User login
Pediatric Primary Cutaneous Marginal Zone Lymphoma Treated With Doxycycline
Case Report
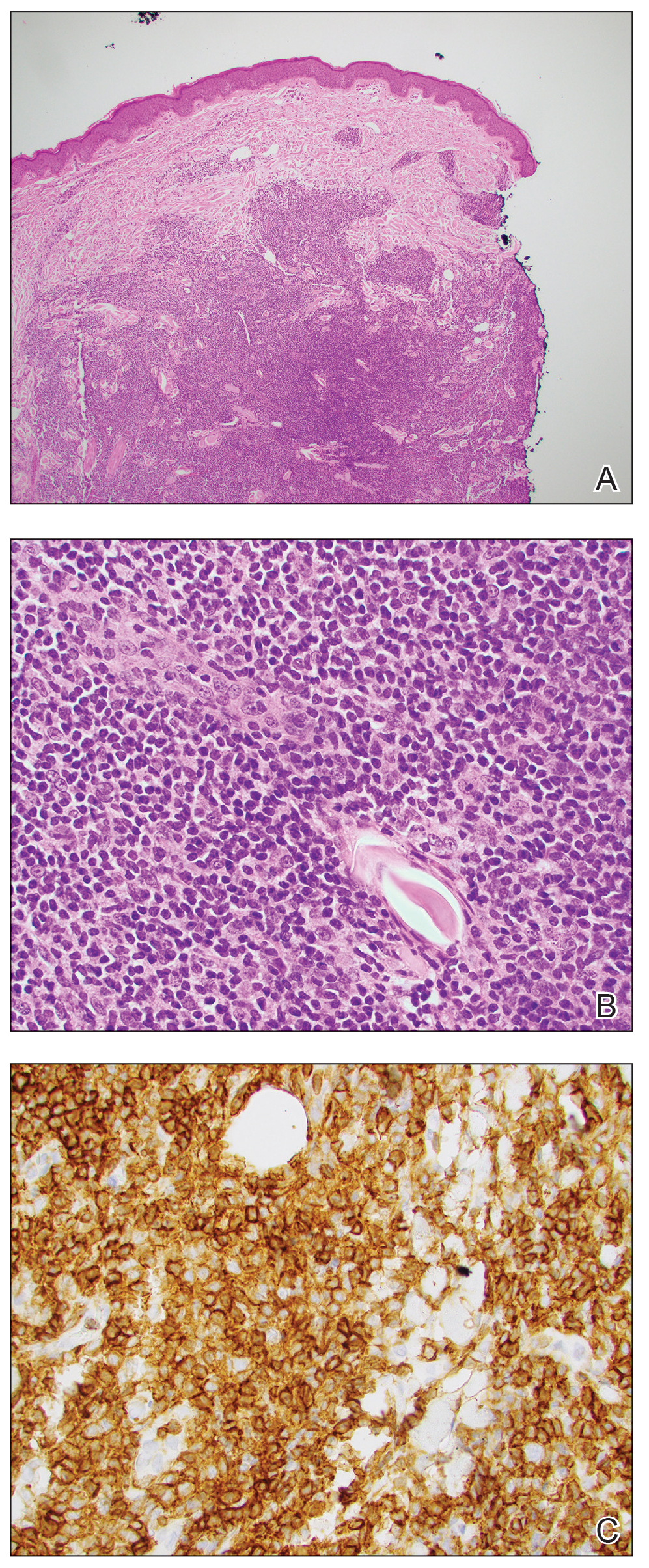
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.
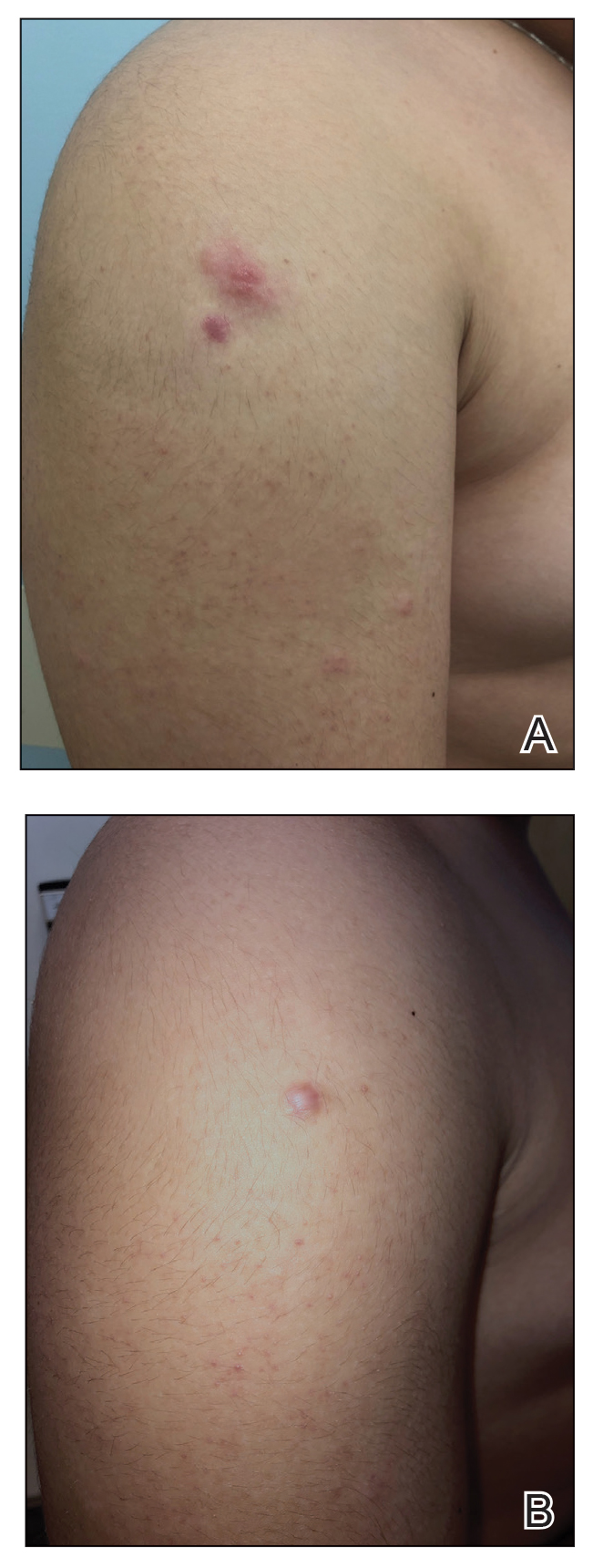
The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.
Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Practice Points
- When skin biopsy reveals marginal zone lymphoma, laboratory workup should include a complete blood cell count, chemistry, and serum lactate dehydrogenase levels. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed.
- For patients with multifocal skin lesions, positive emission tomography with computed tomography is utilized to exclude systemic disease and assess lymph node involvement.
- Treatments for primary cutaneous marginal zone lymphoma include excision, topical steroids, intralesional steroids, intralesional rituximab, radiation therapy, and antibiotics.
- Doxycycline can be considered as a treatment option for pediatric patients with widespread cutaneous involvement.
