User login
In the Literature
Literature at a Glance
A guide to this month’s studies.
- Physiologic doses of corticosteroids provide no clear benefit to patients with septic shock.
- Endovascular vs. open repair of abdominal aortic aneurysms is associated with fewer short-term deaths but complications and higher late reinterventions.
- Neither intensive insulin nor use of colloid over crystalloid improves mortality or organ failure outcomes in sepsis.
- Surgical cure for diabetes shows promise at two-year follow-up.
- Delayed defibrillation negatively affects survival.
- Right-ventricular enlargement in patients with acute PE is not associated with increased mortality.
- Periprocedural interruption of warfarin therapy presents low risk of thromboembolism.
- Minor leg injury increases risk of developing venous thrombosis threefold.
- Oral vs. parenteral antibiotics for pediatric pyelonephritis.
Do Physiologic Doses of Hydrocortisone Benefit Patients With Septic Shock?
Background: Meta-analyses and guidelines advocate the use of physiologic dose steroids in patients exhibiting septic shock. However, recommendations are largely based on the results of a single trial where benefits were seen only in patients without a response to corticotropin.
Study design: Multicenter, randomized, double-blind, placebo-controlled study.
Setting: Fifty-two participating ICUs in nine countries.
Synopsis: A total of 499 patients with evidence of infection or a systemic inflammatory response characterized by refractory hypotension were randomly selected to receive either an 11-day tapering dose of hydrocortisone or a placebo. The primary outcome was death from any cause at 28 days. A corticotropin stimulation test was conducted on every patient to assess adrenal function. There were no differences in death rates or duration of hospitalization between study arms. Overall, there were 86 deaths in the hydrocortisone group and 78 deaths in the placebo group (p=0.51). Also, response to corticotropin appeared to have little bearing on outcomes.
The study was underpowered due to low enrollment and a lower-than-expected death rate. Nevertheless, this is the largest trial to date examining the role of steroids in the management of septic shock and calls into question the strength of prior data and published guidelines.
Bottom line: This study failed to demonstrate a clinically or statistically significant treatment effect from the administration of physiologic-dose steroids in patients with septic shock.
Citation: Sprung C, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124.
Does Open or Endovascular Repair of Abdominal Aortic Aneurysm Benefit the Medicare Population?
Background: Randomized controlled trials (RCT) have shown a perioperative survival benefit of endovascular repair over open repair with fewer complications and shorter recovery. There is concern that late morbidity may be increased with endovascular repair. Patients enrolled in the trials were highly selected at specialty centers, so the results may not reflect actual practice.
Study design: Retrospective, propensity-matched, observational cohort study.
Synopsis: 22,830 patients were matched in each cohort. Patients were eligible if they had an abdominal aortic aneurysm repair without rupture and excluded if they were enrolled in health maintenance organizations.
Outcomes included death within 30 days and late survival, perioperative complications, aneurysm rupture, reintervention, and laparotomy-related complications. The average age was 76, and 20% were women. Perioperative mortality was lower after endovascular repair (1.2% vs. 4.8%, p<0.001), and older patients had a greater benefit. Late survival was similar. By four years, rupture was more likely in the endovascular group (1.8% vs. 0.5%, p<0.001), as was reintervention (9% vs. 1.7%, p<0.001).
In contrast, by four years, surgery for laparotomy-related complications was more likely in the open-repair group (9.7% v 4.1%, p<0.001), as was hospitalization for bowel obstruction or abdominal-wall hernia (14.2% v 8.1%, p<0.001). Limitations included the non-randomized design and use of administrative data for important categorical variables including medical co-morbidities.
Bottom line: As compared with open repair, endovascular repair of abdominal aortic aneurysm is associated with lower short-term death and complications and higher late reinterventions. This is balanced by an increase in laparotomy-related reinterventions after open repair.
Citation: Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008 Jan 31;358(5):464-474.
What Therapy Improves Outcomes in ICU Patients With Severe Sepsis or Septic Shock?
Background: Evidence suggests lower mortality with intensive insulin therapy in post-surgical cardiac patients. There is no proven benefit for non-surgical ICU patients. Despite lack of data, intensive insulin in severe sepsis has been widely advocated. Little is known to guide the use of colloid or crystalloid for fluid resuscitation in sepsis.
Study design: Multicenter, two-by-two factorial, open-label trial.
Setting: Multidisciplinary ICUs at 18 academic tertiary hospitals in Germany.
Synopsis: Data were analyzed for 537 patients with severe sepsis. They were randomly selected to receive intensive insulin therapy (n=247) or conventional insulin therapy (290), with either 10% hydroxyethyl starch (HES) (262) or modified Ringer’s lactate (LR) (275) for fluid resuscitation.
Co-primary endpoints were all-cause mortality at 28 days and morbidity as measured by the mean score on the Sequential Organ Failure Assessment (SOFA). The trial was stopped early for safety reasons. Intensive insulin therapy was terminated due to an increased number of hypoglycemic events in the intensive-therapy group compared with conventional therapy (12.1% vs. 2.1%, p<0.001), and there was no difference in mortality between groups at 28 and 90 days.
Interim analysis of 600 patients showed patients given HES had higher incidence of renal failure compared with LR (34.9% vs. 22.8%, p=0.002), required more days of renal replacement therapy, had lower median platelets and received more units of packed red cells. There was a trend toward higher rate of death at 90 days in those treated with HES (41% vs. 33.9%, p=0.09).
Bottom line: Intensive insulin therapy in ICU patients with severe sepsis and septic shock does not improve mortality and increases hypoglycemia and ICU length of stay. Use of colloid over crystalloid should be avoided, showing a trend toward increased death at 90 days, higher rates of acute renal failure, and need for renal replacement therapy..
Citation: Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139.
How Does Laparoscopic Adjustable Gastric Banding Affect Obese Adults With Type 2 Diabetes?
Background: Observational studies related surgical weight loss to improved glycemic control, but clinical trials did not test this relationship. The current trial examined this hypothesis.
Study design: Unmasked, randomized controlled trial.
Setting: University Obesity Research Center, Australia.
Synopsis: Sixty adults age 20-60 with body-mass index (BMI) of 30-40 and diagnosed with diabetes mellitus type 2 (DM2) within two years of recruitment were randomized into conventional therapy and surgical groups.
While both groups were treated similarly, only the surgical group received laparoscopic adjustable gastric banding. Primary outcome was remission of DM2 (a fasting glucose less than 126 mg/dl, HbA1C less than 6.2%, and off all hypoglycemic agents). At two years, 73% in the surgical group compared with 13% in the conventional group attained this outcome (relative risk [RR] 5.5, 95% confidence interval [CI] 2.2-14.0; p<0.001). Compared with the conventional group, the surgical group demonstrated statistically significant improvements in several secondary outcomes including mean body weight, waist circumference, insulin resistance, and lipids.
The limitations of the study are that it examined a small number of patients with shorter duration of DM2 and a shorter follow-up. The lower surgical complication rates cannot be generalized to other centers.
Bottom line: This study is a step forward in examining the relationship of surgical weight loss and remission of DM2. However, large multicenter trials with longer periods of follow-up in diverse group of patients would result in a better understanding of this relationship.
Citation: Dixon JB, O’Brien PE, Playfair J, et. al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316-323.
What is the Prevalence of Delayed Defibrillation and its Association With Survival to Discharge?
Background: Despite advances in resuscitation, survival rates following cardiac arrest remain low. Previous studies observed the effect of the timing of defibrillation on survival. This study examined the magnitude of delayed defibrillation and its association with survival in adults who sustained cardiac arrest specifically from ventricular fibrillation and pulseless ventricular tachycardia.
Study design: National Registry of Cardiopulmonary Resuscitation (NRCPR), a multicenter prospective cohort.
Setting: 369 U.S. hospitals providing acute care.
Synopsis: Data from NRCPR relating to 6,789 cardiac arrests secondary to ventricular fibrillation or pulseless ventricular tachycardia, at 369 hospitals in hospitalized adults were analyzed. Delayed defibrillation was defined as occurring more than two minutes from the identification of ventricular fibrillation or pulseless ventricular tachycardia to the administration of the first shock to the patient.
Delayed defibrillation occurred in 2,045 (30.1%) subjects. A lower proportion of subjects who received delayed defibrillation (22.2%) compared with those who received defibrillation in two minutes or less (39.3%) survived to hospital discharge. This was statistically significant (adjusted odds ratio [OR] 0.48, 95% CI 0.42 to 0.54; p<0.01).
Bottom line: This study not only reported that delayed defibrillation was prevalent in adult hospitalized patients, but also reinforced the importance of defibrillation within two minutes of identification of cardiac arrest secondary to ventricular fibrillation and pulseless ventricular tachycardia for better survival outcomes.
Citation: Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17.
Does Right-Ventricle Enlargement in Acute PE Increase In-hospital Death From PE or All-cause Mortality?
Background: Previous studies have shown conflicting results regarding the risk of death with right-ventricular enlargement in acute pulmonary embolism (PE). The role of thrombolysis in hemodynamically stable patients with acute PE and right-ventricular enlargement remains controversial.
Study design: Retrospective analysis of prospective cohort study.
Setting: Academic centers housing inpatients and outpatients in the United States and Canada.
Synopsis: Patients enrolled in PIOPED II who were diagnosed with acute PE and had multidetector computed tomographic (CT) angiography were retrospectively reviewed for the presence of right-ventricular enlargement. Study determined that 181 patients had PE and a CT, and 157 were adequate for measurement of right-ventricular size. PE treatment was anticoagulation in 138, anticoagulation and inferior vena cava filter in 15, inferior vena cava filter alone in two, and thrombolysis in two.
Right-ventricular enlargement was found in 78 (50%) patients; 76 were treated with anticoagulation alone or in combination with inferior vena cava filter. For patients with and without right-ventricular enlargement, there was no difference in in-hospital death from PE (0% vs. 1.3%) or all-cause mortality (2.6% vs. 2.5%). The results were unchanged when examined for septal motion abnormality and previous cardiopulmonary disease.
Bottom line: In hemodynamically stable patients with acute pulmonary embolism, right ventricular enlargement does not increase mortality. Further, thrombolytic therapy is unlikely to improve outcomes.
Citation: Stein PD, Beemath A, Matti F, et al. Enlarged right ventricle without shock in acute pulmonary embolus: prognosis. Am J Med. 2008;121:34-42.
What Are Short-term Thromboembolism, Hemorrhage Risks When Interrupting Warfarin Therapy for Procedures?
Background: The risks of thromboembolism and hemorrhage during the periprocedural interruption of warfarin therapy are not known. The risks and benefits of heparin bridging therapy are not well described.
Study design: Multicenter, prospective, observational cohort study.
Setting: Community-based physician practices.
Synopsis: Patients were eligible if they were on long-term warfarin and underwent outpatient procedures requiring interruption of therapy. The primary outcomes were thromboembolism or hemorrhage within 30 days of therapy interruption. In all, 1,024 eligible patients (7.1% considered high risk) had 1,293 interruptions of warfarin therapy. The most common procedures were colonoscopy (25.1%), oral or dental surgery (24.9%), and ophthalmologic surgery (8.9%). Warfarin interruption was five or fewer days in 83.8% of episodes.
Thromboembolism occurred in seven (0.7%) patients, and major or clinically significant bleeding occurred in 23 (0.6%, and 1.7%, respectively) patients. Periprocedural bridging with heparin was used in 88 (8.6%) patients. Of the patients who received periprocedural heparin therapy, none had thromboembolism, and 14 (13%) had bleeding episodes.
Bottom line: In patients whose warfarin therapy is interrupted to undergo outpatient procedures, the risk of thromboembolism is low and the hemorrhagic risk of heparin bridging therapy is significant.
Citation: Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168(1):63-69.
Are Minor Injuries an Independent Risk Factor For Development of DVT?
Background: Prior studies focus on major injuries as a risk factor for deep-vein thrombosis (DVT) and PE. However, major injury is often associated with other risks for venous thrombosis, such as surgery, plaster casting, hospitalization, and extended bed rest. Risk of DVT with minor injuries that don’t lead to these factors is unknown.
Study design: Large population-based case-control study.
Setting: Six anticoagulation clinics in the Netherlands.
Synopsis: 2,471 consecutive cases (patients with first episode of DVT or PE) and 3,534 controls (partners of cases or random digit dialing contacts) were enrolled. Participants were mailed a questionnaire, including a list of eight common injuries.
Participants with history of cast, surgery, recent hospitalization, extended bed rest, or prior history of cancer were excluded. A subset of patients and controls underwent DNA and blood collection to evaluate for presence of a hypercoagulable state. Of the cases, 289 (11.7%) had a minor injury within three months of the index date, compared with 154 (4.4%) of controls, representing a threefold increased risk of DVT/PE with minor injury (OR 3.1). Partial ruptures of muscles or ligaments in the leg (OR 10.9), multiple simultaneous injuries (OR 9.9), and injury within four weeks of presentation (OR 4.0), were associated with increased risk of DVT/PE.
Patients found to be Factor V Leiden carriers with injury had an almost 50-fold increased risk of venous thromboembolism (VTE) compared with non-carriers without injury (OR 49.7). Authors appropriately address possible limitations, including recall and referral bias.
Bottom line: Minor leg injury is associated with threefold risk of DVT/PE, especially in the four weeks following injury. Providers should consider short-term prophylactic treatment in patients with Factor V Leiden or high-risk injuries.
Citation: Van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168(1):21-26.
Is Oral Amox-Clav Non-inferior to IV Antibiotics in Pediatric Pyelonephritis?
Background: Present guidelines recommend initial treatment for pediatric pyelonephritis to be a parenteral third-generation cephalosporin followed by oral antibiotics. One prior randomly selected controlled trial compared oral antibiotics only with antibiotics started parenterally, but there was a higher-than-usual incidence of vesicoureteral reflux and female gender in the study.
Study design: Non-inferiority, multicenter, random, open label, controlled trial.
Setting: Twenty-eight pediatric units in northeast Italy from 2000-2005
Synopsis: 502 children age 1 month to less than 7 years with a clinical diagnosis of first occurrence of acute pyelonephritis according to urinalysis and urine culture (requiring two concordant consecutive tests) with at least two of the following conditions: fever of 38 degrees C or more or elevated erythrocyte sedimentation rate (ESR) or c-reactive protein (CRP), and elevated neutrophil count were randomized to receive oral amoxicillin-clavulanate (AC) or parenteral ceftriaxone followed by oral AC. Exclusion criteria were sepsis, dehydration, vomiting, and creatinine clearance of 70 ml/min or less.
Also, 400 children had dimercaptosuccinic acid (DMSA) scintigraphy within 10 days of study entry. Meantime, 223 had repeat DMSA at one year, and 177 had normal scans at study entry so were not repeated. At one year, 20% of patients were lost to follow-up. The primary outcome was renal scarring at one year. Secondary outcomes included time to fever defervescence, reduction in inflammatory indices, and percentage with sterile urine after 72 hours. Intention to treat analysis showed no significant differences between oral (n=244) and parenteral (n=258) treatment, both in the primary outcome 13.7% vs. 17.7% (95% CI, -11.1% to 3.1%), and secondary outcomes.
Bottom line: Treatment with oral antibiotics is as effective as parenteral then oral treatment for first episode of acute pediatric pyelonephritis.
Citation: Montini G, Toffolo A, Zucchetta P, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled non-inferiority trial. BMJ. 2007 Aug 25;335(7616):386.
Literature at a Glance
A guide to this month’s studies.
- Physiologic doses of corticosteroids provide no clear benefit to patients with septic shock.
- Endovascular vs. open repair of abdominal aortic aneurysms is associated with fewer short-term deaths but complications and higher late reinterventions.
- Neither intensive insulin nor use of colloid over crystalloid improves mortality or organ failure outcomes in sepsis.
- Surgical cure for diabetes shows promise at two-year follow-up.
- Delayed defibrillation negatively affects survival.
- Right-ventricular enlargement in patients with acute PE is not associated with increased mortality.
- Periprocedural interruption of warfarin therapy presents low risk of thromboembolism.
- Minor leg injury increases risk of developing venous thrombosis threefold.
- Oral vs. parenteral antibiotics for pediatric pyelonephritis.
Do Physiologic Doses of Hydrocortisone Benefit Patients With Septic Shock?
Background: Meta-analyses and guidelines advocate the use of physiologic dose steroids in patients exhibiting septic shock. However, recommendations are largely based on the results of a single trial where benefits were seen only in patients without a response to corticotropin.
Study design: Multicenter, randomized, double-blind, placebo-controlled study.
Setting: Fifty-two participating ICUs in nine countries.
Synopsis: A total of 499 patients with evidence of infection or a systemic inflammatory response characterized by refractory hypotension were randomly selected to receive either an 11-day tapering dose of hydrocortisone or a placebo. The primary outcome was death from any cause at 28 days. A corticotropin stimulation test was conducted on every patient to assess adrenal function. There were no differences in death rates or duration of hospitalization between study arms. Overall, there were 86 deaths in the hydrocortisone group and 78 deaths in the placebo group (p=0.51). Also, response to corticotropin appeared to have little bearing on outcomes.
The study was underpowered due to low enrollment and a lower-than-expected death rate. Nevertheless, this is the largest trial to date examining the role of steroids in the management of septic shock and calls into question the strength of prior data and published guidelines.
Bottom line: This study failed to demonstrate a clinically or statistically significant treatment effect from the administration of physiologic-dose steroids in patients with septic shock.
Citation: Sprung C, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124.
Does Open or Endovascular Repair of Abdominal Aortic Aneurysm Benefit the Medicare Population?
Background: Randomized controlled trials (RCT) have shown a perioperative survival benefit of endovascular repair over open repair with fewer complications and shorter recovery. There is concern that late morbidity may be increased with endovascular repair. Patients enrolled in the trials were highly selected at specialty centers, so the results may not reflect actual practice.
Study design: Retrospective, propensity-matched, observational cohort study.
Synopsis: 22,830 patients were matched in each cohort. Patients were eligible if they had an abdominal aortic aneurysm repair without rupture and excluded if they were enrolled in health maintenance organizations.
Outcomes included death within 30 days and late survival, perioperative complications, aneurysm rupture, reintervention, and laparotomy-related complications. The average age was 76, and 20% were women. Perioperative mortality was lower after endovascular repair (1.2% vs. 4.8%, p<0.001), and older patients had a greater benefit. Late survival was similar. By four years, rupture was more likely in the endovascular group (1.8% vs. 0.5%, p<0.001), as was reintervention (9% vs. 1.7%, p<0.001).
In contrast, by four years, surgery for laparotomy-related complications was more likely in the open-repair group (9.7% v 4.1%, p<0.001), as was hospitalization for bowel obstruction or abdominal-wall hernia (14.2% v 8.1%, p<0.001). Limitations included the non-randomized design and use of administrative data for important categorical variables including medical co-morbidities.
Bottom line: As compared with open repair, endovascular repair of abdominal aortic aneurysm is associated with lower short-term death and complications and higher late reinterventions. This is balanced by an increase in laparotomy-related reinterventions after open repair.
Citation: Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008 Jan 31;358(5):464-474.
What Therapy Improves Outcomes in ICU Patients With Severe Sepsis or Septic Shock?
Background: Evidence suggests lower mortality with intensive insulin therapy in post-surgical cardiac patients. There is no proven benefit for non-surgical ICU patients. Despite lack of data, intensive insulin in severe sepsis has been widely advocated. Little is known to guide the use of colloid or crystalloid for fluid resuscitation in sepsis.
Study design: Multicenter, two-by-two factorial, open-label trial.
Setting: Multidisciplinary ICUs at 18 academic tertiary hospitals in Germany.
Synopsis: Data were analyzed for 537 patients with severe sepsis. They were randomly selected to receive intensive insulin therapy (n=247) or conventional insulin therapy (290), with either 10% hydroxyethyl starch (HES) (262) or modified Ringer’s lactate (LR) (275) for fluid resuscitation.
Co-primary endpoints were all-cause mortality at 28 days and morbidity as measured by the mean score on the Sequential Organ Failure Assessment (SOFA). The trial was stopped early for safety reasons. Intensive insulin therapy was terminated due to an increased number of hypoglycemic events in the intensive-therapy group compared with conventional therapy (12.1% vs. 2.1%, p<0.001), and there was no difference in mortality between groups at 28 and 90 days.
Interim analysis of 600 patients showed patients given HES had higher incidence of renal failure compared with LR (34.9% vs. 22.8%, p=0.002), required more days of renal replacement therapy, had lower median platelets and received more units of packed red cells. There was a trend toward higher rate of death at 90 days in those treated with HES (41% vs. 33.9%, p=0.09).
Bottom line: Intensive insulin therapy in ICU patients with severe sepsis and septic shock does not improve mortality and increases hypoglycemia and ICU length of stay. Use of colloid over crystalloid should be avoided, showing a trend toward increased death at 90 days, higher rates of acute renal failure, and need for renal replacement therapy..
Citation: Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139.
How Does Laparoscopic Adjustable Gastric Banding Affect Obese Adults With Type 2 Diabetes?
Background: Observational studies related surgical weight loss to improved glycemic control, but clinical trials did not test this relationship. The current trial examined this hypothesis.
Study design: Unmasked, randomized controlled trial.
Setting: University Obesity Research Center, Australia.
Synopsis: Sixty adults age 20-60 with body-mass index (BMI) of 30-40 and diagnosed with diabetes mellitus type 2 (DM2) within two years of recruitment were randomized into conventional therapy and surgical groups.
While both groups were treated similarly, only the surgical group received laparoscopic adjustable gastric banding. Primary outcome was remission of DM2 (a fasting glucose less than 126 mg/dl, HbA1C less than 6.2%, and off all hypoglycemic agents). At two years, 73% in the surgical group compared with 13% in the conventional group attained this outcome (relative risk [RR] 5.5, 95% confidence interval [CI] 2.2-14.0; p<0.001). Compared with the conventional group, the surgical group demonstrated statistically significant improvements in several secondary outcomes including mean body weight, waist circumference, insulin resistance, and lipids.
The limitations of the study are that it examined a small number of patients with shorter duration of DM2 and a shorter follow-up. The lower surgical complication rates cannot be generalized to other centers.
Bottom line: This study is a step forward in examining the relationship of surgical weight loss and remission of DM2. However, large multicenter trials with longer periods of follow-up in diverse group of patients would result in a better understanding of this relationship.
Citation: Dixon JB, O’Brien PE, Playfair J, et. al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316-323.
What is the Prevalence of Delayed Defibrillation and its Association With Survival to Discharge?
Background: Despite advances in resuscitation, survival rates following cardiac arrest remain low. Previous studies observed the effect of the timing of defibrillation on survival. This study examined the magnitude of delayed defibrillation and its association with survival in adults who sustained cardiac arrest specifically from ventricular fibrillation and pulseless ventricular tachycardia.
Study design: National Registry of Cardiopulmonary Resuscitation (NRCPR), a multicenter prospective cohort.
Setting: 369 U.S. hospitals providing acute care.
Synopsis: Data from NRCPR relating to 6,789 cardiac arrests secondary to ventricular fibrillation or pulseless ventricular tachycardia, at 369 hospitals in hospitalized adults were analyzed. Delayed defibrillation was defined as occurring more than two minutes from the identification of ventricular fibrillation or pulseless ventricular tachycardia to the administration of the first shock to the patient.
Delayed defibrillation occurred in 2,045 (30.1%) subjects. A lower proportion of subjects who received delayed defibrillation (22.2%) compared with those who received defibrillation in two minutes or less (39.3%) survived to hospital discharge. This was statistically significant (adjusted odds ratio [OR] 0.48, 95% CI 0.42 to 0.54; p<0.01).
Bottom line: This study not only reported that delayed defibrillation was prevalent in adult hospitalized patients, but also reinforced the importance of defibrillation within two minutes of identification of cardiac arrest secondary to ventricular fibrillation and pulseless ventricular tachycardia for better survival outcomes.
Citation: Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17.
Does Right-Ventricle Enlargement in Acute PE Increase In-hospital Death From PE or All-cause Mortality?
Background: Previous studies have shown conflicting results regarding the risk of death with right-ventricular enlargement in acute pulmonary embolism (PE). The role of thrombolysis in hemodynamically stable patients with acute PE and right-ventricular enlargement remains controversial.
Study design: Retrospective analysis of prospective cohort study.
Setting: Academic centers housing inpatients and outpatients in the United States and Canada.
Synopsis: Patients enrolled in PIOPED II who were diagnosed with acute PE and had multidetector computed tomographic (CT) angiography were retrospectively reviewed for the presence of right-ventricular enlargement. Study determined that 181 patients had PE and a CT, and 157 were adequate for measurement of right-ventricular size. PE treatment was anticoagulation in 138, anticoagulation and inferior vena cava filter in 15, inferior vena cava filter alone in two, and thrombolysis in two.
Right-ventricular enlargement was found in 78 (50%) patients; 76 were treated with anticoagulation alone or in combination with inferior vena cava filter. For patients with and without right-ventricular enlargement, there was no difference in in-hospital death from PE (0% vs. 1.3%) or all-cause mortality (2.6% vs. 2.5%). The results were unchanged when examined for septal motion abnormality and previous cardiopulmonary disease.
Bottom line: In hemodynamically stable patients with acute pulmonary embolism, right ventricular enlargement does not increase mortality. Further, thrombolytic therapy is unlikely to improve outcomes.
Citation: Stein PD, Beemath A, Matti F, et al. Enlarged right ventricle without shock in acute pulmonary embolus: prognosis. Am J Med. 2008;121:34-42.
What Are Short-term Thromboembolism, Hemorrhage Risks When Interrupting Warfarin Therapy for Procedures?
Background: The risks of thromboembolism and hemorrhage during the periprocedural interruption of warfarin therapy are not known. The risks and benefits of heparin bridging therapy are not well described.
Study design: Multicenter, prospective, observational cohort study.
Setting: Community-based physician practices.
Synopsis: Patients were eligible if they were on long-term warfarin and underwent outpatient procedures requiring interruption of therapy. The primary outcomes were thromboembolism or hemorrhage within 30 days of therapy interruption. In all, 1,024 eligible patients (7.1% considered high risk) had 1,293 interruptions of warfarin therapy. The most common procedures were colonoscopy (25.1%), oral or dental surgery (24.9%), and ophthalmologic surgery (8.9%). Warfarin interruption was five or fewer days in 83.8% of episodes.
Thromboembolism occurred in seven (0.7%) patients, and major or clinically significant bleeding occurred in 23 (0.6%, and 1.7%, respectively) patients. Periprocedural bridging with heparin was used in 88 (8.6%) patients. Of the patients who received periprocedural heparin therapy, none had thromboembolism, and 14 (13%) had bleeding episodes.
Bottom line: In patients whose warfarin therapy is interrupted to undergo outpatient procedures, the risk of thromboembolism is low and the hemorrhagic risk of heparin bridging therapy is significant.
Citation: Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168(1):63-69.
Are Minor Injuries an Independent Risk Factor For Development of DVT?
Background: Prior studies focus on major injuries as a risk factor for deep-vein thrombosis (DVT) and PE. However, major injury is often associated with other risks for venous thrombosis, such as surgery, plaster casting, hospitalization, and extended bed rest. Risk of DVT with minor injuries that don’t lead to these factors is unknown.
Study design: Large population-based case-control study.
Setting: Six anticoagulation clinics in the Netherlands.
Synopsis: 2,471 consecutive cases (patients with first episode of DVT or PE) and 3,534 controls (partners of cases or random digit dialing contacts) were enrolled. Participants were mailed a questionnaire, including a list of eight common injuries.
Participants with history of cast, surgery, recent hospitalization, extended bed rest, or prior history of cancer were excluded. A subset of patients and controls underwent DNA and blood collection to evaluate for presence of a hypercoagulable state. Of the cases, 289 (11.7%) had a minor injury within three months of the index date, compared with 154 (4.4%) of controls, representing a threefold increased risk of DVT/PE with minor injury (OR 3.1). Partial ruptures of muscles or ligaments in the leg (OR 10.9), multiple simultaneous injuries (OR 9.9), and injury within four weeks of presentation (OR 4.0), were associated with increased risk of DVT/PE.
Patients found to be Factor V Leiden carriers with injury had an almost 50-fold increased risk of venous thromboembolism (VTE) compared with non-carriers without injury (OR 49.7). Authors appropriately address possible limitations, including recall and referral bias.
Bottom line: Minor leg injury is associated with threefold risk of DVT/PE, especially in the four weeks following injury. Providers should consider short-term prophylactic treatment in patients with Factor V Leiden or high-risk injuries.
Citation: Van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168(1):21-26.
Is Oral Amox-Clav Non-inferior to IV Antibiotics in Pediatric Pyelonephritis?
Background: Present guidelines recommend initial treatment for pediatric pyelonephritis to be a parenteral third-generation cephalosporin followed by oral antibiotics. One prior randomly selected controlled trial compared oral antibiotics only with antibiotics started parenterally, but there was a higher-than-usual incidence of vesicoureteral reflux and female gender in the study.
Study design: Non-inferiority, multicenter, random, open label, controlled trial.
Setting: Twenty-eight pediatric units in northeast Italy from 2000-2005
Synopsis: 502 children age 1 month to less than 7 years with a clinical diagnosis of first occurrence of acute pyelonephritis according to urinalysis and urine culture (requiring two concordant consecutive tests) with at least two of the following conditions: fever of 38 degrees C or more or elevated erythrocyte sedimentation rate (ESR) or c-reactive protein (CRP), and elevated neutrophil count were randomized to receive oral amoxicillin-clavulanate (AC) or parenteral ceftriaxone followed by oral AC. Exclusion criteria were sepsis, dehydration, vomiting, and creatinine clearance of 70 ml/min or less.
Also, 400 children had dimercaptosuccinic acid (DMSA) scintigraphy within 10 days of study entry. Meantime, 223 had repeat DMSA at one year, and 177 had normal scans at study entry so were not repeated. At one year, 20% of patients were lost to follow-up. The primary outcome was renal scarring at one year. Secondary outcomes included time to fever defervescence, reduction in inflammatory indices, and percentage with sterile urine after 72 hours. Intention to treat analysis showed no significant differences between oral (n=244) and parenteral (n=258) treatment, both in the primary outcome 13.7% vs. 17.7% (95% CI, -11.1% to 3.1%), and secondary outcomes.
Bottom line: Treatment with oral antibiotics is as effective as parenteral then oral treatment for first episode of acute pediatric pyelonephritis.
Citation: Montini G, Toffolo A, Zucchetta P, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled non-inferiority trial. BMJ. 2007 Aug 25;335(7616):386.
Literature at a Glance
A guide to this month’s studies.
- Physiologic doses of corticosteroids provide no clear benefit to patients with septic shock.
- Endovascular vs. open repair of abdominal aortic aneurysms is associated with fewer short-term deaths but complications and higher late reinterventions.
- Neither intensive insulin nor use of colloid over crystalloid improves mortality or organ failure outcomes in sepsis.
- Surgical cure for diabetes shows promise at two-year follow-up.
- Delayed defibrillation negatively affects survival.
- Right-ventricular enlargement in patients with acute PE is not associated with increased mortality.
- Periprocedural interruption of warfarin therapy presents low risk of thromboembolism.
- Minor leg injury increases risk of developing venous thrombosis threefold.
- Oral vs. parenteral antibiotics for pediatric pyelonephritis.
Do Physiologic Doses of Hydrocortisone Benefit Patients With Septic Shock?
Background: Meta-analyses and guidelines advocate the use of physiologic dose steroids in patients exhibiting septic shock. However, recommendations are largely based on the results of a single trial where benefits were seen only in patients without a response to corticotropin.
Study design: Multicenter, randomized, double-blind, placebo-controlled study.
Setting: Fifty-two participating ICUs in nine countries.
Synopsis: A total of 499 patients with evidence of infection or a systemic inflammatory response characterized by refractory hypotension were randomly selected to receive either an 11-day tapering dose of hydrocortisone or a placebo. The primary outcome was death from any cause at 28 days. A corticotropin stimulation test was conducted on every patient to assess adrenal function. There were no differences in death rates or duration of hospitalization between study arms. Overall, there were 86 deaths in the hydrocortisone group and 78 deaths in the placebo group (p=0.51). Also, response to corticotropin appeared to have little bearing on outcomes.
The study was underpowered due to low enrollment and a lower-than-expected death rate. Nevertheless, this is the largest trial to date examining the role of steroids in the management of septic shock and calls into question the strength of prior data and published guidelines.
Bottom line: This study failed to demonstrate a clinically or statistically significant treatment effect from the administration of physiologic-dose steroids in patients with septic shock.
Citation: Sprung C, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124.
Does Open or Endovascular Repair of Abdominal Aortic Aneurysm Benefit the Medicare Population?
Background: Randomized controlled trials (RCT) have shown a perioperative survival benefit of endovascular repair over open repair with fewer complications and shorter recovery. There is concern that late morbidity may be increased with endovascular repair. Patients enrolled in the trials were highly selected at specialty centers, so the results may not reflect actual practice.
Study design: Retrospective, propensity-matched, observational cohort study.
Synopsis: 22,830 patients were matched in each cohort. Patients were eligible if they had an abdominal aortic aneurysm repair without rupture and excluded if they were enrolled in health maintenance organizations.
Outcomes included death within 30 days and late survival, perioperative complications, aneurysm rupture, reintervention, and laparotomy-related complications. The average age was 76, and 20% were women. Perioperative mortality was lower after endovascular repair (1.2% vs. 4.8%, p<0.001), and older patients had a greater benefit. Late survival was similar. By four years, rupture was more likely in the endovascular group (1.8% vs. 0.5%, p<0.001), as was reintervention (9% vs. 1.7%, p<0.001).
In contrast, by four years, surgery for laparotomy-related complications was more likely in the open-repair group (9.7% v 4.1%, p<0.001), as was hospitalization for bowel obstruction or abdominal-wall hernia (14.2% v 8.1%, p<0.001). Limitations included the non-randomized design and use of administrative data for important categorical variables including medical co-morbidities.
Bottom line: As compared with open repair, endovascular repair of abdominal aortic aneurysm is associated with lower short-term death and complications and higher late reinterventions. This is balanced by an increase in laparotomy-related reinterventions after open repair.
Citation: Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008 Jan 31;358(5):464-474.
What Therapy Improves Outcomes in ICU Patients With Severe Sepsis or Septic Shock?
Background: Evidence suggests lower mortality with intensive insulin therapy in post-surgical cardiac patients. There is no proven benefit for non-surgical ICU patients. Despite lack of data, intensive insulin in severe sepsis has been widely advocated. Little is known to guide the use of colloid or crystalloid for fluid resuscitation in sepsis.
Study design: Multicenter, two-by-two factorial, open-label trial.
Setting: Multidisciplinary ICUs at 18 academic tertiary hospitals in Germany.
Synopsis: Data were analyzed for 537 patients with severe sepsis. They were randomly selected to receive intensive insulin therapy (n=247) or conventional insulin therapy (290), with either 10% hydroxyethyl starch (HES) (262) or modified Ringer’s lactate (LR) (275) for fluid resuscitation.
Co-primary endpoints were all-cause mortality at 28 days and morbidity as measured by the mean score on the Sequential Organ Failure Assessment (SOFA). The trial was stopped early for safety reasons. Intensive insulin therapy was terminated due to an increased number of hypoglycemic events in the intensive-therapy group compared with conventional therapy (12.1% vs. 2.1%, p<0.001), and there was no difference in mortality between groups at 28 and 90 days.
Interim analysis of 600 patients showed patients given HES had higher incidence of renal failure compared with LR (34.9% vs. 22.8%, p=0.002), required more days of renal replacement therapy, had lower median platelets and received more units of packed red cells. There was a trend toward higher rate of death at 90 days in those treated with HES (41% vs. 33.9%, p=0.09).
Bottom line: Intensive insulin therapy in ICU patients with severe sepsis and septic shock does not improve mortality and increases hypoglycemia and ICU length of stay. Use of colloid over crystalloid should be avoided, showing a trend toward increased death at 90 days, higher rates of acute renal failure, and need for renal replacement therapy..
Citation: Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139.
How Does Laparoscopic Adjustable Gastric Banding Affect Obese Adults With Type 2 Diabetes?
Background: Observational studies related surgical weight loss to improved glycemic control, but clinical trials did not test this relationship. The current trial examined this hypothesis.
Study design: Unmasked, randomized controlled trial.
Setting: University Obesity Research Center, Australia.
Synopsis: Sixty adults age 20-60 with body-mass index (BMI) of 30-40 and diagnosed with diabetes mellitus type 2 (DM2) within two years of recruitment were randomized into conventional therapy and surgical groups.
While both groups were treated similarly, only the surgical group received laparoscopic adjustable gastric banding. Primary outcome was remission of DM2 (a fasting glucose less than 126 mg/dl, HbA1C less than 6.2%, and off all hypoglycemic agents). At two years, 73% in the surgical group compared with 13% in the conventional group attained this outcome (relative risk [RR] 5.5, 95% confidence interval [CI] 2.2-14.0; p<0.001). Compared with the conventional group, the surgical group demonstrated statistically significant improvements in several secondary outcomes including mean body weight, waist circumference, insulin resistance, and lipids.
The limitations of the study are that it examined a small number of patients with shorter duration of DM2 and a shorter follow-up. The lower surgical complication rates cannot be generalized to other centers.
Bottom line: This study is a step forward in examining the relationship of surgical weight loss and remission of DM2. However, large multicenter trials with longer periods of follow-up in diverse group of patients would result in a better understanding of this relationship.
Citation: Dixon JB, O’Brien PE, Playfair J, et. al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316-323.
What is the Prevalence of Delayed Defibrillation and its Association With Survival to Discharge?
Background: Despite advances in resuscitation, survival rates following cardiac arrest remain low. Previous studies observed the effect of the timing of defibrillation on survival. This study examined the magnitude of delayed defibrillation and its association with survival in adults who sustained cardiac arrest specifically from ventricular fibrillation and pulseless ventricular tachycardia.
Study design: National Registry of Cardiopulmonary Resuscitation (NRCPR), a multicenter prospective cohort.
Setting: 369 U.S. hospitals providing acute care.
Synopsis: Data from NRCPR relating to 6,789 cardiac arrests secondary to ventricular fibrillation or pulseless ventricular tachycardia, at 369 hospitals in hospitalized adults were analyzed. Delayed defibrillation was defined as occurring more than two minutes from the identification of ventricular fibrillation or pulseless ventricular tachycardia to the administration of the first shock to the patient.
Delayed defibrillation occurred in 2,045 (30.1%) subjects. A lower proportion of subjects who received delayed defibrillation (22.2%) compared with those who received defibrillation in two minutes or less (39.3%) survived to hospital discharge. This was statistically significant (adjusted odds ratio [OR] 0.48, 95% CI 0.42 to 0.54; p<0.01).
Bottom line: This study not only reported that delayed defibrillation was prevalent in adult hospitalized patients, but also reinforced the importance of defibrillation within two minutes of identification of cardiac arrest secondary to ventricular fibrillation and pulseless ventricular tachycardia for better survival outcomes.
Citation: Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17.
Does Right-Ventricle Enlargement in Acute PE Increase In-hospital Death From PE or All-cause Mortality?
Background: Previous studies have shown conflicting results regarding the risk of death with right-ventricular enlargement in acute pulmonary embolism (PE). The role of thrombolysis in hemodynamically stable patients with acute PE and right-ventricular enlargement remains controversial.
Study design: Retrospective analysis of prospective cohort study.
Setting: Academic centers housing inpatients and outpatients in the United States and Canada.
Synopsis: Patients enrolled in PIOPED II who were diagnosed with acute PE and had multidetector computed tomographic (CT) angiography were retrospectively reviewed for the presence of right-ventricular enlargement. Study determined that 181 patients had PE and a CT, and 157 were adequate for measurement of right-ventricular size. PE treatment was anticoagulation in 138, anticoagulation and inferior vena cava filter in 15, inferior vena cava filter alone in two, and thrombolysis in two.
Right-ventricular enlargement was found in 78 (50%) patients; 76 were treated with anticoagulation alone or in combination with inferior vena cava filter. For patients with and without right-ventricular enlargement, there was no difference in in-hospital death from PE (0% vs. 1.3%) or all-cause mortality (2.6% vs. 2.5%). The results were unchanged when examined for septal motion abnormality and previous cardiopulmonary disease.
Bottom line: In hemodynamically stable patients with acute pulmonary embolism, right ventricular enlargement does not increase mortality. Further, thrombolytic therapy is unlikely to improve outcomes.
Citation: Stein PD, Beemath A, Matti F, et al. Enlarged right ventricle without shock in acute pulmonary embolus: prognosis. Am J Med. 2008;121:34-42.
What Are Short-term Thromboembolism, Hemorrhage Risks When Interrupting Warfarin Therapy for Procedures?
Background: The risks of thromboembolism and hemorrhage during the periprocedural interruption of warfarin therapy are not known. The risks and benefits of heparin bridging therapy are not well described.
Study design: Multicenter, prospective, observational cohort study.
Setting: Community-based physician practices.
Synopsis: Patients were eligible if they were on long-term warfarin and underwent outpatient procedures requiring interruption of therapy. The primary outcomes were thromboembolism or hemorrhage within 30 days of therapy interruption. In all, 1,024 eligible patients (7.1% considered high risk) had 1,293 interruptions of warfarin therapy. The most common procedures were colonoscopy (25.1%), oral or dental surgery (24.9%), and ophthalmologic surgery (8.9%). Warfarin interruption was five or fewer days in 83.8% of episodes.
Thromboembolism occurred in seven (0.7%) patients, and major or clinically significant bleeding occurred in 23 (0.6%, and 1.7%, respectively) patients. Periprocedural bridging with heparin was used in 88 (8.6%) patients. Of the patients who received periprocedural heparin therapy, none had thromboembolism, and 14 (13%) had bleeding episodes.
Bottom line: In patients whose warfarin therapy is interrupted to undergo outpatient procedures, the risk of thromboembolism is low and the hemorrhagic risk of heparin bridging therapy is significant.
Citation: Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168(1):63-69.
Are Minor Injuries an Independent Risk Factor For Development of DVT?
Background: Prior studies focus on major injuries as a risk factor for deep-vein thrombosis (DVT) and PE. However, major injury is often associated with other risks for venous thrombosis, such as surgery, plaster casting, hospitalization, and extended bed rest. Risk of DVT with minor injuries that don’t lead to these factors is unknown.
Study design: Large population-based case-control study.
Setting: Six anticoagulation clinics in the Netherlands.
Synopsis: 2,471 consecutive cases (patients with first episode of DVT or PE) and 3,534 controls (partners of cases or random digit dialing contacts) were enrolled. Participants were mailed a questionnaire, including a list of eight common injuries.
Participants with history of cast, surgery, recent hospitalization, extended bed rest, or prior history of cancer were excluded. A subset of patients and controls underwent DNA and blood collection to evaluate for presence of a hypercoagulable state. Of the cases, 289 (11.7%) had a minor injury within three months of the index date, compared with 154 (4.4%) of controls, representing a threefold increased risk of DVT/PE with minor injury (OR 3.1). Partial ruptures of muscles or ligaments in the leg (OR 10.9), multiple simultaneous injuries (OR 9.9), and injury within four weeks of presentation (OR 4.0), were associated with increased risk of DVT/PE.
Patients found to be Factor V Leiden carriers with injury had an almost 50-fold increased risk of venous thromboembolism (VTE) compared with non-carriers without injury (OR 49.7). Authors appropriately address possible limitations, including recall and referral bias.
Bottom line: Minor leg injury is associated with threefold risk of DVT/PE, especially in the four weeks following injury. Providers should consider short-term prophylactic treatment in patients with Factor V Leiden or high-risk injuries.
Citation: Van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168(1):21-26.
Is Oral Amox-Clav Non-inferior to IV Antibiotics in Pediatric Pyelonephritis?
Background: Present guidelines recommend initial treatment for pediatric pyelonephritis to be a parenteral third-generation cephalosporin followed by oral antibiotics. One prior randomly selected controlled trial compared oral antibiotics only with antibiotics started parenterally, but there was a higher-than-usual incidence of vesicoureteral reflux and female gender in the study.
Study design: Non-inferiority, multicenter, random, open label, controlled trial.
Setting: Twenty-eight pediatric units in northeast Italy from 2000-2005
Synopsis: 502 children age 1 month to less than 7 years with a clinical diagnosis of first occurrence of acute pyelonephritis according to urinalysis and urine culture (requiring two concordant consecutive tests) with at least two of the following conditions: fever of 38 degrees C or more or elevated erythrocyte sedimentation rate (ESR) or c-reactive protein (CRP), and elevated neutrophil count were randomized to receive oral amoxicillin-clavulanate (AC) or parenteral ceftriaxone followed by oral AC. Exclusion criteria were sepsis, dehydration, vomiting, and creatinine clearance of 70 ml/min or less.
Also, 400 children had dimercaptosuccinic acid (DMSA) scintigraphy within 10 days of study entry. Meantime, 223 had repeat DMSA at one year, and 177 had normal scans at study entry so were not repeated. At one year, 20% of patients were lost to follow-up. The primary outcome was renal scarring at one year. Secondary outcomes included time to fever defervescence, reduction in inflammatory indices, and percentage with sterile urine after 72 hours. Intention to treat analysis showed no significant differences between oral (n=244) and parenteral (n=258) treatment, both in the primary outcome 13.7% vs. 17.7% (95% CI, -11.1% to 3.1%), and secondary outcomes.
Bottom line: Treatment with oral antibiotics is as effective as parenteral then oral treatment for first episode of acute pediatric pyelonephritis.
Citation: Montini G, Toffolo A, Zucchetta P, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled non-inferiority trial. BMJ. 2007 Aug 25;335(7616):386.
Statins/Beta‐Blockers and Mortality after Vascular Surgery
Vascular surgery has higher morbidity and mortality than other noncardiac surgeries. Despite the identification of vascular surgery as higher risk, 30‐day mortality for this surgery has remained at 3%10%. Few studies have examined longer‐term outcomes, but higher mortality rates have been reported, for example, 10%30% 6 months after surgery, 20%40% 1 year after surgery, and 30%50% 5 years after surgery.15 Postoperative adverse events have been found to be highly correlated with perioperative ischemia and infarction.68 Perioperative beta‐blockers have been widely studied and have been shown to benefit patients undergoing noncardiac surgery generally and vascular surgery specifically.9, 10 However, 2 recent trials of perioperative beta‐blockers in noncardiac and vascular surgery patients failed to show an association with 18‐month and 30‐day postoperative morbidity and mortality, respectively.11, 12 In addition, the authors of a recent meta‐analysis of perioperative beta‐blockers suggested more studies were needed.13 Furthermore, there have been promising new data on the use of perioperative statins.1418 Finally, as a recent clinical trial of revascularization before vascular surgery did not demonstrate an advantage over medical management, the identification of which perioperative medicines improve postoperative outcomes and in what combinations becomes even more important.19 We sought to ascertain if the ambulatory use of statins and/or beta‐blockers within 30 days of surgery was associated with a reduction in long‐term mortality.
METHODS
Setting and Subjects
We conducted a retrospective cohort study using a regional Department of Veterans Affairs (VA) administrative and relational database, the Consumer Health Information and Performance Sets (CHIPs), which automatically extracts data from electronic medical records of all facilities in the Veterans Integrated Services Network 20, which encompasses Alaska, Washington, Oregon, and Idaho. CHIPs contains information on both outpatient and inpatient environments, and a record is generated for every contact a patient makes with the VA health care system, which includes picking up prescription medications, laboratory values, demographic information, International Classification of Diseases, 9th Revision (ICD‐9), codes, and vital status. In addition, we used the Beneficiary Identification and Records Locator Subsystem database, which is the national VA death index and includes Social Security Administration data that has been shown to be 90%95% complete for assessing vital status.20
Data for all patients who had vascular surgery at 5 VA medical centers in the region from January 1998 to March 2005 was ascertained. If a patient had a second operation within 2 years of the first, the patient was censored at the date of the second operation. A patient was defined as taking a statin or beta‐blocker if a prescription for either of these medications had been picked up within 30 days before or after surgery. The IRB at the Portland VA Medical Center approved the study with a waiver of informed consent.
Data Elements
For every patient we noted the type of vascular surgery (carotid, aortic, lower extremity bypass, or lower extremity amputation), age, sex, comorbid conditions (hypertension, cerebrovascular disease, cancer, diabetes, hyperlipidemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], coronary artery disease [CAD], heart failure), tobacco use, ethnicity, nutritional status (serum albumin), and medication use, defined as filling a prescription within 30 days before surgery (insulin, aspirin, angiotensin‐converting enzyme [ACE] inhibitor, and clonidine). Each patient was assigned a revised cardiac risk index (RCRI) score.21 For each the risk factors: use of insulin, CAD, heart failure, cerebrovascular disease, CKD, and high‐risk surgery (intrathoracic, intraperitoneal, or suprainguinal vascular procedures) 1 point was assigned. These variables were defined according to ICD‐9 codes. CKD was defined as either an ICD‐9 code for CKD or a serum creatinine > 2 mg/dL. Patients were identified by the index vascular surgery using ICD‐9 codes in the CHIPs database, and both inpatient and outpatient data were extracted.
Statistical Analysis
All patients were censored at the point of last contact up to 5 years after surgery to focus on more clinically relevant long‐term outcomes possibly associated with vascular surgery. We conducted 3 separate analyses: (1) statin exposure regardless of beta‐blocker exposure; (2) beta‐blocker exposure regardless of statin exposure, and; (3) combined exposure to statins and beta‐blockers.
Propensity score methods were used to adjust for imbalance in the baseline characteristics between statin users and nonusers, beta‐blocker users and nonusers, and combination statin and beta‐blocker users and nonusers.22, 23 The range of the propensity score distribution was similar in drug users and nonusers in the individual analyses. There was sufficient overlap between the 2 groups in each stratum. To derive propensity scores for the individual drug analyses, statin use and beta‐blocker use were modeled independently with the demographic and clinical variables using stepwise logistic regression with a relaxed entry criterion of = 0.20. Only 1 variable (hyperlipidemia) remained significantly different between statin users and nonusers, and it was included in the subsequent analyses as a potential confounder. The variable albumin had 511 missing values. To keep this variable in the propensity scores, the missing values were replaced by the predicted values of albumin from the multiple linear regression model that included the other demographic variables. The propensity scores were grouped into quintiles and used as a stratification variable in the subsequent analyses. To confirm that the propensity score method reduced the imbalances, the demographic and clinical characteristics of statin and beta‐blocker users and nonusers and combination users and nonusers were compared using Cochran‐Mantel‐Haenzel tests with the respective propensity score as a stratification variable.
For the combined use of both study drugs, we performed univariate analysis with adjustment only for RCRI (as this was a powerful predictor of mortality in our dataset; Table 1) as well as a propensity score analysis in an exploratory manner. There have been limited applications of propensity score methods to multiple treatment groups. Similar to that in the study by Huang et al.,24 we developed a multinomial baseline response logit model to obtain 3 separate propensity scores (statin only vs. none, beta‐blocker only vs. none, and both vs. none). Because of the limited sample size, the data were stratified according to the median split of each propensity score. Each score had similar ranges for each treatment group. All but 5 variables (CAD, hypertension, hyperlipidemia, ACE inhibitor use, and type of surgery) were balanced after accounting for strata. These 5 variables were then included in the final stratified Cox regression model as potential confounders.
| Variable | Level | N (%) Overall N = 3062 | Hazard ratio (95% CI) | Chi‐square P value |
|---|---|---|---|---|
| ||||
| Age in years, median (IQR) | 67 (5974) | 1.04 (1.04, 1.05)a | <.0001 | |
| Sex | Female | 45 (1) | 0.89 (0.53, 1.51) | .6704 |
| Male | 3017 (99) | 1 | 1.0000 | |
| Preoperative medical conditions | HTN | 2415 (79) | 1.32 (1.13, 1.55) | .0006 |
| CVA/TIA | 589 (19) | 1.05 (0.90, 1.22) | .5753 | |
| CA | 679 (22) | 1.55 (1.36, 1.78) | <.0001 | |
| DM | 1474 (48) | 1.75 (1.54, 1.98) | <.0001 | |
| Lipid | 872 (28) | 0.84 (0.74, 0.97) | .0187 | |
| COPD | 913 (30) | 1.68 (1.48, 1.90) | <.0001 | |
| CAD | 1491 (49) | 1.46 (1.29, 1.66) | <.0001 | |
| CHF | 747 (24) | 2.44 (2.15, 2.77) | <.0001 | |
| CKD | 443 (14) | 2.32 (2.00, 2.69) | <.0001 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 2.73 (2.28, 3.28) | <.0001 |
| Albumin 3.5 | 596 (23) | 2.70 (2.35, 3.10) | <.0001 | |
| Medication use | Aspirin | 1789 (58) | 1.10 (0.97, 1.25) | .1389 |
| ACE inhibitor | 1250 (41) | 0.93 (0.82, 1.06) | .2894 | |
| Insulin | 478 (16) | 1.31 (1.12, 1.54) | .0007 | |
| Clonidine | 115 (4) | 1.68 (1.29, 2.20) | .0001 | |
| Perioperative medication | Statinb | 1346 (44) | 0.66 (0.58, 0.75) | <.0001 |
| Beta‐blockerc | 1617 (53) | 0.74 (0.66, 0.84) | <.0001 | |
| Statin only | 414 (14) | 0.69 (0.56, 0.84) | .0002 | |
| Beta‐blocker only | 685 (22) | 0.81 (0.69, 0.95) | .0079 | |
| Statin and beta‐blocker | 932 (30) | 0.57 (0.49, 0.67) | <.0001 | |
| Noned | 1031 (34) | 1 | 1.0000 | |
| Type of surgery | Aorta | 232 (8) | 1.34 (1.01, 1.77) | <.0001 |
| Carotid | 875 (29) | 1 | ||
| Amputation | 867 (28) | 2.80 (2.36, 3.32) | ||
| Bypass | 1088 (36) | 1.57 (1.32, 1.87) | ||
| RCRI | 0 | 1223 (40) | 1 | <.0001 |
| 1 | 1005 (33) | 1.33 (1.13, 1.55) | ||
| 2 | 598 (20) | 2.22 (1.88, 2.62) | ||
| 3 | 200 (7) | 3.16 (2.54, 3.93) | ||
| 4 | 36 (1) | 4.82 (3.15, 7.37) | ||
| Year surgery occurred | 1998 | 544 (18) | 1 | .6509 |
| 1999 | 463 (15) | 0.91 (0.75, 1.10) | ||
| 2000 | 420 (14) | 0.93 (0.77, 1.13) | ||
| 2001 | 407 (13) | 0.93 (0.75, 1.14) | ||
| 2002 | 374 (12) | 1.12 (0.90, 1.40) | ||
| 2003 | 371 (12) | 1.15 (0.90, 1.47) | ||
| 2004 | 407 (13) | 0.97 (0.72, 1.31) | ||
| 2005 | 76 (3) | 0.68 (0.28, 1.65) | ||
| Tobacco user | Yes | 971 (32) | 0.90 (0.76, 1.08) | .4762 |
| No | 649 (21) | 1 | ||
| Null | 1442 (47) | 0.96 (0.81, 1.13) | ||
| Ethnicity | White | 563 (18) | 1 | .0366 |
| Other | 39 (1) | 0.98 (0.55, 1.76) | ||
| Unknown | 2460 (80) | 1.24 (1.05, 1.46) | ||
To comment on patient‐specific risk by stratification with the RCRI, we used a fixed time point of the 2‐year mortality estimated from the Cox regression model to analyze use of study drugs singly or in combination compared with use of neither.
Chi‐square tests were used to categorize and compare demographic and clinical characteristics of statin users and nonusers, of beta‐blocker users and nonusers, and combination users and nonusers. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Stratified or unstratified Cox regression was used to estimate the hazard ratios of statins and beta‐blockers, with or without adjustment for the propensity score. All analyses were performed using SAS (Statistical Analysis System) software, version 9.1.
RESULTS
Patient Characteristics
The study included 3062 patients whose median age was 67 (interquartile range, 5974; Table 1). Ninety‐nine percent of the study patients were men. Overall, ambulatory use of statins and beta‐blockers was found in 44% and 53% of patients, respectively, and combination use occurred in 30%. Sixty‐one percent of patients had an RCRI of 1 or greater; among them 71% were statin users (Table 2), 68% were beta‐blocker users (Table 3), and 75% were combination users (Table 4). Sixty‐four percent of surgeries were either lower extremity bypass or amputation, 29% were carotid, and 8% aortic. Median follow‐up for all patients was 2.7 years (interquartile range, 1.24.6). Of the whole study cohort, 53% and 62% filled a prescription for a statin or beta‐blocker within 1 year of surgery, respectively, and 58% and 67% filled a prescription within 2 years of surgery, respectively. Overall mortality at 30 days was 3%, at 1 year 14%, and at 2 years 22%.
| Variable, N (%) | Level | Overall (N = 3062) | Statin users (N = 1346 [44]) | Statin nonusers (N = 1716 [56]) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 66 (5973) | 68 (6075) | <.0001 | .9934 | |
| Sex | Female | 45 (1) | 15 (1) | 30 (2) | .1480 | .7822 |
| Male | 3017 (99) | 1331 (99) | 1686 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1176 (87) | 1239 (72) | <.0001 | .2984 |
| CVA/TIA | 589 (19) | 328 (24) | 261 (15) | <.0001 | .3935 | |
| CA | 679 (22) | 307 (23) | 372 (22) | .4550 | .8404 | |
| DM | 1474 (48) | 666 (49) | 808 (47) | .1883 | .5504 | |
| Lipid | 872 (28) | 629 (47) | 243 (14) | <.0001 | .0246 | |
| COPD | 913 (30) | 411 (31) | 502 (29) | .4419 | .8435 | |
| CAD | 1491 (49) | 837 (62) | 654 (38) | <.0001 | .4720 | |
| CHF | 747 (24) | 370 (27) | 377 (22) | .0004 | .4839 | |
| CKD | 443 (14) | 208 (15) | 235 (14) | .1698 | .9990 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 101 (8) | 128 (7) | .9629 | .6911 |
| Albumin 3.5 | 596 (23) | 191 (16) | 405 (30) | <.0001 | .5917 | |
| Medication use | Aspirin | 1789 (58) | 904 (67) | 885 (52) | <.0001 | .6409 |
| Ace inhibitor | 1250 (41) | 712 (53) | 538 (31) | <.0001 | .6075 | |
| Beta‐blocker | 1220 (40) | 767 (57) | 453 (26) | <.0001 | .4058 | |
| Insulin | 478 (16) | 254 (19) | 224 (13) | <.0001 | .7919 | |
| Clonidine | 115 (4) | 61 (5) | 54 (3) | .0454 | .6141 | |
| Type of surgery | Aorta | 232 (8) | 106 (8) | 126 (7) | <.0001 | .9899 |
| Carotid | 875 (29) | 510 (38) | 365 (21) | |||
| Amputation | 867 (28) | 274 (20) | 593 (35) | |||
| Bypass | 1088 (36) | 456 (34) | 632 (37) | |||
| RCRI | 0 | 1223 (40) | 389 (29) | 834 (49) | <.0001 | .9831 |
| 1 | 1005 (33) | 507 (38) | 498 (29) | |||
| 2 | 598 (20) | 318 (24) | 280 (16) | |||
| 3 | 200 (7) | 109 (8) | 91 (5) | |||
| 4 | 36 (1) | 23 (1) | 13 (0.76) | |||
| Year of surgery | 1998 | 544 (18) | 134 (10) | 410 (24) | <.0001 | 1 |
| 1999 | 463 (15) | 163 (12) | 300 (17) | |||
| 2000 | 420 (13) | 178 (13) | 242 (14) | |||
| 2001 | 407 (13) | 188 (14) | 219 (13) | |||
| 2002 | 374 (12) | 194 (14) | 180 (10) | |||
| 2003 | 371 (12) | 209 (16) | 162 (9) | |||
| 2004 | 407 (13) | 229 (17) | 178 (10) | |||
| 2005 | 76 (3) | 51 (4) | 25 (1.5) | |||
| Tobacco user | Yes | 971 (32) | 494 (37) | 477 (28) | <.0001 | .9809 |
| No | 649 (21) | 335 (25) | 314 (18) | |||
| Null | 1442 (47) | 517 (38) | 925 (54) | |||
| Ethnicity | White | 563 (18) | 263 (20) | 300 (17) | .1544 | .9475 |
| Other | 39 (1) | 13 (1) | 26 (1.5) | |||
| Unknown | 2460 (80) | 1070 (79) | 1390 (81) | |||
| Variable, N (%) | Level | Overall N = 3062 | BB users N = 1617 (53) | Non‐BB users N = 1445 (47) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 67 (5975) | 68 (6076) | .0526 | .7671 | |
| Sex | Female | 45 (1) | 12 (1) | 33 (2) | .0004 | .585 |
| Male | 3017 (99) | 1605 (99) | 1412 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1398 (86) | 1017 (70) | <.0001 | .1837 |
| CVA/TIA | 589 (19) | 364 (23) | 225 (16) | <.0001 | .3206 | |
| CA | 679 (22) | 359 (22) | 320 (22) | .9701 | .4288 | |
| DM | 1474 (48) | 739 (46) | 735 (51) | .0043 | .6329 | |
| Lipid | 872 (28) | 555 (34) | 317 (22) | <.0001 | .7180 | |
| COPD | 913 (30) | 487 (30) | 426 (29) | .7007 | .8022 | |
| CAD | 1491 (49) | 975 (60) | 516 (36) | <.0001 | .3496 | |
| CHF | 747 (24) | 439 (27) | 308 (21) | .0002 | .6509 | |
| CKD | 443 (14) | 248 (15) | 195 (13) | .1480 | .8544 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 132 (8) | 97 (7) | .1277 | .5867 |
| Albumin 3.5 | 596 (23) | 252 (18) | 344 (30) | <.0001 | .5347 | |
| Medication use | Aspirin | 1789 (58) | 1046 (65) | 743 (51) | <.0001 | .4942 |
| Ace inhibitor | 1250 (41) | 760 (47) | 490 (34) | <.0001 | .4727 | |
| Statin | 1220 (40) | 932 (58) | 414 (29) | <.0001 | .3706 | |
| Insulin | 478 (16) | 255 (16) | 223 (15) | .7973 | .5991 | |
| Clonidine | 115 (4) | 77 (5) | 38 (3) | .0019 | .8241 | |
| Type of surgery | Aorta | 232 (8) | 176 (11) | 56 (4) | <.0001 | .5664 |
| Carotid | 875 (29) | 515 (32) | 360 (25) | |||
| Amputation | 867 (28) | 339 (21) | 528 (37) | |||
| Bypass | 1088 (36) | 587 (36) | 501 (35) | |||
| RCRI | 0 | 1223 (40) | 518 (32) | 705 (49) | <.0001 | .5489 |
| 1 | 1005 (33) | 583 (36) | 422 (29) | |||
| 2 | 598 (20) | 358 (22) | 240 (17) | |||
| 3 | 200 (7) | 130 (8) | 70 (5) | |||
| 4 | 36 (1) | 28 (2) | 8 (1) | |||
| Year of surgery | 1998 | 544 (18) | 200 (12) | 344 (24) | <.0001 | .3832 |
| 1999 | 463 (15) | 211 (13) | 252 (17) | |||
| 2000 | 420 (13) | 210 (13) | 210 (15) | |||
| 2001 | 407 (13) | 209 (13) | 198 (14) | |||
| 2002 | 374 (12) | 220 (14) | 154 (11) | |||
| 2003 | 371 (12) | 238 (15) | 133 (9) | |||
| 2004 | 407 (13) | 279 (17) | 128 (9) | |||
| 2005 | 76 (3) | 50 (3) | 26 (2) | |||
| Tobacco user | Yes | 971 (32) | 569 (35) | 402 (28) | <.0001 | .9025 |
| No | 649 (21) | 370 (23) | 279 (19) | |||
| Null | 1442 (47) | 678 (42) | 764 (53) | |||
| Ethnicity | White | 563 (18) | 309 (19) | 254 (18) | .4962 | .8762 |
| Other | 39 (1) | 19 (1) | 20 (1) | |||
| Unknown | 2460 (80) | 1289 (80) | 1171 (81) | |||
| N (%) Variable | Level | Overall N = 3062 | BB alone N = 685 (22) | Statin alone N = 414 (14) | Both drugs N = 932 (30) | Neither drug N = 1031 (34) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age in years, median (IQR) | 67 (5974) | 68 (6075) | 67 (6075) | 66 (5973) | 69 (6076) | .0029 | .9824 | |
| Sex | Female | 45 (1) | 7 (1) | 10 (2) | 5 (1) | 23 (2) | .0042 | .5815 |
| Male | 3017 (99) | 678 (99) | 404 (98) | 927 (99) | 1008 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 560 (82) | 338 (82) | 838 (90) | 679 (66) | <.0001 | .0251 |
| CVA/TIA | 589 (19) | 127 (19) | 91 (22) | 237 (25) | 134 (13) | <.0001 | .4543 | |
| CA | 679 (22) | 150 (22) | 98 (24) | 209 (22) | 222 (22) | .8379 | .9749 | |
| DM | 1474 (48) | 291 (43) | 218 (53) | 448 (48) | 517 (50) | .0031 | .3943 | |
| Lipid | 872 (28) | 125 (18) | 199 (48) | 430 (46) | 118 (11) | <.0001 | <.0001 | |
| COPD | 913 (30) | 199 (29) | 123 (30) | 288 (9) | 303 (29) | .8475. | .9769 | |
| CAD | 1491 (49) | 327 (48) | 189 (46) | 648 (70) | 327 (32) | <.0001 | <.0001 | |
| CHF | 747 (24) | 163 (24) | 94 (23) | 276 (30) | 214 (21) | <.0001 | .7031 | |
| CKD | 443 (14) | 92 (13) | 52 (13) | 156 (17) | 143 (14) | .1120 | .8364 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 52 (8) | 21 (5) | 80 (9) | 76 (7) | .1619 | .7184 |
| Albumin 3.5 | 596 (23) | 134 (20) | 73 (20) | 118 (14) | 271 (34) | <.0001 | .2846 | |
| Medication use | Aspirin | 1789 (58) | 398 (58) | 256 (62) | 648 (70) | 487 (47) | <.0001 | .2334 |
| Ace inhibitor | 1250 (41) | 264 (39) | 216 (52) | 496 (53) | 274 (27) | <.0001 | .0216 | |
| Insulin | 478 (16) | 93 (14) | 92 (22) | 162 (17) | 131 (13) | <.0001 | .2952 | |
| Clonidine | 115 (4) | 28 (4) | 12 (3) | 49 (5) | 26 (3) | .0107 | .8035 | |
| Type of surgery | Aorta | 232 (8) | 78 (11) | 8 (2) | 98 (11) | 48 (5) | <.0001 | .008 |
| Carotid | 875 (29) | 165 (24) | 160 (39) | 350 (38) | 200 (19) | |||
| Amputation | 867 (28) | 164 (24) | 99 (24) | 175 (19) | 429 (42) | |||
| Bypass | 1088 (36) | 278 (41) | 147 (36) | 309 (33) | 354 (34) | |||
| RCRI | 0 | 1223 (40) | 288 (42) | 159 (38) | 230 (25) | 546 (53) | <.0001 | .5392 |
| 1 | 1005 (33) | 219 (32) | 143 (35) | 364 (39) | 279 (27) | |||
| 2 | 598 (20) | 125 (18) | 85 (21) | 233 (25) | 155 (15) | |||
| 3 | 200 (7) | 46 (7) | 25 (6) | 84 (9) | 45 (4) | |||
| 4 | 36 (1) | 7 (1) | 2 (0) | 21 (2) | 6 (1) | |||
| Year of surgery | 1998 | 544 (18) | 126 (18) | 60 (14) | 74 (8) | 284 (28) | <.0001 | .3105 |
| 1999 | 463 (15) | 111 (16) | 63 (15) | 100 (11) | 189 (18) | |||
| 2000 | 420 (13) | 87 (13) | 55 (13) | 123 (13) | 155 (15) | |||
| 2001 | 407 (13) | 84 (12) | 63 (15) | 125 (13) | 135 (13) | |||
| 2002 | 374 (12) | 81 (12) | 55 (13) | 139 (15) | 99 (10 | |||
| 2003 | 371 (12) | 85 (13) | 56 (14) | 153 (16) | 77 (7) | |||
| 2004 | 407 (13) | 96 (14) | 46 (11) | 183 (20) | 82 (8) | |||
| 2005 | 76 (3) | 15 (2) | 16 (4) | 35 (4) | 10 (1) | |||
| Tobacco user | Yes | 971 (32) | 227 (33) | 152 (37) | 342 (37) | 250 (24) | <.0001 | .3914 |
| No | 649 (21) | 134 (20) | 99 (24) | 236 (25) | 180 (17) | |||
| Null | 1442 (47) | 324 (47) | 163 (39) | 354 (38) | 601 (58) | |||
| Ethnicity | White | 563 (18) | 115 (17) | 69 (17) | 194 (21) | 185 (18) | .2821 | .9771 |
| Other | 39 (1) | 10 (1) | 4 (1) | 9 (1) | 16 (2) | |||
| Unknown | 2460 (80) | 560 (82) | 341 (82) | 729 (78) | 830 (81) | |||
Univariate Survival Analysis
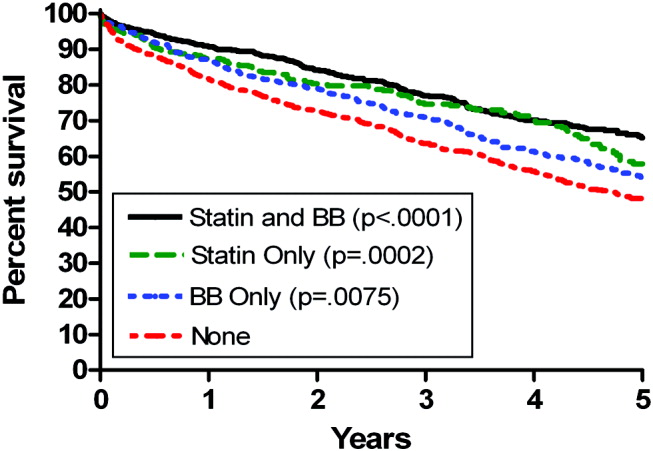
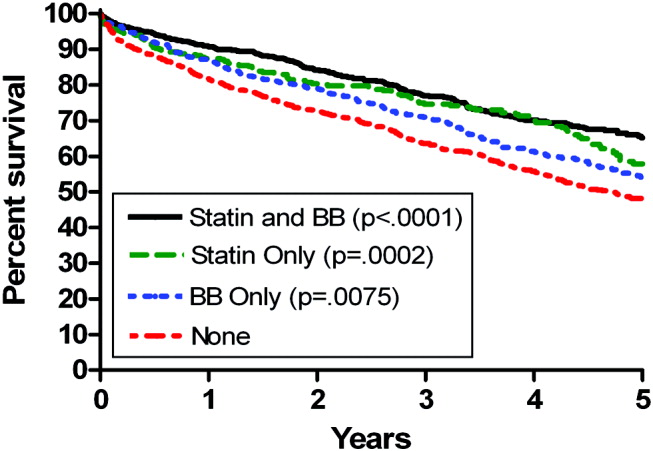
Univariate Cox regression analysis revealed a strong effect of the composite RCRI, which was predictive of mortality in a linear fashion over the course of the study compared with an RCRI of 0 (Table 1). Univariate analysis showed significant associations with decreased mortality for statins (hazard ratio [HR] = 0.66 [95% CI 0.580.75], P < .0001) and beta‐blockers (HR = 0.74 [95% CI 0.660.84], P = .0001); see Table 1. Of note, compared with that in 1998, mortality did not change for all the years for which data were complete. In addition, compared with taking neither study drug, taking a statin only, a beta‐blocker only, or both was associated with decreased mortality (P = .0002, P = .0079, and P < .0001, respectively; Fig. 1).
Propensity Score Analysis for Single Study Drug
There were significant differences in demographic and clinical characteristics between statin‐users versus statin nonusers, and between beta‐blocker users versus beta‐blocker nonusers. These differences became insignificant after the propensity score adjustment, with the exception of hyperlipidemia for statins, P = .02, which was added to the model as a confounder (Table 2). The distribution of the propensity scores was similar for study drug users and nonusers within each stratum. The association with decreased mortality remained significant after adjusting for propensity score (for statins, HR = 0.78 [95% CI 0.670.92, P = .0050], number needed to treat [NNT] = 22; for beta‐blockers HR = 0.84 [95% CI 0.730.96, P = .0103], NNT = 30; Fig. 2).
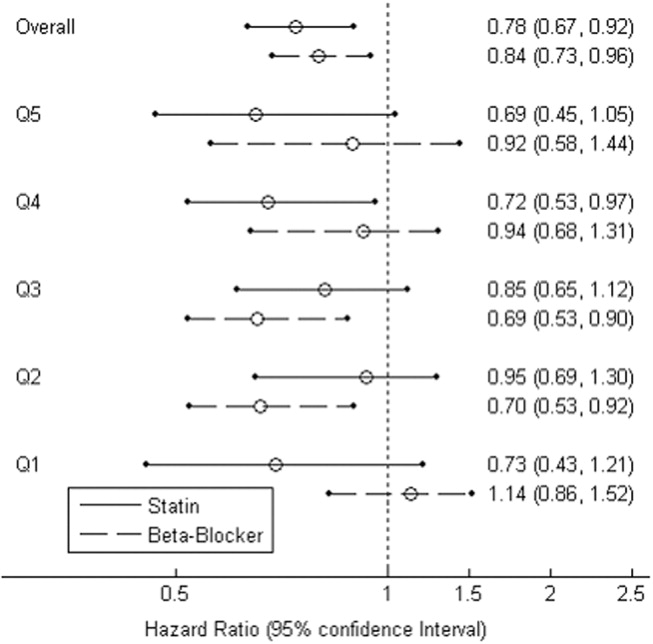
Combination Study Drugs and Revised Cardiac Risk Index: Univariate Analysis
We wanted our results to closely model those of combination use of the study drugs by patients in a clinical situation. Therefore, we first examined the effects of ambulatory statins alone, beta‐blockers alone, and a combination of statins and beta‐blockers by univariate analysis. Grouping patients by study drug use has not commonly been reported in the literature. We also examined the statistical interaction between the study drugs and the RCRI. The main‐effects model adequately explained all‐cause mortality, and the statistical interaction between the study drugs and the RCRI was not significant.
The final univariate Cox regression model, which compared use of a statin alone, a beta‐blocker alone, and a statin and beta‐blocker in combination with using neither study drug, demonstrated that the combination of statins and beta‐blockers had an HR over the whole study period of 0.43 (95% CI 0.360.51, P < .0001), statins alone had an HR of 0.59 (95% CI 0.480.72, P < .0001), and beta‐blockers alone had an HR of 0.71 (95% CI 0.610.83, P < .0001).
To clarify the effects of the study drugs on patients at different levels of risk, we stratified patients by the RCRI and evaluated the effects of the study drugs on mortality at 2 years, comparing the results to a referent of taking no study drugs. The use of both a statin and a beta‐blocker consistently produced a relative risk reduction (RRR) of approximately 50% with an NNT of 410, with highly statistically significant results for patients at all levels of risk (Table 5). As patient risk level increased, the NNT decreased, consistent with higher‐risk patients benefiting most from combination therapy with statins and beta‐blockers.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.19 | |||
| BB | 288 (73) | 0.14 | 20 | 0.27 | .0023 | |
| Statin | 159 (30) | 0.12 | 14 | 0.39 | <.0001 | |
| Statin+BB | 230 (23) | 0.09 | 10 | 0.54 | <.0001 | |
| 1 | None | 279 (130) | 0.28 | |||
| BB | 219 (71) | 0.21 | 14 | 0.26 | .0028 | |
| Statin | 143 (41) | 0.17 | 10 | 0.37 | <.0001 | |
| Statin+BB | 364 (73) | 0.13 | 7 | 0.53 | <.0001 | |
| 2 | None | 155 (100) | 0.43 | |||
| BB | 125 (60) | 0.33 | 10 | 0.23 | .0045 | |
| Statin | 85 (42) | 0.28 | 7 | 0.35 | <.0001 | |
| Statin+BB | 233 (72) | 0.22 | 5 | 0.50 | <.0001 | |
| 3 | None | 51 (39) | 0.59 | |||
| BB | 53 (29) | 0.47 | 9 | 0.20 | .0296 | |
| Statin | 27 (14) | 0.41 | 6 | 0.31 | .0014 | |
| Statin+BB | 105 (52) | 0.32 | 4 | 0.46 | <.0001 | |
In addition, the range of outcomes can be clearly seen for both patient‐specific risk level and study drug use. For example, overall mortality at 2 years for all patients was 22%. For the study drugs, mortality ranged from 16% for patient who used both a statin and a beta‐blocker to 27% for those patients who used neither study drug. The use of the RCRI showed that the healthiest patients who were taking both a statin and a beta‐blocker did the best, with a 2‐year mortality of 9%, compared with the sickest patients who were taking neither study drug, whose 2‐year mortality was 59%. Use of both study drugs by the sickest patients was associated with a reduction in 2‐year mortality to 32% (P < .0001; Table 5).
Propensity Score Analysis of Use of Combination Study Drugs
Because there was very limited literature to guide us in the use of propensity score analysis of multiple treatment groups, we performed these analyses in an exploratory manner. There were significant differences between combination statin and beta‐blocker users and nonusers. These differences became insignificant after adjusting for propensity score, except for the 5 variables previously mentioned, which were added to the model as potential confounders (Table 4). The propensity‐adjusted Cox regression model comparing use of each study drug alone and in combination with taking neither over the whole study period still showed an association with decreased mortality. The combination of statins and beta‐blockers had an HR of 0.56 (95% CI 0.420.74), P < .0001; statins alone had an HR of 0.79 (95% CI 0.620.99), P = .0472; and beta‐blockers alone had an HR of 0.80 (95% CI 0.670.94), P = .0183.
Combination Study Drugs and Revised Cardiac Risk Index: Propensity Analysis
We performed the stratified Cox regression adjusted for the propensity scores for each level of RCRI and estimated 2‐year mortality. The use of both a statin and a beta‐blocker compared with using none was still consistently statistically significant, with an RRR of approximately 36% and an NNT of 820 for all levels of patient risk (Table 6). Possibly because of the reduced number of patients in each RCRI category, neither single‐agent study drug compared with none showed a statistically significant decrease in mortality at any level of patient‐specific risk (Table 6). Again, higher‐risk patients benefited most from combination therapy.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.14 | |||
| BB | 288 (73) | 0.11 | 47 | 0.16 | .3778 | |
| Statin | 159 (30) | 0.11 | 40 | 0.19 | .2902 | |
| Statin+BB | 230 (23) | 0.08 | 20 | 0.38 | .0184 | |
| 1 | None | 279 (130) | 0.21 | |||
| BB | 219 (71) | 0.17 | 32 | 0.15 | .2837 | |
| Statin | 143 (41) | 0.17 | 27 | 0.18 | .1969 | |
| Statin+BB | 364 (73) | 0.13 | 14 | 0.37 | .0038 | |
| 2 | None | 155 (100) | 0.29 | |||
| BB | 125 (60) | 0.25 | 24 | 0.15 | .3295 | |
| Statin | 85 (42) | 0.24 | 20 | 0.17 | .2396 | |
| Statin+BB | 233 (72) | 0.18 | 10 | 0.36 | .0077 | |
| 3 | None | 51 (39) | 0.42 | |||
| BB | 53 (29) | 0.37 | 19 | 0.13 | .3553 | |
| Statin | 27 (14) | 0.36 | 16 | 0.15 | .2653 | |
| Statin+BB | 105 (52) | 0.28 | 8 | 0.33 | .0106 | |
Study Drug Timing: Subcohort Analysis
A subcohort analysis was performed to clarify the timing of the study drugs. Of the patients taking statins, 69 of 1346 (5.1%) took the drug before surgery only, 119 of 1346 (8.8%) took the drug after surgery only, and 1158 of 1346 (86%) took the drug both before and after surgery. Of the patients taking beta‐blockers, 54 of 1617 (3.3%) took the drug before surgery only, 397 of 1617 (24.6%) took the drug after surgery only, and 1166 of 1617 (72.1%) took the drug both before and after surgery. The use of statins and beta‐blockers had a correlation of 0.29 (contingency coefficient).
DISCUSSION
In this retrospective observational study we found that after vascular surgery the use of propensity‐adjusted statins compared with no use of statins reduced long‐term mortality over the study period by 22%, with a number needed to treat of 22, and the use of propensity‐adjusted beta‐blockers compared with no use also reduced long‐term mortality, by 16%, with a number needed to treat of 30. There were no statistically significant differences between outcomes of statin users and beta‐blocker users. In addition, using a propensity‐adjusted combination of statin and beta‐blockers compared with using neither decreased mortality overall by 44%, with a number needed to treat of 9. We focused on the use of outpatient drugs 30 days before or after surgery, as the timing of potentially beneficial medications has not been clearly established. Over time, more patients originally categorized as not taking a study drug began taking one, so that by 2 years after surgery, 58% of the patients were taking a statin, and 67% were taking a beta‐blocker, compared with 44% and 53%, respectively, of the study cohort initially. This would have made it more difficult to demonstrate a difference between these 2 groups. As more patients ended up taking the study drugs over time than the originally identified study drug users, and a mortality difference was still demonstrated, there may be an increased advantage in taking the study drugs around the time of surgery. As our focus was on long‐term postoperative mortality, which has not commonly been studied according to the literature, we preferred to also focus on long‐term, chronic ambulatory use of the study drugs. We did perform a subcohort analysis of the timing of study drug use. This confirmed that this cohort predominately comprised long‐term users of the study drugs who took the drug both before and after surgery. This study was not powered to comment on 30‐day mortality.
Perioperative beta‐blockers have been shown in retrospective cohort studies, case‐control studies, randomized clinical trials, meta‐analyses, and systematic reviews to decrease mortality and morbidity after noncardiac surgery. Although recent studies have not shown a benefit for more moderate‐ to low‐risk subjects,11, 12 perioperative beta‐blockers are still considered an indicator of health care quality in the United States.25 At present, perioperative beta‐blockers have an ACC/AHA class I indication (should be administered; Evidence level C) for patients undergoing vascular surgery with a positive stress test, and class IIa indication (reasonable to administer; Evidence level B) for vascular surgery patients with coronary heart disease or multiple clinical risk factors.26 A recent observational study in noncardiac surgery patients demonstrated perioperative beta‐blockers may be most helpful to prevent in‐hospital death after surgery of patients with an RCRI 2 and may be unhelpful or harmful for patients with an RCRI 1.27 Our univariate RCRI findings did not agree, as we found all patients whatever their level of risk benefited from perioperative use of beta‐blockers, alone or in combination. Our study population was older, had a higher RCRI, and underwent comparatively higher‐risk surgery, we were investigating longer‐term outcome, and we concentrated on ambulatory use of beta‐blockers, which may have contributed to the divergence in findings. Our propensity‐adjusted RCRI analysis did not show beta‐blockers associated with any change in mortality at any patient risk level. This may be, in part, because of the reduced number of patients in the RCRI strata. RCRI stratum‐specific analysis is limited by the number of patients and deaths in each RCRI stratum. For example, the power to detect a 2‐year difference of 10% (or 5%) between statin users and nonusers is approximately 99% (66%), 99% (59%), 92% (42%), and 61% (23%) for RCRI = 0, 1, 2, and 3, respectively.
Case‐control and retrospective cohort studies and one randomized clinical trial have shown perioperative statins to decrease either short‐term cardiovascular morbidity or mortality up to 30 days after surgery, and a limited number of retrospective cohort studies have shown reduced mortality for longer‐term follow‐up.1418, 28 There was one previous preliminary study of vascular surgery patients that demonstrated an additive benefit of using statins and beta‐blockers up to 30 days after surgery. This additive effect was only observed in patients with an RCRI 3.29 The results of our longer‐term follow‐up study of a larger cohort did not agree. Compared with patients who did not take a statin or a beta‐blocker, those patients who took both study drugs decreased their relative risk of mortality by approximately 36% in propensity‐ adjusted analysis and by about 50% in univariate analysis, regardless of patient‐specific risk level. For example, in the propensity‐adjusted analysis, the healthiest patients with an RCRI of 0 who took both study drugs had lower mortality than patients who took neither study drug, 8% versus 14%, a 38% relative reduction in mortality, with a number needed to treat of 20 (P = .0184).
In addition, the use of the RCRI for the first time highlighted the divergent long‐term mortality rates for patient‐specific risk levels and the striking long‐term associations of the perioperative use of ambulatory statins, beta‐blockers, and both drugs in combination with improved long‐term mortality. The long‐term use of the study drugs may indeed help all patients with atherosclerotic vascular disease, regardless of surgery. However, vascular surgery presents an opportunity for medical intervention, and our results are most applicable for these patients. In addition, the perioperative state has a unique physiology of acute and intense inflammation and thrombosis. Beta‐blockers and statins have antiadrenergic, anti‐inflammatory, and antithrombotic properties that may be beneficial during this high‐risk state.
Our findings should be viewed with some caution. The use of ICD‐9 codes and demographic data is dependent on the documentation and coding of comorbidities in the medical record and database. The use of statins and beta‐blockers was not random, and patients who took statins and beta‐blockers were different than those who did not. We used rigorous propensity and multivariate analysis, including controlling for clonidine, which has been shown to decrease death after vascular surgery.30 We also controlled for serum albumin level, which has been shown to be a leading predictor of postoperative death.31 We further separately stratified patients by RCRI, as this was a powerful predictor of death in the univariate analysis, but because of the retrospective nature of the study, unmeasured confounders may exist. Only 1% of the study patients were women, which is a limitation of the study. This administrative database is also limited by not having information on tobacco use for 47% of the patients and by not knowing ethnicity for 80% of the patients.
The use of perioperative statins and beta‐blockers used alone or in combination was associated with a reduction in long‐term mortality for vascular surgery patients, and combination use benefited patients at all levels of risk. Higher‐risk patients benefited most by taking both study drugs. These findings extend prior data, add to the natural history of long‐term postoperative outcomes, and also support clinical trials that would evaluate the prospective use of both these medications in vascular surgery patients with attention to patient‐specific risk level. Until the results of 2 randomized controlled trials become available, which may further clarify the use of perioperative statins and beta‐blockers in noncardiac, and noncardiac vascular surgery,13, 32 the use of statins and beta‐blockers should be considered for all patients undergoing vascular surgery. In addition, long‐term use of statins and beta‐blockers for all patients with atherosclerotic vascular disease should be considered.33
Acknowledgements
The authors thank LeAnn Snodgrass for assistance with data extraction and management. This work was funded by the Oregon Health & Science University Medical Research Foundation.
- ,,,,,.The influence of perioperative myocardial infarction on long‐term prognosis following elective vascular surgery.Chest.1998;113:681–686.
- ,,, et al.Postoperative and amputation‐free survival outcomes after femorodistal bypass grafting surgery: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program.J Vasc Surg.2001;34:283–290.
- ,,,.Perioperative‐ and long‐term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population.Anesth Analg1999;89:849–855.
- ,.Very late survival after vascular surgery.J Surg Res.2002;105(2):109–114.
- ,,, et al.Women have increased risk of perioperative myocardial infarction and higher long‐term mortality rates after lower extremity arterial bypass grafting.J Vasc Surg.1999;29:807–812; discussion12–13.
- ,,,,,.Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery.The Study of Perioperative Ischemia Research Group.N Engl J Med.1990;323:1781–1788.
- ,,,,.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:851–857.
- ,,, et al.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—I: Incidence and severity during the 4 day perioperative period.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:843–850.
- ,,,.Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.Multicenter Study of Perioperative Ischemia Research Group.N Engl J Med.1996;335:1713–1720.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.Effect of perioperative beta blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial.BMJ.2006;332:1482.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA2004;291:2092–2099.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion75–6.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,,,,.The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery.Int J Cardiol.2005;104:264–268.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,,.Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration.Ann Epidemiol.1996;6(2):102–109.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,.A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use.Stat Med.2006;25:2084–2106.
- ,,,,.Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care.Health Serv Res2005;40(1):253–78.
- ,,.Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment.Rockville, Md:AHRQ;2001. Report No. 43.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,, et al.Association between long‐term statin use and mortality after successful abdominal aortic aneurysm surgery.Am J Med.2004;116(2):96–103.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,,,,.Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study.Arch Surg.1999;134(1):36–42.
- ,,, et al.Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study.Am Heart J.2004;148:1047–1052.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation.Circulation.2006;113:e463–e654.
Vascular surgery has higher morbidity and mortality than other noncardiac surgeries. Despite the identification of vascular surgery as higher risk, 30‐day mortality for this surgery has remained at 3%10%. Few studies have examined longer‐term outcomes, but higher mortality rates have been reported, for example, 10%30% 6 months after surgery, 20%40% 1 year after surgery, and 30%50% 5 years after surgery.15 Postoperative adverse events have been found to be highly correlated with perioperative ischemia and infarction.68 Perioperative beta‐blockers have been widely studied and have been shown to benefit patients undergoing noncardiac surgery generally and vascular surgery specifically.9, 10 However, 2 recent trials of perioperative beta‐blockers in noncardiac and vascular surgery patients failed to show an association with 18‐month and 30‐day postoperative morbidity and mortality, respectively.11, 12 In addition, the authors of a recent meta‐analysis of perioperative beta‐blockers suggested more studies were needed.13 Furthermore, there have been promising new data on the use of perioperative statins.1418 Finally, as a recent clinical trial of revascularization before vascular surgery did not demonstrate an advantage over medical management, the identification of which perioperative medicines improve postoperative outcomes and in what combinations becomes even more important.19 We sought to ascertain if the ambulatory use of statins and/or beta‐blockers within 30 days of surgery was associated with a reduction in long‐term mortality.
METHODS
Setting and Subjects
We conducted a retrospective cohort study using a regional Department of Veterans Affairs (VA) administrative and relational database, the Consumer Health Information and Performance Sets (CHIPs), which automatically extracts data from electronic medical records of all facilities in the Veterans Integrated Services Network 20, which encompasses Alaska, Washington, Oregon, and Idaho. CHIPs contains information on both outpatient and inpatient environments, and a record is generated for every contact a patient makes with the VA health care system, which includes picking up prescription medications, laboratory values, demographic information, International Classification of Diseases, 9th Revision (ICD‐9), codes, and vital status. In addition, we used the Beneficiary Identification and Records Locator Subsystem database, which is the national VA death index and includes Social Security Administration data that has been shown to be 90%95% complete for assessing vital status.20
Data for all patients who had vascular surgery at 5 VA medical centers in the region from January 1998 to March 2005 was ascertained. If a patient had a second operation within 2 years of the first, the patient was censored at the date of the second operation. A patient was defined as taking a statin or beta‐blocker if a prescription for either of these medications had been picked up within 30 days before or after surgery. The IRB at the Portland VA Medical Center approved the study with a waiver of informed consent.
Data Elements
For every patient we noted the type of vascular surgery (carotid, aortic, lower extremity bypass, or lower extremity amputation), age, sex, comorbid conditions (hypertension, cerebrovascular disease, cancer, diabetes, hyperlipidemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], coronary artery disease [CAD], heart failure), tobacco use, ethnicity, nutritional status (serum albumin), and medication use, defined as filling a prescription within 30 days before surgery (insulin, aspirin, angiotensin‐converting enzyme [ACE] inhibitor, and clonidine). Each patient was assigned a revised cardiac risk index (RCRI) score.21 For each the risk factors: use of insulin, CAD, heart failure, cerebrovascular disease, CKD, and high‐risk surgery (intrathoracic, intraperitoneal, or suprainguinal vascular procedures) 1 point was assigned. These variables were defined according to ICD‐9 codes. CKD was defined as either an ICD‐9 code for CKD or a serum creatinine > 2 mg/dL. Patients were identified by the index vascular surgery using ICD‐9 codes in the CHIPs database, and both inpatient and outpatient data were extracted.
Statistical Analysis
All patients were censored at the point of last contact up to 5 years after surgery to focus on more clinically relevant long‐term outcomes possibly associated with vascular surgery. We conducted 3 separate analyses: (1) statin exposure regardless of beta‐blocker exposure; (2) beta‐blocker exposure regardless of statin exposure, and; (3) combined exposure to statins and beta‐blockers.
Propensity score methods were used to adjust for imbalance in the baseline characteristics between statin users and nonusers, beta‐blocker users and nonusers, and combination statin and beta‐blocker users and nonusers.22, 23 The range of the propensity score distribution was similar in drug users and nonusers in the individual analyses. There was sufficient overlap between the 2 groups in each stratum. To derive propensity scores for the individual drug analyses, statin use and beta‐blocker use were modeled independently with the demographic and clinical variables using stepwise logistic regression with a relaxed entry criterion of = 0.20. Only 1 variable (hyperlipidemia) remained significantly different between statin users and nonusers, and it was included in the subsequent analyses as a potential confounder. The variable albumin had 511 missing values. To keep this variable in the propensity scores, the missing values were replaced by the predicted values of albumin from the multiple linear regression model that included the other demographic variables. The propensity scores were grouped into quintiles and used as a stratification variable in the subsequent analyses. To confirm that the propensity score method reduced the imbalances, the demographic and clinical characteristics of statin and beta‐blocker users and nonusers and combination users and nonusers were compared using Cochran‐Mantel‐Haenzel tests with the respective propensity score as a stratification variable.
For the combined use of both study drugs, we performed univariate analysis with adjustment only for RCRI (as this was a powerful predictor of mortality in our dataset; Table 1) as well as a propensity score analysis in an exploratory manner. There have been limited applications of propensity score methods to multiple treatment groups. Similar to that in the study by Huang et al.,24 we developed a multinomial baseline response logit model to obtain 3 separate propensity scores (statin only vs. none, beta‐blocker only vs. none, and both vs. none). Because of the limited sample size, the data were stratified according to the median split of each propensity score. Each score had similar ranges for each treatment group. All but 5 variables (CAD, hypertension, hyperlipidemia, ACE inhibitor use, and type of surgery) were balanced after accounting for strata. These 5 variables were then included in the final stratified Cox regression model as potential confounders.
| Variable | Level | N (%) Overall N = 3062 | Hazard ratio (95% CI) | Chi‐square P value |
|---|---|---|---|---|
| ||||
| Age in years, median (IQR) | 67 (5974) | 1.04 (1.04, 1.05)a | <.0001 | |
| Sex | Female | 45 (1) | 0.89 (0.53, 1.51) | .6704 |
| Male | 3017 (99) | 1 | 1.0000 | |
| Preoperative medical conditions | HTN | 2415 (79) | 1.32 (1.13, 1.55) | .0006 |
| CVA/TIA | 589 (19) | 1.05 (0.90, 1.22) | .5753 | |
| CA | 679 (22) | 1.55 (1.36, 1.78) | <.0001 | |
| DM | 1474 (48) | 1.75 (1.54, 1.98) | <.0001 | |
| Lipid | 872 (28) | 0.84 (0.74, 0.97) | .0187 | |
| COPD | 913 (30) | 1.68 (1.48, 1.90) | <.0001 | |
| CAD | 1491 (49) | 1.46 (1.29, 1.66) | <.0001 | |
| CHF | 747 (24) | 2.44 (2.15, 2.77) | <.0001 | |
| CKD | 443 (14) | 2.32 (2.00, 2.69) | <.0001 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 2.73 (2.28, 3.28) | <.0001 |
| Albumin 3.5 | 596 (23) | 2.70 (2.35, 3.10) | <.0001 | |
| Medication use | Aspirin | 1789 (58) | 1.10 (0.97, 1.25) | .1389 |
| ACE inhibitor | 1250 (41) | 0.93 (0.82, 1.06) | .2894 | |
| Insulin | 478 (16) | 1.31 (1.12, 1.54) | .0007 | |
| Clonidine | 115 (4) | 1.68 (1.29, 2.20) | .0001 | |
| Perioperative medication | Statinb | 1346 (44) | 0.66 (0.58, 0.75) | <.0001 |
| Beta‐blockerc | 1617 (53) | 0.74 (0.66, 0.84) | <.0001 | |
| Statin only | 414 (14) | 0.69 (0.56, 0.84) | .0002 | |
| Beta‐blocker only | 685 (22) | 0.81 (0.69, 0.95) | .0079 | |
| Statin and beta‐blocker | 932 (30) | 0.57 (0.49, 0.67) | <.0001 | |
| Noned | 1031 (34) | 1 | 1.0000 | |
| Type of surgery | Aorta | 232 (8) | 1.34 (1.01, 1.77) | <.0001 |
| Carotid | 875 (29) | 1 | ||
| Amputation | 867 (28) | 2.80 (2.36, 3.32) | ||
| Bypass | 1088 (36) | 1.57 (1.32, 1.87) | ||
| RCRI | 0 | 1223 (40) | 1 | <.0001 |
| 1 | 1005 (33) | 1.33 (1.13, 1.55) | ||
| 2 | 598 (20) | 2.22 (1.88, 2.62) | ||
| 3 | 200 (7) | 3.16 (2.54, 3.93) | ||
| 4 | 36 (1) | 4.82 (3.15, 7.37) | ||
| Year surgery occurred | 1998 | 544 (18) | 1 | .6509 |
| 1999 | 463 (15) | 0.91 (0.75, 1.10) | ||
| 2000 | 420 (14) | 0.93 (0.77, 1.13) | ||
| 2001 | 407 (13) | 0.93 (0.75, 1.14) | ||
| 2002 | 374 (12) | 1.12 (0.90, 1.40) | ||
| 2003 | 371 (12) | 1.15 (0.90, 1.47) | ||
| 2004 | 407 (13) | 0.97 (0.72, 1.31) | ||
| 2005 | 76 (3) | 0.68 (0.28, 1.65) | ||
| Tobacco user | Yes | 971 (32) | 0.90 (0.76, 1.08) | .4762 |
| No | 649 (21) | 1 | ||
| Null | 1442 (47) | 0.96 (0.81, 1.13) | ||
| Ethnicity | White | 563 (18) | 1 | .0366 |
| Other | 39 (1) | 0.98 (0.55, 1.76) | ||
| Unknown | 2460 (80) | 1.24 (1.05, 1.46) | ||
To comment on patient‐specific risk by stratification with the RCRI, we used a fixed time point of the 2‐year mortality estimated from the Cox regression model to analyze use of study drugs singly or in combination compared with use of neither.
Chi‐square tests were used to categorize and compare demographic and clinical characteristics of statin users and nonusers, of beta‐blocker users and nonusers, and combination users and nonusers. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Stratified or unstratified Cox regression was used to estimate the hazard ratios of statins and beta‐blockers, with or without adjustment for the propensity score. All analyses were performed using SAS (Statistical Analysis System) software, version 9.1.
RESULTS
Patient Characteristics
The study included 3062 patients whose median age was 67 (interquartile range, 5974; Table 1). Ninety‐nine percent of the study patients were men. Overall, ambulatory use of statins and beta‐blockers was found in 44% and 53% of patients, respectively, and combination use occurred in 30%. Sixty‐one percent of patients had an RCRI of 1 or greater; among them 71% were statin users (Table 2), 68% were beta‐blocker users (Table 3), and 75% were combination users (Table 4). Sixty‐four percent of surgeries were either lower extremity bypass or amputation, 29% were carotid, and 8% aortic. Median follow‐up for all patients was 2.7 years (interquartile range, 1.24.6). Of the whole study cohort, 53% and 62% filled a prescription for a statin or beta‐blocker within 1 year of surgery, respectively, and 58% and 67% filled a prescription within 2 years of surgery, respectively. Overall mortality at 30 days was 3%, at 1 year 14%, and at 2 years 22%.
| Variable, N (%) | Level | Overall (N = 3062) | Statin users (N = 1346 [44]) | Statin nonusers (N = 1716 [56]) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 66 (5973) | 68 (6075) | <.0001 | .9934 | |
| Sex | Female | 45 (1) | 15 (1) | 30 (2) | .1480 | .7822 |
| Male | 3017 (99) | 1331 (99) | 1686 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1176 (87) | 1239 (72) | <.0001 | .2984 |
| CVA/TIA | 589 (19) | 328 (24) | 261 (15) | <.0001 | .3935 | |
| CA | 679 (22) | 307 (23) | 372 (22) | .4550 | .8404 | |
| DM | 1474 (48) | 666 (49) | 808 (47) | .1883 | .5504 | |
| Lipid | 872 (28) | 629 (47) | 243 (14) | <.0001 | .0246 | |
| COPD | 913 (30) | 411 (31) | 502 (29) | .4419 | .8435 | |
| CAD | 1491 (49) | 837 (62) | 654 (38) | <.0001 | .4720 | |
| CHF | 747 (24) | 370 (27) | 377 (22) | .0004 | .4839 | |
| CKD | 443 (14) | 208 (15) | 235 (14) | .1698 | .9990 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 101 (8) | 128 (7) | .9629 | .6911 |
| Albumin 3.5 | 596 (23) | 191 (16) | 405 (30) | <.0001 | .5917 | |
| Medication use | Aspirin | 1789 (58) | 904 (67) | 885 (52) | <.0001 | .6409 |
| Ace inhibitor | 1250 (41) | 712 (53) | 538 (31) | <.0001 | .6075 | |
| Beta‐blocker | 1220 (40) | 767 (57) | 453 (26) | <.0001 | .4058 | |
| Insulin | 478 (16) | 254 (19) | 224 (13) | <.0001 | .7919 | |
| Clonidine | 115 (4) | 61 (5) | 54 (3) | .0454 | .6141 | |
| Type of surgery | Aorta | 232 (8) | 106 (8) | 126 (7) | <.0001 | .9899 |
| Carotid | 875 (29) | 510 (38) | 365 (21) | |||
| Amputation | 867 (28) | 274 (20) | 593 (35) | |||
| Bypass | 1088 (36) | 456 (34) | 632 (37) | |||
| RCRI | 0 | 1223 (40) | 389 (29) | 834 (49) | <.0001 | .9831 |
| 1 | 1005 (33) | 507 (38) | 498 (29) | |||
| 2 | 598 (20) | 318 (24) | 280 (16) | |||
| 3 | 200 (7) | 109 (8) | 91 (5) | |||
| 4 | 36 (1) | 23 (1) | 13 (0.76) | |||
| Year of surgery | 1998 | 544 (18) | 134 (10) | 410 (24) | <.0001 | 1 |
| 1999 | 463 (15) | 163 (12) | 300 (17) | |||
| 2000 | 420 (13) | 178 (13) | 242 (14) | |||
| 2001 | 407 (13) | 188 (14) | 219 (13) | |||
| 2002 | 374 (12) | 194 (14) | 180 (10) | |||
| 2003 | 371 (12) | 209 (16) | 162 (9) | |||
| 2004 | 407 (13) | 229 (17) | 178 (10) | |||
| 2005 | 76 (3) | 51 (4) | 25 (1.5) | |||
| Tobacco user | Yes | 971 (32) | 494 (37) | 477 (28) | <.0001 | .9809 |
| No | 649 (21) | 335 (25) | 314 (18) | |||
| Null | 1442 (47) | 517 (38) | 925 (54) | |||
| Ethnicity | White | 563 (18) | 263 (20) | 300 (17) | .1544 | .9475 |
| Other | 39 (1) | 13 (1) | 26 (1.5) | |||
| Unknown | 2460 (80) | 1070 (79) | 1390 (81) | |||
| Variable, N (%) | Level | Overall N = 3062 | BB users N = 1617 (53) | Non‐BB users N = 1445 (47) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 67 (5975) | 68 (6076) | .0526 | .7671 | |
| Sex | Female | 45 (1) | 12 (1) | 33 (2) | .0004 | .585 |
| Male | 3017 (99) | 1605 (99) | 1412 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1398 (86) | 1017 (70) | <.0001 | .1837 |
| CVA/TIA | 589 (19) | 364 (23) | 225 (16) | <.0001 | .3206 | |
| CA | 679 (22) | 359 (22) | 320 (22) | .9701 | .4288 | |
| DM | 1474 (48) | 739 (46) | 735 (51) | .0043 | .6329 | |
| Lipid | 872 (28) | 555 (34) | 317 (22) | <.0001 | .7180 | |
| COPD | 913 (30) | 487 (30) | 426 (29) | .7007 | .8022 | |
| CAD | 1491 (49) | 975 (60) | 516 (36) | <.0001 | .3496 | |
| CHF | 747 (24) | 439 (27) | 308 (21) | .0002 | .6509 | |
| CKD | 443 (14) | 248 (15) | 195 (13) | .1480 | .8544 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 132 (8) | 97 (7) | .1277 | .5867 |
| Albumin 3.5 | 596 (23) | 252 (18) | 344 (30) | <.0001 | .5347 | |
| Medication use | Aspirin | 1789 (58) | 1046 (65) | 743 (51) | <.0001 | .4942 |
| Ace inhibitor | 1250 (41) | 760 (47) | 490 (34) | <.0001 | .4727 | |
| Statin | 1220 (40) | 932 (58) | 414 (29) | <.0001 | .3706 | |
| Insulin | 478 (16) | 255 (16) | 223 (15) | .7973 | .5991 | |
| Clonidine | 115 (4) | 77 (5) | 38 (3) | .0019 | .8241 | |
| Type of surgery | Aorta | 232 (8) | 176 (11) | 56 (4) | <.0001 | .5664 |
| Carotid | 875 (29) | 515 (32) | 360 (25) | |||
| Amputation | 867 (28) | 339 (21) | 528 (37) | |||
| Bypass | 1088 (36) | 587 (36) | 501 (35) | |||
| RCRI | 0 | 1223 (40) | 518 (32) | 705 (49) | <.0001 | .5489 |
| 1 | 1005 (33) | 583 (36) | 422 (29) | |||
| 2 | 598 (20) | 358 (22) | 240 (17) | |||
| 3 | 200 (7) | 130 (8) | 70 (5) | |||
| 4 | 36 (1) | 28 (2) | 8 (1) | |||
| Year of surgery | 1998 | 544 (18) | 200 (12) | 344 (24) | <.0001 | .3832 |
| 1999 | 463 (15) | 211 (13) | 252 (17) | |||
| 2000 | 420 (13) | 210 (13) | 210 (15) | |||
| 2001 | 407 (13) | 209 (13) | 198 (14) | |||
| 2002 | 374 (12) | 220 (14) | 154 (11) | |||
| 2003 | 371 (12) | 238 (15) | 133 (9) | |||
| 2004 | 407 (13) | 279 (17) | 128 (9) | |||
| 2005 | 76 (3) | 50 (3) | 26 (2) | |||
| Tobacco user | Yes | 971 (32) | 569 (35) | 402 (28) | <.0001 | .9025 |
| No | 649 (21) | 370 (23) | 279 (19) | |||
| Null | 1442 (47) | 678 (42) | 764 (53) | |||
| Ethnicity | White | 563 (18) | 309 (19) | 254 (18) | .4962 | .8762 |
| Other | 39 (1) | 19 (1) | 20 (1) | |||
| Unknown | 2460 (80) | 1289 (80) | 1171 (81) | |||
| N (%) Variable | Level | Overall N = 3062 | BB alone N = 685 (22) | Statin alone N = 414 (14) | Both drugs N = 932 (30) | Neither drug N = 1031 (34) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age in years, median (IQR) | 67 (5974) | 68 (6075) | 67 (6075) | 66 (5973) | 69 (6076) | .0029 | .9824 | |
| Sex | Female | 45 (1) | 7 (1) | 10 (2) | 5 (1) | 23 (2) | .0042 | .5815 |
| Male | 3017 (99) | 678 (99) | 404 (98) | 927 (99) | 1008 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 560 (82) | 338 (82) | 838 (90) | 679 (66) | <.0001 | .0251 |
| CVA/TIA | 589 (19) | 127 (19) | 91 (22) | 237 (25) | 134 (13) | <.0001 | .4543 | |
| CA | 679 (22) | 150 (22) | 98 (24) | 209 (22) | 222 (22) | .8379 | .9749 | |
| DM | 1474 (48) | 291 (43) | 218 (53) | 448 (48) | 517 (50) | .0031 | .3943 | |
| Lipid | 872 (28) | 125 (18) | 199 (48) | 430 (46) | 118 (11) | <.0001 | <.0001 | |
| COPD | 913 (30) | 199 (29) | 123 (30) | 288 (9) | 303 (29) | .8475. | .9769 | |
| CAD | 1491 (49) | 327 (48) | 189 (46) | 648 (70) | 327 (32) | <.0001 | <.0001 | |
| CHF | 747 (24) | 163 (24) | 94 (23) | 276 (30) | 214 (21) | <.0001 | .7031 | |
| CKD | 443 (14) | 92 (13) | 52 (13) | 156 (17) | 143 (14) | .1120 | .8364 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 52 (8) | 21 (5) | 80 (9) | 76 (7) | .1619 | .7184 |
| Albumin 3.5 | 596 (23) | 134 (20) | 73 (20) | 118 (14) | 271 (34) | <.0001 | .2846 | |
| Medication use | Aspirin | 1789 (58) | 398 (58) | 256 (62) | 648 (70) | 487 (47) | <.0001 | .2334 |
| Ace inhibitor | 1250 (41) | 264 (39) | 216 (52) | 496 (53) | 274 (27) | <.0001 | .0216 | |
| Insulin | 478 (16) | 93 (14) | 92 (22) | 162 (17) | 131 (13) | <.0001 | .2952 | |
| Clonidine | 115 (4) | 28 (4) | 12 (3) | 49 (5) | 26 (3) | .0107 | .8035 | |
| Type of surgery | Aorta | 232 (8) | 78 (11) | 8 (2) | 98 (11) | 48 (5) | <.0001 | .008 |
| Carotid | 875 (29) | 165 (24) | 160 (39) | 350 (38) | 200 (19) | |||
| Amputation | 867 (28) | 164 (24) | 99 (24) | 175 (19) | 429 (42) | |||
| Bypass | 1088 (36) | 278 (41) | 147 (36) | 309 (33) | 354 (34) | |||
| RCRI | 0 | 1223 (40) | 288 (42) | 159 (38) | 230 (25) | 546 (53) | <.0001 | .5392 |
| 1 | 1005 (33) | 219 (32) | 143 (35) | 364 (39) | 279 (27) | |||
| 2 | 598 (20) | 125 (18) | 85 (21) | 233 (25) | 155 (15) | |||
| 3 | 200 (7) | 46 (7) | 25 (6) | 84 (9) | 45 (4) | |||
| 4 | 36 (1) | 7 (1) | 2 (0) | 21 (2) | 6 (1) | |||
| Year of surgery | 1998 | 544 (18) | 126 (18) | 60 (14) | 74 (8) | 284 (28) | <.0001 | .3105 |
| 1999 | 463 (15) | 111 (16) | 63 (15) | 100 (11) | 189 (18) | |||
| 2000 | 420 (13) | 87 (13) | 55 (13) | 123 (13) | 155 (15) | |||
| 2001 | 407 (13) | 84 (12) | 63 (15) | 125 (13) | 135 (13) | |||
| 2002 | 374 (12) | 81 (12) | 55 (13) | 139 (15) | 99 (10 | |||
| 2003 | 371 (12) | 85 (13) | 56 (14) | 153 (16) | 77 (7) | |||
| 2004 | 407 (13) | 96 (14) | 46 (11) | 183 (20) | 82 (8) | |||
| 2005 | 76 (3) | 15 (2) | 16 (4) | 35 (4) | 10 (1) | |||
| Tobacco user | Yes | 971 (32) | 227 (33) | 152 (37) | 342 (37) | 250 (24) | <.0001 | .3914 |
| No | 649 (21) | 134 (20) | 99 (24) | 236 (25) | 180 (17) | |||
| Null | 1442 (47) | 324 (47) | 163 (39) | 354 (38) | 601 (58) | |||
| Ethnicity | White | 563 (18) | 115 (17) | 69 (17) | 194 (21) | 185 (18) | .2821 | .9771 |
| Other | 39 (1) | 10 (1) | 4 (1) | 9 (1) | 16 (2) | |||
| Unknown | 2460 (80) | 560 (82) | 341 (82) | 729 (78) | 830 (81) | |||
Univariate Survival Analysis
Univariate Cox regression analysis revealed a strong effect of the composite RCRI, which was predictive of mortality in a linear fashion over the course of the study compared with an RCRI of 0 (Table 1). Univariate analysis showed significant associations with decreased mortality for statins (hazard ratio [HR] = 0.66 [95% CI 0.580.75], P < .0001) and beta‐blockers (HR = 0.74 [95% CI 0.660.84], P = .0001); see Table 1. Of note, compared with that in 1998, mortality did not change for all the years for which data were complete. In addition, compared with taking neither study drug, taking a statin only, a beta‐blocker only, or both was associated with decreased mortality (P = .0002, P = .0079, and P < .0001, respectively; Fig. 1).

Propensity Score Analysis for Single Study Drug
There were significant differences in demographic and clinical characteristics between statin‐users versus statin nonusers, and between beta‐blocker users versus beta‐blocker nonusers. These differences became insignificant after the propensity score adjustment, with the exception of hyperlipidemia for statins, P = .02, which was added to the model as a confounder (Table 2). The distribution of the propensity scores was similar for study drug users and nonusers within each stratum. The association with decreased mortality remained significant after adjusting for propensity score (for statins, HR = 0.78 [95% CI 0.670.92, P = .0050], number needed to treat [NNT] = 22; for beta‐blockers HR = 0.84 [95% CI 0.730.96, P = .0103], NNT = 30; Fig. 2).

Combination Study Drugs and Revised Cardiac Risk Index: Univariate Analysis
We wanted our results to closely model those of combination use of the study drugs by patients in a clinical situation. Therefore, we first examined the effects of ambulatory statins alone, beta‐blockers alone, and a combination of statins and beta‐blockers by univariate analysis. Grouping patients by study drug use has not commonly been reported in the literature. We also examined the statistical interaction between the study drugs and the RCRI. The main‐effects model adequately explained all‐cause mortality, and the statistical interaction between the study drugs and the RCRI was not significant.
The final univariate Cox regression model, which compared use of a statin alone, a beta‐blocker alone, and a statin and beta‐blocker in combination with using neither study drug, demonstrated that the combination of statins and beta‐blockers had an HR over the whole study period of 0.43 (95% CI 0.360.51, P < .0001), statins alone had an HR of 0.59 (95% CI 0.480.72, P < .0001), and beta‐blockers alone had an HR of 0.71 (95% CI 0.610.83, P < .0001).
To clarify the effects of the study drugs on patients at different levels of risk, we stratified patients by the RCRI and evaluated the effects of the study drugs on mortality at 2 years, comparing the results to a referent of taking no study drugs. The use of both a statin and a beta‐blocker consistently produced a relative risk reduction (RRR) of approximately 50% with an NNT of 410, with highly statistically significant results for patients at all levels of risk (Table 5). As patient risk level increased, the NNT decreased, consistent with higher‐risk patients benefiting most from combination therapy with statins and beta‐blockers.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.19 | |||
| BB | 288 (73) | 0.14 | 20 | 0.27 | .0023 | |
| Statin | 159 (30) | 0.12 | 14 | 0.39 | <.0001 | |
| Statin+BB | 230 (23) | 0.09 | 10 | 0.54 | <.0001 | |
| 1 | None | 279 (130) | 0.28 | |||
| BB | 219 (71) | 0.21 | 14 | 0.26 | .0028 | |
| Statin | 143 (41) | 0.17 | 10 | 0.37 | <.0001 | |
| Statin+BB | 364 (73) | 0.13 | 7 | 0.53 | <.0001 | |
| 2 | None | 155 (100) | 0.43 | |||
| BB | 125 (60) | 0.33 | 10 | 0.23 | .0045 | |
| Statin | 85 (42) | 0.28 | 7 | 0.35 | <.0001 | |
| Statin+BB | 233 (72) | 0.22 | 5 | 0.50 | <.0001 | |
| 3 | None | 51 (39) | 0.59 | |||
| BB | 53 (29) | 0.47 | 9 | 0.20 | .0296 | |
| Statin | 27 (14) | 0.41 | 6 | 0.31 | .0014 | |
| Statin+BB | 105 (52) | 0.32 | 4 | 0.46 | <.0001 | |
In addition, the range of outcomes can be clearly seen for both patient‐specific risk level and study drug use. For example, overall mortality at 2 years for all patients was 22%. For the study drugs, mortality ranged from 16% for patient who used both a statin and a beta‐blocker to 27% for those patients who used neither study drug. The use of the RCRI showed that the healthiest patients who were taking both a statin and a beta‐blocker did the best, with a 2‐year mortality of 9%, compared with the sickest patients who were taking neither study drug, whose 2‐year mortality was 59%. Use of both study drugs by the sickest patients was associated with a reduction in 2‐year mortality to 32% (P < .0001; Table 5).
Propensity Score Analysis of Use of Combination Study Drugs
Because there was very limited literature to guide us in the use of propensity score analysis of multiple treatment groups, we performed these analyses in an exploratory manner. There were significant differences between combination statin and beta‐blocker users and nonusers. These differences became insignificant after adjusting for propensity score, except for the 5 variables previously mentioned, which were added to the model as potential confounders (Table 4). The propensity‐adjusted Cox regression model comparing use of each study drug alone and in combination with taking neither over the whole study period still showed an association with decreased mortality. The combination of statins and beta‐blockers had an HR of 0.56 (95% CI 0.420.74), P < .0001; statins alone had an HR of 0.79 (95% CI 0.620.99), P = .0472; and beta‐blockers alone had an HR of 0.80 (95% CI 0.670.94), P = .0183.
Combination Study Drugs and Revised Cardiac Risk Index: Propensity Analysis
We performed the stratified Cox regression adjusted for the propensity scores for each level of RCRI and estimated 2‐year mortality. The use of both a statin and a beta‐blocker compared with using none was still consistently statistically significant, with an RRR of approximately 36% and an NNT of 820 for all levels of patient risk (Table 6). Possibly because of the reduced number of patients in each RCRI category, neither single‐agent study drug compared with none showed a statistically significant decrease in mortality at any level of patient‐specific risk (Table 6). Again, higher‐risk patients benefited most from combination therapy.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.14 | |||
| BB | 288 (73) | 0.11 | 47 | 0.16 | .3778 | |
| Statin | 159 (30) | 0.11 | 40 | 0.19 | .2902 | |
| Statin+BB | 230 (23) | 0.08 | 20 | 0.38 | .0184 | |
| 1 | None | 279 (130) | 0.21 | |||
| BB | 219 (71) | 0.17 | 32 | 0.15 | .2837 | |
| Statin | 143 (41) | 0.17 | 27 | 0.18 | .1969 | |
| Statin+BB | 364 (73) | 0.13 | 14 | 0.37 | .0038 | |
| 2 | None | 155 (100) | 0.29 | |||
| BB | 125 (60) | 0.25 | 24 | 0.15 | .3295 | |
| Statin | 85 (42) | 0.24 | 20 | 0.17 | .2396 | |
| Statin+BB | 233 (72) | 0.18 | 10 | 0.36 | .0077 | |
| 3 | None | 51 (39) | 0.42 | |||
| BB | 53 (29) | 0.37 | 19 | 0.13 | .3553 | |
| Statin | 27 (14) | 0.36 | 16 | 0.15 | .2653 | |
| Statin+BB | 105 (52) | 0.28 | 8 | 0.33 | .0106 | |
Study Drug Timing: Subcohort Analysis
A subcohort analysis was performed to clarify the timing of the study drugs. Of the patients taking statins, 69 of 1346 (5.1%) took the drug before surgery only, 119 of 1346 (8.8%) took the drug after surgery only, and 1158 of 1346 (86%) took the drug both before and after surgery. Of the patients taking beta‐blockers, 54 of 1617 (3.3%) took the drug before surgery only, 397 of 1617 (24.6%) took the drug after surgery only, and 1166 of 1617 (72.1%) took the drug both before and after surgery. The use of statins and beta‐blockers had a correlation of 0.29 (contingency coefficient).
DISCUSSION
In this retrospective observational study we found that after vascular surgery the use of propensity‐adjusted statins compared with no use of statins reduced long‐term mortality over the study period by 22%, with a number needed to treat of 22, and the use of propensity‐adjusted beta‐blockers compared with no use also reduced long‐term mortality, by 16%, with a number needed to treat of 30. There were no statistically significant differences between outcomes of statin users and beta‐blocker users. In addition, using a propensity‐adjusted combination of statin and beta‐blockers compared with using neither decreased mortality overall by 44%, with a number needed to treat of 9. We focused on the use of outpatient drugs 30 days before or after surgery, as the timing of potentially beneficial medications has not been clearly established. Over time, more patients originally categorized as not taking a study drug began taking one, so that by 2 years after surgery, 58% of the patients were taking a statin, and 67% were taking a beta‐blocker, compared with 44% and 53%, respectively, of the study cohort initially. This would have made it more difficult to demonstrate a difference between these 2 groups. As more patients ended up taking the study drugs over time than the originally identified study drug users, and a mortality difference was still demonstrated, there may be an increased advantage in taking the study drugs around the time of surgery. As our focus was on long‐term postoperative mortality, which has not commonly been studied according to the literature, we preferred to also focus on long‐term, chronic ambulatory use of the study drugs. We did perform a subcohort analysis of the timing of study drug use. This confirmed that this cohort predominately comprised long‐term users of the study drugs who took the drug both before and after surgery. This study was not powered to comment on 30‐day mortality.
Perioperative beta‐blockers have been shown in retrospective cohort studies, case‐control studies, randomized clinical trials, meta‐analyses, and systematic reviews to decrease mortality and morbidity after noncardiac surgery. Although recent studies have not shown a benefit for more moderate‐ to low‐risk subjects,11, 12 perioperative beta‐blockers are still considered an indicator of health care quality in the United States.25 At present, perioperative beta‐blockers have an ACC/AHA class I indication (should be administered; Evidence level C) for patients undergoing vascular surgery with a positive stress test, and class IIa indication (reasonable to administer; Evidence level B) for vascular surgery patients with coronary heart disease or multiple clinical risk factors.26 A recent observational study in noncardiac surgery patients demonstrated perioperative beta‐blockers may be most helpful to prevent in‐hospital death after surgery of patients with an RCRI 2 and may be unhelpful or harmful for patients with an RCRI 1.27 Our univariate RCRI findings did not agree, as we found all patients whatever their level of risk benefited from perioperative use of beta‐blockers, alone or in combination. Our study population was older, had a higher RCRI, and underwent comparatively higher‐risk surgery, we were investigating longer‐term outcome, and we concentrated on ambulatory use of beta‐blockers, which may have contributed to the divergence in findings. Our propensity‐adjusted RCRI analysis did not show beta‐blockers associated with any change in mortality at any patient risk level. This may be, in part, because of the reduced number of patients in the RCRI strata. RCRI stratum‐specific analysis is limited by the number of patients and deaths in each RCRI stratum. For example, the power to detect a 2‐year difference of 10% (or 5%) between statin users and nonusers is approximately 99% (66%), 99% (59%), 92% (42%), and 61% (23%) for RCRI = 0, 1, 2, and 3, respectively.
Case‐control and retrospective cohort studies and one randomized clinical trial have shown perioperative statins to decrease either short‐term cardiovascular morbidity or mortality up to 30 days after surgery, and a limited number of retrospective cohort studies have shown reduced mortality for longer‐term follow‐up.1418, 28 There was one previous preliminary study of vascular surgery patients that demonstrated an additive benefit of using statins and beta‐blockers up to 30 days after surgery. This additive effect was only observed in patients with an RCRI 3.29 The results of our longer‐term follow‐up study of a larger cohort did not agree. Compared with patients who did not take a statin or a beta‐blocker, those patients who took both study drugs decreased their relative risk of mortality by approximately 36% in propensity‐ adjusted analysis and by about 50% in univariate analysis, regardless of patient‐specific risk level. For example, in the propensity‐adjusted analysis, the healthiest patients with an RCRI of 0 who took both study drugs had lower mortality than patients who took neither study drug, 8% versus 14%, a 38% relative reduction in mortality, with a number needed to treat of 20 (P = .0184).
In addition, the use of the RCRI for the first time highlighted the divergent long‐term mortality rates for patient‐specific risk levels and the striking long‐term associations of the perioperative use of ambulatory statins, beta‐blockers, and both drugs in combination with improved long‐term mortality. The long‐term use of the study drugs may indeed help all patients with atherosclerotic vascular disease, regardless of surgery. However, vascular surgery presents an opportunity for medical intervention, and our results are most applicable for these patients. In addition, the perioperative state has a unique physiology of acute and intense inflammation and thrombosis. Beta‐blockers and statins have antiadrenergic, anti‐inflammatory, and antithrombotic properties that may be beneficial during this high‐risk state.
Our findings should be viewed with some caution. The use of ICD‐9 codes and demographic data is dependent on the documentation and coding of comorbidities in the medical record and database. The use of statins and beta‐blockers was not random, and patients who took statins and beta‐blockers were different than those who did not. We used rigorous propensity and multivariate analysis, including controlling for clonidine, which has been shown to decrease death after vascular surgery.30 We also controlled for serum albumin level, which has been shown to be a leading predictor of postoperative death.31 We further separately stratified patients by RCRI, as this was a powerful predictor of death in the univariate analysis, but because of the retrospective nature of the study, unmeasured confounders may exist. Only 1% of the study patients were women, which is a limitation of the study. This administrative database is also limited by not having information on tobacco use for 47% of the patients and by not knowing ethnicity for 80% of the patients.
The use of perioperative statins and beta‐blockers used alone or in combination was associated with a reduction in long‐term mortality for vascular surgery patients, and combination use benefited patients at all levels of risk. Higher‐risk patients benefited most by taking both study drugs. These findings extend prior data, add to the natural history of long‐term postoperative outcomes, and also support clinical trials that would evaluate the prospective use of both these medications in vascular surgery patients with attention to patient‐specific risk level. Until the results of 2 randomized controlled trials become available, which may further clarify the use of perioperative statins and beta‐blockers in noncardiac, and noncardiac vascular surgery,13, 32 the use of statins and beta‐blockers should be considered for all patients undergoing vascular surgery. In addition, long‐term use of statins and beta‐blockers for all patients with atherosclerotic vascular disease should be considered.33
Acknowledgements
The authors thank LeAnn Snodgrass for assistance with data extraction and management. This work was funded by the Oregon Health & Science University Medical Research Foundation.
Vascular surgery has higher morbidity and mortality than other noncardiac surgeries. Despite the identification of vascular surgery as higher risk, 30‐day mortality for this surgery has remained at 3%10%. Few studies have examined longer‐term outcomes, but higher mortality rates have been reported, for example, 10%30% 6 months after surgery, 20%40% 1 year after surgery, and 30%50% 5 years after surgery.15 Postoperative adverse events have been found to be highly correlated with perioperative ischemia and infarction.68 Perioperative beta‐blockers have been widely studied and have been shown to benefit patients undergoing noncardiac surgery generally and vascular surgery specifically.9, 10 However, 2 recent trials of perioperative beta‐blockers in noncardiac and vascular surgery patients failed to show an association with 18‐month and 30‐day postoperative morbidity and mortality, respectively.11, 12 In addition, the authors of a recent meta‐analysis of perioperative beta‐blockers suggested more studies were needed.13 Furthermore, there have been promising new data on the use of perioperative statins.1418 Finally, as a recent clinical trial of revascularization before vascular surgery did not demonstrate an advantage over medical management, the identification of which perioperative medicines improve postoperative outcomes and in what combinations becomes even more important.19 We sought to ascertain if the ambulatory use of statins and/or beta‐blockers within 30 days of surgery was associated with a reduction in long‐term mortality.
METHODS
Setting and Subjects
We conducted a retrospective cohort study using a regional Department of Veterans Affairs (VA) administrative and relational database, the Consumer Health Information and Performance Sets (CHIPs), which automatically extracts data from electronic medical records of all facilities in the Veterans Integrated Services Network 20, which encompasses Alaska, Washington, Oregon, and Idaho. CHIPs contains information on both outpatient and inpatient environments, and a record is generated for every contact a patient makes with the VA health care system, which includes picking up prescription medications, laboratory values, demographic information, International Classification of Diseases, 9th Revision (ICD‐9), codes, and vital status. In addition, we used the Beneficiary Identification and Records Locator Subsystem database, which is the national VA death index and includes Social Security Administration data that has been shown to be 90%95% complete for assessing vital status.20
Data for all patients who had vascular surgery at 5 VA medical centers in the region from January 1998 to March 2005 was ascertained. If a patient had a second operation within 2 years of the first, the patient was censored at the date of the second operation. A patient was defined as taking a statin or beta‐blocker if a prescription for either of these medications had been picked up within 30 days before or after surgery. The IRB at the Portland VA Medical Center approved the study with a waiver of informed consent.
Data Elements
For every patient we noted the type of vascular surgery (carotid, aortic, lower extremity bypass, or lower extremity amputation), age, sex, comorbid conditions (hypertension, cerebrovascular disease, cancer, diabetes, hyperlipidemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], coronary artery disease [CAD], heart failure), tobacco use, ethnicity, nutritional status (serum albumin), and medication use, defined as filling a prescription within 30 days before surgery (insulin, aspirin, angiotensin‐converting enzyme [ACE] inhibitor, and clonidine). Each patient was assigned a revised cardiac risk index (RCRI) score.21 For each the risk factors: use of insulin, CAD, heart failure, cerebrovascular disease, CKD, and high‐risk surgery (intrathoracic, intraperitoneal, or suprainguinal vascular procedures) 1 point was assigned. These variables were defined according to ICD‐9 codes. CKD was defined as either an ICD‐9 code for CKD or a serum creatinine > 2 mg/dL. Patients were identified by the index vascular surgery using ICD‐9 codes in the CHIPs database, and both inpatient and outpatient data were extracted.
Statistical Analysis
All patients were censored at the point of last contact up to 5 years after surgery to focus on more clinically relevant long‐term outcomes possibly associated with vascular surgery. We conducted 3 separate analyses: (1) statin exposure regardless of beta‐blocker exposure; (2) beta‐blocker exposure regardless of statin exposure, and; (3) combined exposure to statins and beta‐blockers.
Propensity score methods were used to adjust for imbalance in the baseline characteristics between statin users and nonusers, beta‐blocker users and nonusers, and combination statin and beta‐blocker users and nonusers.22, 23 The range of the propensity score distribution was similar in drug users and nonusers in the individual analyses. There was sufficient overlap between the 2 groups in each stratum. To derive propensity scores for the individual drug analyses, statin use and beta‐blocker use were modeled independently with the demographic and clinical variables using stepwise logistic regression with a relaxed entry criterion of = 0.20. Only 1 variable (hyperlipidemia) remained significantly different between statin users and nonusers, and it was included in the subsequent analyses as a potential confounder. The variable albumin had 511 missing values. To keep this variable in the propensity scores, the missing values were replaced by the predicted values of albumin from the multiple linear regression model that included the other demographic variables. The propensity scores were grouped into quintiles and used as a stratification variable in the subsequent analyses. To confirm that the propensity score method reduced the imbalances, the demographic and clinical characteristics of statin and beta‐blocker users and nonusers and combination users and nonusers were compared using Cochran‐Mantel‐Haenzel tests with the respective propensity score as a stratification variable.
For the combined use of both study drugs, we performed univariate analysis with adjustment only for RCRI (as this was a powerful predictor of mortality in our dataset; Table 1) as well as a propensity score analysis in an exploratory manner. There have been limited applications of propensity score methods to multiple treatment groups. Similar to that in the study by Huang et al.,24 we developed a multinomial baseline response logit model to obtain 3 separate propensity scores (statin only vs. none, beta‐blocker only vs. none, and both vs. none). Because of the limited sample size, the data were stratified according to the median split of each propensity score. Each score had similar ranges for each treatment group. All but 5 variables (CAD, hypertension, hyperlipidemia, ACE inhibitor use, and type of surgery) were balanced after accounting for strata. These 5 variables were then included in the final stratified Cox regression model as potential confounders.
| Variable | Level | N (%) Overall N = 3062 | Hazard ratio (95% CI) | Chi‐square P value |
|---|---|---|---|---|
| ||||
| Age in years, median (IQR) | 67 (5974) | 1.04 (1.04, 1.05)a | <.0001 | |
| Sex | Female | 45 (1) | 0.89 (0.53, 1.51) | .6704 |
| Male | 3017 (99) | 1 | 1.0000 | |
| Preoperative medical conditions | HTN | 2415 (79) | 1.32 (1.13, 1.55) | .0006 |
| CVA/TIA | 589 (19) | 1.05 (0.90, 1.22) | .5753 | |
| CA | 679 (22) | 1.55 (1.36, 1.78) | <.0001 | |
| DM | 1474 (48) | 1.75 (1.54, 1.98) | <.0001 | |
| Lipid | 872 (28) | 0.84 (0.74, 0.97) | .0187 | |
| COPD | 913 (30) | 1.68 (1.48, 1.90) | <.0001 | |
| CAD | 1491 (49) | 1.46 (1.29, 1.66) | <.0001 | |
| CHF | 747 (24) | 2.44 (2.15, 2.77) | <.0001 | |
| CKD | 443 (14) | 2.32 (2.00, 2.69) | <.0001 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 2.73 (2.28, 3.28) | <.0001 |
| Albumin 3.5 | 596 (23) | 2.70 (2.35, 3.10) | <.0001 | |
| Medication use | Aspirin | 1789 (58) | 1.10 (0.97, 1.25) | .1389 |
| ACE inhibitor | 1250 (41) | 0.93 (0.82, 1.06) | .2894 | |
| Insulin | 478 (16) | 1.31 (1.12, 1.54) | .0007 | |
| Clonidine | 115 (4) | 1.68 (1.29, 2.20) | .0001 | |
| Perioperative medication | Statinb | 1346 (44) | 0.66 (0.58, 0.75) | <.0001 |
| Beta‐blockerc | 1617 (53) | 0.74 (0.66, 0.84) | <.0001 | |
| Statin only | 414 (14) | 0.69 (0.56, 0.84) | .0002 | |
| Beta‐blocker only | 685 (22) | 0.81 (0.69, 0.95) | .0079 | |
| Statin and beta‐blocker | 932 (30) | 0.57 (0.49, 0.67) | <.0001 | |
| Noned | 1031 (34) | 1 | 1.0000 | |
| Type of surgery | Aorta | 232 (8) | 1.34 (1.01, 1.77) | <.0001 |
| Carotid | 875 (29) | 1 | ||
| Amputation | 867 (28) | 2.80 (2.36, 3.32) | ||
| Bypass | 1088 (36) | 1.57 (1.32, 1.87) | ||
| RCRI | 0 | 1223 (40) | 1 | <.0001 |
| 1 | 1005 (33) | 1.33 (1.13, 1.55) | ||
| 2 | 598 (20) | 2.22 (1.88, 2.62) | ||
| 3 | 200 (7) | 3.16 (2.54, 3.93) | ||
| 4 | 36 (1) | 4.82 (3.15, 7.37) | ||
| Year surgery occurred | 1998 | 544 (18) | 1 | .6509 |
| 1999 | 463 (15) | 0.91 (0.75, 1.10) | ||
| 2000 | 420 (14) | 0.93 (0.77, 1.13) | ||
| 2001 | 407 (13) | 0.93 (0.75, 1.14) | ||
| 2002 | 374 (12) | 1.12 (0.90, 1.40) | ||
| 2003 | 371 (12) | 1.15 (0.90, 1.47) | ||
| 2004 | 407 (13) | 0.97 (0.72, 1.31) | ||
| 2005 | 76 (3) | 0.68 (0.28, 1.65) | ||
| Tobacco user | Yes | 971 (32) | 0.90 (0.76, 1.08) | .4762 |
| No | 649 (21) | 1 | ||
| Null | 1442 (47) | 0.96 (0.81, 1.13) | ||
| Ethnicity | White | 563 (18) | 1 | .0366 |
| Other | 39 (1) | 0.98 (0.55, 1.76) | ||
| Unknown | 2460 (80) | 1.24 (1.05, 1.46) | ||
To comment on patient‐specific risk by stratification with the RCRI, we used a fixed time point of the 2‐year mortality estimated from the Cox regression model to analyze use of study drugs singly or in combination compared with use of neither.
Chi‐square tests were used to categorize and compare demographic and clinical characteristics of statin users and nonusers, of beta‐blocker users and nonusers, and combination users and nonusers. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Stratified or unstratified Cox regression was used to estimate the hazard ratios of statins and beta‐blockers, with or without adjustment for the propensity score. All analyses were performed using SAS (Statistical Analysis System) software, version 9.1.
RESULTS
Patient Characteristics
The study included 3062 patients whose median age was 67 (interquartile range, 5974; Table 1). Ninety‐nine percent of the study patients were men. Overall, ambulatory use of statins and beta‐blockers was found in 44% and 53% of patients, respectively, and combination use occurred in 30%. Sixty‐one percent of patients had an RCRI of 1 or greater; among them 71% were statin users (Table 2), 68% were beta‐blocker users (Table 3), and 75% were combination users (Table 4). Sixty‐four percent of surgeries were either lower extremity bypass or amputation, 29% were carotid, and 8% aortic. Median follow‐up for all patients was 2.7 years (interquartile range, 1.24.6). Of the whole study cohort, 53% and 62% filled a prescription for a statin or beta‐blocker within 1 year of surgery, respectively, and 58% and 67% filled a prescription within 2 years of surgery, respectively. Overall mortality at 30 days was 3%, at 1 year 14%, and at 2 years 22%.
| Variable, N (%) | Level | Overall (N = 3062) | Statin users (N = 1346 [44]) | Statin nonusers (N = 1716 [56]) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 66 (5973) | 68 (6075) | <.0001 | .9934 | |
| Sex | Female | 45 (1) | 15 (1) | 30 (2) | .1480 | .7822 |
| Male | 3017 (99) | 1331 (99) | 1686 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1176 (87) | 1239 (72) | <.0001 | .2984 |
| CVA/TIA | 589 (19) | 328 (24) | 261 (15) | <.0001 | .3935 | |
| CA | 679 (22) | 307 (23) | 372 (22) | .4550 | .8404 | |
| DM | 1474 (48) | 666 (49) | 808 (47) | .1883 | .5504 | |
| Lipid | 872 (28) | 629 (47) | 243 (14) | <.0001 | .0246 | |
| COPD | 913 (30) | 411 (31) | 502 (29) | .4419 | .8435 | |
| CAD | 1491 (49) | 837 (62) | 654 (38) | <.0001 | .4720 | |
| CHF | 747 (24) | 370 (27) | 377 (22) | .0004 | .4839 | |
| CKD | 443 (14) | 208 (15) | 235 (14) | .1698 | .9990 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 101 (8) | 128 (7) | .9629 | .6911 |
| Albumin 3.5 | 596 (23) | 191 (16) | 405 (30) | <.0001 | .5917 | |
| Medication use | Aspirin | 1789 (58) | 904 (67) | 885 (52) | <.0001 | .6409 |
| Ace inhibitor | 1250 (41) | 712 (53) | 538 (31) | <.0001 | .6075 | |
| Beta‐blocker | 1220 (40) | 767 (57) | 453 (26) | <.0001 | .4058 | |
| Insulin | 478 (16) | 254 (19) | 224 (13) | <.0001 | .7919 | |
| Clonidine | 115 (4) | 61 (5) | 54 (3) | .0454 | .6141 | |
| Type of surgery | Aorta | 232 (8) | 106 (8) | 126 (7) | <.0001 | .9899 |
| Carotid | 875 (29) | 510 (38) | 365 (21) | |||
| Amputation | 867 (28) | 274 (20) | 593 (35) | |||
| Bypass | 1088 (36) | 456 (34) | 632 (37) | |||
| RCRI | 0 | 1223 (40) | 389 (29) | 834 (49) | <.0001 | .9831 |
| 1 | 1005 (33) | 507 (38) | 498 (29) | |||
| 2 | 598 (20) | 318 (24) | 280 (16) | |||
| 3 | 200 (7) | 109 (8) | 91 (5) | |||
| 4 | 36 (1) | 23 (1) | 13 (0.76) | |||
| Year of surgery | 1998 | 544 (18) | 134 (10) | 410 (24) | <.0001 | 1 |
| 1999 | 463 (15) | 163 (12) | 300 (17) | |||
| 2000 | 420 (13) | 178 (13) | 242 (14) | |||
| 2001 | 407 (13) | 188 (14) | 219 (13) | |||
| 2002 | 374 (12) | 194 (14) | 180 (10) | |||
| 2003 | 371 (12) | 209 (16) | 162 (9) | |||
| 2004 | 407 (13) | 229 (17) | 178 (10) | |||
| 2005 | 76 (3) | 51 (4) | 25 (1.5) | |||
| Tobacco user | Yes | 971 (32) | 494 (37) | 477 (28) | <.0001 | .9809 |
| No | 649 (21) | 335 (25) | 314 (18) | |||
| Null | 1442 (47) | 517 (38) | 925 (54) | |||
| Ethnicity | White | 563 (18) | 263 (20) | 300 (17) | .1544 | .9475 |
| Other | 39 (1) | 13 (1) | 26 (1.5) | |||
| Unknown | 2460 (80) | 1070 (79) | 1390 (81) | |||
| Variable, N (%) | Level | Overall N = 3062 | BB users N = 1617 (53) | Non‐BB users N = 1445 (47) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 67 (5975) | 68 (6076) | .0526 | .7671 | |
| Sex | Female | 45 (1) | 12 (1) | 33 (2) | .0004 | .585 |
| Male | 3017 (99) | 1605 (99) | 1412 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1398 (86) | 1017 (70) | <.0001 | .1837 |
| CVA/TIA | 589 (19) | 364 (23) | 225 (16) | <.0001 | .3206 | |
| CA | 679 (22) | 359 (22) | 320 (22) | .9701 | .4288 | |
| DM | 1474 (48) | 739 (46) | 735 (51) | .0043 | .6329 | |
| Lipid | 872 (28) | 555 (34) | 317 (22) | <.0001 | .7180 | |
| COPD | 913 (30) | 487 (30) | 426 (29) | .7007 | .8022 | |
| CAD | 1491 (49) | 975 (60) | 516 (36) | <.0001 | .3496 | |
| CHF | 747 (24) | 439 (27) | 308 (21) | .0002 | .6509 | |
| CKD | 443 (14) | 248 (15) | 195 (13) | .1480 | .8544 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 132 (8) | 97 (7) | .1277 | .5867 |
| Albumin 3.5 | 596 (23) | 252 (18) | 344 (30) | <.0001 | .5347 | |
| Medication use | Aspirin | 1789 (58) | 1046 (65) | 743 (51) | <.0001 | .4942 |
| Ace inhibitor | 1250 (41) | 760 (47) | 490 (34) | <.0001 | .4727 | |
| Statin | 1220 (40) | 932 (58) | 414 (29) | <.0001 | .3706 | |
| Insulin | 478 (16) | 255 (16) | 223 (15) | .7973 | .5991 | |
| Clonidine | 115 (4) | 77 (5) | 38 (3) | .0019 | .8241 | |
| Type of surgery | Aorta | 232 (8) | 176 (11) | 56 (4) | <.0001 | .5664 |
| Carotid | 875 (29) | 515 (32) | 360 (25) | |||
| Amputation | 867 (28) | 339 (21) | 528 (37) | |||
| Bypass | 1088 (36) | 587 (36) | 501 (35) | |||
| RCRI | 0 | 1223 (40) | 518 (32) | 705 (49) | <.0001 | .5489 |
| 1 | 1005 (33) | 583 (36) | 422 (29) | |||
| 2 | 598 (20) | 358 (22) | 240 (17) | |||
| 3 | 200 (7) | 130 (8) | 70 (5) | |||
| 4 | 36 (1) | 28 (2) | 8 (1) | |||
| Year of surgery | 1998 | 544 (18) | 200 (12) | 344 (24) | <.0001 | .3832 |
| 1999 | 463 (15) | 211 (13) | 252 (17) | |||
| 2000 | 420 (13) | 210 (13) | 210 (15) | |||
| 2001 | 407 (13) | 209 (13) | 198 (14) | |||
| 2002 | 374 (12) | 220 (14) | 154 (11) | |||
| 2003 | 371 (12) | 238 (15) | 133 (9) | |||
| 2004 | 407 (13) | 279 (17) | 128 (9) | |||
| 2005 | 76 (3) | 50 (3) | 26 (2) | |||
| Tobacco user | Yes | 971 (32) | 569 (35) | 402 (28) | <.0001 | .9025 |
| No | 649 (21) | 370 (23) | 279 (19) | |||
| Null | 1442 (47) | 678 (42) | 764 (53) | |||
| Ethnicity | White | 563 (18) | 309 (19) | 254 (18) | .4962 | .8762 |
| Other | 39 (1) | 19 (1) | 20 (1) | |||
| Unknown | 2460 (80) | 1289 (80) | 1171 (81) | |||
| N (%) Variable | Level | Overall N = 3062 | BB alone N = 685 (22) | Statin alone N = 414 (14) | Both drugs N = 932 (30) | Neither drug N = 1031 (34) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age in years, median (IQR) | 67 (5974) | 68 (6075) | 67 (6075) | 66 (5973) | 69 (6076) | .0029 | .9824 | |
| Sex | Female | 45 (1) | 7 (1) | 10 (2) | 5 (1) | 23 (2) | .0042 | .5815 |
| Male | 3017 (99) | 678 (99) | 404 (98) | 927 (99) | 1008 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 560 (82) | 338 (82) | 838 (90) | 679 (66) | <.0001 | .0251 |
| CVA/TIA | 589 (19) | 127 (19) | 91 (22) | 237 (25) | 134 (13) | <.0001 | .4543 | |
| CA | 679 (22) | 150 (22) | 98 (24) | 209 (22) | 222 (22) | .8379 | .9749 | |
| DM | 1474 (48) | 291 (43) | 218 (53) | 448 (48) | 517 (50) | .0031 | .3943 | |
| Lipid | 872 (28) | 125 (18) | 199 (48) | 430 (46) | 118 (11) | <.0001 | <.0001 | |
| COPD | 913 (30) | 199 (29) | 123 (30) | 288 (9) | 303 (29) | .8475. | .9769 | |
| CAD | 1491 (49) | 327 (48) | 189 (46) | 648 (70) | 327 (32) | <.0001 | <.0001 | |
| CHF | 747 (24) | 163 (24) | 94 (23) | 276 (30) | 214 (21) | <.0001 | .7031 | |
| CKD | 443 (14) | 92 (13) | 52 (13) | 156 (17) | 143 (14) | .1120 | .8364 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 52 (8) | 21 (5) | 80 (9) | 76 (7) | .1619 | .7184 |
| Albumin 3.5 | 596 (23) | 134 (20) | 73 (20) | 118 (14) | 271 (34) | <.0001 | .2846 | |
| Medication use | Aspirin | 1789 (58) | 398 (58) | 256 (62) | 648 (70) | 487 (47) | <.0001 | .2334 |
| Ace inhibitor | 1250 (41) | 264 (39) | 216 (52) | 496 (53) | 274 (27) | <.0001 | .0216 | |
| Insulin | 478 (16) | 93 (14) | 92 (22) | 162 (17) | 131 (13) | <.0001 | .2952 | |
| Clonidine | 115 (4) | 28 (4) | 12 (3) | 49 (5) | 26 (3) | .0107 | .8035 | |
| Type of surgery | Aorta | 232 (8) | 78 (11) | 8 (2) | 98 (11) | 48 (5) | <.0001 | .008 |
| Carotid | 875 (29) | 165 (24) | 160 (39) | 350 (38) | 200 (19) | |||
| Amputation | 867 (28) | 164 (24) | 99 (24) | 175 (19) | 429 (42) | |||
| Bypass | 1088 (36) | 278 (41) | 147 (36) | 309 (33) | 354 (34) | |||
| RCRI | 0 | 1223 (40) | 288 (42) | 159 (38) | 230 (25) | 546 (53) | <.0001 | .5392 |
| 1 | 1005 (33) | 219 (32) | 143 (35) | 364 (39) | 279 (27) | |||
| 2 | 598 (20) | 125 (18) | 85 (21) | 233 (25) | 155 (15) | |||
| 3 | 200 (7) | 46 (7) | 25 (6) | 84 (9) | 45 (4) | |||
| 4 | 36 (1) | 7 (1) | 2 (0) | 21 (2) | 6 (1) | |||
| Year of surgery | 1998 | 544 (18) | 126 (18) | 60 (14) | 74 (8) | 284 (28) | <.0001 | .3105 |
| 1999 | 463 (15) | 111 (16) | 63 (15) | 100 (11) | 189 (18) | |||
| 2000 | 420 (13) | 87 (13) | 55 (13) | 123 (13) | 155 (15) | |||
| 2001 | 407 (13) | 84 (12) | 63 (15) | 125 (13) | 135 (13) | |||
| 2002 | 374 (12) | 81 (12) | 55 (13) | 139 (15) | 99 (10 | |||
| 2003 | 371 (12) | 85 (13) | 56 (14) | 153 (16) | 77 (7) | |||
| 2004 | 407 (13) | 96 (14) | 46 (11) | 183 (20) | 82 (8) | |||
| 2005 | 76 (3) | 15 (2) | 16 (4) | 35 (4) | 10 (1) | |||
| Tobacco user | Yes | 971 (32) | 227 (33) | 152 (37) | 342 (37) | 250 (24) | <.0001 | .3914 |
| No | 649 (21) | 134 (20) | 99 (24) | 236 (25) | 180 (17) | |||
| Null | 1442 (47) | 324 (47) | 163 (39) | 354 (38) | 601 (58) | |||
| Ethnicity | White | 563 (18) | 115 (17) | 69 (17) | 194 (21) | 185 (18) | .2821 | .9771 |
| Other | 39 (1) | 10 (1) | 4 (1) | 9 (1) | 16 (2) | |||
| Unknown | 2460 (80) | 560 (82) | 341 (82) | 729 (78) | 830 (81) | |||
Univariate Survival Analysis
Univariate Cox regression analysis revealed a strong effect of the composite RCRI, which was predictive of mortality in a linear fashion over the course of the study compared with an RCRI of 0 (Table 1). Univariate analysis showed significant associations with decreased mortality for statins (hazard ratio [HR] = 0.66 [95% CI 0.580.75], P < .0001) and beta‐blockers (HR = 0.74 [95% CI 0.660.84], P = .0001); see Table 1. Of note, compared with that in 1998, mortality did not change for all the years for which data were complete. In addition, compared with taking neither study drug, taking a statin only, a beta‐blocker only, or both was associated with decreased mortality (P = .0002, P = .0079, and P < .0001, respectively; Fig. 1).

Propensity Score Analysis for Single Study Drug
There were significant differences in demographic and clinical characteristics between statin‐users versus statin nonusers, and between beta‐blocker users versus beta‐blocker nonusers. These differences became insignificant after the propensity score adjustment, with the exception of hyperlipidemia for statins, P = .02, which was added to the model as a confounder (Table 2). The distribution of the propensity scores was similar for study drug users and nonusers within each stratum. The association with decreased mortality remained significant after adjusting for propensity score (for statins, HR = 0.78 [95% CI 0.670.92, P = .0050], number needed to treat [NNT] = 22; for beta‐blockers HR = 0.84 [95% CI 0.730.96, P = .0103], NNT = 30; Fig. 2).

Combination Study Drugs and Revised Cardiac Risk Index: Univariate Analysis
We wanted our results to closely model those of combination use of the study drugs by patients in a clinical situation. Therefore, we first examined the effects of ambulatory statins alone, beta‐blockers alone, and a combination of statins and beta‐blockers by univariate analysis. Grouping patients by study drug use has not commonly been reported in the literature. We also examined the statistical interaction between the study drugs and the RCRI. The main‐effects model adequately explained all‐cause mortality, and the statistical interaction between the study drugs and the RCRI was not significant.
The final univariate Cox regression model, which compared use of a statin alone, a beta‐blocker alone, and a statin and beta‐blocker in combination with using neither study drug, demonstrated that the combination of statins and beta‐blockers had an HR over the whole study period of 0.43 (95% CI 0.360.51, P < .0001), statins alone had an HR of 0.59 (95% CI 0.480.72, P < .0001), and beta‐blockers alone had an HR of 0.71 (95% CI 0.610.83, P < .0001).
To clarify the effects of the study drugs on patients at different levels of risk, we stratified patients by the RCRI and evaluated the effects of the study drugs on mortality at 2 years, comparing the results to a referent of taking no study drugs. The use of both a statin and a beta‐blocker consistently produced a relative risk reduction (RRR) of approximately 50% with an NNT of 410, with highly statistically significant results for patients at all levels of risk (Table 5). As patient risk level increased, the NNT decreased, consistent with higher‐risk patients benefiting most from combination therapy with statins and beta‐blockers.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.19 | |||
| BB | 288 (73) | 0.14 | 20 | 0.27 | .0023 | |
| Statin | 159 (30) | 0.12 | 14 | 0.39 | <.0001 | |
| Statin+BB | 230 (23) | 0.09 | 10 | 0.54 | <.0001 | |
| 1 | None | 279 (130) | 0.28 | |||
| BB | 219 (71) | 0.21 | 14 | 0.26 | .0028 | |
| Statin | 143 (41) | 0.17 | 10 | 0.37 | <.0001 | |
| Statin+BB | 364 (73) | 0.13 | 7 | 0.53 | <.0001 | |
| 2 | None | 155 (100) | 0.43 | |||
| BB | 125 (60) | 0.33 | 10 | 0.23 | .0045 | |
| Statin | 85 (42) | 0.28 | 7 | 0.35 | <.0001 | |
| Statin+BB | 233 (72) | 0.22 | 5 | 0.50 | <.0001 | |
| 3 | None | 51 (39) | 0.59 | |||
| BB | 53 (29) | 0.47 | 9 | 0.20 | .0296 | |
| Statin | 27 (14) | 0.41 | 6 | 0.31 | .0014 | |
| Statin+BB | 105 (52) | 0.32 | 4 | 0.46 | <.0001 | |
In addition, the range of outcomes can be clearly seen for both patient‐specific risk level and study drug use. For example, overall mortality at 2 years for all patients was 22%. For the study drugs, mortality ranged from 16% for patient who used both a statin and a beta‐blocker to 27% for those patients who used neither study drug. The use of the RCRI showed that the healthiest patients who were taking both a statin and a beta‐blocker did the best, with a 2‐year mortality of 9%, compared with the sickest patients who were taking neither study drug, whose 2‐year mortality was 59%. Use of both study drugs by the sickest patients was associated with a reduction in 2‐year mortality to 32% (P < .0001; Table 5).
Propensity Score Analysis of Use of Combination Study Drugs
Because there was very limited literature to guide us in the use of propensity score analysis of multiple treatment groups, we performed these analyses in an exploratory manner. There were significant differences between combination statin and beta‐blocker users and nonusers. These differences became insignificant after adjusting for propensity score, except for the 5 variables previously mentioned, which were added to the model as potential confounders (Table 4). The propensity‐adjusted Cox regression model comparing use of each study drug alone and in combination with taking neither over the whole study period still showed an association with decreased mortality. The combination of statins and beta‐blockers had an HR of 0.56 (95% CI 0.420.74), P < .0001; statins alone had an HR of 0.79 (95% CI 0.620.99), P = .0472; and beta‐blockers alone had an HR of 0.80 (95% CI 0.670.94), P = .0183.
Combination Study Drugs and Revised Cardiac Risk Index: Propensity Analysis
We performed the stratified Cox regression adjusted for the propensity scores for each level of RCRI and estimated 2‐year mortality. The use of both a statin and a beta‐blocker compared with using none was still consistently statistically significant, with an RRR of approximately 36% and an NNT of 820 for all levels of patient risk (Table 6). Possibly because of the reduced number of patients in each RCRI category, neither single‐agent study drug compared with none showed a statistically significant decrease in mortality at any level of patient‐specific risk (Table 6). Again, higher‐risk patients benefited most from combination therapy.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.14 | |||
| BB | 288 (73) | 0.11 | 47 | 0.16 | .3778 | |
| Statin | 159 (30) | 0.11 | 40 | 0.19 | .2902 | |
| Statin+BB | 230 (23) | 0.08 | 20 | 0.38 | .0184 | |
| 1 | None | 279 (130) | 0.21 | |||
| BB | 219 (71) | 0.17 | 32 | 0.15 | .2837 | |
| Statin | 143 (41) | 0.17 | 27 | 0.18 | .1969 | |
| Statin+BB | 364 (73) | 0.13 | 14 | 0.37 | .0038 | |
| 2 | None | 155 (100) | 0.29 | |||
| BB | 125 (60) | 0.25 | 24 | 0.15 | .3295 | |
| Statin | 85 (42) | 0.24 | 20 | 0.17 | .2396 | |
| Statin+BB | 233 (72) | 0.18 | 10 | 0.36 | .0077 | |
| 3 | None | 51 (39) | 0.42 | |||
| BB | 53 (29) | 0.37 | 19 | 0.13 | .3553 | |
| Statin | 27 (14) | 0.36 | 16 | 0.15 | .2653 | |
| Statin+BB | 105 (52) | 0.28 | 8 | 0.33 | .0106 | |
Study Drug Timing: Subcohort Analysis
A subcohort analysis was performed to clarify the timing of the study drugs. Of the patients taking statins, 69 of 1346 (5.1%) took the drug before surgery only, 119 of 1346 (8.8%) took the drug after surgery only, and 1158 of 1346 (86%) took the drug both before and after surgery. Of the patients taking beta‐blockers, 54 of 1617 (3.3%) took the drug before surgery only, 397 of 1617 (24.6%) took the drug after surgery only, and 1166 of 1617 (72.1%) took the drug both before and after surgery. The use of statins and beta‐blockers had a correlation of 0.29 (contingency coefficient).
DISCUSSION
In this retrospective observational study we found that after vascular surgery the use of propensity‐adjusted statins compared with no use of statins reduced long‐term mortality over the study period by 22%, with a number needed to treat of 22, and the use of propensity‐adjusted beta‐blockers compared with no use also reduced long‐term mortality, by 16%, with a number needed to treat of 30. There were no statistically significant differences between outcomes of statin users and beta‐blocker users. In addition, using a propensity‐adjusted combination of statin and beta‐blockers compared with using neither decreased mortality overall by 44%, with a number needed to treat of 9. We focused on the use of outpatient drugs 30 days before or after surgery, as the timing of potentially beneficial medications has not been clearly established. Over time, more patients originally categorized as not taking a study drug began taking one, so that by 2 years after surgery, 58% of the patients were taking a statin, and 67% were taking a beta‐blocker, compared with 44% and 53%, respectively, of the study cohort initially. This would have made it more difficult to demonstrate a difference between these 2 groups. As more patients ended up taking the study drugs over time than the originally identified study drug users, and a mortality difference was still demonstrated, there may be an increased advantage in taking the study drugs around the time of surgery. As our focus was on long‐term postoperative mortality, which has not commonly been studied according to the literature, we preferred to also focus on long‐term, chronic ambulatory use of the study drugs. We did perform a subcohort analysis of the timing of study drug use. This confirmed that this cohort predominately comprised long‐term users of the study drugs who took the drug both before and after surgery. This study was not powered to comment on 30‐day mortality.
Perioperative beta‐blockers have been shown in retrospective cohort studies, case‐control studies, randomized clinical trials, meta‐analyses, and systematic reviews to decrease mortality and morbidity after noncardiac surgery. Although recent studies have not shown a benefit for more moderate‐ to low‐risk subjects,11, 12 perioperative beta‐blockers are still considered an indicator of health care quality in the United States.25 At present, perioperative beta‐blockers have an ACC/AHA class I indication (should be administered; Evidence level C) for patients undergoing vascular surgery with a positive stress test, and class IIa indication (reasonable to administer; Evidence level B) for vascular surgery patients with coronary heart disease or multiple clinical risk factors.26 A recent observational study in noncardiac surgery patients demonstrated perioperative beta‐blockers may be most helpful to prevent in‐hospital death after surgery of patients with an RCRI 2 and may be unhelpful or harmful for patients with an RCRI 1.27 Our univariate RCRI findings did not agree, as we found all patients whatever their level of risk benefited from perioperative use of beta‐blockers, alone or in combination. Our study population was older, had a higher RCRI, and underwent comparatively higher‐risk surgery, we were investigating longer‐term outcome, and we concentrated on ambulatory use of beta‐blockers, which may have contributed to the divergence in findings. Our propensity‐adjusted RCRI analysis did not show beta‐blockers associated with any change in mortality at any patient risk level. This may be, in part, because of the reduced number of patients in the RCRI strata. RCRI stratum‐specific analysis is limited by the number of patients and deaths in each RCRI stratum. For example, the power to detect a 2‐year difference of 10% (or 5%) between statin users and nonusers is approximately 99% (66%), 99% (59%), 92% (42%), and 61% (23%) for RCRI = 0, 1, 2, and 3, respectively.
Case‐control and retrospective cohort studies and one randomized clinical trial have shown perioperative statins to decrease either short‐term cardiovascular morbidity or mortality up to 30 days after surgery, and a limited number of retrospective cohort studies have shown reduced mortality for longer‐term follow‐up.1418, 28 There was one previous preliminary study of vascular surgery patients that demonstrated an additive benefit of using statins and beta‐blockers up to 30 days after surgery. This additive effect was only observed in patients with an RCRI 3.29 The results of our longer‐term follow‐up study of a larger cohort did not agree. Compared with patients who did not take a statin or a beta‐blocker, those patients who took both study drugs decreased their relative risk of mortality by approximately 36% in propensity‐ adjusted analysis and by about 50% in univariate analysis, regardless of patient‐specific risk level. For example, in the propensity‐adjusted analysis, the healthiest patients with an RCRI of 0 who took both study drugs had lower mortality than patients who took neither study drug, 8% versus 14%, a 38% relative reduction in mortality, with a number needed to treat of 20 (P = .0184).
In addition, the use of the RCRI for the first time highlighted the divergent long‐term mortality rates for patient‐specific risk levels and the striking long‐term associations of the perioperative use of ambulatory statins, beta‐blockers, and both drugs in combination with improved long‐term mortality. The long‐term use of the study drugs may indeed help all patients with atherosclerotic vascular disease, regardless of surgery. However, vascular surgery presents an opportunity for medical intervention, and our results are most applicable for these patients. In addition, the perioperative state has a unique physiology of acute and intense inflammation and thrombosis. Beta‐blockers and statins have antiadrenergic, anti‐inflammatory, and antithrombotic properties that may be beneficial during this high‐risk state.
Our findings should be viewed with some caution. The use of ICD‐9 codes and demographic data is dependent on the documentation and coding of comorbidities in the medical record and database. The use of statins and beta‐blockers was not random, and patients who took statins and beta‐blockers were different than those who did not. We used rigorous propensity and multivariate analysis, including controlling for clonidine, which has been shown to decrease death after vascular surgery.30 We also controlled for serum albumin level, which has been shown to be a leading predictor of postoperative death.31 We further separately stratified patients by RCRI, as this was a powerful predictor of death in the univariate analysis, but because of the retrospective nature of the study, unmeasured confounders may exist. Only 1% of the study patients were women, which is a limitation of the study. This administrative database is also limited by not having information on tobacco use for 47% of the patients and by not knowing ethnicity for 80% of the patients.
The use of perioperative statins and beta‐blockers used alone or in combination was associated with a reduction in long‐term mortality for vascular surgery patients, and combination use benefited patients at all levels of risk. Higher‐risk patients benefited most by taking both study drugs. These findings extend prior data, add to the natural history of long‐term postoperative outcomes, and also support clinical trials that would evaluate the prospective use of both these medications in vascular surgery patients with attention to patient‐specific risk level. Until the results of 2 randomized controlled trials become available, which may further clarify the use of perioperative statins and beta‐blockers in noncardiac, and noncardiac vascular surgery,13, 32 the use of statins and beta‐blockers should be considered for all patients undergoing vascular surgery. In addition, long‐term use of statins and beta‐blockers for all patients with atherosclerotic vascular disease should be considered.33
Acknowledgements
The authors thank LeAnn Snodgrass for assistance with data extraction and management. This work was funded by the Oregon Health & Science University Medical Research Foundation.
- ,,,,,.The influence of perioperative myocardial infarction on long‐term prognosis following elective vascular surgery.Chest.1998;113:681–686.
- ,,, et al.Postoperative and amputation‐free survival outcomes after femorodistal bypass grafting surgery: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program.J Vasc Surg.2001;34:283–290.
- ,,,.Perioperative‐ and long‐term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population.Anesth Analg1999;89:849–855.
- ,.Very late survival after vascular surgery.J Surg Res.2002;105(2):109–114.
- ,,, et al.Women have increased risk of perioperative myocardial infarction and higher long‐term mortality rates after lower extremity arterial bypass grafting.J Vasc Surg.1999;29:807–812; discussion12–13.
- ,,,,,.Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery.The Study of Perioperative Ischemia Research Group.N Engl J Med.1990;323:1781–1788.
- ,,,,.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:851–857.
- ,,, et al.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—I: Incidence and severity during the 4 day perioperative period.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:843–850.
- ,,,.Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.Multicenter Study of Perioperative Ischemia Research Group.N Engl J Med.1996;335:1713–1720.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.Effect of perioperative beta blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial.BMJ.2006;332:1482.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA2004;291:2092–2099.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion75–6.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,,,,.The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery.Int J Cardiol.2005;104:264–268.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,,.Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration.Ann Epidemiol.1996;6(2):102–109.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,.A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use.Stat Med.2006;25:2084–2106.
- ,,,,.Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care.Health Serv Res2005;40(1):253–78.
- ,,.Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment.Rockville, Md:AHRQ;2001. Report No. 43.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,, et al.Association between long‐term statin use and mortality after successful abdominal aortic aneurysm surgery.Am J Med.2004;116(2):96–103.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,,,,.Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study.Arch Surg.1999;134(1):36–42.
- ,,, et al.Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study.Am Heart J.2004;148:1047–1052.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation.Circulation.2006;113:e463–e654.
- ,,,,,.The influence of perioperative myocardial infarction on long‐term prognosis following elective vascular surgery.Chest.1998;113:681–686.
- ,,, et al.Postoperative and amputation‐free survival outcomes after femorodistal bypass grafting surgery: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program.J Vasc Surg.2001;34:283–290.
- ,,,.Perioperative‐ and long‐term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population.Anesth Analg1999;89:849–855.
- ,.Very late survival after vascular surgery.J Surg Res.2002;105(2):109–114.
- ,,, et al.Women have increased risk of perioperative myocardial infarction and higher long‐term mortality rates after lower extremity arterial bypass grafting.J Vasc Surg.1999;29:807–812; discussion12–13.
- ,,,,,.Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery.The Study of Perioperative Ischemia Research Group.N Engl J Med.1990;323:1781–1788.
- ,,,,.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:851–857.
- ,,, et al.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—I: Incidence and severity during the 4 day perioperative period.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:843–850.
- ,,,.Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.Multicenter Study of Perioperative Ischemia Research Group.N Engl J Med.1996;335:1713–1720.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.Effect of perioperative beta blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial.BMJ.2006;332:1482.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA2004;291:2092–2099.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion75–6.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,,,,.The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery.Int J Cardiol.2005;104:264–268.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,,.Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration.Ann Epidemiol.1996;6(2):102–109.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,.A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use.Stat Med.2006;25:2084–2106.
- ,,,,.Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care.Health Serv Res2005;40(1):253–78.
- ,,.Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment.Rockville, Md:AHRQ;2001. Report No. 43.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,, et al.Association between long‐term statin use and mortality after successful abdominal aortic aneurysm surgery.Am J Med.2004;116(2):96–103.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,,,,.Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study.Arch Surg.1999;134(1):36–42.
- ,,, et al.Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study.Am Heart J.2004;148:1047–1052.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation.Circulation.2006;113:e463–e654.
Copyright © 2007 Society of Hospital Medicine