User login
Update on constipation: One treatment does not fit all
Constipation is both a symptom and, when chronic, a multisymptom disorder, and it can overlap with other gastrointestinal tract disorders such as dyspepsia and gastroesophageal reflux disease. Furthermore, one should keep in mind the possibility of cancer and be alert for its warning signs.
Since constipation has a variety of causes and forms, one treatment does not fit all patients. Conservative measures such as recommending that the patient increase his or her intake of dietary fiber and water and engage in more physical activity are still the cornerstone of treatment, but they do not help all patients. On the other hand, polyethylene glycol and stimulant laxatives, which are traditionally given only for a short time, can be safe and effective when given long-term if other agents fail. New agents have become available or are in development.
In this article we outline our approach to constipation, as a guide for internists.
CONSTIPATION IS COMMON, BUT HOW SHOULD WE DEFINE IT?
Constipation affects 2% to 27% (average 14.8%) of the North American adult population—approximately 63 million people.1 It is more common than many other chronic diseases, including hypertension (48 million people), migraine (33 million), obesity (50 million), and diabetes mellitus (15 million).1–3
Constipation affects more women than men (2.1:1 ratio) and more nonwhites than whites (1.68:1).1 It occurs in all age groups but is more common in those older than 65 years and younger than 4 years.4,5
Constipation accounts for more than 2.5 million office visits and more than $500 million spent on laxatives per year.6,7 Also, people with constipation may report decreased productivity and increased absenteeism.8
The broad range in the prevalence of constipation cited above reflects differences in how it is defined and, in particular, a lack of agreement between how patients and physicians perceive it.1,9 Physicians mainly define constipation on the basis of stool frequency, considering fewer than three bowel movements per week to be abnormal.1 In contrast, patients typically define it on the basis of bothersome symptoms such as straining, passage of hard stool, unproductive urges, inability to defecate at will, and sensations of incomplete evacuation or abdominal bloating.1,9,10
The Rome III diagnostic criteria were developed to provide a consistent diagnostic approach for use in clinical practice and clinical trials.11 The Rome III criteria define functional chronic constipation as a chronic bowel disorder characterized by two or more of the following:
- Straining
- Lumpy or hard stools
- Sensations of incomplete evacuation
- Sensations of anorectal obstruction or blockage
- Use of manual maneuvers to facilitate defecation (eg, digital evacuation, support of the pelvic floor) during at least 25% of defecations
- Fewer than three bowel movements per week.
In addition, loose stools should rarely occur without the use of laxatives, and there should be insufficient criteria for irritable bowel syndrome.11 Chronicity is established by symptom onset within the previous 6 months and symptom duration of at least 3 months.
In contrast, patients with irritable bowel syndrome, also a functional bowel disorder, experience recurrent abdominal pain and discomfort associated with two or more of the following: symptom improvement with defecation, symptom onset associated with a change in the frequency of bowel movements, and a change in the form or appearance of the stool.
THREE TYPES OF IDIOPATHIC CONSTIPATION
There are three types of primary or idiopathic constipation5,9,12,13:
- Functional
- Slow-transit
- Outlet dysfunction.
Functional constipation includes functional chronic idiopathic constipation and constipation-predominant irritable bowel syndrome. It presents with a sense of difficult or delayed evacuation, hard stools, or abdominal bloating or discomfort.6,9,13 The predominant symptom of constipation-predominant irritable bowel syndrome is severe discomfort or pain; in chronic idiopathic constipation, pain and discomfort may be present but are not the primary symptom.
Slow-transit constipation (or delayed-transit constipation) is associated with a prolonged time between bowel movements. Its symptoms include low stool frequency, lack of urge to defecate, abdominal distention, bloating, and abdominal discomfort.14
Outlet dysfunction. Disorders of defecation can be due to mechanical causes such as Hirschsprung disease, anal stricture, cancer, prolapse, and large rectoceles, or from pelvic floor dysfunction. Pelvic floor dysfunction may be due to inadequate or excessive perineal descent or to inadequate propulsive forces, as may occur in neurologic or neuromuscular conditions and dyssynergia.
Pelvic floor dyssynergia, also called anorectal dyssynergia, dyssynergic defecation, and anismus, results from a functional defect in coordinated evacuation. The characteristic symptom is a feeling of being unable to adequately empty the rectum.14 Other symptoms such as excessive straining and manual disimpaction indicate but are not unique to pelvic floor dyssynergia.14,15
Combined forms. Patients may have more than one type of primary constipation and presentation, and pelvic floor dyssynergia has been shown to prolong intestinal transit, which may improve with treatment.
Secondary constipation can be due to causes such as diet, lifestyle, certain medications (calcium channel blockers, beta-blockers, opioids, diuretics, antidepressants, anticonvulsants, antacids, anticholinergics, and antispasmodics),5,16 underlying medical conditions (diabetes, hypothyroidism, multiple sclerosis, parkinsonism),16,17 pregnancy, and advanced age.18
NEUROTRANSMITTERS MAY PLAY A ROLE
Among the mechanisms thought to cause chronic constipation are impaired gastrointestinal motility,19–22 reduced intestinal secretions,21–23 and inadequate reflex relaxation of the pelvic floor muscles.22,24
Neurotransmitters such as serotonin, somatostatin, peptide YY, and vasoactive intestinal peptide affect intestinal secretion and motility.25,26 Hyperactivity of these neurotransmitters associated with increased secretion and motility results in diarrhea, whereas hypoactivity leads to decreased secretion, delayed transit, and constipation.23
Serotonin has a role in regulating visceral pain perception and intestinal motility, as well as secretion.26–28 Clinical trials have shown that activation of serotonin receptors in the gut enhances gastrointestinal motility, inhibits visceral sensitivity, and stimulates intestinal secretion.26,27,29
A hypothesis has recently been proposed that degeneration of enteric neurons may also play a role in the development of severe idiopathic constipation.30
DIAGNOSIS IS MOSTLY CLINICAL
The history and physical examination remain the cornerstones in the diagnosis and subsequent treatment of chronic constipation.
History
Risk factors for primary and secondary constipation to note during the interview include age (< 4 years, > 65 years); low-fiber diet; female sex; lack of physical activity; history of childhood constipation, endocrine and neuromuscular disorders, abuse, depression, or anxiety; family history of cancer; and personal history of pelvic surgery.
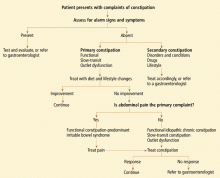
Since drugs can also cause chronic constipation, especially in elderly or immobile patients, medication lists should be reviewed and adjustments should be made if necessary (or possible) before recommending laxatives or invasive testing, if no alarm signs are present.
Alarm signs such as weight loss, hematochezia, melena, change in bowel habits, and symptoms refractory to therapy may represent colon cancer and indicate the need for early diagnostic testing.
Physical examination
Physical examination should always include inspection of the perianal area for evidence of hemorrhoids or fissures. Digital rectal examination may reveal a contracted sphincter or a puborectalis muscle that contracts with the Valsalva maneuver, suggesting dysfunction.
Laboratory testing
If the history and physical examination suggest that the constipation may be secondary, or if the patient is 50 years of age or older, then laboratory studies such as a complete blood cell count, serum electrolyte levels, blood sugar level, and thyroid function studies may help rule out a metabolic, endocrine, or organic cause.
Colonoscopy, other tests
At present, little evidence suggests that routine testing is warranted in patients without evidence of secondary constipation and without alarm signs. However, diagnostic studies are indicated in patients 50 years of age and older, as well as in those with alarm symptoms such as hematochezia, anemia, a positive fecal occult blood test, unintentional loss of more than 10 pounds, family history of colon cancer or inflammatory bowel disease, fever, nausea, vomiting, acute onset (especially in the elderly), and lack of improvement with conventional therapies regardless of age.2
The full length of the colon should be inspected by colonoscopy or by flexible sigmoidoscopy paired with a barium enema study to rule out structural disease. Of note, all patients 50 years of age or older should be screened for colon cancer.
If the patient does not respond to therapy, further tests such as colonic transit studies, anorectal manometry with balloon expulsion, and, possibly, defecating proctography or dynamic pelvic magnetic resonance imaging may be considered. These patients would likely also benefit from referral to a gastroenterologist for further management
DIET AND LIFESTYLE AS TREATMENT
For many years, health care providers have provided reassurance and recommended diet and lifestyle modifications as treatment for constipation. Increased water intake, increased activity, and a scheduled attempt at defecation when motor activity in the colon is highest, ie, in the morning or after eating, have all been recommended.
Data on the efficacy of these recommendations are scarce and often contradictory. Studies have shown that increasing water intake or daily exercise is not always helpful.32–34 Nevertheless, many patients who comply with dietary and exercise recommendations have improvement in symptoms. Eating fewer meals per day (and hence taking in fewer calories) has been shown to be associated with constipation in the elderly. However, no relationships between fiber or fluid intake and constipation were noted.35
In a study in which chronically constipated patients were fed a standardized diet that contained 25 g of fiber a day, stool frequency increased significantly and laxative use decreased.36 While on a high-fiber diet, the patients were divided into two groups, one that drank 1.1 L of fluid per day and one that drank 2.1 L of mineral water per day. Both groups experienced further improvements in stool frequency and decreases in laxative use, with the mineral-water group benefiting the most.36
Recently, Murakami and others37 found, in a cross-sectional study in young Japanese women with low daily fiber intake (6.4 g/day), that low water intake from foods and low magnesium intake were associated with an increasing prevalence of functional constipation as defined by the Rome III criteria. Constipation was also found to be significantly associated with low intake of fruits and vegetables in a study from Singapore.38
Moderate physical activity and high fiber intake may be associated with a lower prevalence of constipation in women. In the Nurses’ Health Study, more than 62,000 women between the ages of 36 and 61 were surveyed, and those who said they engaged in daily physical activity had a lower prevalence of constipation (prevalence ratio [PR] = 0.56, 95% confidence interval [CI] 0.44–0.70), as did those with a median fiber intake of 20 g/day (PR = 0.64, 95% CI 0.57–0.73).39
BULK LAXATIVES (FIBER SUPPLEMENTS): THE FIRST-LINE TREATMENT
Fiber remains the first-line treatment for constipation. It may relieve or improve symptoms in functional constipation. However, fewer than 30% of patients with either slow-transit constipation or pelvic floor dysfunction have improvement in symptoms with fiber, and in these types of constipation it can even worsen symptoms.40
There is much confusion about what types of fiber should be recommended and how the various types of fiber perform in resolving constipation.
Insoluble fiber
Insoluble fiber resists bacterial degradation in the colon and can retain more water than soluble fiber can.
Bran 20 g/day increased the frequency of bowel movements by 55%, increased fecal weight by 157%, and decreased intestinal transit time by 50% in women who had three or fewer bowel movements per week.41
Muller-Lissner42 and others performed a meta-analysis and found that bran (25 g/day) increased stool weight and decreased transit time in both healthy controls and patients with chronic constipation. Yet constipated patients taking bran still had lower stool weights and slower transit times than did healthy subjects.
When bran 20 g/day was compared with placebo in chronically constipated patients, bowel frequency and stool weight increased with both treatments,43 suggesting that factors other than intake may affect bowel function and transit time. However, bran was more effective than placebo in decreasing oroanal transit time.
Elderly constipated patients who received bran 10 g twice a day had significantly shorter transit times (89 hours vs 126 hours) than did those who received psyllium (a soluble fiber) 6 g twice daily. They also needed less additional laxative.44
Soluble fiber
Soluble fiber also affects the bowel habits of both healthy and constipated patients.
Methylcellulose, given to healthy volunteers at a dose of 4 g/day, resulted in statistically significant increases in stool weight, fecal water weight, and fecal solids.45 In constipated patients, methylcellulose 1 g/day was as effective as psyllium 3.4 g/day at increasing stool frequency, fecal water weight, and fecal solids.45
Konjac glucomannan was also shown to significantly increase stool frequency, water weight, and fecal solids.46
Psyllium. In a study that randomly assigned 22 patients with chronic constipation to receive either psyllium 5 g twice daily or placebo for 8 weeks, followed by a 4-week washout phase in which placebo was given,47 those who received psyllium reported significant improvements in stool consistency and pain with defecation, as well as significant increases in both stool frequency (3.8 vs 2.9 per week, P < .05) and stool weight (665 g vs 405 g, P < .05). However, colonic transit times and anorectal manometric measurements did not differ significantly between those who received psyllium vs placebo.47
Fiber may not help everyone
Others have also shown that while fiber may improve stool characteristics, it may not significantly alter the sensorimotor functions of the colon and pelvic floor.
Cheskin et al48 performed a crossover study in 10 constipated men and women in the community. Patients received either 24 g of psyllium fiber daily or a placebo fiber for 1 month and then crossed over to the other treatment for the next month. The most common cause of constipation in this study was pelvic floor dysfunction. Total gut transit time was significantly increased by psyllium fiber, and there was a trend toward increased stool frequency, demonstrating that psyllium clinically improved constipation. However, pelvic floor dysfunction, as measured by rectal manometry, was not improved.
It may be that only people with normal-transit constipation, not those with underlying slow-transit constipation or pelvic floor dysfunction, are helped by additional dietary fiber. Voderholzer and others40 studied 149 consecutive patients with chronic constipation and evaluated their response to at least 6 weeks of psyllium (Plantago ovata seeds 15 to 30 g/day) by serial symptom measurements, oroanal transit times, and functional rectoanal evaluation with defecography, manometry, and sigmoidoscopy. Of the patients with no evidence of pelvic floor dysfunction or slow-transit constipation, 85% improved. However, 80% of those with slow-transit constipation and 63% of those with pelvic floor dysfunction did not improve with the use of fiber. The authors concluded that it is reasonable to try dietary fiber in patients with constipation and, if no improvement is noted, to then consider further investigation for other subtypes of constipation (ie, slow-transit or pelvic-floor dysfunction).
Adverse effects may limit the use of fiber and may differ depending on the type of fiber used. Soluble fiber may be better tolerated, especially in patients with constipation-predominant irritable bowel syndrome.49 Side effects include the sensation of bloating and distention, excessive gas production, and abdominal cramping.
Our recommendations on fiber
We recommend the following regarding fiber in constipated patients:
- Increase fiber intake from natural foods up to 20 g/day. This increase should be completed over 2 to 3 weeks to minimize adverse effects.
- Consider adding a fiber supplement, such as psyllium, if increasing the intake of natural fiber does not relieve constipation-related symptoms.
- If symptoms persist despite the use of fiber supplements and diet and lifestyle modification, then further structural and functional investigation of the colon (anorectal manometry, colonoscopy, defecography, colon manometry) should be considered.
OSMOTIC LAXATIVES
Osmotic laxatives are molecules that are either not absorbed or poorly absorbed and that draw water into the intestinal lumen to maintain isotonicity between the intestinal contents and the serum. Examples are polyethylene glycol, sodium phosphate (Fleet phosphosoda), magnesium hydroxide, magnesium citrate, the sugars lactulose and sorbitol, and glycerin.
Certain formulations of this class of laxative can cause bloating, diarrhea, electrolyte disturbances, volume overload, or dehydration. These effects limit their use, and these medications should be used with caution in patients prone to renal insufficiency or cardiac abnormalities.
Polyethylene glycol
Polyethylene glycol is an exception. It is not absorbed and lacks electrolytes, making it an attractive option in patients with underlying renal or cardiac dysfunction. In several placebo-controlled trials,50–52 various formulations significantly increased stool frequency while significantly decreasing straining, use of other laxatives, and colonic transit. No increase in adverse effects was noted compared with placebo.
Compared with lactulose, polyethylene glycol at about 21 g/day significantly increased bowel movement frequency while significantly decreasing the sense of straining with bowel movements and flatus due to laxative use.51 Both polyethylene glycol and lactulose accelerate colonic transit, although polyethylene glycol does so to a greater extent.53
Polyethylene glycol has been safe and effective when used for up to 6 months.54
Lactulose and sorbitol
Carbohydrate or sugar-based laxatives, if taken in sufficient doses, have a cathartic effect through two mechanisms: a primary osmotic effect of the sugar itself and a secondary osmotic effect as a substrate for colonic bacteria to cleave to acid metabolites, which exert an osmotic effect in the colon. This secondary effect will be discussed in a later section.
Lactulose and sorbitol are sugars that are poorly absorbed by the intestine. Lactulose has been shown to be more effective than placebo in increasing stool frequency, volume, weight, and consistency in chronically constipated patients.55 In a head-to-head comparison between sugar laxatives, 70% sorbitol was as effective as lactulose in increasing the frequency of bowel movements, and it was similar in its adverse effects56; 70% sorbitol is a cost-effective alternative to lactulose in the elderly nursing home population.57
Compared with fiber alone, lactulose use leads to a significantly higher number of bowel movements and better stool consistency.58 However, when lactulose was compared with a combination of fiber and a stimulant laxative, it was less effective than the combination therapy.59,60
Sugar laxatives, while effective, may have dose-limiting or use-limiting adverse effects such as abdominal bloating and flatulence.
Phosphate, magnesium
Sodium phosphate, like polyethylene glycol, is often used as a bowel preparation before colonoscopy, for which it is about as good or slightly better than polyethylene glycol.61,62
Although magnesium and sodium phosphate preparations are effective, there are multiple reports of clinically significant electrolyte abnormalities, renal failure, and congestive heart failure occurring with these preparations. Therefore, they must be used with discretion and caution in appropriate patients with frequent monitoring.
STIMULANT (IRRITANT) LAXATIVES
Stimulant laxatives are usually reserved for use when bulking agents and osmotic laxatives fail. Their mechanism of action involves the alteration of intestinal motility and intestinal fluid secretion.
Anthraquinones (cascara, aloe, and senna), castor oil, and diphenylmethanes (bisacodyl) are the most commonly used stimulant laxatives. They work relatively quickly, often eliciting a bowel movement 2 to 8 hours after they are taken.
This class of laxatives has historically been underused or given for only short periods of time, owing to concern about impairing colonic function, damaging the enteric nervous system, causing laxative dependency, causing cathartic colon, and even causing colon cancer. However, there is very little evidence to support these concerns. Stimulant laxatives can be used on a more regular basis when bulking or osmotic agents fail.63
Possibly of greatest concern is the potential for the overuse and abuse of stimulant laxatives. Excessive use can cause electrolyte disturbances brought about by high-volume watery diarrhea. Risk factors for overuse and abuse include underlying psychiatric disturbances and eating disorders. Prescribing other types of laxatives or cathartic agents may reduce risk, but the potential for abuse exists with all categories of laxatives.
TEGASEROD: GONE BUT STILL AVAILABLE, ON A CONTROLLED BASIS
Tegaserod (Zelnorm), a serotonin (5-HT4) agonist, was used predominantly in women with constipation-predominant irritable bowel syndrome and in men and women with chronic constipation. However, it was suspended from the market in the United States in March 2007 owing to concern about a high risk of adverse cardiovascular effects compared with placebo.
In a double-blind, randomized controlled trial, men with chronic constipation who received tegaserod 6 mg twice a day for 12 weeks had more spontaneous bowel movements than those receiving placebo (P = .04).64
Lin et al65 evaluated the use of tegaserod 6 mg twice daily for 4 weeks in both men and women with chronic constipation. Those receiving tegaserod had significantly more spontaneous bowel movements per week, less straining, and better stool consistency than those receiving placebo.
Tegaserod can still be obtained for appropriate patients via a treatment investigational new drug application. Safety data are under further review by the US Food and Drug Administration. Studies of other serotonin agonists are under way.
LUBIPROSTONE
Lubiprostone (Amitiza) is an agonist of the chloride channel subtype 2, found on the apical membrane of intestinal epithelial cells. It causes increased chloride secretion into the intestinal lumen, enhancing intestinal fluid secretion. It has been shown to be effective in chronic constipation by improving stool consistency and increasing the motility of the small intestine and colon.66 It is approved for treating chronic constipation in adults.
In randomized, double-blind trials, patients receiving lubiprostone 24 fig twice daily for 4 weeks had significantly more bowel movements per week, reported significantly better stool consistency and less abdominal bloating and straining, and rated their constipation as less severe than did patients receiving placebo.67–69
More recently, in an open-label study, lubiprostone improved constipation symptoms when taken for up to 48 weeks.70
The drug is well tolerated, but its adverse effects include nausea (which appears to be dose-dependent and may diminish over time or if the drug is taken with food), diarrhea, and headache.68 Of note, the drug appears to be well tolerated by older people (65 years of age and older), in whom adverse effects occur less often than in younger users.71 However, adverse events may cause up to 20% of patients to stop taking the drug.69 When lubiprostone is discontinued, patients may once again revert to their baseline bowel habit.72
Lubiprostone has not been compared with conventional laxatives, and cost may prohibit it from becoming a first-line drug for chronic constipation.73
OTHER PROMOTILITY AGENTS
Several promotility agents have been studied for treating chronic idiopathic constipation.
Cisapride (Propulsid), a 5-HT3 receptor antagonist and 5-HT4 receptor agonist, and prucalopride, a 5-HT4 agonist, were effective in relieving symptoms associated with chronic constipation.74–76 However, safety issues (cardiac arrhythmias) necessitated withdrawal of cisapride from the US market in 2000. Prucalopride is undergoing clinical trials.77
Renzapride, a mixed 5-HT4 receptor agonist and 5-HT3 receptor antagonist, has been shown to improve stool consistency and to increase colonic transit in patients with constipation-predominant irritable bowel syndrome.78 Renzapride has been studied in patients with this condition,78–81 but not in patients with chronic constipation. Renzapride is in phase III clinical development in the United States for treating constipation-predominant irritable bowel syndrome.
EMERGING TREATMENTS
New drugs with novel mechanisms of action are being investigated for the treatment of chronic idiopathic constipation.
Neurotrophin-3, a neurotrophic factor, modulates the development of the nervous system by regulating the survival and differentiation of nerves.82 In patients with functional constipation, subcutaneous doses of neurotrophin-3 improved stool frequency, the number of complete spontaneous bowel movements, and stool consistency.83
Alvimopan is a selective antagonist of the muopioid receptor that is being studied for opiate-related constipation and postoperative ileus.84,85 Little of this drug is systemically absorbed and it does not cross the blood-brain barrier; thus, it relieves the opiate-related side effects, ie, bloating, abdominal discomfort, and reduced stool frequency, without interfering with the central analgesic effects.
Linaclotide (MD 1100), a poorly absorbed guanylate cyclase agonist, is also being investigated as a treatment for chronic constipation.86 Linaclotide increases intestinal fluid secretion and transit via stimulation of cyclic guanosine monophosphate production and activation of the cystic fibrosis transmembrane conductance regulator.86,87 In preliminary studies, linaclotide increased stool frequency and the Bristol Stool Form Scale consistency score (Table 1) by increasing intestinal fluid secretion and transit.86
Chenodeoxycholic acid is a bile acid that is synthesized from cholesterol.88 Treatment of constipation with chenodeoxycholic acid has been proposed, given its laxative effect. A study by Bazzoli et al89 showed increased stool frequency and a decrease in stool consistency in chronic constipation patients given chenodeoxycholic acid 10 mg/kg/day. The main side effect was diarrhea. Chenodeoxycholic acid may be worthwhile in the management of constipation, but more studies are needed.
PROBIOTICS AND PREBIOTICS
The bacteria of the colon influence peristalsis of the colon.90 Probiotics (live bacterial preparations) and prebiotics (nondigestible preparations that stimulate the growth or activity of beneficial colonic bacteria) have been gaining interest as potential therapies for constipation.91,92
Probiotic bacterial preparations are generally composed of strains of Bifodobacterium,93,94Lactobacillus,95 and combinations thereof, and are available as mixed preparations of multiple bacterial strains of Lactobacillus, Bifodobacterium, and Streptococcus species, such as VSL#3.96
Probiotics may help relieve constipation, but their effect may depend on the strain of bacteria used and the population being studied.97 In a double-blind parallel study in 70 healthy adults, ingestion of 375 g/day of milk fermented with B animalis strain DN-173 010 for 11 days reduced colon transit time by 20% from baseline. The effect was more pronounced in women, particularly in those with longer baseline transit.98
Lactic acid-producing bacteria are considered commensal organisms with essentially no pathogenic potential.99 A review of the safety of bifodobacteria and lactobacilli concluded there was no health risk to consumers.100
Prebiotics are short-chain carbohydrates such as lactulose that stimulate the activity of beneficial colonic bacteria.91 They are thought to have a small laxative effect that is likely both osmotic and due to beneficial actions of bacteria for which they are a substrate. Both konjac glucomannan and lactulose, sugar-based laxatives and prebiotics, have been shown to significantly increase the fecal concentrations of lactobacilli and total bacteria, possibly through increases in stool bulk.46 Prebiotics that have been the focus of research include inulin, fructo-oligosaccharides, and galacto-oligosaccharides.91 Evidence on the efficacy of probiotics and prebiotics at relieving symptoms of constipation, however, is inconclusive because few well-controlled clinical studies have been done.91,92
STRATEGIES FOR MANAGING CHRONIC CONSTIPATION
In the absence of secondary causes, treatment of chronic constipation is focused on relieving symptoms.
If symptoms are refractory to these traditional treatments, agents such as lactulose and polyethylene glycol may provide relief.11,21 Although they do not address the underlying cause of constipation, these agents increase the fluid content of the intestine, contributing to improved stool consistency, and consequently increase the frequency of bowel movements.
Lubiprostone similarly increases the fluid content of the colon, contributing to improved stool consistency, reduced fecal transit time, and increased frequency of bowel movements.66,70,102 Unlike lactulose and polyethylene glycol, which are indicated only for short-term use, lubiprostone has been found to be safe and effective when used for up to 48 weeks.70,71
Biofeedback is the preferred treatment for pelvic floor dyssynergia, in which it has a success rate of 70% to 81% and in which it is superior to standard treatment (laxatives, fiber, and education).103–105 In an instrument-based training program, patients receive auditory or visual feedback or both to help train the pelvic floor and relax the anal sphincter while simulating defecation. It also improves rectal sensation to assist in proper evacuation. The best outcomes are achieved when committed patients receive instruction from empathetic, properly trained physical therapists or other technicians. Studies show that the benefits of biofeedback are long-lasting.104 It does not improve slow-transit constipation, though pelvic floor dyssynergia and slow-transit constipation can overlap.
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004; 99:750–759.
- Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005; 100 suppl 1:S5–S21.
- Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2005. Vital Health Stat 10 2006; 232:1–153.
- Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005; 23:461–476.
- Arce DA, Ermocilla CA, Costa H. Evaluation of constipation. Am Fam Physician 2002; 65:2283–2290.
- Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol 1989; 11:525–536.
- Harari D, Gurwitz JH, Minaker KL. Constipation in the elderly. J Am Geriatr Soc 1993; 41:1130–1140.
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007; 25:599–608.
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003; 349:1360–1368.
- Sandler RS, Drossman DA. Bowel habits in young adults not seeking health care. Dig Dis Sci 1987; 32:841–845.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130:1480–1491.
- Locke GR, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology 2000; 119:1766–1778.
- Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003; 32:659–683.
- Locke GR, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000; 119:1761–1766.
- Prather CM. Subtypes of constipation: sorting out the confusion. Rev Gastroenterol Disord 2004; 4 suppl 2:S11–S16.
- Talley NJ, Jones M, Nuyts G, Dubois D. Risk factors for chronic constipation based on a general practice sample. Am J Gastroenterol 2003; 98:1107–1111.
- Bharucha AE. Treatment of severe and intractable constipation. Curr Treat Options Gastroenterol 2004; 7:291–298.
- Borum ML. Constipation: evaluation and management. Prim Care 2001; 28:577–590.
- Camilleri M, Ford MJ. Review article: colonic sensorimotor physiology in health, and its alteration in constipation and diarrhoeal disorders. Aliment Pharmacol Ther 1998; 12:287–302.
- Knowles CH, Martin JE. Slow transit constipation: a model of human gut dysmotility. Review of possible aetiologies. Neurogastroenterology Motility 2000; 12:181–196.
- Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther 2001; 15:749–763.
- Crowell MD. Pathogenesis of slow transit and pelvic floor dysfunction: from bench to bedside. Rev Gastroenterol Disord 2004; 4 suppl 2:S17–S27.
- Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol 2002; 35 suppl 1:S11–S22.
- Chitkara DK, Bredenoord AJ, Cremonini F, et al. The role of pelvic floor dysfunction and slow colonic transit in adolescents with refractory constipation. Am J Gastroenterol 2004; 99:1579–1584.
- El-Salhy M. Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci 2002; 107:101–110.
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 2004; 141:1285–1293.
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 1999; 13 suppl 2:15–30.
- Gershon MD. Serotonin and its implication for the management of irritable bowel syndrome. Rev Gastroenterol Disord 2003; 3 suppl 2:S25–S34.
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 1998; 115:370–380.
- Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE. Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology 1985; 88:26–34.
- O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990; 300:439–440.
- Meshkinpour H, Selod S, Movahedi H, Nami N, James N, Wilson A. Effects of regular exercise in management of chronic idopathic constipation. Dig Dis Sci 1998; 43:2379–2383.
- Young RJ, Beerman LE, Vanderhoff JA. Increasing oral fluids in chronic constipation in children. Gastroenterol Nurs 1998; 21:156–161.
- Tuteja AK, Talley NJ, Joos SK, Woehl JV, Hickam DH. Is constipation associated with decreased physical activity in normally active subjects? Am J Gastroenterol 2005; 100:124–129.
- Towers AL, Burgio KL, Locher JL, Merkel IS, Safaeian M, Wald A. Constipation in the elderly: influence of dietary, psychological, and physiological factors. J Am Geriatr Soc 1994; 42:701–706.
- Anti J, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998; 45:727–732.
- Murakami K, Sasaki S, Okubo H, et al. Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur J Clin Nutr 2007; 61:616–622.
- Wong ML, Wee S, Pin CH, Gan GL, Ye HC. Sociodemographic and lifestyle factors associated with constipation in an elderly Asian community. Am J Gastroenterol 1999; 94:1283–1291.
- Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003; 98:1790–1796.
- Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol 1997; 92:95–98.
- Graham DY, Moser SE, Estes MK. The effect of bran on bowel function in constipation. Am J Gastroenterol 1982; 77:599–603.
- Muller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. Br Med J (Clin Res Ed) 1988; 296:615–617.
- Badiali D, Corazziari E, Habib FI, et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci 1995; 40:349–356.
- Andersson H, Basaeus I, Falkheden T, Melkersson M. Transit time in constipated geriatric patients during treatment with bulk laxative and bran: a comparison. Scand J Gastroenterol 1979; 14:821–826.
- Hamilton JW, Wagner J, Burdick BB, Bass P. Clinical evaluation of methyl-cellulose as a bulk laxative. Dig Dis Sci 1988; 33:993–998.
- Chen HL, Cheng HC, Liu YJ, Liu SY, Wu WT. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition 2006; 22:1112–1119.
- Ashraf W, Park F, Lof J, Quigley EM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther 1995; 9:639–647.
- Cheskin LJ, Kamal N, Crowell MD, Schuster MM, Whitehead WE. Mechanisms of constipation in older persons and effects of fiber compared with placebo. J Am Geriatr Soc 1995; 43:666–669.
- Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fiber in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004; 19:245–251.
- Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF–100) in treatment of chronic nonorganic constipation. Dig Dis Sci 1996; 41:1636–1642.
- Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999; 44:226–230.
- Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerabilitity of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF–100) in the treatment of functional chronic constipation. Gut 2000; 46:522–526.
- Fritz E, Hammer HF, Lipp RW, Högenauer C, Stauber R, Hammer J. Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther 2005; 21:259–268.
- DiPalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007; 102:1436–1441.
- Bass P, Dennis S. The laxative effects of lactulose in normal and constipated subjects. J Clin Gastroenterol 1981; 3 suppl 1:23–28.
- Lederle FA, Busch DL, Mattox KM, West MJ, Aske DM. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990; 89:597–601.
- Volicer L, Lane P, Panke J, Lyman P. Management of constipation in residents with dementia: sorbitol effectiveness and cost. J Am Med Dir Assoc 2004; 5:239–241.
- Quah HM, Ooi BS, Seow-Choen F, Sng KK, Ho KS. Prospective randomized crossover trial comparing fibre with lactulose in the treatment of idiopathic chronic constipation. Tech Coloproctol 2006; 10:111–114.
- Passmore AP, Davies KW, Flanagan PG, Stoker C, Scott MG. A comparison of Agiolax and lactulose in elderly patients with chronic constipation. Pharmacology 1993; 47 suppl 1:249–252.
- Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ 1993; 307:769–771.
- Poon CM, Lee DW, Mak SK, et al. Two liters of polyethylene glycol-electrolyte lavage solution versus sodium phosphate as bowel cleansing regimen for colonoscopy: a prospective randomized controlled trial. Endoscopy 2002; 34:560–563.
- Rostom A, Jolicoeur E, Dube C, et al. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc 2006; 64:544–552.
- Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol 2003; 36:386–389.
- Fried M, Johanson JF, Gwee KA, Wagner A, Pecher E, Rueegg P. Efficacy of tegaserod in chronic constipation in men. Am J Gastroenterol 2007; 102:362–370.
- Lin SR, Ke MY, Luo JY, et al. A randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of tegaserod in patients from China with chronic constipation. World J Gastroenterol 2007; 13:732–739.
- Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G942–G947.
- McKeage K, Plosker GL, Siddiqui MA. Lubiprostone. Drugs 2006; 66:873–879.
- Johanson JF, Gargano MA, Patchen ML, Ueno R. Efficacy and safety of a novel compound, RU-0211, for the treatment of constipation [abstract]. Gastroenterology 2002; 122 suppl 1:A315.
- Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Multicenter open-label study of oral lubiprostone for the treatment of chronic constipation [abstract]. Am J Gastroenterol 2005; 100 suppl:S331.
- Johanson JF, Panas R, Holland P, Ueno R. Long-term efficacy of lubiprostone for the treatment of chronic constipation [abstract]. Gastroenterology 2006; 130 suppl 2:A317.
- Ueno R, Panas R, Wahle A, Zhu Y, Holland P. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in the elderly [abstract]. Gastroenterology 2006; 130 suppl 2:A188.
- Johanson JF, Gargano MA, Holland P, Patchen ML, Ueno R. Phase III, randomized withdrawal study of RU-0211, a novel chloride channel activator for the treatment of constipation [abstract]. Gastroenterology 2004; 126 suppl 2:A100.
- Rivkin A, Chagan L. Lubiprostone: chloride channel activator for chronic constipation. Clin Ther 2006; 28:2008–2021.
- Johanson JF, Miner PB, Parkman HP, et al. Prucalopride improves bowel movement frequency and symptoms in patients with chronic constipation: results of two double-blind, placebo-controlled trials [abstract]. Gastroenterology 2000; 118 suppl 2:A175.
- Cash BD, Chey WD. Review article: the role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment Pharmacol Ther 2005; 22:1047–1060.
- Altabas K, Biliæ A, Jurciæ D, et al. The efficacy of cisapride vs. placebo and diet in patients with chronic constipation. Coll Antropol 2003; 27:197–204.
- Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 2008; 358:2344–2354.
- Camilleri M, McKinzie S, Fox J, et al. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2004; 2:895–904.
- Tack J, Middleton SJ, Horne MC, et al. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23:1655–1665.
- Henderson JC, Palmer RM, Meyers NL, Spiller RC. A phase IIb clinical study of renzapride in mixed symptom (alternating) irritable bowel syndrome [abstract]. Gastroenterology 2004; 126 suppl 2:A644.
- Meyers NL, Palmer RMJ, George A. Efficacy and safety of renzapride in patients with constipation-predominant IBS: a phase IIb study in the UK primary healthcare setting [abstract]. Gastroenterology 2004; 126 suppl 2:A640.
- Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 2000; 119:41–50.
- Parkman HP, Rao SS, Reynolds JC, et al. Neurotrophin-3 improves functional constipation. Am J Gastroenterol 2003; 98:1338–1347.
- Camilleri M. Alvimopan, a selective peripherally acting muopioid antagonist. Neurogastroenterol Motil 2005; 17:157–165.
- Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 2004; 361:192–195.
- Kurtz C, Fitch D, Busby R, et al. Effects of multidose administration of MD-1100 on safety, tolerability, exposure and pharmacodynamics in healthy subjects [abstract]. Gastroenterology 2006; 130 suppl 2:A26.
- Eutamene H, Theodorou V, Tondereau V, et al. Influence of guanylate cyclase C binding ligand MD-1100 on TNBS-induced visceral hypersensitivity in WT vs. KO guanylate cyclase C deficient mice [abstract]. Gastroenterology 2006; 130 suppl 2:A597.
- Broughton G. Chenodeoxycholate: the bile acid. The drug. a review. Am J Med Sci 1994; 307:54–63.
- Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res 1983; 11:120–123.
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther 2005; 22:495–512.
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 2006; 24:701–714.
- Ouwehand A, Lagstrom H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab 2002; 46:159–162.
- Whorwell P, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101:1581–1590.
- O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128:541–551.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft H. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol 2003; 17:655–659.
- Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2003; 17:895–904.
- Fernández-Bañares F. Nutritional care of the patient with constipation. Best Pract Res Clin Gastroenterol 2006; 20:575–587.
- Bouvier M, Meance S, Bouley C, Berta J, Grimaud J. Effects of consumption of milk fermented by the probiotic strain Bifidobacterium animalis DN-173 010 on colonit transit times in healthy humans. Biosci Microflor 2001; 20 2:43–48.
- Makelainen H, Tahvonen R, Salminen S, Ouwehand AC. In vivo safety assessment of two Bifidobacterium longum strains. Microbiol Immunol 2003; 47:911–914.
- Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 2003; 36:775–780.
- Ramkumar D, Rao S. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005; 100:936–971.
- Ueno R, Osama H, Habe T, Engelke K, Patchen M. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels [abstract]. Gastroenterology 2004; 126 suppl 2:A298.
- Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham biofeedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007; 5:331–338.
- Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006; 130:657–664.
- Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 2007; 50:428–441.
Constipation is both a symptom and, when chronic, a multisymptom disorder, and it can overlap with other gastrointestinal tract disorders such as dyspepsia and gastroesophageal reflux disease. Furthermore, one should keep in mind the possibility of cancer and be alert for its warning signs.
Since constipation has a variety of causes and forms, one treatment does not fit all patients. Conservative measures such as recommending that the patient increase his or her intake of dietary fiber and water and engage in more physical activity are still the cornerstone of treatment, but they do not help all patients. On the other hand, polyethylene glycol and stimulant laxatives, which are traditionally given only for a short time, can be safe and effective when given long-term if other agents fail. New agents have become available or are in development.
In this article we outline our approach to constipation, as a guide for internists.
CONSTIPATION IS COMMON, BUT HOW SHOULD WE DEFINE IT?
Constipation affects 2% to 27% (average 14.8%) of the North American adult population—approximately 63 million people.1 It is more common than many other chronic diseases, including hypertension (48 million people), migraine (33 million), obesity (50 million), and diabetes mellitus (15 million).1–3
Constipation affects more women than men (2.1:1 ratio) and more nonwhites than whites (1.68:1).1 It occurs in all age groups but is more common in those older than 65 years and younger than 4 years.4,5
Constipation accounts for more than 2.5 million office visits and more than $500 million spent on laxatives per year.6,7 Also, people with constipation may report decreased productivity and increased absenteeism.8
The broad range in the prevalence of constipation cited above reflects differences in how it is defined and, in particular, a lack of agreement between how patients and physicians perceive it.1,9 Physicians mainly define constipation on the basis of stool frequency, considering fewer than three bowel movements per week to be abnormal.1 In contrast, patients typically define it on the basis of bothersome symptoms such as straining, passage of hard stool, unproductive urges, inability to defecate at will, and sensations of incomplete evacuation or abdominal bloating.1,9,10
The Rome III diagnostic criteria were developed to provide a consistent diagnostic approach for use in clinical practice and clinical trials.11 The Rome III criteria define functional chronic constipation as a chronic bowel disorder characterized by two or more of the following:
- Straining
- Lumpy or hard stools
- Sensations of incomplete evacuation
- Sensations of anorectal obstruction or blockage
- Use of manual maneuvers to facilitate defecation (eg, digital evacuation, support of the pelvic floor) during at least 25% of defecations
- Fewer than three bowel movements per week.
In addition, loose stools should rarely occur without the use of laxatives, and there should be insufficient criteria for irritable bowel syndrome.11 Chronicity is established by symptom onset within the previous 6 months and symptom duration of at least 3 months.
In contrast, patients with irritable bowel syndrome, also a functional bowel disorder, experience recurrent abdominal pain and discomfort associated with two or more of the following: symptom improvement with defecation, symptom onset associated with a change in the frequency of bowel movements, and a change in the form or appearance of the stool.
THREE TYPES OF IDIOPATHIC CONSTIPATION
There are three types of primary or idiopathic constipation5,9,12,13:
- Functional
- Slow-transit
- Outlet dysfunction.
Functional constipation includes functional chronic idiopathic constipation and constipation-predominant irritable bowel syndrome. It presents with a sense of difficult or delayed evacuation, hard stools, or abdominal bloating or discomfort.6,9,13 The predominant symptom of constipation-predominant irritable bowel syndrome is severe discomfort or pain; in chronic idiopathic constipation, pain and discomfort may be present but are not the primary symptom.
Slow-transit constipation (or delayed-transit constipation) is associated with a prolonged time between bowel movements. Its symptoms include low stool frequency, lack of urge to defecate, abdominal distention, bloating, and abdominal discomfort.14
Outlet dysfunction. Disorders of defecation can be due to mechanical causes such as Hirschsprung disease, anal stricture, cancer, prolapse, and large rectoceles, or from pelvic floor dysfunction. Pelvic floor dysfunction may be due to inadequate or excessive perineal descent or to inadequate propulsive forces, as may occur in neurologic or neuromuscular conditions and dyssynergia.
Pelvic floor dyssynergia, also called anorectal dyssynergia, dyssynergic defecation, and anismus, results from a functional defect in coordinated evacuation. The characteristic symptom is a feeling of being unable to adequately empty the rectum.14 Other symptoms such as excessive straining and manual disimpaction indicate but are not unique to pelvic floor dyssynergia.14,15
Combined forms. Patients may have more than one type of primary constipation and presentation, and pelvic floor dyssynergia has been shown to prolong intestinal transit, which may improve with treatment.
Secondary constipation can be due to causes such as diet, lifestyle, certain medications (calcium channel blockers, beta-blockers, opioids, diuretics, antidepressants, anticonvulsants, antacids, anticholinergics, and antispasmodics),5,16 underlying medical conditions (diabetes, hypothyroidism, multiple sclerosis, parkinsonism),16,17 pregnancy, and advanced age.18
NEUROTRANSMITTERS MAY PLAY A ROLE
Among the mechanisms thought to cause chronic constipation are impaired gastrointestinal motility,19–22 reduced intestinal secretions,21–23 and inadequate reflex relaxation of the pelvic floor muscles.22,24
Neurotransmitters such as serotonin, somatostatin, peptide YY, and vasoactive intestinal peptide affect intestinal secretion and motility.25,26 Hyperactivity of these neurotransmitters associated with increased secretion and motility results in diarrhea, whereas hypoactivity leads to decreased secretion, delayed transit, and constipation.23
Serotonin has a role in regulating visceral pain perception and intestinal motility, as well as secretion.26–28 Clinical trials have shown that activation of serotonin receptors in the gut enhances gastrointestinal motility, inhibits visceral sensitivity, and stimulates intestinal secretion.26,27,29
A hypothesis has recently been proposed that degeneration of enteric neurons may also play a role in the development of severe idiopathic constipation.30
DIAGNOSIS IS MOSTLY CLINICAL
The history and physical examination remain the cornerstones in the diagnosis and subsequent treatment of chronic constipation.
History
Risk factors for primary and secondary constipation to note during the interview include age (< 4 years, > 65 years); low-fiber diet; female sex; lack of physical activity; history of childhood constipation, endocrine and neuromuscular disorders, abuse, depression, or anxiety; family history of cancer; and personal history of pelvic surgery.
Since drugs can also cause chronic constipation, especially in elderly or immobile patients, medication lists should be reviewed and adjustments should be made if necessary (or possible) before recommending laxatives or invasive testing, if no alarm signs are present.
Alarm signs such as weight loss, hematochezia, melena, change in bowel habits, and symptoms refractory to therapy may represent colon cancer and indicate the need for early diagnostic testing.
Physical examination
Physical examination should always include inspection of the perianal area for evidence of hemorrhoids or fissures. Digital rectal examination may reveal a contracted sphincter or a puborectalis muscle that contracts with the Valsalva maneuver, suggesting dysfunction.
Laboratory testing
If the history and physical examination suggest that the constipation may be secondary, or if the patient is 50 years of age or older, then laboratory studies such as a complete blood cell count, serum electrolyte levels, blood sugar level, and thyroid function studies may help rule out a metabolic, endocrine, or organic cause.
Colonoscopy, other tests
At present, little evidence suggests that routine testing is warranted in patients without evidence of secondary constipation and without alarm signs. However, diagnostic studies are indicated in patients 50 years of age and older, as well as in those with alarm symptoms such as hematochezia, anemia, a positive fecal occult blood test, unintentional loss of more than 10 pounds, family history of colon cancer or inflammatory bowel disease, fever, nausea, vomiting, acute onset (especially in the elderly), and lack of improvement with conventional therapies regardless of age.2
The full length of the colon should be inspected by colonoscopy or by flexible sigmoidoscopy paired with a barium enema study to rule out structural disease. Of note, all patients 50 years of age or older should be screened for colon cancer.
If the patient does not respond to therapy, further tests such as colonic transit studies, anorectal manometry with balloon expulsion, and, possibly, defecating proctography or dynamic pelvic magnetic resonance imaging may be considered. These patients would likely also benefit from referral to a gastroenterologist for further management
DIET AND LIFESTYLE AS TREATMENT
For many years, health care providers have provided reassurance and recommended diet and lifestyle modifications as treatment for constipation. Increased water intake, increased activity, and a scheduled attempt at defecation when motor activity in the colon is highest, ie, in the morning or after eating, have all been recommended.
Data on the efficacy of these recommendations are scarce and often contradictory. Studies have shown that increasing water intake or daily exercise is not always helpful.32–34 Nevertheless, many patients who comply with dietary and exercise recommendations have improvement in symptoms. Eating fewer meals per day (and hence taking in fewer calories) has been shown to be associated with constipation in the elderly. However, no relationships between fiber or fluid intake and constipation were noted.35
In a study in which chronically constipated patients were fed a standardized diet that contained 25 g of fiber a day, stool frequency increased significantly and laxative use decreased.36 While on a high-fiber diet, the patients were divided into two groups, one that drank 1.1 L of fluid per day and one that drank 2.1 L of mineral water per day. Both groups experienced further improvements in stool frequency and decreases in laxative use, with the mineral-water group benefiting the most.36
Recently, Murakami and others37 found, in a cross-sectional study in young Japanese women with low daily fiber intake (6.4 g/day), that low water intake from foods and low magnesium intake were associated with an increasing prevalence of functional constipation as defined by the Rome III criteria. Constipation was also found to be significantly associated with low intake of fruits and vegetables in a study from Singapore.38
Moderate physical activity and high fiber intake may be associated with a lower prevalence of constipation in women. In the Nurses’ Health Study, more than 62,000 women between the ages of 36 and 61 were surveyed, and those who said they engaged in daily physical activity had a lower prevalence of constipation (prevalence ratio [PR] = 0.56, 95% confidence interval [CI] 0.44–0.70), as did those with a median fiber intake of 20 g/day (PR = 0.64, 95% CI 0.57–0.73).39
BULK LAXATIVES (FIBER SUPPLEMENTS): THE FIRST-LINE TREATMENT
Fiber remains the first-line treatment for constipation. It may relieve or improve symptoms in functional constipation. However, fewer than 30% of patients with either slow-transit constipation or pelvic floor dysfunction have improvement in symptoms with fiber, and in these types of constipation it can even worsen symptoms.40
There is much confusion about what types of fiber should be recommended and how the various types of fiber perform in resolving constipation.
Insoluble fiber
Insoluble fiber resists bacterial degradation in the colon and can retain more water than soluble fiber can.
Bran 20 g/day increased the frequency of bowel movements by 55%, increased fecal weight by 157%, and decreased intestinal transit time by 50% in women who had three or fewer bowel movements per week.41
Muller-Lissner42 and others performed a meta-analysis and found that bran (25 g/day) increased stool weight and decreased transit time in both healthy controls and patients with chronic constipation. Yet constipated patients taking bran still had lower stool weights and slower transit times than did healthy subjects.
When bran 20 g/day was compared with placebo in chronically constipated patients, bowel frequency and stool weight increased with both treatments,43 suggesting that factors other than intake may affect bowel function and transit time. However, bran was more effective than placebo in decreasing oroanal transit time.
Elderly constipated patients who received bran 10 g twice a day had significantly shorter transit times (89 hours vs 126 hours) than did those who received psyllium (a soluble fiber) 6 g twice daily. They also needed less additional laxative.44
Soluble fiber
Soluble fiber also affects the bowel habits of both healthy and constipated patients.
Methylcellulose, given to healthy volunteers at a dose of 4 g/day, resulted in statistically significant increases in stool weight, fecal water weight, and fecal solids.45 In constipated patients, methylcellulose 1 g/day was as effective as psyllium 3.4 g/day at increasing stool frequency, fecal water weight, and fecal solids.45
Konjac glucomannan was also shown to significantly increase stool frequency, water weight, and fecal solids.46
Psyllium. In a study that randomly assigned 22 patients with chronic constipation to receive either psyllium 5 g twice daily or placebo for 8 weeks, followed by a 4-week washout phase in which placebo was given,47 those who received psyllium reported significant improvements in stool consistency and pain with defecation, as well as significant increases in both stool frequency (3.8 vs 2.9 per week, P < .05) and stool weight (665 g vs 405 g, P < .05). However, colonic transit times and anorectal manometric measurements did not differ significantly between those who received psyllium vs placebo.47
Fiber may not help everyone
Others have also shown that while fiber may improve stool characteristics, it may not significantly alter the sensorimotor functions of the colon and pelvic floor.
Cheskin et al48 performed a crossover study in 10 constipated men and women in the community. Patients received either 24 g of psyllium fiber daily or a placebo fiber for 1 month and then crossed over to the other treatment for the next month. The most common cause of constipation in this study was pelvic floor dysfunction. Total gut transit time was significantly increased by psyllium fiber, and there was a trend toward increased stool frequency, demonstrating that psyllium clinically improved constipation. However, pelvic floor dysfunction, as measured by rectal manometry, was not improved.
It may be that only people with normal-transit constipation, not those with underlying slow-transit constipation or pelvic floor dysfunction, are helped by additional dietary fiber. Voderholzer and others40 studied 149 consecutive patients with chronic constipation and evaluated their response to at least 6 weeks of psyllium (Plantago ovata seeds 15 to 30 g/day) by serial symptom measurements, oroanal transit times, and functional rectoanal evaluation with defecography, manometry, and sigmoidoscopy. Of the patients with no evidence of pelvic floor dysfunction or slow-transit constipation, 85% improved. However, 80% of those with slow-transit constipation and 63% of those with pelvic floor dysfunction did not improve with the use of fiber. The authors concluded that it is reasonable to try dietary fiber in patients with constipation and, if no improvement is noted, to then consider further investigation for other subtypes of constipation (ie, slow-transit or pelvic-floor dysfunction).
Adverse effects may limit the use of fiber and may differ depending on the type of fiber used. Soluble fiber may be better tolerated, especially in patients with constipation-predominant irritable bowel syndrome.49 Side effects include the sensation of bloating and distention, excessive gas production, and abdominal cramping.
Our recommendations on fiber
We recommend the following regarding fiber in constipated patients:
- Increase fiber intake from natural foods up to 20 g/day. This increase should be completed over 2 to 3 weeks to minimize adverse effects.
- Consider adding a fiber supplement, such as psyllium, if increasing the intake of natural fiber does not relieve constipation-related symptoms.
- If symptoms persist despite the use of fiber supplements and diet and lifestyle modification, then further structural and functional investigation of the colon (anorectal manometry, colonoscopy, defecography, colon manometry) should be considered.
OSMOTIC LAXATIVES
Osmotic laxatives are molecules that are either not absorbed or poorly absorbed and that draw water into the intestinal lumen to maintain isotonicity between the intestinal contents and the serum. Examples are polyethylene glycol, sodium phosphate (Fleet phosphosoda), magnesium hydroxide, magnesium citrate, the sugars lactulose and sorbitol, and glycerin.
Certain formulations of this class of laxative can cause bloating, diarrhea, electrolyte disturbances, volume overload, or dehydration. These effects limit their use, and these medications should be used with caution in patients prone to renal insufficiency or cardiac abnormalities.
Polyethylene glycol
Polyethylene glycol is an exception. It is not absorbed and lacks electrolytes, making it an attractive option in patients with underlying renal or cardiac dysfunction. In several placebo-controlled trials,50–52 various formulations significantly increased stool frequency while significantly decreasing straining, use of other laxatives, and colonic transit. No increase in adverse effects was noted compared with placebo.
Compared with lactulose, polyethylene glycol at about 21 g/day significantly increased bowel movement frequency while significantly decreasing the sense of straining with bowel movements and flatus due to laxative use.51 Both polyethylene glycol and lactulose accelerate colonic transit, although polyethylene glycol does so to a greater extent.53
Polyethylene glycol has been safe and effective when used for up to 6 months.54
Lactulose and sorbitol
Carbohydrate or sugar-based laxatives, if taken in sufficient doses, have a cathartic effect through two mechanisms: a primary osmotic effect of the sugar itself and a secondary osmotic effect as a substrate for colonic bacteria to cleave to acid metabolites, which exert an osmotic effect in the colon. This secondary effect will be discussed in a later section.
Lactulose and sorbitol are sugars that are poorly absorbed by the intestine. Lactulose has been shown to be more effective than placebo in increasing stool frequency, volume, weight, and consistency in chronically constipated patients.55 In a head-to-head comparison between sugar laxatives, 70% sorbitol was as effective as lactulose in increasing the frequency of bowel movements, and it was similar in its adverse effects56; 70% sorbitol is a cost-effective alternative to lactulose in the elderly nursing home population.57
Compared with fiber alone, lactulose use leads to a significantly higher number of bowel movements and better stool consistency.58 However, when lactulose was compared with a combination of fiber and a stimulant laxative, it was less effective than the combination therapy.59,60
Sugar laxatives, while effective, may have dose-limiting or use-limiting adverse effects such as abdominal bloating and flatulence.
Phosphate, magnesium
Sodium phosphate, like polyethylene glycol, is often used as a bowel preparation before colonoscopy, for which it is about as good or slightly better than polyethylene glycol.61,62
Although magnesium and sodium phosphate preparations are effective, there are multiple reports of clinically significant electrolyte abnormalities, renal failure, and congestive heart failure occurring with these preparations. Therefore, they must be used with discretion and caution in appropriate patients with frequent monitoring.
STIMULANT (IRRITANT) LAXATIVES
Stimulant laxatives are usually reserved for use when bulking agents and osmotic laxatives fail. Their mechanism of action involves the alteration of intestinal motility and intestinal fluid secretion.
Anthraquinones (cascara, aloe, and senna), castor oil, and diphenylmethanes (bisacodyl) are the most commonly used stimulant laxatives. They work relatively quickly, often eliciting a bowel movement 2 to 8 hours after they are taken.
This class of laxatives has historically been underused or given for only short periods of time, owing to concern about impairing colonic function, damaging the enteric nervous system, causing laxative dependency, causing cathartic colon, and even causing colon cancer. However, there is very little evidence to support these concerns. Stimulant laxatives can be used on a more regular basis when bulking or osmotic agents fail.63
Possibly of greatest concern is the potential for the overuse and abuse of stimulant laxatives. Excessive use can cause electrolyte disturbances brought about by high-volume watery diarrhea. Risk factors for overuse and abuse include underlying psychiatric disturbances and eating disorders. Prescribing other types of laxatives or cathartic agents may reduce risk, but the potential for abuse exists with all categories of laxatives.
TEGASEROD: GONE BUT STILL AVAILABLE, ON A CONTROLLED BASIS
Tegaserod (Zelnorm), a serotonin (5-HT4) agonist, was used predominantly in women with constipation-predominant irritable bowel syndrome and in men and women with chronic constipation. However, it was suspended from the market in the United States in March 2007 owing to concern about a high risk of adverse cardiovascular effects compared with placebo.
In a double-blind, randomized controlled trial, men with chronic constipation who received tegaserod 6 mg twice a day for 12 weeks had more spontaneous bowel movements than those receiving placebo (P = .04).64
Lin et al65 evaluated the use of tegaserod 6 mg twice daily for 4 weeks in both men and women with chronic constipation. Those receiving tegaserod had significantly more spontaneous bowel movements per week, less straining, and better stool consistency than those receiving placebo.
Tegaserod can still be obtained for appropriate patients via a treatment investigational new drug application. Safety data are under further review by the US Food and Drug Administration. Studies of other serotonin agonists are under way.
LUBIPROSTONE
Lubiprostone (Amitiza) is an agonist of the chloride channel subtype 2, found on the apical membrane of intestinal epithelial cells. It causes increased chloride secretion into the intestinal lumen, enhancing intestinal fluid secretion. It has been shown to be effective in chronic constipation by improving stool consistency and increasing the motility of the small intestine and colon.66 It is approved for treating chronic constipation in adults.
In randomized, double-blind trials, patients receiving lubiprostone 24 fig twice daily for 4 weeks had significantly more bowel movements per week, reported significantly better stool consistency and less abdominal bloating and straining, and rated their constipation as less severe than did patients receiving placebo.67–69
More recently, in an open-label study, lubiprostone improved constipation symptoms when taken for up to 48 weeks.70
The drug is well tolerated, but its adverse effects include nausea (which appears to be dose-dependent and may diminish over time or if the drug is taken with food), diarrhea, and headache.68 Of note, the drug appears to be well tolerated by older people (65 years of age and older), in whom adverse effects occur less often than in younger users.71 However, adverse events may cause up to 20% of patients to stop taking the drug.69 When lubiprostone is discontinued, patients may once again revert to their baseline bowel habit.72
Lubiprostone has not been compared with conventional laxatives, and cost may prohibit it from becoming a first-line drug for chronic constipation.73
OTHER PROMOTILITY AGENTS
Several promotility agents have been studied for treating chronic idiopathic constipation.
Cisapride (Propulsid), a 5-HT3 receptor antagonist and 5-HT4 receptor agonist, and prucalopride, a 5-HT4 agonist, were effective in relieving symptoms associated with chronic constipation.74–76 However, safety issues (cardiac arrhythmias) necessitated withdrawal of cisapride from the US market in 2000. Prucalopride is undergoing clinical trials.77
Renzapride, a mixed 5-HT4 receptor agonist and 5-HT3 receptor antagonist, has been shown to improve stool consistency and to increase colonic transit in patients with constipation-predominant irritable bowel syndrome.78 Renzapride has been studied in patients with this condition,78–81 but not in patients with chronic constipation. Renzapride is in phase III clinical development in the United States for treating constipation-predominant irritable bowel syndrome.
EMERGING TREATMENTS
New drugs with novel mechanisms of action are being investigated for the treatment of chronic idiopathic constipation.
Neurotrophin-3, a neurotrophic factor, modulates the development of the nervous system by regulating the survival and differentiation of nerves.82 In patients with functional constipation, subcutaneous doses of neurotrophin-3 improved stool frequency, the number of complete spontaneous bowel movements, and stool consistency.83
Alvimopan is a selective antagonist of the muopioid receptor that is being studied for opiate-related constipation and postoperative ileus.84,85 Little of this drug is systemically absorbed and it does not cross the blood-brain barrier; thus, it relieves the opiate-related side effects, ie, bloating, abdominal discomfort, and reduced stool frequency, without interfering with the central analgesic effects.
Linaclotide (MD 1100), a poorly absorbed guanylate cyclase agonist, is also being investigated as a treatment for chronic constipation.86 Linaclotide increases intestinal fluid secretion and transit via stimulation of cyclic guanosine monophosphate production and activation of the cystic fibrosis transmembrane conductance regulator.86,87 In preliminary studies, linaclotide increased stool frequency and the Bristol Stool Form Scale consistency score (Table 1) by increasing intestinal fluid secretion and transit.86
Chenodeoxycholic acid is a bile acid that is synthesized from cholesterol.88 Treatment of constipation with chenodeoxycholic acid has been proposed, given its laxative effect. A study by Bazzoli et al89 showed increased stool frequency and a decrease in stool consistency in chronic constipation patients given chenodeoxycholic acid 10 mg/kg/day. The main side effect was diarrhea. Chenodeoxycholic acid may be worthwhile in the management of constipation, but more studies are needed.
PROBIOTICS AND PREBIOTICS
The bacteria of the colon influence peristalsis of the colon.90 Probiotics (live bacterial preparations) and prebiotics (nondigestible preparations that stimulate the growth or activity of beneficial colonic bacteria) have been gaining interest as potential therapies for constipation.91,92
Probiotic bacterial preparations are generally composed of strains of Bifodobacterium,93,94Lactobacillus,95 and combinations thereof, and are available as mixed preparations of multiple bacterial strains of Lactobacillus, Bifodobacterium, and Streptococcus species, such as VSL#3.96
Probiotics may help relieve constipation, but their effect may depend on the strain of bacteria used and the population being studied.97 In a double-blind parallel study in 70 healthy adults, ingestion of 375 g/day of milk fermented with B animalis strain DN-173 010 for 11 days reduced colon transit time by 20% from baseline. The effect was more pronounced in women, particularly in those with longer baseline transit.98
Lactic acid-producing bacteria are considered commensal organisms with essentially no pathogenic potential.99 A review of the safety of bifodobacteria and lactobacilli concluded there was no health risk to consumers.100
Prebiotics are short-chain carbohydrates such as lactulose that stimulate the activity of beneficial colonic bacteria.91 They are thought to have a small laxative effect that is likely both osmotic and due to beneficial actions of bacteria for which they are a substrate. Both konjac glucomannan and lactulose, sugar-based laxatives and prebiotics, have been shown to significantly increase the fecal concentrations of lactobacilli and total bacteria, possibly through increases in stool bulk.46 Prebiotics that have been the focus of research include inulin, fructo-oligosaccharides, and galacto-oligosaccharides.91 Evidence on the efficacy of probiotics and prebiotics at relieving symptoms of constipation, however, is inconclusive because few well-controlled clinical studies have been done.91,92
STRATEGIES FOR MANAGING CHRONIC CONSTIPATION
In the absence of secondary causes, treatment of chronic constipation is focused on relieving symptoms.
If symptoms are refractory to these traditional treatments, agents such as lactulose and polyethylene glycol may provide relief.11,21 Although they do not address the underlying cause of constipation, these agents increase the fluid content of the intestine, contributing to improved stool consistency, and consequently increase the frequency of bowel movements.
Lubiprostone similarly increases the fluid content of the colon, contributing to improved stool consistency, reduced fecal transit time, and increased frequency of bowel movements.66,70,102 Unlike lactulose and polyethylene glycol, which are indicated only for short-term use, lubiprostone has been found to be safe and effective when used for up to 48 weeks.70,71
Biofeedback is the preferred treatment for pelvic floor dyssynergia, in which it has a success rate of 70% to 81% and in which it is superior to standard treatment (laxatives, fiber, and education).103–105 In an instrument-based training program, patients receive auditory or visual feedback or both to help train the pelvic floor and relax the anal sphincter while simulating defecation. It also improves rectal sensation to assist in proper evacuation. The best outcomes are achieved when committed patients receive instruction from empathetic, properly trained physical therapists or other technicians. Studies show that the benefits of biofeedback are long-lasting.104 It does not improve slow-transit constipation, though pelvic floor dyssynergia and slow-transit constipation can overlap.
Constipation is both a symptom and, when chronic, a multisymptom disorder, and it can overlap with other gastrointestinal tract disorders such as dyspepsia and gastroesophageal reflux disease. Furthermore, one should keep in mind the possibility of cancer and be alert for its warning signs.
Since constipation has a variety of causes and forms, one treatment does not fit all patients. Conservative measures such as recommending that the patient increase his or her intake of dietary fiber and water and engage in more physical activity are still the cornerstone of treatment, but they do not help all patients. On the other hand, polyethylene glycol and stimulant laxatives, which are traditionally given only for a short time, can be safe and effective when given long-term if other agents fail. New agents have become available or are in development.
In this article we outline our approach to constipation, as a guide for internists.
CONSTIPATION IS COMMON, BUT HOW SHOULD WE DEFINE IT?
Constipation affects 2% to 27% (average 14.8%) of the North American adult population—approximately 63 million people.1 It is more common than many other chronic diseases, including hypertension (48 million people), migraine (33 million), obesity (50 million), and diabetes mellitus (15 million).1–3
Constipation affects more women than men (2.1:1 ratio) and more nonwhites than whites (1.68:1).1 It occurs in all age groups but is more common in those older than 65 years and younger than 4 years.4,5
Constipation accounts for more than 2.5 million office visits and more than $500 million spent on laxatives per year.6,7 Also, people with constipation may report decreased productivity and increased absenteeism.8
The broad range in the prevalence of constipation cited above reflects differences in how it is defined and, in particular, a lack of agreement between how patients and physicians perceive it.1,9 Physicians mainly define constipation on the basis of stool frequency, considering fewer than three bowel movements per week to be abnormal.1 In contrast, patients typically define it on the basis of bothersome symptoms such as straining, passage of hard stool, unproductive urges, inability to defecate at will, and sensations of incomplete evacuation or abdominal bloating.1,9,10
The Rome III diagnostic criteria were developed to provide a consistent diagnostic approach for use in clinical practice and clinical trials.11 The Rome III criteria define functional chronic constipation as a chronic bowel disorder characterized by two or more of the following:
- Straining
- Lumpy or hard stools
- Sensations of incomplete evacuation
- Sensations of anorectal obstruction or blockage
- Use of manual maneuvers to facilitate defecation (eg, digital evacuation, support of the pelvic floor) during at least 25% of defecations
- Fewer than three bowel movements per week.
In addition, loose stools should rarely occur without the use of laxatives, and there should be insufficient criteria for irritable bowel syndrome.11 Chronicity is established by symptom onset within the previous 6 months and symptom duration of at least 3 months.
In contrast, patients with irritable bowel syndrome, also a functional bowel disorder, experience recurrent abdominal pain and discomfort associated with two or more of the following: symptom improvement with defecation, symptom onset associated with a change in the frequency of bowel movements, and a change in the form or appearance of the stool.
THREE TYPES OF IDIOPATHIC CONSTIPATION
There are three types of primary or idiopathic constipation5,9,12,13:
- Functional
- Slow-transit
- Outlet dysfunction.
Functional constipation includes functional chronic idiopathic constipation and constipation-predominant irritable bowel syndrome. It presents with a sense of difficult or delayed evacuation, hard stools, or abdominal bloating or discomfort.6,9,13 The predominant symptom of constipation-predominant irritable bowel syndrome is severe discomfort or pain; in chronic idiopathic constipation, pain and discomfort may be present but are not the primary symptom.
Slow-transit constipation (or delayed-transit constipation) is associated with a prolonged time between bowel movements. Its symptoms include low stool frequency, lack of urge to defecate, abdominal distention, bloating, and abdominal discomfort.14
Outlet dysfunction. Disorders of defecation can be due to mechanical causes such as Hirschsprung disease, anal stricture, cancer, prolapse, and large rectoceles, or from pelvic floor dysfunction. Pelvic floor dysfunction may be due to inadequate or excessive perineal descent or to inadequate propulsive forces, as may occur in neurologic or neuromuscular conditions and dyssynergia.
Pelvic floor dyssynergia, also called anorectal dyssynergia, dyssynergic defecation, and anismus, results from a functional defect in coordinated evacuation. The characteristic symptom is a feeling of being unable to adequately empty the rectum.14 Other symptoms such as excessive straining and manual disimpaction indicate but are not unique to pelvic floor dyssynergia.14,15
Combined forms. Patients may have more than one type of primary constipation and presentation, and pelvic floor dyssynergia has been shown to prolong intestinal transit, which may improve with treatment.
Secondary constipation can be due to causes such as diet, lifestyle, certain medications (calcium channel blockers, beta-blockers, opioids, diuretics, antidepressants, anticonvulsants, antacids, anticholinergics, and antispasmodics),5,16 underlying medical conditions (diabetes, hypothyroidism, multiple sclerosis, parkinsonism),16,17 pregnancy, and advanced age.18
NEUROTRANSMITTERS MAY PLAY A ROLE
Among the mechanisms thought to cause chronic constipation are impaired gastrointestinal motility,19–22 reduced intestinal secretions,21–23 and inadequate reflex relaxation of the pelvic floor muscles.22,24
Neurotransmitters such as serotonin, somatostatin, peptide YY, and vasoactive intestinal peptide affect intestinal secretion and motility.25,26 Hyperactivity of these neurotransmitters associated with increased secretion and motility results in diarrhea, whereas hypoactivity leads to decreased secretion, delayed transit, and constipation.23
Serotonin has a role in regulating visceral pain perception and intestinal motility, as well as secretion.26–28 Clinical trials have shown that activation of serotonin receptors in the gut enhances gastrointestinal motility, inhibits visceral sensitivity, and stimulates intestinal secretion.26,27,29
A hypothesis has recently been proposed that degeneration of enteric neurons may also play a role in the development of severe idiopathic constipation.30
DIAGNOSIS IS MOSTLY CLINICAL
The history and physical examination remain the cornerstones in the diagnosis and subsequent treatment of chronic constipation.
History
Risk factors for primary and secondary constipation to note during the interview include age (< 4 years, > 65 years); low-fiber diet; female sex; lack of physical activity; history of childhood constipation, endocrine and neuromuscular disorders, abuse, depression, or anxiety; family history of cancer; and personal history of pelvic surgery.
Since drugs can also cause chronic constipation, especially in elderly or immobile patients, medication lists should be reviewed and adjustments should be made if necessary (or possible) before recommending laxatives or invasive testing, if no alarm signs are present.
Alarm signs such as weight loss, hematochezia, melena, change in bowel habits, and symptoms refractory to therapy may represent colon cancer and indicate the need for early diagnostic testing.
Physical examination
Physical examination should always include inspection of the perianal area for evidence of hemorrhoids or fissures. Digital rectal examination may reveal a contracted sphincter or a puborectalis muscle that contracts with the Valsalva maneuver, suggesting dysfunction.
Laboratory testing
If the history and physical examination suggest that the constipation may be secondary, or if the patient is 50 years of age or older, then laboratory studies such as a complete blood cell count, serum electrolyte levels, blood sugar level, and thyroid function studies may help rule out a metabolic, endocrine, or organic cause.
Colonoscopy, other tests
At present, little evidence suggests that routine testing is warranted in patients without evidence of secondary constipation and without alarm signs. However, diagnostic studies are indicated in patients 50 years of age and older, as well as in those with alarm symptoms such as hematochezia, anemia, a positive fecal occult blood test, unintentional loss of more than 10 pounds, family history of colon cancer or inflammatory bowel disease, fever, nausea, vomiting, acute onset (especially in the elderly), and lack of improvement with conventional therapies regardless of age.2
The full length of the colon should be inspected by colonoscopy or by flexible sigmoidoscopy paired with a barium enema study to rule out structural disease. Of note, all patients 50 years of age or older should be screened for colon cancer.
If the patient does not respond to therapy, further tests such as colonic transit studies, anorectal manometry with balloon expulsion, and, possibly, defecating proctography or dynamic pelvic magnetic resonance imaging may be considered. These patients would likely also benefit from referral to a gastroenterologist for further management
DIET AND LIFESTYLE AS TREATMENT
For many years, health care providers have provided reassurance and recommended diet and lifestyle modifications as treatment for constipation. Increased water intake, increased activity, and a scheduled attempt at defecation when motor activity in the colon is highest, ie, in the morning or after eating, have all been recommended.
Data on the efficacy of these recommendations are scarce and often contradictory. Studies have shown that increasing water intake or daily exercise is not always helpful.32–34 Nevertheless, many patients who comply with dietary and exercise recommendations have improvement in symptoms. Eating fewer meals per day (and hence taking in fewer calories) has been shown to be associated with constipation in the elderly. However, no relationships between fiber or fluid intake and constipation were noted.35
In a study in which chronically constipated patients were fed a standardized diet that contained 25 g of fiber a day, stool frequency increased significantly and laxative use decreased.36 While on a high-fiber diet, the patients were divided into two groups, one that drank 1.1 L of fluid per day and one that drank 2.1 L of mineral water per day. Both groups experienced further improvements in stool frequency and decreases in laxative use, with the mineral-water group benefiting the most.36
Recently, Murakami and others37 found, in a cross-sectional study in young Japanese women with low daily fiber intake (6.4 g/day), that low water intake from foods and low magnesium intake were associated with an increasing prevalence of functional constipation as defined by the Rome III criteria. Constipation was also found to be significantly associated with low intake of fruits and vegetables in a study from Singapore.38
Moderate physical activity and high fiber intake may be associated with a lower prevalence of constipation in women. In the Nurses’ Health Study, more than 62,000 women between the ages of 36 and 61 were surveyed, and those who said they engaged in daily physical activity had a lower prevalence of constipation (prevalence ratio [PR] = 0.56, 95% confidence interval [CI] 0.44–0.70), as did those with a median fiber intake of 20 g/day (PR = 0.64, 95% CI 0.57–0.73).39
BULK LAXATIVES (FIBER SUPPLEMENTS): THE FIRST-LINE TREATMENT
Fiber remains the first-line treatment for constipation. It may relieve or improve symptoms in functional constipation. However, fewer than 30% of patients with either slow-transit constipation or pelvic floor dysfunction have improvement in symptoms with fiber, and in these types of constipation it can even worsen symptoms.40
There is much confusion about what types of fiber should be recommended and how the various types of fiber perform in resolving constipation.
Insoluble fiber
Insoluble fiber resists bacterial degradation in the colon and can retain more water than soluble fiber can.
Bran 20 g/day increased the frequency of bowel movements by 55%, increased fecal weight by 157%, and decreased intestinal transit time by 50% in women who had three or fewer bowel movements per week.41
Muller-Lissner42 and others performed a meta-analysis and found that bran (25 g/day) increased stool weight and decreased transit time in both healthy controls and patients with chronic constipation. Yet constipated patients taking bran still had lower stool weights and slower transit times than did healthy subjects.
When bran 20 g/day was compared with placebo in chronically constipated patients, bowel frequency and stool weight increased with both treatments,43 suggesting that factors other than intake may affect bowel function and transit time. However, bran was more effective than placebo in decreasing oroanal transit time.
Elderly constipated patients who received bran 10 g twice a day had significantly shorter transit times (89 hours vs 126 hours) than did those who received psyllium (a soluble fiber) 6 g twice daily. They also needed less additional laxative.44
Soluble fiber
Soluble fiber also affects the bowel habits of both healthy and constipated patients.
Methylcellulose, given to healthy volunteers at a dose of 4 g/day, resulted in statistically significant increases in stool weight, fecal water weight, and fecal solids.45 In constipated patients, methylcellulose 1 g/day was as effective as psyllium 3.4 g/day at increasing stool frequency, fecal water weight, and fecal solids.45
Konjac glucomannan was also shown to significantly increase stool frequency, water weight, and fecal solids.46
Psyllium. In a study that randomly assigned 22 patients with chronic constipation to receive either psyllium 5 g twice daily or placebo for 8 weeks, followed by a 4-week washout phase in which placebo was given,47 those who received psyllium reported significant improvements in stool consistency and pain with defecation, as well as significant increases in both stool frequency (3.8 vs 2.9 per week, P < .05) and stool weight (665 g vs 405 g, P < .05). However, colonic transit times and anorectal manometric measurements did not differ significantly between those who received psyllium vs placebo.47
Fiber may not help everyone
Others have also shown that while fiber may improve stool characteristics, it may not significantly alter the sensorimotor functions of the colon and pelvic floor.
Cheskin et al48 performed a crossover study in 10 constipated men and women in the community. Patients received either 24 g of psyllium fiber daily or a placebo fiber for 1 month and then crossed over to the other treatment for the next month. The most common cause of constipation in this study was pelvic floor dysfunction. Total gut transit time was significantly increased by psyllium fiber, and there was a trend toward increased stool frequency, demonstrating that psyllium clinically improved constipation. However, pelvic floor dysfunction, as measured by rectal manometry, was not improved.
It may be that only people with normal-transit constipation, not those with underlying slow-transit constipation or pelvic floor dysfunction, are helped by additional dietary fiber. Voderholzer and others40 studied 149 consecutive patients with chronic constipation and evaluated their response to at least 6 weeks of psyllium (Plantago ovata seeds 15 to 30 g/day) by serial symptom measurements, oroanal transit times, and functional rectoanal evaluation with defecography, manometry, and sigmoidoscopy. Of the patients with no evidence of pelvic floor dysfunction or slow-transit constipation, 85% improved. However, 80% of those with slow-transit constipation and 63% of those with pelvic floor dysfunction did not improve with the use of fiber. The authors concluded that it is reasonable to try dietary fiber in patients with constipation and, if no improvement is noted, to then consider further investigation for other subtypes of constipation (ie, slow-transit or pelvic-floor dysfunction).
Adverse effects may limit the use of fiber and may differ depending on the type of fiber used. Soluble fiber may be better tolerated, especially in patients with constipation-predominant irritable bowel syndrome.49 Side effects include the sensation of bloating and distention, excessive gas production, and abdominal cramping.
Our recommendations on fiber
We recommend the following regarding fiber in constipated patients:
- Increase fiber intake from natural foods up to 20 g/day. This increase should be completed over 2 to 3 weeks to minimize adverse effects.
- Consider adding a fiber supplement, such as psyllium, if increasing the intake of natural fiber does not relieve constipation-related symptoms.
- If symptoms persist despite the use of fiber supplements and diet and lifestyle modification, then further structural and functional investigation of the colon (anorectal manometry, colonoscopy, defecography, colon manometry) should be considered.
OSMOTIC LAXATIVES
Osmotic laxatives are molecules that are either not absorbed or poorly absorbed and that draw water into the intestinal lumen to maintain isotonicity between the intestinal contents and the serum. Examples are polyethylene glycol, sodium phosphate (Fleet phosphosoda), magnesium hydroxide, magnesium citrate, the sugars lactulose and sorbitol, and glycerin.
Certain formulations of this class of laxative can cause bloating, diarrhea, electrolyte disturbances, volume overload, or dehydration. These effects limit their use, and these medications should be used with caution in patients prone to renal insufficiency or cardiac abnormalities.
Polyethylene glycol
Polyethylene glycol is an exception. It is not absorbed and lacks electrolytes, making it an attractive option in patients with underlying renal or cardiac dysfunction. In several placebo-controlled trials,50–52 various formulations significantly increased stool frequency while significantly decreasing straining, use of other laxatives, and colonic transit. No increase in adverse effects was noted compared with placebo.
Compared with lactulose, polyethylene glycol at about 21 g/day significantly increased bowel movement frequency while significantly decreasing the sense of straining with bowel movements and flatus due to laxative use.51 Both polyethylene glycol and lactulose accelerate colonic transit, although polyethylene glycol does so to a greater extent.53
Polyethylene glycol has been safe and effective when used for up to 6 months.54
Lactulose and sorbitol
Carbohydrate or sugar-based laxatives, if taken in sufficient doses, have a cathartic effect through two mechanisms: a primary osmotic effect of the sugar itself and a secondary osmotic effect as a substrate for colonic bacteria to cleave to acid metabolites, which exert an osmotic effect in the colon. This secondary effect will be discussed in a later section.
Lactulose and sorbitol are sugars that are poorly absorbed by the intestine. Lactulose has been shown to be more effective than placebo in increasing stool frequency, volume, weight, and consistency in chronically constipated patients.55 In a head-to-head comparison between sugar laxatives, 70% sorbitol was as effective as lactulose in increasing the frequency of bowel movements, and it was similar in its adverse effects56; 70% sorbitol is a cost-effective alternative to lactulose in the elderly nursing home population.57
Compared with fiber alone, lactulose use leads to a significantly higher number of bowel movements and better stool consistency.58 However, when lactulose was compared with a combination of fiber and a stimulant laxative, it was less effective than the combination therapy.59,60
Sugar laxatives, while effective, may have dose-limiting or use-limiting adverse effects such as abdominal bloating and flatulence.
Phosphate, magnesium
Sodium phosphate, like polyethylene glycol, is often used as a bowel preparation before colonoscopy, for which it is about as good or slightly better than polyethylene glycol.61,62
Although magnesium and sodium phosphate preparations are effective, there are multiple reports of clinically significant electrolyte abnormalities, renal failure, and congestive heart failure occurring with these preparations. Therefore, they must be used with discretion and caution in appropriate patients with frequent monitoring.
STIMULANT (IRRITANT) LAXATIVES
Stimulant laxatives are usually reserved for use when bulking agents and osmotic laxatives fail. Their mechanism of action involves the alteration of intestinal motility and intestinal fluid secretion.
Anthraquinones (cascara, aloe, and senna), castor oil, and diphenylmethanes (bisacodyl) are the most commonly used stimulant laxatives. They work relatively quickly, often eliciting a bowel movement 2 to 8 hours after they are taken.
This class of laxatives has historically been underused or given for only short periods of time, owing to concern about impairing colonic function, damaging the enteric nervous system, causing laxative dependency, causing cathartic colon, and even causing colon cancer. However, there is very little evidence to support these concerns. Stimulant laxatives can be used on a more regular basis when bulking or osmotic agents fail.63
Possibly of greatest concern is the potential for the overuse and abuse of stimulant laxatives. Excessive use can cause electrolyte disturbances brought about by high-volume watery diarrhea. Risk factors for overuse and abuse include underlying psychiatric disturbances and eating disorders. Prescribing other types of laxatives or cathartic agents may reduce risk, but the potential for abuse exists with all categories of laxatives.
TEGASEROD: GONE BUT STILL AVAILABLE, ON A CONTROLLED BASIS
Tegaserod (Zelnorm), a serotonin (5-HT4) agonist, was used predominantly in women with constipation-predominant irritable bowel syndrome and in men and women with chronic constipation. However, it was suspended from the market in the United States in March 2007 owing to concern about a high risk of adverse cardiovascular effects compared with placebo.
In a double-blind, randomized controlled trial, men with chronic constipation who received tegaserod 6 mg twice a day for 12 weeks had more spontaneous bowel movements than those receiving placebo (P = .04).64
Lin et al65 evaluated the use of tegaserod 6 mg twice daily for 4 weeks in both men and women with chronic constipation. Those receiving tegaserod had significantly more spontaneous bowel movements per week, less straining, and better stool consistency than those receiving placebo.
Tegaserod can still be obtained for appropriate patients via a treatment investigational new drug application. Safety data are under further review by the US Food and Drug Administration. Studies of other serotonin agonists are under way.
LUBIPROSTONE
Lubiprostone (Amitiza) is an agonist of the chloride channel subtype 2, found on the apical membrane of intestinal epithelial cells. It causes increased chloride secretion into the intestinal lumen, enhancing intestinal fluid secretion. It has been shown to be effective in chronic constipation by improving stool consistency and increasing the motility of the small intestine and colon.66 It is approved for treating chronic constipation in adults.
In randomized, double-blind trials, patients receiving lubiprostone 24 fig twice daily for 4 weeks had significantly more bowel movements per week, reported significantly better stool consistency and less abdominal bloating and straining, and rated their constipation as less severe than did patients receiving placebo.67–69
More recently, in an open-label study, lubiprostone improved constipation symptoms when taken for up to 48 weeks.70
The drug is well tolerated, but its adverse effects include nausea (which appears to be dose-dependent and may diminish over time or if the drug is taken with food), diarrhea, and headache.68 Of note, the drug appears to be well tolerated by older people (65 years of age and older), in whom adverse effects occur less often than in younger users.71 However, adverse events may cause up to 20% of patients to stop taking the drug.69 When lubiprostone is discontinued, patients may once again revert to their baseline bowel habit.72
Lubiprostone has not been compared with conventional laxatives, and cost may prohibit it from becoming a first-line drug for chronic constipation.73
OTHER PROMOTILITY AGENTS
Several promotility agents have been studied for treating chronic idiopathic constipation.
Cisapride (Propulsid), a 5-HT3 receptor antagonist and 5-HT4 receptor agonist, and prucalopride, a 5-HT4 agonist, were effective in relieving symptoms associated with chronic constipation.74–76 However, safety issues (cardiac arrhythmias) necessitated withdrawal of cisapride from the US market in 2000. Prucalopride is undergoing clinical trials.77
Renzapride, a mixed 5-HT4 receptor agonist and 5-HT3 receptor antagonist, has been shown to improve stool consistency and to increase colonic transit in patients with constipation-predominant irritable bowel syndrome.78 Renzapride has been studied in patients with this condition,78–81 but not in patients with chronic constipation. Renzapride is in phase III clinical development in the United States for treating constipation-predominant irritable bowel syndrome.
EMERGING TREATMENTS
New drugs with novel mechanisms of action are being investigated for the treatment of chronic idiopathic constipation.
Neurotrophin-3, a neurotrophic factor, modulates the development of the nervous system by regulating the survival and differentiation of nerves.82 In patients with functional constipation, subcutaneous doses of neurotrophin-3 improved stool frequency, the number of complete spontaneous bowel movements, and stool consistency.83
Alvimopan is a selective antagonist of the muopioid receptor that is being studied for opiate-related constipation and postoperative ileus.84,85 Little of this drug is systemically absorbed and it does not cross the blood-brain barrier; thus, it relieves the opiate-related side effects, ie, bloating, abdominal discomfort, and reduced stool frequency, without interfering with the central analgesic effects.
Linaclotide (MD 1100), a poorly absorbed guanylate cyclase agonist, is also being investigated as a treatment for chronic constipation.86 Linaclotide increases intestinal fluid secretion and transit via stimulation of cyclic guanosine monophosphate production and activation of the cystic fibrosis transmembrane conductance regulator.86,87 In preliminary studies, linaclotide increased stool frequency and the Bristol Stool Form Scale consistency score (Table 1) by increasing intestinal fluid secretion and transit.86
Chenodeoxycholic acid is a bile acid that is synthesized from cholesterol.88 Treatment of constipation with chenodeoxycholic acid has been proposed, given its laxative effect. A study by Bazzoli et al89 showed increased stool frequency and a decrease in stool consistency in chronic constipation patients given chenodeoxycholic acid 10 mg/kg/day. The main side effect was diarrhea. Chenodeoxycholic acid may be worthwhile in the management of constipation, but more studies are needed.
PROBIOTICS AND PREBIOTICS
The bacteria of the colon influence peristalsis of the colon.90 Probiotics (live bacterial preparations) and prebiotics (nondigestible preparations that stimulate the growth or activity of beneficial colonic bacteria) have been gaining interest as potential therapies for constipation.91,92
Probiotic bacterial preparations are generally composed of strains of Bifodobacterium,93,94Lactobacillus,95 and combinations thereof, and are available as mixed preparations of multiple bacterial strains of Lactobacillus, Bifodobacterium, and Streptococcus species, such as VSL#3.96
Probiotics may help relieve constipation, but their effect may depend on the strain of bacteria used and the population being studied.97 In a double-blind parallel study in 70 healthy adults, ingestion of 375 g/day of milk fermented with B animalis strain DN-173 010 for 11 days reduced colon transit time by 20% from baseline. The effect was more pronounced in women, particularly in those with longer baseline transit.98
Lactic acid-producing bacteria are considered commensal organisms with essentially no pathogenic potential.99 A review of the safety of bifodobacteria and lactobacilli concluded there was no health risk to consumers.100
Prebiotics are short-chain carbohydrates such as lactulose that stimulate the activity of beneficial colonic bacteria.91 They are thought to have a small laxative effect that is likely both osmotic and due to beneficial actions of bacteria for which they are a substrate. Both konjac glucomannan and lactulose, sugar-based laxatives and prebiotics, have been shown to significantly increase the fecal concentrations of lactobacilli and total bacteria, possibly through increases in stool bulk.46 Prebiotics that have been the focus of research include inulin, fructo-oligosaccharides, and galacto-oligosaccharides.91 Evidence on the efficacy of probiotics and prebiotics at relieving symptoms of constipation, however, is inconclusive because few well-controlled clinical studies have been done.91,92
STRATEGIES FOR MANAGING CHRONIC CONSTIPATION
In the absence of secondary causes, treatment of chronic constipation is focused on relieving symptoms.
If symptoms are refractory to these traditional treatments, agents such as lactulose and polyethylene glycol may provide relief.11,21 Although they do not address the underlying cause of constipation, these agents increase the fluid content of the intestine, contributing to improved stool consistency, and consequently increase the frequency of bowel movements.
Lubiprostone similarly increases the fluid content of the colon, contributing to improved stool consistency, reduced fecal transit time, and increased frequency of bowel movements.66,70,102 Unlike lactulose and polyethylene glycol, which are indicated only for short-term use, lubiprostone has been found to be safe and effective when used for up to 48 weeks.70,71
Biofeedback is the preferred treatment for pelvic floor dyssynergia, in which it has a success rate of 70% to 81% and in which it is superior to standard treatment (laxatives, fiber, and education).103–105 In an instrument-based training program, patients receive auditory or visual feedback or both to help train the pelvic floor and relax the anal sphincter while simulating defecation. It also improves rectal sensation to assist in proper evacuation. The best outcomes are achieved when committed patients receive instruction from empathetic, properly trained physical therapists or other technicians. Studies show that the benefits of biofeedback are long-lasting.104 It does not improve slow-transit constipation, though pelvic floor dyssynergia and slow-transit constipation can overlap.
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004; 99:750–759.
- Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005; 100 suppl 1:S5–S21.
- Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2005. Vital Health Stat 10 2006; 232:1–153.
- Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005; 23:461–476.
- Arce DA, Ermocilla CA, Costa H. Evaluation of constipation. Am Fam Physician 2002; 65:2283–2290.
- Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol 1989; 11:525–536.
- Harari D, Gurwitz JH, Minaker KL. Constipation in the elderly. J Am Geriatr Soc 1993; 41:1130–1140.
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007; 25:599–608.
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003; 349:1360–1368.
- Sandler RS, Drossman DA. Bowel habits in young adults not seeking health care. Dig Dis Sci 1987; 32:841–845.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130:1480–1491.
- Locke GR, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology 2000; 119:1766–1778.
- Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003; 32:659–683.
- Locke GR, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000; 119:1761–1766.
- Prather CM. Subtypes of constipation: sorting out the confusion. Rev Gastroenterol Disord 2004; 4 suppl 2:S11–S16.
- Talley NJ, Jones M, Nuyts G, Dubois D. Risk factors for chronic constipation based on a general practice sample. Am J Gastroenterol 2003; 98:1107–1111.
- Bharucha AE. Treatment of severe and intractable constipation. Curr Treat Options Gastroenterol 2004; 7:291–298.
- Borum ML. Constipation: evaluation and management. Prim Care 2001; 28:577–590.
- Camilleri M, Ford MJ. Review article: colonic sensorimotor physiology in health, and its alteration in constipation and diarrhoeal disorders. Aliment Pharmacol Ther 1998; 12:287–302.
- Knowles CH, Martin JE. Slow transit constipation: a model of human gut dysmotility. Review of possible aetiologies. Neurogastroenterology Motility 2000; 12:181–196.
- Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther 2001; 15:749–763.
- Crowell MD. Pathogenesis of slow transit and pelvic floor dysfunction: from bench to bedside. Rev Gastroenterol Disord 2004; 4 suppl 2:S17–S27.
- Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol 2002; 35 suppl 1:S11–S22.
- Chitkara DK, Bredenoord AJ, Cremonini F, et al. The role of pelvic floor dysfunction and slow colonic transit in adolescents with refractory constipation. Am J Gastroenterol 2004; 99:1579–1584.
- El-Salhy M. Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci 2002; 107:101–110.
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 2004; 141:1285–1293.
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 1999; 13 suppl 2:15–30.
- Gershon MD. Serotonin and its implication for the management of irritable bowel syndrome. Rev Gastroenterol Disord 2003; 3 suppl 2:S25–S34.
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 1998; 115:370–380.
- Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE. Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology 1985; 88:26–34.
- O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990; 300:439–440.
- Meshkinpour H, Selod S, Movahedi H, Nami N, James N, Wilson A. Effects of regular exercise in management of chronic idopathic constipation. Dig Dis Sci 1998; 43:2379–2383.
- Young RJ, Beerman LE, Vanderhoff JA. Increasing oral fluids in chronic constipation in children. Gastroenterol Nurs 1998; 21:156–161.
- Tuteja AK, Talley NJ, Joos SK, Woehl JV, Hickam DH. Is constipation associated with decreased physical activity in normally active subjects? Am J Gastroenterol 2005; 100:124–129.
- Towers AL, Burgio KL, Locher JL, Merkel IS, Safaeian M, Wald A. Constipation in the elderly: influence of dietary, psychological, and physiological factors. J Am Geriatr Soc 1994; 42:701–706.
- Anti J, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998; 45:727–732.
- Murakami K, Sasaki S, Okubo H, et al. Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur J Clin Nutr 2007; 61:616–622.
- Wong ML, Wee S, Pin CH, Gan GL, Ye HC. Sociodemographic and lifestyle factors associated with constipation in an elderly Asian community. Am J Gastroenterol 1999; 94:1283–1291.
- Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003; 98:1790–1796.
- Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol 1997; 92:95–98.
- Graham DY, Moser SE, Estes MK. The effect of bran on bowel function in constipation. Am J Gastroenterol 1982; 77:599–603.
- Muller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. Br Med J (Clin Res Ed) 1988; 296:615–617.
- Badiali D, Corazziari E, Habib FI, et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci 1995; 40:349–356.
- Andersson H, Basaeus I, Falkheden T, Melkersson M. Transit time in constipated geriatric patients during treatment with bulk laxative and bran: a comparison. Scand J Gastroenterol 1979; 14:821–826.
- Hamilton JW, Wagner J, Burdick BB, Bass P. Clinical evaluation of methyl-cellulose as a bulk laxative. Dig Dis Sci 1988; 33:993–998.
- Chen HL, Cheng HC, Liu YJ, Liu SY, Wu WT. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition 2006; 22:1112–1119.
- Ashraf W, Park F, Lof J, Quigley EM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther 1995; 9:639–647.
- Cheskin LJ, Kamal N, Crowell MD, Schuster MM, Whitehead WE. Mechanisms of constipation in older persons and effects of fiber compared with placebo. J Am Geriatr Soc 1995; 43:666–669.
- Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fiber in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004; 19:245–251.
- Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF–100) in treatment of chronic nonorganic constipation. Dig Dis Sci 1996; 41:1636–1642.
- Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999; 44:226–230.
- Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerabilitity of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF–100) in the treatment of functional chronic constipation. Gut 2000; 46:522–526.
- Fritz E, Hammer HF, Lipp RW, Högenauer C, Stauber R, Hammer J. Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther 2005; 21:259–268.
- DiPalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007; 102:1436–1441.
- Bass P, Dennis S. The laxative effects of lactulose in normal and constipated subjects. J Clin Gastroenterol 1981; 3 suppl 1:23–28.
- Lederle FA, Busch DL, Mattox KM, West MJ, Aske DM. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990; 89:597–601.
- Volicer L, Lane P, Panke J, Lyman P. Management of constipation in residents with dementia: sorbitol effectiveness and cost. J Am Med Dir Assoc 2004; 5:239–241.
- Quah HM, Ooi BS, Seow-Choen F, Sng KK, Ho KS. Prospective randomized crossover trial comparing fibre with lactulose in the treatment of idiopathic chronic constipation. Tech Coloproctol 2006; 10:111–114.
- Passmore AP, Davies KW, Flanagan PG, Stoker C, Scott MG. A comparison of Agiolax and lactulose in elderly patients with chronic constipation. Pharmacology 1993; 47 suppl 1:249–252.
- Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ 1993; 307:769–771.
- Poon CM, Lee DW, Mak SK, et al. Two liters of polyethylene glycol-electrolyte lavage solution versus sodium phosphate as bowel cleansing regimen for colonoscopy: a prospective randomized controlled trial. Endoscopy 2002; 34:560–563.
- Rostom A, Jolicoeur E, Dube C, et al. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc 2006; 64:544–552.
- Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol 2003; 36:386–389.
- Fried M, Johanson JF, Gwee KA, Wagner A, Pecher E, Rueegg P. Efficacy of tegaserod in chronic constipation in men. Am J Gastroenterol 2007; 102:362–370.
- Lin SR, Ke MY, Luo JY, et al. A randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of tegaserod in patients from China with chronic constipation. World J Gastroenterol 2007; 13:732–739.
- Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G942–G947.
- McKeage K, Plosker GL, Siddiqui MA. Lubiprostone. Drugs 2006; 66:873–879.
- Johanson JF, Gargano MA, Patchen ML, Ueno R. Efficacy and safety of a novel compound, RU-0211, for the treatment of constipation [abstract]. Gastroenterology 2002; 122 suppl 1:A315.
- Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Multicenter open-label study of oral lubiprostone for the treatment of chronic constipation [abstract]. Am J Gastroenterol 2005; 100 suppl:S331.
- Johanson JF, Panas R, Holland P, Ueno R. Long-term efficacy of lubiprostone for the treatment of chronic constipation [abstract]. Gastroenterology 2006; 130 suppl 2:A317.
- Ueno R, Panas R, Wahle A, Zhu Y, Holland P. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in the elderly [abstract]. Gastroenterology 2006; 130 suppl 2:A188.
- Johanson JF, Gargano MA, Holland P, Patchen ML, Ueno R. Phase III, randomized withdrawal study of RU-0211, a novel chloride channel activator for the treatment of constipation [abstract]. Gastroenterology 2004; 126 suppl 2:A100.
- Rivkin A, Chagan L. Lubiprostone: chloride channel activator for chronic constipation. Clin Ther 2006; 28:2008–2021.
- Johanson JF, Miner PB, Parkman HP, et al. Prucalopride improves bowel movement frequency and symptoms in patients with chronic constipation: results of two double-blind, placebo-controlled trials [abstract]. Gastroenterology 2000; 118 suppl 2:A175.
- Cash BD, Chey WD. Review article: the role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment Pharmacol Ther 2005; 22:1047–1060.
- Altabas K, Biliæ A, Jurciæ D, et al. The efficacy of cisapride vs. placebo and diet in patients with chronic constipation. Coll Antropol 2003; 27:197–204.
- Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 2008; 358:2344–2354.
- Camilleri M, McKinzie S, Fox J, et al. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2004; 2:895–904.
- Tack J, Middleton SJ, Horne MC, et al. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23:1655–1665.
- Henderson JC, Palmer RM, Meyers NL, Spiller RC. A phase IIb clinical study of renzapride in mixed symptom (alternating) irritable bowel syndrome [abstract]. Gastroenterology 2004; 126 suppl 2:A644.
- Meyers NL, Palmer RMJ, George A. Efficacy and safety of renzapride in patients with constipation-predominant IBS: a phase IIb study in the UK primary healthcare setting [abstract]. Gastroenterology 2004; 126 suppl 2:A640.
- Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 2000; 119:41–50.
- Parkman HP, Rao SS, Reynolds JC, et al. Neurotrophin-3 improves functional constipation. Am J Gastroenterol 2003; 98:1338–1347.
- Camilleri M. Alvimopan, a selective peripherally acting muopioid antagonist. Neurogastroenterol Motil 2005; 17:157–165.
- Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 2004; 361:192–195.
- Kurtz C, Fitch D, Busby R, et al. Effects of multidose administration of MD-1100 on safety, tolerability, exposure and pharmacodynamics in healthy subjects [abstract]. Gastroenterology 2006; 130 suppl 2:A26.
- Eutamene H, Theodorou V, Tondereau V, et al. Influence of guanylate cyclase C binding ligand MD-1100 on TNBS-induced visceral hypersensitivity in WT vs. KO guanylate cyclase C deficient mice [abstract]. Gastroenterology 2006; 130 suppl 2:A597.
- Broughton G. Chenodeoxycholate: the bile acid. The drug. a review. Am J Med Sci 1994; 307:54–63.
- Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res 1983; 11:120–123.
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther 2005; 22:495–512.
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 2006; 24:701–714.
- Ouwehand A, Lagstrom H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab 2002; 46:159–162.
- Whorwell P, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101:1581–1590.
- O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128:541–551.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft H. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol 2003; 17:655–659.
- Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2003; 17:895–904.
- Fernández-Bañares F. Nutritional care of the patient with constipation. Best Pract Res Clin Gastroenterol 2006; 20:575–587.
- Bouvier M, Meance S, Bouley C, Berta J, Grimaud J. Effects of consumption of milk fermented by the probiotic strain Bifidobacterium animalis DN-173 010 on colonit transit times in healthy humans. Biosci Microflor 2001; 20 2:43–48.
- Makelainen H, Tahvonen R, Salminen S, Ouwehand AC. In vivo safety assessment of two Bifidobacterium longum strains. Microbiol Immunol 2003; 47:911–914.
- Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 2003; 36:775–780.
- Ramkumar D, Rao S. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005; 100:936–971.
- Ueno R, Osama H, Habe T, Engelke K, Patchen M. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels [abstract]. Gastroenterology 2004; 126 suppl 2:A298.
- Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham biofeedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007; 5:331–338.
- Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006; 130:657–664.
- Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 2007; 50:428–441.
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004; 99:750–759.
- Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005; 100 suppl 1:S5–S21.
- Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2005. Vital Health Stat 10 2006; 232:1–153.
- Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005; 23:461–476.
- Arce DA, Ermocilla CA, Costa H. Evaluation of constipation. Am Fam Physician 2002; 65:2283–2290.
- Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol 1989; 11:525–536.
- Harari D, Gurwitz JH, Minaker KL. Constipation in the elderly. J Am Geriatr Soc 1993; 41:1130–1140.
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007; 25:599–608.
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003; 349:1360–1368.
- Sandler RS, Drossman DA. Bowel habits in young adults not seeking health care. Dig Dis Sci 1987; 32:841–845.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130:1480–1491.
- Locke GR, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology 2000; 119:1766–1778.
- Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003; 32:659–683.
- Locke GR, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000; 119:1761–1766.
- Prather CM. Subtypes of constipation: sorting out the confusion. Rev Gastroenterol Disord 2004; 4 suppl 2:S11–S16.
- Talley NJ, Jones M, Nuyts G, Dubois D. Risk factors for chronic constipation based on a general practice sample. Am J Gastroenterol 2003; 98:1107–1111.
- Bharucha AE. Treatment of severe and intractable constipation. Curr Treat Options Gastroenterol 2004; 7:291–298.
- Borum ML. Constipation: evaluation and management. Prim Care 2001; 28:577–590.
- Camilleri M, Ford MJ. Review article: colonic sensorimotor physiology in health, and its alteration in constipation and diarrhoeal disorders. Aliment Pharmacol Ther 1998; 12:287–302.
- Knowles CH, Martin JE. Slow transit constipation: a model of human gut dysmotility. Review of possible aetiologies. Neurogastroenterology Motility 2000; 12:181–196.
- Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther 2001; 15:749–763.
- Crowell MD. Pathogenesis of slow transit and pelvic floor dysfunction: from bench to bedside. Rev Gastroenterol Disord 2004; 4 suppl 2:S17–S27.
- Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol 2002; 35 suppl 1:S11–S22.
- Chitkara DK, Bredenoord AJ, Cremonini F, et al. The role of pelvic floor dysfunction and slow colonic transit in adolescents with refractory constipation. Am J Gastroenterol 2004; 99:1579–1584.
- El-Salhy M. Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci 2002; 107:101–110.
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 2004; 141:1285–1293.
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 1999; 13 suppl 2:15–30.
- Gershon MD. Serotonin and its implication for the management of irritable bowel syndrome. Rev Gastroenterol Disord 2003; 3 suppl 2:S25–S34.
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 1998; 115:370–380.
- Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE. Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology 1985; 88:26–34.
- O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990; 300:439–440.
- Meshkinpour H, Selod S, Movahedi H, Nami N, James N, Wilson A. Effects of regular exercise in management of chronic idopathic constipation. Dig Dis Sci 1998; 43:2379–2383.
- Young RJ, Beerman LE, Vanderhoff JA. Increasing oral fluids in chronic constipation in children. Gastroenterol Nurs 1998; 21:156–161.
- Tuteja AK, Talley NJ, Joos SK, Woehl JV, Hickam DH. Is constipation associated with decreased physical activity in normally active subjects? Am J Gastroenterol 2005; 100:124–129.
- Towers AL, Burgio KL, Locher JL, Merkel IS, Safaeian M, Wald A. Constipation in the elderly: influence of dietary, psychological, and physiological factors. J Am Geriatr Soc 1994; 42:701–706.
- Anti J, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998; 45:727–732.
- Murakami K, Sasaki S, Okubo H, et al. Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur J Clin Nutr 2007; 61:616–622.
- Wong ML, Wee S, Pin CH, Gan GL, Ye HC. Sociodemographic and lifestyle factors associated with constipation in an elderly Asian community. Am J Gastroenterol 1999; 94:1283–1291.
- Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003; 98:1790–1796.
- Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol 1997; 92:95–98.
- Graham DY, Moser SE, Estes MK. The effect of bran on bowel function in constipation. Am J Gastroenterol 1982; 77:599–603.
- Muller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. Br Med J (Clin Res Ed) 1988; 296:615–617.
- Badiali D, Corazziari E, Habib FI, et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci 1995; 40:349–356.
- Andersson H, Basaeus I, Falkheden T, Melkersson M. Transit time in constipated geriatric patients during treatment with bulk laxative and bran: a comparison. Scand J Gastroenterol 1979; 14:821–826.
- Hamilton JW, Wagner J, Burdick BB, Bass P. Clinical evaluation of methyl-cellulose as a bulk laxative. Dig Dis Sci 1988; 33:993–998.
- Chen HL, Cheng HC, Liu YJ, Liu SY, Wu WT. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition 2006; 22:1112–1119.
- Ashraf W, Park F, Lof J, Quigley EM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther 1995; 9:639–647.
- Cheskin LJ, Kamal N, Crowell MD, Schuster MM, Whitehead WE. Mechanisms of constipation in older persons and effects of fiber compared with placebo. J Am Geriatr Soc 1995; 43:666–669.
- Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fiber in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004; 19:245–251.
- Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF–100) in treatment of chronic nonorganic constipation. Dig Dis Sci 1996; 41:1636–1642.
- Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999; 44:226–230.
- Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerabilitity of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF–100) in the treatment of functional chronic constipation. Gut 2000; 46:522–526.
- Fritz E, Hammer HF, Lipp RW, Högenauer C, Stauber R, Hammer J. Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther 2005; 21:259–268.
- DiPalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007; 102:1436–1441.
- Bass P, Dennis S. The laxative effects of lactulose in normal and constipated subjects. J Clin Gastroenterol 1981; 3 suppl 1:23–28.
- Lederle FA, Busch DL, Mattox KM, West MJ, Aske DM. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990; 89:597–601.
- Volicer L, Lane P, Panke J, Lyman P. Management of constipation in residents with dementia: sorbitol effectiveness and cost. J Am Med Dir Assoc 2004; 5:239–241.
- Quah HM, Ooi BS, Seow-Choen F, Sng KK, Ho KS. Prospective randomized crossover trial comparing fibre with lactulose in the treatment of idiopathic chronic constipation. Tech Coloproctol 2006; 10:111–114.
- Passmore AP, Davies KW, Flanagan PG, Stoker C, Scott MG. A comparison of Agiolax and lactulose in elderly patients with chronic constipation. Pharmacology 1993; 47 suppl 1:249–252.
- Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ 1993; 307:769–771.
- Poon CM, Lee DW, Mak SK, et al. Two liters of polyethylene glycol-electrolyte lavage solution versus sodium phosphate as bowel cleansing regimen for colonoscopy: a prospective randomized controlled trial. Endoscopy 2002; 34:560–563.
- Rostom A, Jolicoeur E, Dube C, et al. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc 2006; 64:544–552.
- Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol 2003; 36:386–389.
- Fried M, Johanson JF, Gwee KA, Wagner A, Pecher E, Rueegg P. Efficacy of tegaserod in chronic constipation in men. Am J Gastroenterol 2007; 102:362–370.
- Lin SR, Ke MY, Luo JY, et al. A randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of tegaserod in patients from China with chronic constipation. World J Gastroenterol 2007; 13:732–739.
- Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G942–G947.
- McKeage K, Plosker GL, Siddiqui MA. Lubiprostone. Drugs 2006; 66:873–879.
- Johanson JF, Gargano MA, Patchen ML, Ueno R. Efficacy and safety of a novel compound, RU-0211, for the treatment of constipation [abstract]. Gastroenterology 2002; 122 suppl 1:A315.
- Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Multicenter open-label study of oral lubiprostone for the treatment of chronic constipation [abstract]. Am J Gastroenterol 2005; 100 suppl:S331.
- Johanson JF, Panas R, Holland P, Ueno R. Long-term efficacy of lubiprostone for the treatment of chronic constipation [abstract]. Gastroenterology 2006; 130 suppl 2:A317.
- Ueno R, Panas R, Wahle A, Zhu Y, Holland P. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in the elderly [abstract]. Gastroenterology 2006; 130 suppl 2:A188.
- Johanson JF, Gargano MA, Holland P, Patchen ML, Ueno R. Phase III, randomized withdrawal study of RU-0211, a novel chloride channel activator for the treatment of constipation [abstract]. Gastroenterology 2004; 126 suppl 2:A100.
- Rivkin A, Chagan L. Lubiprostone: chloride channel activator for chronic constipation. Clin Ther 2006; 28:2008–2021.
- Johanson JF, Miner PB, Parkman HP, et al. Prucalopride improves bowel movement frequency and symptoms in patients with chronic constipation: results of two double-blind, placebo-controlled trials [abstract]. Gastroenterology 2000; 118 suppl 2:A175.
- Cash BD, Chey WD. Review article: the role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment Pharmacol Ther 2005; 22:1047–1060.
- Altabas K, Biliæ A, Jurciæ D, et al. The efficacy of cisapride vs. placebo and diet in patients with chronic constipation. Coll Antropol 2003; 27:197–204.
- Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 2008; 358:2344–2354.
- Camilleri M, McKinzie S, Fox J, et al. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2004; 2:895–904.
- Tack J, Middleton SJ, Horne MC, et al. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23:1655–1665.
- Henderson JC, Palmer RM, Meyers NL, Spiller RC. A phase IIb clinical study of renzapride in mixed symptom (alternating) irritable bowel syndrome [abstract]. Gastroenterology 2004; 126 suppl 2:A644.
- Meyers NL, Palmer RMJ, George A. Efficacy and safety of renzapride in patients with constipation-predominant IBS: a phase IIb study in the UK primary healthcare setting [abstract]. Gastroenterology 2004; 126 suppl 2:A640.
- Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 2000; 119:41–50.
- Parkman HP, Rao SS, Reynolds JC, et al. Neurotrophin-3 improves functional constipation. Am J Gastroenterol 2003; 98:1338–1347.
- Camilleri M. Alvimopan, a selective peripherally acting muopioid antagonist. Neurogastroenterol Motil 2005; 17:157–165.
- Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 2004; 361:192–195.
- Kurtz C, Fitch D, Busby R, et al. Effects of multidose administration of MD-1100 on safety, tolerability, exposure and pharmacodynamics in healthy subjects [abstract]. Gastroenterology 2006; 130 suppl 2:A26.
- Eutamene H, Theodorou V, Tondereau V, et al. Influence of guanylate cyclase C binding ligand MD-1100 on TNBS-induced visceral hypersensitivity in WT vs. KO guanylate cyclase C deficient mice [abstract]. Gastroenterology 2006; 130 suppl 2:A597.
- Broughton G. Chenodeoxycholate: the bile acid. The drug. a review. Am J Med Sci 1994; 307:54–63.
- Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res 1983; 11:120–123.
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther 2005; 22:495–512.
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 2006; 24:701–714.
- Ouwehand A, Lagstrom H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab 2002; 46:159–162.
- Whorwell P, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101:1581–1590.
- O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128:541–551.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft H. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol 2003; 17:655–659.
- Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2003; 17:895–904.
- Fernández-Bañares F. Nutritional care of the patient with constipation. Best Pract Res Clin Gastroenterol 2006; 20:575–587.
- Bouvier M, Meance S, Bouley C, Berta J, Grimaud J. Effects of consumption of milk fermented by the probiotic strain Bifidobacterium animalis DN-173 010 on colonit transit times in healthy humans. Biosci Microflor 2001; 20 2:43–48.
- Makelainen H, Tahvonen R, Salminen S, Ouwehand AC. In vivo safety assessment of two Bifidobacterium longum strains. Microbiol Immunol 2003; 47:911–914.
- Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 2003; 36:775–780.
- Ramkumar D, Rao S. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005; 100:936–971.
- Ueno R, Osama H, Habe T, Engelke K, Patchen M. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels [abstract]. Gastroenterology 2004; 126 suppl 2:A298.
- Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham biofeedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007; 5:331–338.
- Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006; 130:657–664.
- Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 2007; 50:428–441.
KEY POINTS
- A high-fiber diet often improves functional constipation, but it may worsen slow-transit constipation or dyssynergia (a failure of the pelvic floor muscles to relax). Nevertheless, fiber remains a mainstay of treatment for its ability to provide homogeneous stool consistency.
- Drugs approved for treating constipation increase fluid in the lumen, speed intestinal transit, and improve stool consistency, while tegaserod (Zelnorm) additionally acts as a serotonin agonist.
- Colonoscopy and other tests are reserved for patients with refractory constipation and those with symptoms suggesting colon cancer.
- Prebiotics (short-chain carbohydrates that stimulate activity of beneficial colonic bacterial flora) and probiotics (live bacterial preparations) are under evaluation as treatments for chronic constipation.