualization in the perianal region or more invasively by an anoscopic or colonoscopic examination. However, endoscopic examination may sometimes give false‐negative results as the worms are small, (ie, only a few millimeters in length) and may be missed if the endoscopist is not actively looking for them.
A single oral dose of benzimidazoles (100 mg of mebendazole or 400 mg of albendazole) results in a cure of rate of 95% and 100%, respectively. Despite the high initial cure rates, reinfection remains common; hence, a second dose 12 weeks after the initial treatment is often given to prevent it.4, 5 Pyrantel pamoate and piperazine are alternate treatments. However, they have lower efficacy and are more toxic than benzimidazoles.
Close contacts such as household members are often concurrently infected, and treatment of the remaining household members or of the group institution is also indicated. All bedding and clothes should be laundered. Personal hygiene such as fingernail clipping, frequent hand washing, and bathing should also be encouraged.
Although the pinworm's entire life cycle is in the human intestinal tract, gastrointestinal symptoms have seldom been reported. However, this may be because of underreporting. Given the increasing number of patients living in institutionalized environments such as nursing homes and assisted living, it is important to consider the possibility of E. vermicularis infection early on in a diagnostic workup of patients presenting with symptoms of colitis, even when not accompanied by anal pruritis. In a patient presenting with symptoms of inflammatory bowel disease with histopathological evaluation of nonspecific colitis should prompt clinicians to consider E. vermicularis infection.6 On the other hand, in patients who fail to respond to antiparasitic therapy or those who present with weight loss, change in bowel habits, or melena, colonscopic examination is warranted. Considering pinworm infection early during evaluation of nonspecific abdominal complaints may avoid an unnecessary and expensive diagnostic workup.
KEY POINTS
-
Recognize early on that Enterobius vermicularis infection is an important differential diagnosis for patients presenting with symptoms of colitis, thus avoiding unnecessary, expensive, and potentially harmful invasive testing.
-
Recognize that a simple and inexpensive Scotch tape test and/or direct visualization is an easy and effective way of confirming diagnosis and that stool examination may be unhelpful.
-
Recognize that reinfection may be prevented using a second dose of the antiparasitic drug.
An 84‐year‐old female patient with hypertension, osteoarthritis, hypothyroidism, and remote breast cancer was admitted with complaints of generalized abdominal pain of 2 months' duration. Pain was described as noncolicky in nature and was associated with diarrhea. She reported 78 daily episodes of watery, non‐foul‐smelling diarrhea. She denied any nausea, vomiting, fever, joint pains, oral ulcers, eye redness, stool incontinence, melena, hematochezia, or weight loss. There was no history of recent travel, antibiotic use, or exposure to sick contacts. She had no risk factors for HIV infection or other sexually transmitted infections. Her social history was significant for dining out on a regular basis and living in an assisted living facility. However, she denied any relationship between her abdominal symptoms and any particular food intake or with bowel movements. She denied any anal pruritis but reported seeing white squiggly things on tissue paper after bowel movements. She denied use of over‐the‐counter laxatives or herbal supplements. None of her prescription medications had diarrhea as a major side effect. Her social history was unremarkable for smoking, alcohol use, or illicit drug use. There were no prior abdominal surgeries. The patient's physical exam showed normal vitals on presentation and was unremarkable except for vague, generalized abdominal tenderness with no involuntary guarding or rebound pain. Her initial laboratory evaluation showed normal complete blood counts with no eosinophilia and normal serum electrolytes and liver and thyroid panel. Acute‐phase reactants, erythrocyte sedimentation rate, and C‐reactive protein were not elevated. Stool evaluation was unremarkable for Clostridium difficile toxin, fat droplets, leukocytes, erythrocytes, ova, parasites, or any bacterial growth on cultures. Computed tomography scans of the abdomen and pelvis were nonrevealing. Her colonoscopic examination 1 year prior was significant only for diverticulosis.1, 2


The patient was treated with loperamide as an outpatient with no relief. She was then admitted to the hospital for further diagnostic workup. Hospital workup included a Scotch tape test, which showed adult pinworms. She was treated with a single dose of 400 mg of albendazole with complete resolution of her symptoms within 2 days. No further workup was done. Patient was discharged with advice to contact her primary care doctor for reevaluation if symptoms recurred. However, the patient remained symptom free 1 year after discharge.
DISCUSSION
Enterobius vermicularis is a parasite that infects 2040 million people annually in the United States and about 200 million people worldwide. Equal infection rates are seen in all races, socioeconomic classes, and cultures.1 It is more prevalent among those in crowded living conditions. Humans are the primary natural host for the parasite, although it has been documented in cockroaches and primates. Transmission occurs via the feco‐oral route or via airborne eggs that are dislodged from contaminated clothing or bed linen. Its life cycle begins with parasite eggs hatching in the duodenum, usually within 6 hours of ingestion. They mature into adults in as little as 2 weeks and have a life span of approximately 2 months. Enterobius vermicularis normally inhabits distal small bowel including the terminal ileum, cecum, and vermiform appendix, as well as the proximal ascending colon. After copulation, an adult female will migrate to the perineum, often at night, and lay an average of 10,00015,000 eggs. These eggs mature in about 6 hours and are then transmitted to a new host by the feco‐oral route. The worms live mainly in the intestinal lumen and do not invade tissue. Hence, pinworm infections, unlike many other parasitic infections, are rarely associated with serum eosinophilia or elevated serum IgE levels.
