User login
Assessment and Treatment of Late-Life Depression
From the Department of Neuropsychiatry and Behavioral Sceince, University of South Carolina School of Medicine, Columbia, SC.
Abstract
- Objective: To review the identification, clinical assessment and treatment of patients with late-life depression.
- Methods: Review of the literature.
- Results: Depressive symptoms are present in up to 1 in 4 older adults. Comprehensive evaluation of depressive symptoms in this population often requires a multidisciplinary and collaborative approach between primary care, mental health, and other ancillary providers. Key aspects include a detailed history, physical and mental status examinations, cognitive and functional status assessment, and suicide risk assessment. Treatment options include anti-depressants, psychotherapy, and electroconvulsive therapy.
- Conclusion: A systematic approach to evaluating depressive symptoms in the elderly can enhance timely recognition and treatment.
Key words: Late-life depression; clinical assessment; antidepressants; psychotherapy; electroconvulsive therapy.
The U.S. population is aging, and with this comes the potential for increased health care needs. In 2014, there were over 46 million Americans age 65 and over (14.5% of the U.S. population). This number is projected to increase to 88 million by the year 2050 [1]. One in 4 older adults suffers with depressive symptoms that cause distress and functional impairment [2]. The World Health Organization Global Burden of Disease Study found depressive disorders to be the leading cause of disability-adjusted life years (DALYs) and the second leading cause of years lived with disability (YLDs). The burden of disease due to depressive disorders increased by 37.5% between 1990 and 2010, and 10.4% was attributable to aging [3]. These figures underscore the importance of accurate assessment and treatment of depression in the elderly. In this article, we review the identification, clinical assessment, and treatment of patients with late-life depression.
Diagnostic Criteria
Prevalence
It is estimated that 1% to 4% of community-dwelling adults age 65 and older suffer from MDD, with women more likely to be affected than men (prevalence of 4.4% vs. 2.7) [2,5–7]. This estimate is low compared with lifetime prevalence of almost 20% in the general adult population [8]. However, when depressive symptoms that do not meet criteria for MDD are considered, prevalence rates increase up to 25% [2,9]. These estimates also vary by clinical setting, with the highest rates (up to 40%) among elderly patients in long-term care facilities [10,11]. While individuals with subsyndromal depression may experience fewer symptoms than those with MDD, clinically significant distress persists, impacting health and functional status. Depression is associated with overall poor social or occupational functioning, cognitive decline, increased health care utilization and cost, increased morbidity and mortality from medical illness, and increased suicide mortality [5,9,10,12].
Identifying LLD
Accurate identification of LLD also requires recognition of the differences in the presentation of LLD compared with onset in earlier life. Depression in younger adults is often marked by depressed mood and loss of interest [18]. In contrast, older adults may present with increased anger or irritability [5]. Younger adults are more likely to report suicidal thoughts while older patients report feelings of hopelessness and thoughts of death [18]. LLD is often characterized by increased somatic complaints, hypochondriasis, or pain [5,18,19]. Another major difference lies in the presentation of cognitive difficulties. Younger patients typically complain of poor concentration or indecisiveness. Geriatric patients may present with cognitive changes including objective findings of slower processing speed and executive dysfunction on neuropsychological testing [17].
Depression rating scales may aid in identification of LLD. They are not a substitute for clinical diagnosis but can be useful as screening tools. Two commonly utilized depression rating scales are the Geriatric Depression Scale (GDS) and the Patient Health Questionnaire-9 (PHQ-9). GDS is a 30-item instrument developed specifically for older adults. Shorter 15-item, 5-item, and 4-item versions exist. The scale utilizes a Yes/No format and can be self- or clinician-administered [20]. One advantage of the GDS lies in its focus on psychological and cognitive aspects of depression rather than neurovegetative symptoms that may overlap with medical illnesses common in older adults [21]. The PHQ-9 is a 9-item self- or clinician-administered screening tool designed for use in primary care settings and has also been validated in geriatric populations [22,23]. The 9 items on this scale correspond to the DSM-5 criteria for major depression. A shorter 2-item version (PHQ-2) has also been validated, and a positive screen on this test should prompt administration of the full-length version. Both versions have approximately 80% sensitivity and specificity in detecting depression. An added advantage of PHQ-9 over GDS is that it can be useful in monitoring treatment response over time [22,23]
Comprehensive Assessment of LLD
The comprehensive assessment of patients with LLD can be carried out by health professionals in both mental health or primary care settings. In a multidisciplinary approach, psychiatrists and mental health professionals have collaborated with primary care providers using depression care managers with successful outcomes in managing depression in older adults [24,25]. Complete evaluation of a patient with suspected LLD begins with a history and physical and mental status examination. Other essential components of the evaluation include assessment of cognition, functional status, and suicide risk. Laboratory and neuroimaging studies may be necessary as well. Due to the comprehensive nature of this assessment, a multidisciplinary approach with collaboration between primary care, psychiatry, psychology, and ancillary services such as social work may be necessary. Multiple patient interactions may be required to complete a thorough evaluation.
History and Mental Status Examination
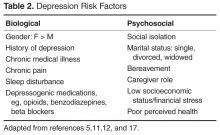
As with many other psychiatric illnesses, LLD is a clinical diagnosis. A careful history should be obtained initially utilizing open-ended questions. This should be followed by more directed questions as indicated to elicit the presence of depressive symptoms. The history should be obtained from the patient. A relevant collateral informant can be invaluable in the assessment, especially in cases where there is a comorbid neurocognitive disorder. However, the patient’s informed consent must be obtained prior to obtaining collateral information whenever possible. Psychosocial stressors that may have precipitated or may be perpetuating symptoms should be explored. Such stressors may include recent changes in living situation, loss of social support, recent deaths, or financial difficulties. Biological precipitants also need to be explored including presence of physical illness, depressogenic medications, and comorbid alcohol or other substance use. The patient’s past psychiatric history, psychiatric hospitalizations, and past medication trials should be ascertained. Any family history of depression, other psychiatric disorders, substance use disorders, and suicide attempts should be documented. A full mental status exam including cognitive assessment should be completed [21,26].
Cognitive Assessment
Cognitive impairment can be associated with LLD and may be due to the underlying depression or represent a comorbid neurocognitive disorder. Furthermore, the burden of medical illness as well as cerebrovascular and cardiovascular risk factors have been linked to executive dysfunction and reduced processing speed in individuals with LDD [27,28]. Distinguishing between these can be challenging; however, chronology of symptom onset is often helpful. Depression is more likely the etiology of cognitive impairment when depressive symptoms precede onset of cognitive deficits. This type of cognitive impairment is termed dementia syndrome of depression and may improve with treatment of depression [5]. Some patients may progress to develop major cognitive decline, and it remains unclear whether LLD represents a risk factor or prodrome to developing a major neurocognitive disorder [29]. On the other hand, if depression develops later in the course of cognitive decline, there may be an underlying neurocognitive disorder [17]. Up to 20% of individuals with major neurocognitive disorder due to Alzheimer’s disease also have major depression [11]. For these reasons, concomitant assessment of cognition is essential to the evaluation of the older adult presenting with depressive symptoms [30]. Cognitive domains that may be affected include learning and memory, language, attention, perceptual motor abilities, social cognition, and executive function [4]. Many of these domains can be assessed during the mental status examination, with brief cognitive screening tools, or with formal neuropsychological testing.
While there are numerous cognitive screening tools, some commonly used, brief tools include the Mini-Cog, the Folstein Mini-Mental State Exam (MMSE), and the Montreal Cognitive Assessment (MoCA). The Mini-Cog consists of a 3-item registration, delayed recall, and clock drawing test and has several advantages over other screening tools. It is a brief test (taking approximately 3 minutes to administer) with good sensitivity and specificity of 80% or greater. Compared with other cognitive screening tools, it is less influenced by level of education, language, or cultural background [31–33]. The MMSE is a longer screening tool consisting of 19 items and requires about 10 minutes to administer. Unlike the Mini-Cog, performance on the MMSE can be affected by level of education and cultural background. However, the MMSE can be administered serially to monitor changes in cognition over time [34,35]. The MoCA is a 10-minute cognitive screening tool first developed to detect mild cognitive impairment (MCI) [36]. The MoCA consists of 7 subscore sections covering visuospatial/executive function, naming, memory (delayed recall), attention, language, abstraction, and orientation. The total score is 30, and 1 point is added to the score if the testing subject has less than high school/12 years of education. The MoCA has demonstrated better sensitivity than the MMSE for the detection of MCI [36]. Elderly patients with depression often perform poorly on these cognitive screening tests due to apathy and poor effort.
Functional Assessment
The diagnosis of LLD requires that symptoms cause significant distress or interfere with functioning. A functional assessment is especially important in the evaluation of the older adult in that it allows clinicians to determine an individual’s ability to live independently and attend to daily needs. Basic activities of daily living (ADLs) include bathing, dressing, grooming, toileting, and self-transferring. Instrumental activities of daily living (IADLs) include more complex daily activities such as preparing meals, administering medications, driving, managing finances, and using simple electronics such as the telephone or remote control [26]. Impairment in IADLs is associated with increased depression severity. Conversely, the severity of depressive symptoms along with associated cognitive impairment predicts IADL impairment [37]. The Philadelphia Multilevel Assessment Instrument is a tool that can aid in the assessment of ADLs and IADLs and has been utilized in studies examining disability in depressed elderly patients [37,38]. Other available scales to quantify functional status include OARS Physical Activities of Daily Living, OARS Instrumental Activities of Daily Living Scale, and Direct Assessment of Functional Status Scale [26].
Suicide Assessment
Assessment for suicidality is an integral part of all psychiatric evaluations and is especially important in the evaluation of the depressed older adult. According to the Centers for Disease Control and Prevention, the suicide rate for individuals age 65 and older is 16.6 per 100,000, a figure that is comparable to that for individuals 18–64 years of age [39]. Non-Hispanic Caucasian males age 85 and older have the highest rate of completed suicide (56.5 per 100,000), underscoring the importance of a thorough suicide assessment [39]. Suicidality can range from passive thoughts of death and wishing that one were not alive, to active thoughts of self-harm with plan and intent. A Canadian study found 2% of community-dwelling adults age 55 and older had suicidal thoughts over a 12-month period and, of these, 28% had major depression [40]. A suicide assessment begins with inquiring about the presence of suicidal thoughts, plans, and intent. The 3 most frequently used methods of completed suicide in the elderly are firearms (28%), hanging (24%) and poisoning (21%) [41]. Access to weapons or other lethal means of self-harm such has hoarding of medications should be ascertained.
A complete suicide assessment requires attention to suicide risk factors, protective factors, and warning signs of impending suicide. Risk factors for suicide in the older adult include mood disorders, chronic medical illnesses and associated functional impairment, chronic pain, and psychosocial factors such as social isolation [42]. Mood disorders are present in 54% to 87% of cases of completed suicide, with major depression being the most common [42]. Chronic medical illness and pain can result in functional impairment leading to feelings of excessive guilt or being a burden to loved ones. Protective factors such as social connectedness, spirituality, religious beliefs, and cultural attitudes against suicide may serve as buffers against these risk factors [43]. Warning signs of impending suicide may indicate preparations for suicide and include feelings of hopelessness or lack of purpose, feeling trapped, talking about death, threatening suicide, agitation, social withdrawal, increased substance use and reckless behavior. Warning signs should prompt action to ensure the safety of the individual [44,45].
Physical Examination, Laboratory Studies, and Neuroimaging
Evaluation of LLD is not complete without a physical examination and ancillary studies to identify underlying medical conditions possibly contributing to or mimicking depressive symptoms. Routine laboratory studies include complete blood count, complete metabolic panel, thyroid studies, and urine drug screen. Signs and symptoms of underlying medical conditions may necessitate further laboratory studies [46]. Neuroimaging may reveal signs of cerebrovascular disease which can predispose, precipitate, or perpetuate depression in older adults [47].
Treatment
Treatment of LLD can take many forms and occur in various settings. Geriatric psychiatrists have expertise in the assessment and treatment of mental illness in the elderly. Workforce estimates for 2010 revealed 1 geriatric psychiatrist per 10,000 adults age 75 and over. This figure is estimated to decrease to 0.5 per 10,000 by the year 2030, underscoring the importance of increasing the knowledge base of clinicians across specialties who provide care to the depressed elderly [48]. The primary care setting is often the locus of care for depression in older adults; however, studies suggest that patients are often left untreated or undertreated [49]. Collaborative care models whereby mental health care is integrated into primary care have been shown to improve outcomes. The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) study found that use of care managers to assist primary care providers in identification of depression, offer algorithm-based treatment recommendations, monitor symptoms and medication side effects, and provide follow-up yielded improvement in outcomes. Patients in the intervention group were more likely to receive pharmacotherapy or psychotherapy, achieve remission, and showed greater decline in suicidal ideation [50]. Similar results were found in the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study in which intervention patients treated under a collaborative care model showed lower depression severity, less functional impairment, and greater reduction in depressive symptoms [25].
Just as a collaborative care model can lead to improved outcomes, the overall strategy of treating depression must be multifaceted. The biopsychosocial model of disease first described in the 1970s emphasizes biological and psychosocial determinants of illness that must be addressed when treatment is considered [51]. This includes nonmodifiable biological factors such as age, gender, and genetic predisposition that may affect treatment options, as well as modifiable biological factors such as comorbid medical illness, medications, or substance use disorders. Psychological factors that can affect depressive symptoms include coping skills and defense mechanisms in the face of stressful life events. Social factors including the role of culture, environment, and family dynamics in disease presentation must be considered as well [52].
Pharmacologic Treatment of LLD
The primary pharmacologic treatment for depression is antidepressants. Treatment consists of 3 phases—acute, continuation, and maintenance. In the acute phase, the goal is remission of current symptoms and restoration of function. The continuation phase, extending up to 6 months after remission, aims to prevent relapse back into a depressive episode. Maintenance therapy is geared at preventing recurrence of future depressive episodes [53]. Studies have found a 50% risk of relapse after 1 episode of depression and 80% after 2 episodes. Up to 20% will develop chronic symptoms. For this reason, maintenance therapy is often necessary for recurrent depression [54].
While cognitive impairment may affect antidepressant efficacy, age does not appear to be a determinant. Gildengers et al examined antidepressant response in young, middle, and older-old patients and found no significant difference in response rates [59]. Early onset versus late onset of first depressive episode also does not predict antidepressant response in patients age 55 and over [60]. There is scant evidence for efficacy of antidepressants in depressed patients with neurocognitive disorders. A 2002 Cochrane review with 4 studies in the meta-analysis (n = 137) concluded that there was weak support for antidepressant efficacy in this population [61]. A 2011 meta-analysis with 330 participants also yielded inconclusive results [62]. The paucity of evidence for antidepressant efficacy in depressed patients with neurocognitive disorders should prompt careful consideration of potential benefits versus adverse effects.
Antidepressants are generally well tolerated in older adults. Side effects vary by medication and contribute to discontinuation in up to 25% of new users (versus 22% for new users who discontinue for reasons other than side effects) [63]. Potential adverse effects shared by most SSRIs and SNRIs include GI disturbance (nausea, diarrhea or constipation), sexual dysfunction, headache, and sleep disturbance [64,65]. In addition, abrupt discontinuation can precipitate serotonin withdrawal syndrome characterized by sensory disturbance (paresthesia, tremor, and irritability) as well as headache, lightheadedness, diaphoresis, insomnia, and agitation. Other medication-specific side effects include risk of seizure with bupropion and sedation with mirtazapine [65].
Despite superiority of antidepressants to placebo in treating depression, up to one-third of patients may not respond to a trial of antidepressants. Sequential treatment protocols such as switching to a different antidepressant or augmentation can increase the proportion of antidepressant responders [66–68]. Studies have found particularly favorable response to augmentation with lithium, with one study achieving a 33% remission rate in treatment- resistant geriatric depression [67,69]. Other pharmacologic augmentation strategies include the addition of mood stabilizers such as lamotrigine, antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone), and psychostimulants [70–73]. Electroconvulsive therapy (ECT) is a nonpharmacologic option for treatment-resistant depression that will be reviewed later.
Psychotherapeutic and Psychosocial Interventions
Psychotherapeutic interventions have demonstrated efficacy in the treatment of geriatric depression, including but not limited to cognitive behavioral therapy (CBT), interpersonal therapy (IPT), problem-solving therapy (PST), reminiscence and life review, and brief psychodynamic psychotherapy [74]. Some older adults may prefer psychotherapy to pharmacologic treatment (57% vs. 43%) [75]. Potential benefits of psychotherapy include ability to directly address psychosocial stressors that may precipitate or perpetuate depressive symptoms. In addition, psychotherapy is associated with few to no side effects and avoids drug interactions. Barriers to employing psychotherapy may include cost and access to trained psychotherapists [76]. Efficacy of several psychotherapeutic approaches in the care of older depressed adults has been examined. CBT, brief psychodynamic psychotherapy, and IPT will be briefly reviewed here.
CBT. Cognitive therapy was first described by Aaron Beck in the 1960s [77]. It is a highly structured therapy built on the premise that beliefs and assumptions an individual holds can influence emotions and behavior. CBT aims to identify maladaptive belief systems, test the validity of these cognitive distortions, and help individuals formulate more realistic cognitions [78]. Symptom improvement results from addressing these cognitive aspects as well as integration of behavioral activation and skills training to overcome maladaptive behavioral patterns [78]. CBT approaches have been applied to older adults with depression and results show acceptability [79] and efficacy in this population [80–82]. A 2008 Cochrane review (n = 153) found CBT to be superior to waitlist controls [82].
Brief psychodynamic psychotherapy. Brief psychodynamic psychotherapy, unlike highly structured CBT, aims to alter behavior by examining how past experiences and unresolved conflicts influence current emotions and behavior. While studies on application to the treatment of geriatric depression are scarce, limited data demonstrate efficacy in treating geriatric depression [81] and no significant difference in outcomes when compared to CBT [82].
IPT. Like CBT, IPT is a structured time-limited psychotherapeutic treatment approach first developed in the late 1960s by Klerman and Weissman [83]. IPT focuses on the impact of interpersonal relationships on depressive symptoms and examines 4 domains: interpersonal conflict, interpersonal deficits, role transitions, and grief [74].
Studies have shown efficacy of IPT in reducing depressive symptoms in the elderly when compared to usual care [84]. Reynolds et al found IPT combined with nortriptyline (a tricyclic antidepressant) to be superior to either nortriptyline alone or IPT alone in preventing recurrent depressive episodes [85]. Interestingly, a similar study investigating the efficacy of IPT in combination with paroxetine (an SSRI) failed to show added benefit of IPT in preventing recurrence, suggesting that further studies are needed [86].
Psychosocial interventions are integral in the care of the elderly depressed patient. Studies have shown positive benefits of aerobic exercise on depressive symptoms [87]. Yoga, Tai Chi, and other mindfulness-based exercises can increase sense of emotional and physical wellbeing [88–90]. Spirituality, religious beliefs, and involvement with a faith group may be protective against development of mental illness while at the same time provide avenues for increased social connectedness [91]. These and other avenues for socialization should be encouraged as part of the treatment plan for older depressed patients [92]
Electroconvulsive Therapy
ECT is indicated for the treatment of mood and psychotic disorders and has demonstrated efficacy in the treatment of severe depression [93]. It is typically initiated when patients fail to respond to pharmacotherapy and psychotherapy. Circumstances in which ECT can be considered first-line treatment include situations that require a rapid response (severe inanition, weight loss, or suicidality), situations where risks of ECT are lower than that of alternative treatments, previous positive response to ECT, or strong patient preference [94]. ECT is performed under general anesthesia and involves the induction of a generalized tonic-clonic seizure, which is theorized to enhance serotonergic, noradrenergic, and dopaminergic neurotransmission. A typical course of ECT involves treatments 3 times a week for an average of 6 to 12 treatments in total [95]. Elderly patients and those suffering from severe depression with psychotic features respond more robustly to ECT [93,96]. Estimated remission rates after an ECT series have been higher than 80% [93], making this modality the most effective treatment for severe depression to date.
Conclusion
As the population continues to age, clinicians are increasingly likely to encounter patients with late-life depression. A thorough evaluation includes not only assessment of depressive symptoms, but also cognitive, functional, and suicide assessment. Treatment options include pharmaco-therapy, psychotherapy, and in some cases electroconvulsive therapy. Utilization of assessment and treatment nuances unique to the geriatric population, with a multidisciplinary and collaborative approach involving primary care, mental health, and other ancillary providers, will serve to ultimately enhance patient care.
Corresponding author: Corresponding author: Juliet Glover, MD, Dept. of Neuropsychiatry and Behavioral Science, Univ. of South Carolina School of Medicine, 15 Medical Park, Suite 301, Columbia, SC 29203, Juliet.Glover@uscmed.sc.edu.
Financial disclosures: None reported.
Author contributions: conception and design, JAG, SS; drafting of article, JAG, SS; critical revision of the article, JAG, SS.
1. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. US Census Bureau: May 2010.
2. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clinc Geriatr Med 1992; 8:235–51.
3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global gurden of disease study 2010. PLoS Med 2013;10:e1001547.
4 .American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
5 .Ellison JM, Kyomen HH, Harper DG. Depression in later life: an overview with treatment recommendations. Psychiatr Clin North Am 2012;35:203–29.
6. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.
7. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000;57:601–7.
8. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.
9. McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry 2013;21:418–32.
10. Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–87.
11. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–65.
12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003;160:1147–56.
13. Ritchie K. Late-life depression. European Psychiatry 2014;29:577.
14. Hegeman JM, de Waal MW, Comijs HC, et al. Depression in later life: a more somatic presentation? J Affect Disord 2015;170:196–202.
15. Lackamp J, Schlachet R, Sajatovic M. Assessment and management of major depressive disorder in older adults. Psychiatria Danubina 2016;28(Suppl 1):95–98.
16. Morichi V, Dell’Aquila G, Trotta F, et al. Diagnosing and treating depression in older and oldest old. Curr Pharm Des 2015;21:1690–8.
17. Fiske AJ, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89.
18. Balsis S, Cully JA. Comparing depression diagnostic symptoms across younger and older adults. Aging Ment Health 2008;12:800–6.
19. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77.
21. Blazer DG. The psychiatric interview of older adults. In: Blazer D, Steffens D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
22. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44.
23. Richardson TM, He H, Podgorski C, et al. Screening for depression in aging services clients. Am J Geriatr Psychiatry 2010;18:1116–23.
24. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012 Oct 17.
25. Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting a randomized controlled trial. JAMA 2002;288:2836–45.
26. Silver I, Herrmann N. Comprehensive psychiatric evaluation. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691–8.
28. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65.
29. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–8.
30. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 2015;1345:36–46.
31. Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7.
32. Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400.
33. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry 2010;25:111–20.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
35. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47: 2018–23.
36. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
37. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005;13:244–9.
38. Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry 1996;153:877–85.
39. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Accessed 9 Feb 2016 at http://webappa.cdc.gov/sasweb/ncipc/dataRestriction_inj.html.
40. Corna LM, Cairney J, Streiner DL. Suicide ideation in older adults: relationship to mental health problems and service use. Gerontologist 2010;50:785–97.
41. Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–84.
42. Van Orden K, Conwell Y. Suicides in late life. Curr Psychiatry Rep 2011;13:234–41.
43. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68, ix.
44. Rudd MD, Berman AL, Joiner TE, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav 2006;36:255–62.
45. Know the warning signs of suicide. American Association of Suicidology. Accessed 9 Feb 2016 at www.suicidology.org/resources/warning-signs.
46. Taylor W, Doraiswamy P. Use of the laboratory in the diagnostic workup of older adults. In: Blazer D, Steffen D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
47. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–22.
48. ADGAP Status of Geriatrics Workforce Study. Accessed 26 Dec 2016 at www.americangeriatrics.org/files/documents/gwps/Table%201_29.pdf.
49. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
50. Alexopoulos GS, Reynolds CF, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
51. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
52. Schotte CKW, Van den Bossche B, Doncker DD, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression Anxiety 2006;23:312–24.
53. Kupfer DJ, Frank E. The interaction of drug-and psychotherapy in the long-term treatment of depression. J Affect Disord 2001;62:131–7.
54. Katon W, Rutter C, Ludman EJ, et al. A randomized trial of relpase prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241–7.
55. Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord 2012;141:103–15.
56. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9.
58. Mukai Y, Tampi RR. Treatment of depression in the elderly: a review of the recent literature on the efficacy of single- versus dual-action antidepressants. Clin Ther 2009;31:945–61.
59. Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord 2002;69:177–84.
60. Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode. Am J Geriatr Psychiatry 2008;16:58–64.
61. Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Databse Syst Rev 2002;(4):CD003944.
62. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–85.
63. Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry 2011;19:211–21.
64. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician 2014;60:121–6.
65. Kennedy GJ, Marcus P. Use of antidepressants in older patients with co-morbid medical conditions: guidance from studies of depression in somatic illness. Drugs Aging 2005;22:273–87.
66. Sackheim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-tern outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
67. Kok RM, Nolen WA, Hereen TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81.
68. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004;65:1634–41.
69. Kok RM, Vink D, Hereen TJ, Nolen WA. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly; an open, randomized, controlled trial. J Clin Psychiatry 2006;68:1177–85.
70. Kok RM. What is the role of medications in late life depression? Psychiatr Clin North Am 2003;36:597–605.
71. Wen XJ, Wang LM, Liu ZL,. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 2014;47:605–16.
72. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet 2015;386:2404–12.
73. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008;16:21–30.
74. Francis JL, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am 2013;36:561–75.
75. Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist 2006;46:14–22.
76. Pinquart M, Duberstein PR, Lyness JM. Treatments for late-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501.
77. Beck Institute for Cognitive Behavior Therapy. Accessed 28 Dec 2016 at www.beckinstitute.org
78. Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970;1:184–200.
79. Landrevile P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontology B Psychol Sci Soc Sci 2001;56:P285–91.
80. Thompson LW, Gallagher D, Breckenridge JS. Comparitive effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385–90.
81. Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. J Consult Clin Psychol 1994;62:543–9.
82. Wilson KC, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;(1):CD004853
83. Markowitz JC, Weissman MM. Interpersonal psychotherapy: past, present and future. Clin Psychol Psychother 2012;19:99–105.
84. Van Schaik A, van Marwijk H, Ader H, et al. Interpersonal psychotherapy for elderly patients in primary care. Am J Geriatr Psychiatry 2006;14:777–86.
85. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39–45.
86. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8.
87. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56.
88. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs 2007;33:17–23.
89. Wang C, Bannuru R, Ramel J, et al. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010;10:23.
90. Cho KL. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Med Sport Sci 2008;52:146–54.
91. Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life- a randomized, controlled trial. Altern Ther Health Med 2006;12:26–35.
92. Nyer M, Doorley J, Durham K, et al. What is the role of alternative treatments in late-life depression? Psychiatr Clin North Am 2013;36:577–96.
93. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244–53.
94. Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical manual of electroconvulsive therapy. American Psychiatric Publishing; 2010.
95. Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry 2012;169:1238–44.
96. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late-life depression. Can J Psychiatry 2002;47:734–41.
From the Department of Neuropsychiatry and Behavioral Sceince, University of South Carolina School of Medicine, Columbia, SC.
Abstract
- Objective: To review the identification, clinical assessment and treatment of patients with late-life depression.
- Methods: Review of the literature.
- Results: Depressive symptoms are present in up to 1 in 4 older adults. Comprehensive evaluation of depressive symptoms in this population often requires a multidisciplinary and collaborative approach between primary care, mental health, and other ancillary providers. Key aspects include a detailed history, physical and mental status examinations, cognitive and functional status assessment, and suicide risk assessment. Treatment options include anti-depressants, psychotherapy, and electroconvulsive therapy.
- Conclusion: A systematic approach to evaluating depressive symptoms in the elderly can enhance timely recognition and treatment.
Key words: Late-life depression; clinical assessment; antidepressants; psychotherapy; electroconvulsive therapy.
The U.S. population is aging, and with this comes the potential for increased health care needs. In 2014, there were over 46 million Americans age 65 and over (14.5% of the U.S. population). This number is projected to increase to 88 million by the year 2050 [1]. One in 4 older adults suffers with depressive symptoms that cause distress and functional impairment [2]. The World Health Organization Global Burden of Disease Study found depressive disorders to be the leading cause of disability-adjusted life years (DALYs) and the second leading cause of years lived with disability (YLDs). The burden of disease due to depressive disorders increased by 37.5% between 1990 and 2010, and 10.4% was attributable to aging [3]. These figures underscore the importance of accurate assessment and treatment of depression in the elderly. In this article, we review the identification, clinical assessment, and treatment of patients with late-life depression.
Diagnostic Criteria
Prevalence
It is estimated that 1% to 4% of community-dwelling adults age 65 and older suffer from MDD, with women more likely to be affected than men (prevalence of 4.4% vs. 2.7) [2,5–7]. This estimate is low compared with lifetime prevalence of almost 20% in the general adult population [8]. However, when depressive symptoms that do not meet criteria for MDD are considered, prevalence rates increase up to 25% [2,9]. These estimates also vary by clinical setting, with the highest rates (up to 40%) among elderly patients in long-term care facilities [10,11]. While individuals with subsyndromal depression may experience fewer symptoms than those with MDD, clinically significant distress persists, impacting health and functional status. Depression is associated with overall poor social or occupational functioning, cognitive decline, increased health care utilization and cost, increased morbidity and mortality from medical illness, and increased suicide mortality [5,9,10,12].
Identifying LLD
Accurate identification of LLD also requires recognition of the differences in the presentation of LLD compared with onset in earlier life. Depression in younger adults is often marked by depressed mood and loss of interest [18]. In contrast, older adults may present with increased anger or irritability [5]. Younger adults are more likely to report suicidal thoughts while older patients report feelings of hopelessness and thoughts of death [18]. LLD is often characterized by increased somatic complaints, hypochondriasis, or pain [5,18,19]. Another major difference lies in the presentation of cognitive difficulties. Younger patients typically complain of poor concentration or indecisiveness. Geriatric patients may present with cognitive changes including objective findings of slower processing speed and executive dysfunction on neuropsychological testing [17].
Depression rating scales may aid in identification of LLD. They are not a substitute for clinical diagnosis but can be useful as screening tools. Two commonly utilized depression rating scales are the Geriatric Depression Scale (GDS) and the Patient Health Questionnaire-9 (PHQ-9). GDS is a 30-item instrument developed specifically for older adults. Shorter 15-item, 5-item, and 4-item versions exist. The scale utilizes a Yes/No format and can be self- or clinician-administered [20]. One advantage of the GDS lies in its focus on psychological and cognitive aspects of depression rather than neurovegetative symptoms that may overlap with medical illnesses common in older adults [21]. The PHQ-9 is a 9-item self- or clinician-administered screening tool designed for use in primary care settings and has also been validated in geriatric populations [22,23]. The 9 items on this scale correspond to the DSM-5 criteria for major depression. A shorter 2-item version (PHQ-2) has also been validated, and a positive screen on this test should prompt administration of the full-length version. Both versions have approximately 80% sensitivity and specificity in detecting depression. An added advantage of PHQ-9 over GDS is that it can be useful in monitoring treatment response over time [22,23]
Comprehensive Assessment of LLD
The comprehensive assessment of patients with LLD can be carried out by health professionals in both mental health or primary care settings. In a multidisciplinary approach, psychiatrists and mental health professionals have collaborated with primary care providers using depression care managers with successful outcomes in managing depression in older adults [24,25]. Complete evaluation of a patient with suspected LLD begins with a history and physical and mental status examination. Other essential components of the evaluation include assessment of cognition, functional status, and suicide risk. Laboratory and neuroimaging studies may be necessary as well. Due to the comprehensive nature of this assessment, a multidisciplinary approach with collaboration between primary care, psychiatry, psychology, and ancillary services such as social work may be necessary. Multiple patient interactions may be required to complete a thorough evaluation.
History and Mental Status Examination
As with many other psychiatric illnesses, LLD is a clinical diagnosis. A careful history should be obtained initially utilizing open-ended questions. This should be followed by more directed questions as indicated to elicit the presence of depressive symptoms. The history should be obtained from the patient. A relevant collateral informant can be invaluable in the assessment, especially in cases where there is a comorbid neurocognitive disorder. However, the patient’s informed consent must be obtained prior to obtaining collateral information whenever possible. Psychosocial stressors that may have precipitated or may be perpetuating symptoms should be explored. Such stressors may include recent changes in living situation, loss of social support, recent deaths, or financial difficulties. Biological precipitants also need to be explored including presence of physical illness, depressogenic medications, and comorbid alcohol or other substance use. The patient’s past psychiatric history, psychiatric hospitalizations, and past medication trials should be ascertained. Any family history of depression, other psychiatric disorders, substance use disorders, and suicide attempts should be documented. A full mental status exam including cognitive assessment should be completed [21,26].
Cognitive Assessment
Cognitive impairment can be associated with LLD and may be due to the underlying depression or represent a comorbid neurocognitive disorder. Furthermore, the burden of medical illness as well as cerebrovascular and cardiovascular risk factors have been linked to executive dysfunction and reduced processing speed in individuals with LDD [27,28]. Distinguishing between these can be challenging; however, chronology of symptom onset is often helpful. Depression is more likely the etiology of cognitive impairment when depressive symptoms precede onset of cognitive deficits. This type of cognitive impairment is termed dementia syndrome of depression and may improve with treatment of depression [5]. Some patients may progress to develop major cognitive decline, and it remains unclear whether LLD represents a risk factor or prodrome to developing a major neurocognitive disorder [29]. On the other hand, if depression develops later in the course of cognitive decline, there may be an underlying neurocognitive disorder [17]. Up to 20% of individuals with major neurocognitive disorder due to Alzheimer’s disease also have major depression [11]. For these reasons, concomitant assessment of cognition is essential to the evaluation of the older adult presenting with depressive symptoms [30]. Cognitive domains that may be affected include learning and memory, language, attention, perceptual motor abilities, social cognition, and executive function [4]. Many of these domains can be assessed during the mental status examination, with brief cognitive screening tools, or with formal neuropsychological testing.
While there are numerous cognitive screening tools, some commonly used, brief tools include the Mini-Cog, the Folstein Mini-Mental State Exam (MMSE), and the Montreal Cognitive Assessment (MoCA). The Mini-Cog consists of a 3-item registration, delayed recall, and clock drawing test and has several advantages over other screening tools. It is a brief test (taking approximately 3 minutes to administer) with good sensitivity and specificity of 80% or greater. Compared with other cognitive screening tools, it is less influenced by level of education, language, or cultural background [31–33]. The MMSE is a longer screening tool consisting of 19 items and requires about 10 minutes to administer. Unlike the Mini-Cog, performance on the MMSE can be affected by level of education and cultural background. However, the MMSE can be administered serially to monitor changes in cognition over time [34,35]. The MoCA is a 10-minute cognitive screening tool first developed to detect mild cognitive impairment (MCI) [36]. The MoCA consists of 7 subscore sections covering visuospatial/executive function, naming, memory (delayed recall), attention, language, abstraction, and orientation. The total score is 30, and 1 point is added to the score if the testing subject has less than high school/12 years of education. The MoCA has demonstrated better sensitivity than the MMSE for the detection of MCI [36]. Elderly patients with depression often perform poorly on these cognitive screening tests due to apathy and poor effort.
Functional Assessment
The diagnosis of LLD requires that symptoms cause significant distress or interfere with functioning. A functional assessment is especially important in the evaluation of the older adult in that it allows clinicians to determine an individual’s ability to live independently and attend to daily needs. Basic activities of daily living (ADLs) include bathing, dressing, grooming, toileting, and self-transferring. Instrumental activities of daily living (IADLs) include more complex daily activities such as preparing meals, administering medications, driving, managing finances, and using simple electronics such as the telephone or remote control [26]. Impairment in IADLs is associated with increased depression severity. Conversely, the severity of depressive symptoms along with associated cognitive impairment predicts IADL impairment [37]. The Philadelphia Multilevel Assessment Instrument is a tool that can aid in the assessment of ADLs and IADLs and has been utilized in studies examining disability in depressed elderly patients [37,38]. Other available scales to quantify functional status include OARS Physical Activities of Daily Living, OARS Instrumental Activities of Daily Living Scale, and Direct Assessment of Functional Status Scale [26].
Suicide Assessment
Assessment for suicidality is an integral part of all psychiatric evaluations and is especially important in the evaluation of the depressed older adult. According to the Centers for Disease Control and Prevention, the suicide rate for individuals age 65 and older is 16.6 per 100,000, a figure that is comparable to that for individuals 18–64 years of age [39]. Non-Hispanic Caucasian males age 85 and older have the highest rate of completed suicide (56.5 per 100,000), underscoring the importance of a thorough suicide assessment [39]. Suicidality can range from passive thoughts of death and wishing that one were not alive, to active thoughts of self-harm with plan and intent. A Canadian study found 2% of community-dwelling adults age 55 and older had suicidal thoughts over a 12-month period and, of these, 28% had major depression [40]. A suicide assessment begins with inquiring about the presence of suicidal thoughts, plans, and intent. The 3 most frequently used methods of completed suicide in the elderly are firearms (28%), hanging (24%) and poisoning (21%) [41]. Access to weapons or other lethal means of self-harm such has hoarding of medications should be ascertained.
A complete suicide assessment requires attention to suicide risk factors, protective factors, and warning signs of impending suicide. Risk factors for suicide in the older adult include mood disorders, chronic medical illnesses and associated functional impairment, chronic pain, and psychosocial factors such as social isolation [42]. Mood disorders are present in 54% to 87% of cases of completed suicide, with major depression being the most common [42]. Chronic medical illness and pain can result in functional impairment leading to feelings of excessive guilt or being a burden to loved ones. Protective factors such as social connectedness, spirituality, religious beliefs, and cultural attitudes against suicide may serve as buffers against these risk factors [43]. Warning signs of impending suicide may indicate preparations for suicide and include feelings of hopelessness or lack of purpose, feeling trapped, talking about death, threatening suicide, agitation, social withdrawal, increased substance use and reckless behavior. Warning signs should prompt action to ensure the safety of the individual [44,45].
Physical Examination, Laboratory Studies, and Neuroimaging
Evaluation of LLD is not complete without a physical examination and ancillary studies to identify underlying medical conditions possibly contributing to or mimicking depressive symptoms. Routine laboratory studies include complete blood count, complete metabolic panel, thyroid studies, and urine drug screen. Signs and symptoms of underlying medical conditions may necessitate further laboratory studies [46]. Neuroimaging may reveal signs of cerebrovascular disease which can predispose, precipitate, or perpetuate depression in older adults [47].
Treatment
Treatment of LLD can take many forms and occur in various settings. Geriatric psychiatrists have expertise in the assessment and treatment of mental illness in the elderly. Workforce estimates for 2010 revealed 1 geriatric psychiatrist per 10,000 adults age 75 and over. This figure is estimated to decrease to 0.5 per 10,000 by the year 2030, underscoring the importance of increasing the knowledge base of clinicians across specialties who provide care to the depressed elderly [48]. The primary care setting is often the locus of care for depression in older adults; however, studies suggest that patients are often left untreated or undertreated [49]. Collaborative care models whereby mental health care is integrated into primary care have been shown to improve outcomes. The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) study found that use of care managers to assist primary care providers in identification of depression, offer algorithm-based treatment recommendations, monitor symptoms and medication side effects, and provide follow-up yielded improvement in outcomes. Patients in the intervention group were more likely to receive pharmacotherapy or psychotherapy, achieve remission, and showed greater decline in suicidal ideation [50]. Similar results were found in the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study in which intervention patients treated under a collaborative care model showed lower depression severity, less functional impairment, and greater reduction in depressive symptoms [25].
Just as a collaborative care model can lead to improved outcomes, the overall strategy of treating depression must be multifaceted. The biopsychosocial model of disease first described in the 1970s emphasizes biological and psychosocial determinants of illness that must be addressed when treatment is considered [51]. This includes nonmodifiable biological factors such as age, gender, and genetic predisposition that may affect treatment options, as well as modifiable biological factors such as comorbid medical illness, medications, or substance use disorders. Psychological factors that can affect depressive symptoms include coping skills and defense mechanisms in the face of stressful life events. Social factors including the role of culture, environment, and family dynamics in disease presentation must be considered as well [52].
Pharmacologic Treatment of LLD
The primary pharmacologic treatment for depression is antidepressants. Treatment consists of 3 phases—acute, continuation, and maintenance. In the acute phase, the goal is remission of current symptoms and restoration of function. The continuation phase, extending up to 6 months after remission, aims to prevent relapse back into a depressive episode. Maintenance therapy is geared at preventing recurrence of future depressive episodes [53]. Studies have found a 50% risk of relapse after 1 episode of depression and 80% after 2 episodes. Up to 20% will develop chronic symptoms. For this reason, maintenance therapy is often necessary for recurrent depression [54].
While cognitive impairment may affect antidepressant efficacy, age does not appear to be a determinant. Gildengers et al examined antidepressant response in young, middle, and older-old patients and found no significant difference in response rates [59]. Early onset versus late onset of first depressive episode also does not predict antidepressant response in patients age 55 and over [60]. There is scant evidence for efficacy of antidepressants in depressed patients with neurocognitive disorders. A 2002 Cochrane review with 4 studies in the meta-analysis (n = 137) concluded that there was weak support for antidepressant efficacy in this population [61]. A 2011 meta-analysis with 330 participants also yielded inconclusive results [62]. The paucity of evidence for antidepressant efficacy in depressed patients with neurocognitive disorders should prompt careful consideration of potential benefits versus adverse effects.
Antidepressants are generally well tolerated in older adults. Side effects vary by medication and contribute to discontinuation in up to 25% of new users (versus 22% for new users who discontinue for reasons other than side effects) [63]. Potential adverse effects shared by most SSRIs and SNRIs include GI disturbance (nausea, diarrhea or constipation), sexual dysfunction, headache, and sleep disturbance [64,65]. In addition, abrupt discontinuation can precipitate serotonin withdrawal syndrome characterized by sensory disturbance (paresthesia, tremor, and irritability) as well as headache, lightheadedness, diaphoresis, insomnia, and agitation. Other medication-specific side effects include risk of seizure with bupropion and sedation with mirtazapine [65].
Despite superiority of antidepressants to placebo in treating depression, up to one-third of patients may not respond to a trial of antidepressants. Sequential treatment protocols such as switching to a different antidepressant or augmentation can increase the proportion of antidepressant responders [66–68]. Studies have found particularly favorable response to augmentation with lithium, with one study achieving a 33% remission rate in treatment- resistant geriatric depression [67,69]. Other pharmacologic augmentation strategies include the addition of mood stabilizers such as lamotrigine, antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone), and psychostimulants [70–73]. Electroconvulsive therapy (ECT) is a nonpharmacologic option for treatment-resistant depression that will be reviewed later.
Psychotherapeutic and Psychosocial Interventions
Psychotherapeutic interventions have demonstrated efficacy in the treatment of geriatric depression, including but not limited to cognitive behavioral therapy (CBT), interpersonal therapy (IPT), problem-solving therapy (PST), reminiscence and life review, and brief psychodynamic psychotherapy [74]. Some older adults may prefer psychotherapy to pharmacologic treatment (57% vs. 43%) [75]. Potential benefits of psychotherapy include ability to directly address psychosocial stressors that may precipitate or perpetuate depressive symptoms. In addition, psychotherapy is associated with few to no side effects and avoids drug interactions. Barriers to employing psychotherapy may include cost and access to trained psychotherapists [76]. Efficacy of several psychotherapeutic approaches in the care of older depressed adults has been examined. CBT, brief psychodynamic psychotherapy, and IPT will be briefly reviewed here.
CBT. Cognitive therapy was first described by Aaron Beck in the 1960s [77]. It is a highly structured therapy built on the premise that beliefs and assumptions an individual holds can influence emotions and behavior. CBT aims to identify maladaptive belief systems, test the validity of these cognitive distortions, and help individuals formulate more realistic cognitions [78]. Symptom improvement results from addressing these cognitive aspects as well as integration of behavioral activation and skills training to overcome maladaptive behavioral patterns [78]. CBT approaches have been applied to older adults with depression and results show acceptability [79] and efficacy in this population [80–82]. A 2008 Cochrane review (n = 153) found CBT to be superior to waitlist controls [82].
Brief psychodynamic psychotherapy. Brief psychodynamic psychotherapy, unlike highly structured CBT, aims to alter behavior by examining how past experiences and unresolved conflicts influence current emotions and behavior. While studies on application to the treatment of geriatric depression are scarce, limited data demonstrate efficacy in treating geriatric depression [81] and no significant difference in outcomes when compared to CBT [82].
IPT. Like CBT, IPT is a structured time-limited psychotherapeutic treatment approach first developed in the late 1960s by Klerman and Weissman [83]. IPT focuses on the impact of interpersonal relationships on depressive symptoms and examines 4 domains: interpersonal conflict, interpersonal deficits, role transitions, and grief [74].
Studies have shown efficacy of IPT in reducing depressive symptoms in the elderly when compared to usual care [84]. Reynolds et al found IPT combined with nortriptyline (a tricyclic antidepressant) to be superior to either nortriptyline alone or IPT alone in preventing recurrent depressive episodes [85]. Interestingly, a similar study investigating the efficacy of IPT in combination with paroxetine (an SSRI) failed to show added benefit of IPT in preventing recurrence, suggesting that further studies are needed [86].
Psychosocial interventions are integral in the care of the elderly depressed patient. Studies have shown positive benefits of aerobic exercise on depressive symptoms [87]. Yoga, Tai Chi, and other mindfulness-based exercises can increase sense of emotional and physical wellbeing [88–90]. Spirituality, religious beliefs, and involvement with a faith group may be protective against development of mental illness while at the same time provide avenues for increased social connectedness [91]. These and other avenues for socialization should be encouraged as part of the treatment plan for older depressed patients [92]
Electroconvulsive Therapy
ECT is indicated for the treatment of mood and psychotic disorders and has demonstrated efficacy in the treatment of severe depression [93]. It is typically initiated when patients fail to respond to pharmacotherapy and psychotherapy. Circumstances in which ECT can be considered first-line treatment include situations that require a rapid response (severe inanition, weight loss, or suicidality), situations where risks of ECT are lower than that of alternative treatments, previous positive response to ECT, or strong patient preference [94]. ECT is performed under general anesthesia and involves the induction of a generalized tonic-clonic seizure, which is theorized to enhance serotonergic, noradrenergic, and dopaminergic neurotransmission. A typical course of ECT involves treatments 3 times a week for an average of 6 to 12 treatments in total [95]. Elderly patients and those suffering from severe depression with psychotic features respond more robustly to ECT [93,96]. Estimated remission rates after an ECT series have been higher than 80% [93], making this modality the most effective treatment for severe depression to date.
Conclusion
As the population continues to age, clinicians are increasingly likely to encounter patients with late-life depression. A thorough evaluation includes not only assessment of depressive symptoms, but also cognitive, functional, and suicide assessment. Treatment options include pharmaco-therapy, psychotherapy, and in some cases electroconvulsive therapy. Utilization of assessment and treatment nuances unique to the geriatric population, with a multidisciplinary and collaborative approach involving primary care, mental health, and other ancillary providers, will serve to ultimately enhance patient care.
Corresponding author: Corresponding author: Juliet Glover, MD, Dept. of Neuropsychiatry and Behavioral Science, Univ. of South Carolina School of Medicine, 15 Medical Park, Suite 301, Columbia, SC 29203, Juliet.Glover@uscmed.sc.edu.
Financial disclosures: None reported.
Author contributions: conception and design, JAG, SS; drafting of article, JAG, SS; critical revision of the article, JAG, SS.
From the Department of Neuropsychiatry and Behavioral Sceince, University of South Carolina School of Medicine, Columbia, SC.
Abstract
- Objective: To review the identification, clinical assessment and treatment of patients with late-life depression.
- Methods: Review of the literature.
- Results: Depressive symptoms are present in up to 1 in 4 older adults. Comprehensive evaluation of depressive symptoms in this population often requires a multidisciplinary and collaborative approach between primary care, mental health, and other ancillary providers. Key aspects include a detailed history, physical and mental status examinations, cognitive and functional status assessment, and suicide risk assessment. Treatment options include anti-depressants, psychotherapy, and electroconvulsive therapy.
- Conclusion: A systematic approach to evaluating depressive symptoms in the elderly can enhance timely recognition and treatment.
Key words: Late-life depression; clinical assessment; antidepressants; psychotherapy; electroconvulsive therapy.
The U.S. population is aging, and with this comes the potential for increased health care needs. In 2014, there were over 46 million Americans age 65 and over (14.5% of the U.S. population). This number is projected to increase to 88 million by the year 2050 [1]. One in 4 older adults suffers with depressive symptoms that cause distress and functional impairment [2]. The World Health Organization Global Burden of Disease Study found depressive disorders to be the leading cause of disability-adjusted life years (DALYs) and the second leading cause of years lived with disability (YLDs). The burden of disease due to depressive disorders increased by 37.5% between 1990 and 2010, and 10.4% was attributable to aging [3]. These figures underscore the importance of accurate assessment and treatment of depression in the elderly. In this article, we review the identification, clinical assessment, and treatment of patients with late-life depression.
Diagnostic Criteria
Prevalence
It is estimated that 1% to 4% of community-dwelling adults age 65 and older suffer from MDD, with women more likely to be affected than men (prevalence of 4.4% vs. 2.7) [2,5–7]. This estimate is low compared with lifetime prevalence of almost 20% in the general adult population [8]. However, when depressive symptoms that do not meet criteria for MDD are considered, prevalence rates increase up to 25% [2,9]. These estimates also vary by clinical setting, with the highest rates (up to 40%) among elderly patients in long-term care facilities [10,11]. While individuals with subsyndromal depression may experience fewer symptoms than those with MDD, clinically significant distress persists, impacting health and functional status. Depression is associated with overall poor social or occupational functioning, cognitive decline, increased health care utilization and cost, increased morbidity and mortality from medical illness, and increased suicide mortality [5,9,10,12].
Identifying LLD
Accurate identification of LLD also requires recognition of the differences in the presentation of LLD compared with onset in earlier life. Depression in younger adults is often marked by depressed mood and loss of interest [18]. In contrast, older adults may present with increased anger or irritability [5]. Younger adults are more likely to report suicidal thoughts while older patients report feelings of hopelessness and thoughts of death [18]. LLD is often characterized by increased somatic complaints, hypochondriasis, or pain [5,18,19]. Another major difference lies in the presentation of cognitive difficulties. Younger patients typically complain of poor concentration or indecisiveness. Geriatric patients may present with cognitive changes including objective findings of slower processing speed and executive dysfunction on neuropsychological testing [17].
Depression rating scales may aid in identification of LLD. They are not a substitute for clinical diagnosis but can be useful as screening tools. Two commonly utilized depression rating scales are the Geriatric Depression Scale (GDS) and the Patient Health Questionnaire-9 (PHQ-9). GDS is a 30-item instrument developed specifically for older adults. Shorter 15-item, 5-item, and 4-item versions exist. The scale utilizes a Yes/No format and can be self- or clinician-administered [20]. One advantage of the GDS lies in its focus on psychological and cognitive aspects of depression rather than neurovegetative symptoms that may overlap with medical illnesses common in older adults [21]. The PHQ-9 is a 9-item self- or clinician-administered screening tool designed for use in primary care settings and has also been validated in geriatric populations [22,23]. The 9 items on this scale correspond to the DSM-5 criteria for major depression. A shorter 2-item version (PHQ-2) has also been validated, and a positive screen on this test should prompt administration of the full-length version. Both versions have approximately 80% sensitivity and specificity in detecting depression. An added advantage of PHQ-9 over GDS is that it can be useful in monitoring treatment response over time [22,23]
Comprehensive Assessment of LLD
The comprehensive assessment of patients with LLD can be carried out by health professionals in both mental health or primary care settings. In a multidisciplinary approach, psychiatrists and mental health professionals have collaborated with primary care providers using depression care managers with successful outcomes in managing depression in older adults [24,25]. Complete evaluation of a patient with suspected LLD begins with a history and physical and mental status examination. Other essential components of the evaluation include assessment of cognition, functional status, and suicide risk. Laboratory and neuroimaging studies may be necessary as well. Due to the comprehensive nature of this assessment, a multidisciplinary approach with collaboration between primary care, psychiatry, psychology, and ancillary services such as social work may be necessary. Multiple patient interactions may be required to complete a thorough evaluation.
History and Mental Status Examination
As with many other psychiatric illnesses, LLD is a clinical diagnosis. A careful history should be obtained initially utilizing open-ended questions. This should be followed by more directed questions as indicated to elicit the presence of depressive symptoms. The history should be obtained from the patient. A relevant collateral informant can be invaluable in the assessment, especially in cases where there is a comorbid neurocognitive disorder. However, the patient’s informed consent must be obtained prior to obtaining collateral information whenever possible. Psychosocial stressors that may have precipitated or may be perpetuating symptoms should be explored. Such stressors may include recent changes in living situation, loss of social support, recent deaths, or financial difficulties. Biological precipitants also need to be explored including presence of physical illness, depressogenic medications, and comorbid alcohol or other substance use. The patient’s past psychiatric history, psychiatric hospitalizations, and past medication trials should be ascertained. Any family history of depression, other psychiatric disorders, substance use disorders, and suicide attempts should be documented. A full mental status exam including cognitive assessment should be completed [21,26].
Cognitive Assessment
Cognitive impairment can be associated with LLD and may be due to the underlying depression or represent a comorbid neurocognitive disorder. Furthermore, the burden of medical illness as well as cerebrovascular and cardiovascular risk factors have been linked to executive dysfunction and reduced processing speed in individuals with LDD [27,28]. Distinguishing between these can be challenging; however, chronology of symptom onset is often helpful. Depression is more likely the etiology of cognitive impairment when depressive symptoms precede onset of cognitive deficits. This type of cognitive impairment is termed dementia syndrome of depression and may improve with treatment of depression [5]. Some patients may progress to develop major cognitive decline, and it remains unclear whether LLD represents a risk factor or prodrome to developing a major neurocognitive disorder [29]. On the other hand, if depression develops later in the course of cognitive decline, there may be an underlying neurocognitive disorder [17]. Up to 20% of individuals with major neurocognitive disorder due to Alzheimer’s disease also have major depression [11]. For these reasons, concomitant assessment of cognition is essential to the evaluation of the older adult presenting with depressive symptoms [30]. Cognitive domains that may be affected include learning and memory, language, attention, perceptual motor abilities, social cognition, and executive function [4]. Many of these domains can be assessed during the mental status examination, with brief cognitive screening tools, or with formal neuropsychological testing.
While there are numerous cognitive screening tools, some commonly used, brief tools include the Mini-Cog, the Folstein Mini-Mental State Exam (MMSE), and the Montreal Cognitive Assessment (MoCA). The Mini-Cog consists of a 3-item registration, delayed recall, and clock drawing test and has several advantages over other screening tools. It is a brief test (taking approximately 3 minutes to administer) with good sensitivity and specificity of 80% or greater. Compared with other cognitive screening tools, it is less influenced by level of education, language, or cultural background [31–33]. The MMSE is a longer screening tool consisting of 19 items and requires about 10 minutes to administer. Unlike the Mini-Cog, performance on the MMSE can be affected by level of education and cultural background. However, the MMSE can be administered serially to monitor changes in cognition over time [34,35]. The MoCA is a 10-minute cognitive screening tool first developed to detect mild cognitive impairment (MCI) [36]. The MoCA consists of 7 subscore sections covering visuospatial/executive function, naming, memory (delayed recall), attention, language, abstraction, and orientation. The total score is 30, and 1 point is added to the score if the testing subject has less than high school/12 years of education. The MoCA has demonstrated better sensitivity than the MMSE for the detection of MCI [36]. Elderly patients with depression often perform poorly on these cognitive screening tests due to apathy and poor effort.
Functional Assessment
The diagnosis of LLD requires that symptoms cause significant distress or interfere with functioning. A functional assessment is especially important in the evaluation of the older adult in that it allows clinicians to determine an individual’s ability to live independently and attend to daily needs. Basic activities of daily living (ADLs) include bathing, dressing, grooming, toileting, and self-transferring. Instrumental activities of daily living (IADLs) include more complex daily activities such as preparing meals, administering medications, driving, managing finances, and using simple electronics such as the telephone or remote control [26]. Impairment in IADLs is associated with increased depression severity. Conversely, the severity of depressive symptoms along with associated cognitive impairment predicts IADL impairment [37]. The Philadelphia Multilevel Assessment Instrument is a tool that can aid in the assessment of ADLs and IADLs and has been utilized in studies examining disability in depressed elderly patients [37,38]. Other available scales to quantify functional status include OARS Physical Activities of Daily Living, OARS Instrumental Activities of Daily Living Scale, and Direct Assessment of Functional Status Scale [26].
Suicide Assessment
Assessment for suicidality is an integral part of all psychiatric evaluations and is especially important in the evaluation of the depressed older adult. According to the Centers for Disease Control and Prevention, the suicide rate for individuals age 65 and older is 16.6 per 100,000, a figure that is comparable to that for individuals 18–64 years of age [39]. Non-Hispanic Caucasian males age 85 and older have the highest rate of completed suicide (56.5 per 100,000), underscoring the importance of a thorough suicide assessment [39]. Suicidality can range from passive thoughts of death and wishing that one were not alive, to active thoughts of self-harm with plan and intent. A Canadian study found 2% of community-dwelling adults age 55 and older had suicidal thoughts over a 12-month period and, of these, 28% had major depression [40]. A suicide assessment begins with inquiring about the presence of suicidal thoughts, plans, and intent. The 3 most frequently used methods of completed suicide in the elderly are firearms (28%), hanging (24%) and poisoning (21%) [41]. Access to weapons or other lethal means of self-harm such has hoarding of medications should be ascertained.
A complete suicide assessment requires attention to suicide risk factors, protective factors, and warning signs of impending suicide. Risk factors for suicide in the older adult include mood disorders, chronic medical illnesses and associated functional impairment, chronic pain, and psychosocial factors such as social isolation [42]. Mood disorders are present in 54% to 87% of cases of completed suicide, with major depression being the most common [42]. Chronic medical illness and pain can result in functional impairment leading to feelings of excessive guilt or being a burden to loved ones. Protective factors such as social connectedness, spirituality, religious beliefs, and cultural attitudes against suicide may serve as buffers against these risk factors [43]. Warning signs of impending suicide may indicate preparations for suicide and include feelings of hopelessness or lack of purpose, feeling trapped, talking about death, threatening suicide, agitation, social withdrawal, increased substance use and reckless behavior. Warning signs should prompt action to ensure the safety of the individual [44,45].
Physical Examination, Laboratory Studies, and Neuroimaging
Evaluation of LLD is not complete without a physical examination and ancillary studies to identify underlying medical conditions possibly contributing to or mimicking depressive symptoms. Routine laboratory studies include complete blood count, complete metabolic panel, thyroid studies, and urine drug screen. Signs and symptoms of underlying medical conditions may necessitate further laboratory studies [46]. Neuroimaging may reveal signs of cerebrovascular disease which can predispose, precipitate, or perpetuate depression in older adults [47].
Treatment
Treatment of LLD can take many forms and occur in various settings. Geriatric psychiatrists have expertise in the assessment and treatment of mental illness in the elderly. Workforce estimates for 2010 revealed 1 geriatric psychiatrist per 10,000 adults age 75 and over. This figure is estimated to decrease to 0.5 per 10,000 by the year 2030, underscoring the importance of increasing the knowledge base of clinicians across specialties who provide care to the depressed elderly [48]. The primary care setting is often the locus of care for depression in older adults; however, studies suggest that patients are often left untreated or undertreated [49]. Collaborative care models whereby mental health care is integrated into primary care have been shown to improve outcomes. The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) study found that use of care managers to assist primary care providers in identification of depression, offer algorithm-based treatment recommendations, monitor symptoms and medication side effects, and provide follow-up yielded improvement in outcomes. Patients in the intervention group were more likely to receive pharmacotherapy or psychotherapy, achieve remission, and showed greater decline in suicidal ideation [50]. Similar results were found in the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study in which intervention patients treated under a collaborative care model showed lower depression severity, less functional impairment, and greater reduction in depressive symptoms [25].
Just as a collaborative care model can lead to improved outcomes, the overall strategy of treating depression must be multifaceted. The biopsychosocial model of disease first described in the 1970s emphasizes biological and psychosocial determinants of illness that must be addressed when treatment is considered [51]. This includes nonmodifiable biological factors such as age, gender, and genetic predisposition that may affect treatment options, as well as modifiable biological factors such as comorbid medical illness, medications, or substance use disorders. Psychological factors that can affect depressive symptoms include coping skills and defense mechanisms in the face of stressful life events. Social factors including the role of culture, environment, and family dynamics in disease presentation must be considered as well [52].
Pharmacologic Treatment of LLD
The primary pharmacologic treatment for depression is antidepressants. Treatment consists of 3 phases—acute, continuation, and maintenance. In the acute phase, the goal is remission of current symptoms and restoration of function. The continuation phase, extending up to 6 months after remission, aims to prevent relapse back into a depressive episode. Maintenance therapy is geared at preventing recurrence of future depressive episodes [53]. Studies have found a 50% risk of relapse after 1 episode of depression and 80% after 2 episodes. Up to 20% will develop chronic symptoms. For this reason, maintenance therapy is often necessary for recurrent depression [54].
While cognitive impairment may affect antidepressant efficacy, age does not appear to be a determinant. Gildengers et al examined antidepressant response in young, middle, and older-old patients and found no significant difference in response rates [59]. Early onset versus late onset of first depressive episode also does not predict antidepressant response in patients age 55 and over [60]. There is scant evidence for efficacy of antidepressants in depressed patients with neurocognitive disorders. A 2002 Cochrane review with 4 studies in the meta-analysis (n = 137) concluded that there was weak support for antidepressant efficacy in this population [61]. A 2011 meta-analysis with 330 participants also yielded inconclusive results [62]. The paucity of evidence for antidepressant efficacy in depressed patients with neurocognitive disorders should prompt careful consideration of potential benefits versus adverse effects.
Antidepressants are generally well tolerated in older adults. Side effects vary by medication and contribute to discontinuation in up to 25% of new users (versus 22% for new users who discontinue for reasons other than side effects) [63]. Potential adverse effects shared by most SSRIs and SNRIs include GI disturbance (nausea, diarrhea or constipation), sexual dysfunction, headache, and sleep disturbance [64,65]. In addition, abrupt discontinuation can precipitate serotonin withdrawal syndrome characterized by sensory disturbance (paresthesia, tremor, and irritability) as well as headache, lightheadedness, diaphoresis, insomnia, and agitation. Other medication-specific side effects include risk of seizure with bupropion and sedation with mirtazapine [65].
Despite superiority of antidepressants to placebo in treating depression, up to one-third of patients may not respond to a trial of antidepressants. Sequential treatment protocols such as switching to a different antidepressant or augmentation can increase the proportion of antidepressant responders [66–68]. Studies have found particularly favorable response to augmentation with lithium, with one study achieving a 33% remission rate in treatment- resistant geriatric depression [67,69]. Other pharmacologic augmentation strategies include the addition of mood stabilizers such as lamotrigine, antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone), and psychostimulants [70–73]. Electroconvulsive therapy (ECT) is a nonpharmacologic option for treatment-resistant depression that will be reviewed later.
Psychotherapeutic and Psychosocial Interventions
Psychotherapeutic interventions have demonstrated efficacy in the treatment of geriatric depression, including but not limited to cognitive behavioral therapy (CBT), interpersonal therapy (IPT), problem-solving therapy (PST), reminiscence and life review, and brief psychodynamic psychotherapy [74]. Some older adults may prefer psychotherapy to pharmacologic treatment (57% vs. 43%) [75]. Potential benefits of psychotherapy include ability to directly address psychosocial stressors that may precipitate or perpetuate depressive symptoms. In addition, psychotherapy is associated with few to no side effects and avoids drug interactions. Barriers to employing psychotherapy may include cost and access to trained psychotherapists [76]. Efficacy of several psychotherapeutic approaches in the care of older depressed adults has been examined. CBT, brief psychodynamic psychotherapy, and IPT will be briefly reviewed here.
CBT. Cognitive therapy was first described by Aaron Beck in the 1960s [77]. It is a highly structured therapy built on the premise that beliefs and assumptions an individual holds can influence emotions and behavior. CBT aims to identify maladaptive belief systems, test the validity of these cognitive distortions, and help individuals formulate more realistic cognitions [78]. Symptom improvement results from addressing these cognitive aspects as well as integration of behavioral activation and skills training to overcome maladaptive behavioral patterns [78]. CBT approaches have been applied to older adults with depression and results show acceptability [79] and efficacy in this population [80–82]. A 2008 Cochrane review (n = 153) found CBT to be superior to waitlist controls [82].
Brief psychodynamic psychotherapy. Brief psychodynamic psychotherapy, unlike highly structured CBT, aims to alter behavior by examining how past experiences and unresolved conflicts influence current emotions and behavior. While studies on application to the treatment of geriatric depression are scarce, limited data demonstrate efficacy in treating geriatric depression [81] and no significant difference in outcomes when compared to CBT [82].
IPT. Like CBT, IPT is a structured time-limited psychotherapeutic treatment approach first developed in the late 1960s by Klerman and Weissman [83]. IPT focuses on the impact of interpersonal relationships on depressive symptoms and examines 4 domains: interpersonal conflict, interpersonal deficits, role transitions, and grief [74].
Studies have shown efficacy of IPT in reducing depressive symptoms in the elderly when compared to usual care [84]. Reynolds et al found IPT combined with nortriptyline (a tricyclic antidepressant) to be superior to either nortriptyline alone or IPT alone in preventing recurrent depressive episodes [85]. Interestingly, a similar study investigating the efficacy of IPT in combination with paroxetine (an SSRI) failed to show added benefit of IPT in preventing recurrence, suggesting that further studies are needed [86].
Psychosocial interventions are integral in the care of the elderly depressed patient. Studies have shown positive benefits of aerobic exercise on depressive symptoms [87]. Yoga, Tai Chi, and other mindfulness-based exercises can increase sense of emotional and physical wellbeing [88–90]. Spirituality, religious beliefs, and involvement with a faith group may be protective against development of mental illness while at the same time provide avenues for increased social connectedness [91]. These and other avenues for socialization should be encouraged as part of the treatment plan for older depressed patients [92]
Electroconvulsive Therapy
ECT is indicated for the treatment of mood and psychotic disorders and has demonstrated efficacy in the treatment of severe depression [93]. It is typically initiated when patients fail to respond to pharmacotherapy and psychotherapy. Circumstances in which ECT can be considered first-line treatment include situations that require a rapid response (severe inanition, weight loss, or suicidality), situations where risks of ECT are lower than that of alternative treatments, previous positive response to ECT, or strong patient preference [94]. ECT is performed under general anesthesia and involves the induction of a generalized tonic-clonic seizure, which is theorized to enhance serotonergic, noradrenergic, and dopaminergic neurotransmission. A typical course of ECT involves treatments 3 times a week for an average of 6 to 12 treatments in total [95]. Elderly patients and those suffering from severe depression with psychotic features respond more robustly to ECT [93,96]. Estimated remission rates after an ECT series have been higher than 80% [93], making this modality the most effective treatment for severe depression to date.
Conclusion
As the population continues to age, clinicians are increasingly likely to encounter patients with late-life depression. A thorough evaluation includes not only assessment of depressive symptoms, but also cognitive, functional, and suicide assessment. Treatment options include pharmaco-therapy, psychotherapy, and in some cases electroconvulsive therapy. Utilization of assessment and treatment nuances unique to the geriatric population, with a multidisciplinary and collaborative approach involving primary care, mental health, and other ancillary providers, will serve to ultimately enhance patient care.
Corresponding author: Corresponding author: Juliet Glover, MD, Dept. of Neuropsychiatry and Behavioral Science, Univ. of South Carolina School of Medicine, 15 Medical Park, Suite 301, Columbia, SC 29203, Juliet.Glover@uscmed.sc.edu.
Financial disclosures: None reported.
Author contributions: conception and design, JAG, SS; drafting of article, JAG, SS; critical revision of the article, JAG, SS.
1. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. US Census Bureau: May 2010.
2. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clinc Geriatr Med 1992; 8:235–51.
3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global gurden of disease study 2010. PLoS Med 2013;10:e1001547.
4 .American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
5 .Ellison JM, Kyomen HH, Harper DG. Depression in later life: an overview with treatment recommendations. Psychiatr Clin North Am 2012;35:203–29.
6. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.
7. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000;57:601–7.
8. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.
9. McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry 2013;21:418–32.
10. Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–87.
11. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–65.
12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003;160:1147–56.
13. Ritchie K. Late-life depression. European Psychiatry 2014;29:577.
14. Hegeman JM, de Waal MW, Comijs HC, et al. Depression in later life: a more somatic presentation? J Affect Disord 2015;170:196–202.
15. Lackamp J, Schlachet R, Sajatovic M. Assessment and management of major depressive disorder in older adults. Psychiatria Danubina 2016;28(Suppl 1):95–98.
16. Morichi V, Dell’Aquila G, Trotta F, et al. Diagnosing and treating depression in older and oldest old. Curr Pharm Des 2015;21:1690–8.
17. Fiske AJ, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89.
18. Balsis S, Cully JA. Comparing depression diagnostic symptoms across younger and older adults. Aging Ment Health 2008;12:800–6.
19. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77.
21. Blazer DG. The psychiatric interview of older adults. In: Blazer D, Steffens D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
22. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44.
23. Richardson TM, He H, Podgorski C, et al. Screening for depression in aging services clients. Am J Geriatr Psychiatry 2010;18:1116–23.
24. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012 Oct 17.
25. Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting a randomized controlled trial. JAMA 2002;288:2836–45.
26. Silver I, Herrmann N. Comprehensive psychiatric evaluation. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691–8.
28. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65.
29. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–8.
30. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 2015;1345:36–46.
31. Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7.
32. Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400.
33. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry 2010;25:111–20.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
35. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47: 2018–23.
36. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
37. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005;13:244–9.
38. Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry 1996;153:877–85.
39. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Accessed 9 Feb 2016 at http://webappa.cdc.gov/sasweb/ncipc/dataRestriction_inj.html.
40. Corna LM, Cairney J, Streiner DL. Suicide ideation in older adults: relationship to mental health problems and service use. Gerontologist 2010;50:785–97.
41. Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–84.
42. Van Orden K, Conwell Y. Suicides in late life. Curr Psychiatry Rep 2011;13:234–41.
43. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68, ix.
44. Rudd MD, Berman AL, Joiner TE, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav 2006;36:255–62.
45. Know the warning signs of suicide. American Association of Suicidology. Accessed 9 Feb 2016 at www.suicidology.org/resources/warning-signs.
46. Taylor W, Doraiswamy P. Use of the laboratory in the diagnostic workup of older adults. In: Blazer D, Steffen D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
47. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–22.
48. ADGAP Status of Geriatrics Workforce Study. Accessed 26 Dec 2016 at www.americangeriatrics.org/files/documents/gwps/Table%201_29.pdf.
49. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
50. Alexopoulos GS, Reynolds CF, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
51. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
52. Schotte CKW, Van den Bossche B, Doncker DD, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression Anxiety 2006;23:312–24.
53. Kupfer DJ, Frank E. The interaction of drug-and psychotherapy in the long-term treatment of depression. J Affect Disord 2001;62:131–7.
54. Katon W, Rutter C, Ludman EJ, et al. A randomized trial of relpase prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241–7.
55. Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord 2012;141:103–15.
56. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9.
58. Mukai Y, Tampi RR. Treatment of depression in the elderly: a review of the recent literature on the efficacy of single- versus dual-action antidepressants. Clin Ther 2009;31:945–61.
59. Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord 2002;69:177–84.
60. Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode. Am J Geriatr Psychiatry 2008;16:58–64.
61. Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Databse Syst Rev 2002;(4):CD003944.
62. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–85.
63. Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry 2011;19:211–21.
64. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician 2014;60:121–6.
65. Kennedy GJ, Marcus P. Use of antidepressants in older patients with co-morbid medical conditions: guidance from studies of depression in somatic illness. Drugs Aging 2005;22:273–87.
66. Sackheim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-tern outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
67. Kok RM, Nolen WA, Hereen TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81.
68. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004;65:1634–41.
69. Kok RM, Vink D, Hereen TJ, Nolen WA. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly; an open, randomized, controlled trial. J Clin Psychiatry 2006;68:1177–85.
70. Kok RM. What is the role of medications in late life depression? Psychiatr Clin North Am 2003;36:597–605.
71. Wen XJ, Wang LM, Liu ZL,. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 2014;47:605–16.
72. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet 2015;386:2404–12.
73. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008;16:21–30.
74. Francis JL, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am 2013;36:561–75.
75. Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist 2006;46:14–22.
76. Pinquart M, Duberstein PR, Lyness JM. Treatments for late-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501.
77. Beck Institute for Cognitive Behavior Therapy. Accessed 28 Dec 2016 at www.beckinstitute.org
78. Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970;1:184–200.
79. Landrevile P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontology B Psychol Sci Soc Sci 2001;56:P285–91.
80. Thompson LW, Gallagher D, Breckenridge JS. Comparitive effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385–90.
81. Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. J Consult Clin Psychol 1994;62:543–9.
82. Wilson KC, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;(1):CD004853
83. Markowitz JC, Weissman MM. Interpersonal psychotherapy: past, present and future. Clin Psychol Psychother 2012;19:99–105.
84. Van Schaik A, van Marwijk H, Ader H, et al. Interpersonal psychotherapy for elderly patients in primary care. Am J Geriatr Psychiatry 2006;14:777–86.
85. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39–45.
86. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8.
87. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56.
88. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs 2007;33:17–23.
89. Wang C, Bannuru R, Ramel J, et al. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010;10:23.
90. Cho KL. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Med Sport Sci 2008;52:146–54.
91. Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life- a randomized, controlled trial. Altern Ther Health Med 2006;12:26–35.
92. Nyer M, Doorley J, Durham K, et al. What is the role of alternative treatments in late-life depression? Psychiatr Clin North Am 2013;36:577–96.
93. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244–53.
94. Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical manual of electroconvulsive therapy. American Psychiatric Publishing; 2010.
95. Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry 2012;169:1238–44.
96. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late-life depression. Can J Psychiatry 2002;47:734–41.
1. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. US Census Bureau: May 2010.
2. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clinc Geriatr Med 1992; 8:235–51.
3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global gurden of disease study 2010. PLoS Med 2013;10:e1001547.
4 .American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
5 .Ellison JM, Kyomen HH, Harper DG. Depression in later life: an overview with treatment recommendations. Psychiatr Clin North Am 2012;35:203–29.
6. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.
7. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000;57:601–7.
8. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.
9. McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry 2013;21:418–32.
10. Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–87.
11. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–65.
12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003;160:1147–56.
13. Ritchie K. Late-life depression. European Psychiatry 2014;29:577.
14. Hegeman JM, de Waal MW, Comijs HC, et al. Depression in later life: a more somatic presentation? J Affect Disord 2015;170:196–202.
15. Lackamp J, Schlachet R, Sajatovic M. Assessment and management of major depressive disorder in older adults. Psychiatria Danubina 2016;28(Suppl 1):95–98.
16. Morichi V, Dell’Aquila G, Trotta F, et al. Diagnosing and treating depression in older and oldest old. Curr Pharm Des 2015;21:1690–8.
17. Fiske AJ, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89.
18. Balsis S, Cully JA. Comparing depression diagnostic symptoms across younger and older adults. Aging Ment Health 2008;12:800–6.
19. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77.
21. Blazer DG. The psychiatric interview of older adults. In: Blazer D, Steffens D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
22. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44.
23. Richardson TM, He H, Podgorski C, et al. Screening for depression in aging services clients. Am J Geriatr Psychiatry 2010;18:1116–23.
24. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012 Oct 17.
25. Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting a randomized controlled trial. JAMA 2002;288:2836–45.
26. Silver I, Herrmann N. Comprehensive psychiatric evaluation. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691–8.
28. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65.
29. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–8.
30. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 2015;1345:36–46.
31. Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7.
32. Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400.
33. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry 2010;25:111–20.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
35. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47: 2018–23.
36. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
37. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005;13:244–9.
38. Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry 1996;153:877–85.
39. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Accessed 9 Feb 2016 at http://webappa.cdc.gov/sasweb/ncipc/dataRestriction_inj.html.
40. Corna LM, Cairney J, Streiner DL. Suicide ideation in older adults: relationship to mental health problems and service use. Gerontologist 2010;50:785–97.
41. Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–84.
42. Van Orden K, Conwell Y. Suicides in late life. Curr Psychiatry Rep 2011;13:234–41.
43. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68, ix.
44. Rudd MD, Berman AL, Joiner TE, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav 2006;36:255–62.
45. Know the warning signs of suicide. American Association of Suicidology. Accessed 9 Feb 2016 at www.suicidology.org/resources/warning-signs.
46. Taylor W, Doraiswamy P. Use of the laboratory in the diagnostic workup of older adults. In: Blazer D, Steffen D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
47. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–22.
48. ADGAP Status of Geriatrics Workforce Study. Accessed 26 Dec 2016 at www.americangeriatrics.org/files/documents/gwps/Table%201_29.pdf.
49. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
50. Alexopoulos GS, Reynolds CF, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
51. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
52. Schotte CKW, Van den Bossche B, Doncker DD, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression Anxiety 2006;23:312–24.
53. Kupfer DJ, Frank E. The interaction of drug-and psychotherapy in the long-term treatment of depression. J Affect Disord 2001;62:131–7.
54. Katon W, Rutter C, Ludman EJ, et al. A randomized trial of relpase prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241–7.
55. Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord 2012;141:103–15.
56. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9.
58. Mukai Y, Tampi RR. Treatment of depression in the elderly: a review of the recent literature on the efficacy of single- versus dual-action antidepressants. Clin Ther 2009;31:945–61.
59. Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord 2002;69:177–84.
60. Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode. Am J Geriatr Psychiatry 2008;16:58–64.
61. Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Databse Syst Rev 2002;(4):CD003944.
62. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–85.
63. Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry 2011;19:211–21.
64. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician 2014;60:121–6.
65. Kennedy GJ, Marcus P. Use of antidepressants in older patients with co-morbid medical conditions: guidance from studies of depression in somatic illness. Drugs Aging 2005;22:273–87.
66. Sackheim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-tern outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
67. Kok RM, Nolen WA, Hereen TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81.
68. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004;65:1634–41.
69. Kok RM, Vink D, Hereen TJ, Nolen WA. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly; an open, randomized, controlled trial. J Clin Psychiatry 2006;68:1177–85.
70. Kok RM. What is the role of medications in late life depression? Psychiatr Clin North Am 2003;36:597–605.
71. Wen XJ, Wang LM, Liu ZL,. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 2014;47:605–16.
72. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet 2015;386:2404–12.
73. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008;16:21–30.
74. Francis JL, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am 2013;36:561–75.
75. Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist 2006;46:14–22.
76. Pinquart M, Duberstein PR, Lyness JM. Treatments for late-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501.
77. Beck Institute for Cognitive Behavior Therapy. Accessed 28 Dec 2016 at www.beckinstitute.org
78. Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970;1:184–200.
79. Landrevile P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontology B Psychol Sci Soc Sci 2001;56:P285–91.
80. Thompson LW, Gallagher D, Breckenridge JS. Comparitive effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385–90.
81. Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. J Consult Clin Psychol 1994;62:543–9.
82. Wilson KC, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;(1):CD004853
83. Markowitz JC, Weissman MM. Interpersonal psychotherapy: past, present and future. Clin Psychol Psychother 2012;19:99–105.
84. Van Schaik A, van Marwijk H, Ader H, et al. Interpersonal psychotherapy for elderly patients in primary care. Am J Geriatr Psychiatry 2006;14:777–86.
85. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39–45.
86. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8.
87. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56.
88. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs 2007;33:17–23.
89. Wang C, Bannuru R, Ramel J, et al. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010;10:23.
90. Cho KL. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Med Sport Sci 2008;52:146–54.
91. Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life- a randomized, controlled trial. Altern Ther Health Med 2006;12:26–35.
92. Nyer M, Doorley J, Durham K, et al. What is the role of alternative treatments in late-life depression? Psychiatr Clin North Am 2013;36:577–96.
93. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244–53.
94. Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical manual of electroconvulsive therapy. American Psychiatric Publishing; 2010.
95. Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry 2012;169:1238–44.
96. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late-life depression. Can J Psychiatry 2002;47:734–41.