E. vermicularis is generally considered to be an innocuous parasite. Perianal pruritis, especially during the nighttime, is the most common symptom. Patients may develop secondary bacterial infection of the irritated anal skin. Rarely, E. vermicularis infection may result in a life‐threatening illness. A literature review showed pinworm infection to be an infrequent cause of eosinophilic enterocolitis, appendicitis, intestinal obstruction, intestinal perforation, hepatic infection, urinary tract infection, sialoadenitis, salpingitis, enterocolitis, eosinophilic ileocolitis, vulvovaginitis, pelvic inflammatory disease, conditions mimicking inflammatory bowel diseases, perianal abscesses, and perianal granulomas. In a retrospective review of 180 colonoscopies done on patients with rectal bleeding or suspected inflammatory bowel disease, E. vermicularis was identified macroscopically in 31 cases (17.2%). Data collected on 23 of these cases showed that symptoms were present for a mean of 17 months; the symptoms with the highest frequency were abdominal pain (73%), rectal bleeding (62%), chronic diarrhea (50%), and weight loss (42%). None of these patients experienced perianal pruritis or developed inflammatory bowel disease during the follow‐up period of up to 5 years, although 21 patients demonstrated histopathological evidence of nonspecific colitis.6
The gold standard for diagnosing E. vermicularis infection is by visualizing the worms directly or by examination of the parasitic eggs under a microscope. The Scotch tape test is a simple, inexpensive, and quick way for confirming the infection. It is performed by doubling clear cellophane Scotch tape onto a wooden stick so that the sticky side points outward and pressing it against the perianal skin. The kidney‐bean‐shaped eggs (50 25 m) will stick to the tape and can then be directly visualized under a microscope. Pinworms are most active during the night, and eggs are deposited around the perianal region and are best recovered before defecation, early in the morning. The sensitivity of this test is 90% if done on 3 consecutive mornings and goes up to 99% when performed on 5 consecutive mornings.2, 3 Female adult worms are pin‐shaped, about 813 mm long, and white in color. They may be seen by direct visualization in the perianal region or more invasively by an anoscopic or colonoscopic examination. However, endoscopic examination may sometimes give false‐negative results as the worms are small, (ie, only a few millimeters in length) and may be missed if the endoscopist is not actively looking for them.
A single oral dose of benzimidazoles (100 mg of mebendazole or 400 mg of albendazole) results in a cure of rate of 95% and 100%, respectively. Despite the high initial cure rates, reinfection remains common; hence, a second dose 12 weeks after the initial treatment is often given to prevent it.4, 5 Pyrantel pamoate and piperazine are alternate treatments. However, they have lower efficacy and are more toxic than benzimidazoles.
Close contacts such as household members are often concurrently infected, and treatment of the remaining household members or of the group institution is also indicated. All bedding and clothes should be laundered. Personal hygiene such as fingernail clipping, frequent hand washing, and bathing should also be encouraged.
Although the pinworm's entire life cycle is in the human intestinal tract, gastrointestinal symptoms have seldom been reported. However, this may be because of underreporting. Given the increasing number of patients living in institutionalized environments such as nursing homes and assisted living, it is important to consider the possibility of E. vermicularis infection early on in a diagnostic workup of patients presenting with symptoms of colitis, even when not accompanied by anal pruritis. In a patient presenting with symptoms of inflammatory bowel disease with histopathological evaluation of nonspecific colitis should prompt clinicians to consider E. vermicularis infection.6 On the other hand, in patients who fail to respond to antiparasitic therapy or those who present with weight loss, change in bowel habits, or melena, colonscopic examination is warranted. Considering pinworm infection early during evaluation of nonspecific abdominal complaints may avoid an unnecessary and expensive diagnostic workup.
KEY POINTS
-
Recognize early on that Enterobius vermicularis infection is an important differential diagnosis for patients presenting with symptoms of colitis, thus avoiding unnecessary, expensive, and potentially harmful invasive testing.
-
Recognize that a simple and inexpensive Scotch tape test and/or direct visualization is an easy and effective way of confirming diagnosis and that stool examination may be unhelpful.
-
Recognize that reinfection may be prevented using a second dose of the antiparasitic drug.
- .The pinworm, Enterobius vermicularis.Prim Care.1991;18:13–24.
- ,,,,.Prevalence of intestinal parasites in three socioeconomically‐different regions of Sivas, Turkey.J Health Popul Nutr.2005;23:184–191.
- ,,,.Pinworm infection.Gastrointest Endosc.2001;53:210.
- ,,.Mebendazole (R 17635) in enterobiasis. A clinical trial in mental retardates.Chemotherapy.1975;21:255–260.
- ,,, et al.Field trials on the efficacy of albendazole composite against intestinal nematodiasis.Chung Kuo Chi Sheng Chung Hsueh Yu Chi Sheng Chung Ping Tsa Chih.1998;16:1–5.
- ,,.Enterobius vermicularis and colitis in children.J Pediatr Gastroenterol Nutr.2006;43:610–612.
- .The pinworm, Enterobius vermicularis.Prim Care.1991;18:13–24.
- ,,,,.Prevalence of intestinal parasites in three socioeconomically‐different regions of Sivas, Turkey.J Health Popul Nutr.2005;23:184–191.
- ,,,.Pinworm infection.Gastrointest Endosc.2001;53:210.
- ,,.Mebendazole (R 17635) in enterobiasis. A clinical trial in mental retardates.Chemotherapy.1975;21:255–260.
- ,,, et al.Field trials on the efficacy of albendazole composite against intestinal nematodiasis.Chung Kuo Chi Sheng Chung Hsueh Yu Chi Sheng Chung Ping Tsa Chih.1998;16:1–5.
- ,,.Enterobius vermicularis and colitis in children.J Pediatr Gastroenterol Nutr.2006;43:610–612.
In the Literature
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Do all patients undergoing bariatric surgery need polysomnography to evaluate for obstructive sleep apnea?
In the Literature
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH