User login
An update on proteinuric chronic kidney disease: The dual-goal approach
When angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor blockers (ARBs) were introduced, we hoped that these drugs would slow or stop the inexorable progression of chronic kidney disease. This hasn’t come to pass: the incidence of end-stage renal disease continued to increase throughout the 1990s, and although it may have finally reached a plateau, it remains unacceptably high.1 One reason may be that, used singly, drugs that block the renin-angiotensin-aldosterone system are only moderately successful, as approximately 20% to 40% of patients still reach unfavorable renal end points such as doubling of the serum creatinine level or dialysis.2–7
In view of these disappointing results, some experts are advocating a new strategy in which they advise that both blood pressure and urinary albumin excretion be lowered to specific goals. To achieve these goals, we will generally have to give higher doses of ACE inhibitors and ARBs alone or use a combination of these and other drugs that block the renin-angiotensin-aldosterone system at various sites.
This article describes how the dual-goal approach, with a focus on renin-angiotensin-aldosterone system inhibition, may be applied in the therapy of proteinuric chronic kidney disease. This appears to be a reasonable approach, based on current evidence, to address the epidemic of renal failure. However, further studies are needed to establish the effectiveness of this approach, and the risk of hyperkalemia following aggressive inhibition of the renin-angiotensin-aldosterone system poses a significant management problem.
ALBUMIN MAY BE TOXIC
While hypertension has long been associated with poor renal outcomes, urinary albumin has more recently been implicated by observational and experimental evidence as a tubular-interstitial toxin that may also accelerate the progression of renal disease.
For example, in both the Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) study8 and the Ramipril Efficacy in Nephropathy study,4 baseline proteinuria was almost linearly related to worse renal outcomes. In RENAAL, patients who excreted more than about 3 g of albumin per day had an 8.1-fold higher risk of progressing to end-stage renal disease.8 Moreover, the more that protein excretion could be reduced, the better the renal outcomes, down to a level of about 500 mg/day.8
Of importance, lowering blood pressure did not always decrease protein excretion—nearly 40% of patients had a dissociation between the two.9 In fact, prescribing a single ACE inhibitor or ARB while targeting only blood pressure has not predictably reduced protein excretion to 500 mg/day (the proposed goal).2–7
Although albumin has not been conclusively proven to be a renal toxin, targeting the reduction of proteinuria may also succeed if urinary albumin simply serves as a marker of the success of chronic kidney disease treatment and reflects prognosis.
A DUAL-GOAL APPROACH
In view of the observational and experimental evidence, many experts10,15–18 are advocating a dual-goal approach that stresses lowering both blood pressure and urinary protein (albumin) excretion. The recommended goal for systolic blood pressure is less than 120 to 125 mm Hg; the goal for proteinuria is less than 300 to 500 mg/24 hours,16,17,19 aiming to slow the decline in glomerular filtration rate to less than 2 mL/ min/year.11,20
The strategy of targeting both proteinuria and blood pressure has recently received further support. In a prospective randomized controlled study,21 nondiabetic patients with proteinuria received either an ACE inhibitor or an ARB. In one group, the dose was adjusted to lower the blood pressure to less than 130/80 mm/Hg; in the other group, the dose was adjusted to lower the blood pressure to 130/80 and to reduce protein excretion maximally. Only about half as many patients in the group with the dual-goal strategy reached the composite primary end point (doubling of serum creatinine, end-stage renal disease, or death) over a median of 3.7 years of follow-up, as compared with those treated by targeting the blood pressure alone.
In retrospect, the suboptimal success in the earlier landmark studies2–7 may have derived from the failure of ACE inhibitors and ARBs, used by themselves at moderate doses, to either lower the blood pressure to the recently advised goal (the actual results obtained varied from about 128 to about 145 mm Hg systolic) or, perhaps, to reduce proteinuria to the goal level.
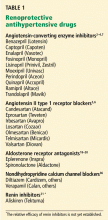
Not all nephrologists currently pursue the stringent proteinuria goal of 500 mg per day—the targeted reduction of proteinuria requires further prospective evidence to support it. However, nephrologists do commonly follow the broad theme that antihypertensive therapy in proteinuric chronic kidney disease should accentuate medicines that protect the kidney beyond their antihypertensive effect (Table 1), and that proteinuria is an important metric that, at the very least, reflects the response to therapy and prognosis.
BLOCKING RENIN-ANGIOTENSIN- ALDOSTERONE MORE COMPLETELY
These issues may be addressed by more complete inhibition of the renin-angiotensin-aldosterone system, now achievable with the addition of aldosterone receptor antagonists and direct renin inhibitors to the ACE inhibitors and ARBs. Although we lack long-term studies of the relative efficacy of these medicines alone or in various combinations, the multistep sequence of the renin-angiotensin-aldosterone system allows for the possibility that more complete suppression via coordinated pharmacologic attention to multiple sites will yield beneficial results.
Combining an ACE inhibitor and an ARB
Even in the absence of ACE, angiotensin II is also produced by other kinases and therefore is not completely suppressed by an ACE inhibitor. For this and other reasons, there are theoretical advantages to adding an ARB to an ACE inhibitor.
In the Combination Treatment of Angiotensin 2 Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-Diabetic Renal Disease (COOPERATE) study,20 the combination of an ACE inhibitor and an ARB protected the kidneys better than either medicine alone, not only in terms of less protein in the urine but also in terms of significantly fewer patients progressing to the primary end points of doubling of serum creatinine or end-stage renal disease after 3 years of follow-up (11% of patients on combination therapy vs 23% on single therapy).
Aldosterone receptor antagonists or renin inhibitors plus ACE inhibitors and ARBs
Aldosterone escape is common during long-term therapy with ACE inhibitors and ARBs, and an aldosterone-receptor antagonist reduces proteinuria11–13 and stabilizes kidney function13 in a manner additive to that of ACE inhibitors and ARBs.
Direct renin inhibitors overcome the reactive rises in renin activity and in angiotensin II that complicate therapy with ACE inhibitors and ARBs, and they also reduce urinary aldosterone excretion.14
When to consider combination therapy
Inhibition of the renin-angiotensin-aldosterone system at multiple sites may be considered in cases of persistent hypertension or proteinuria, or of progression of chronic kidney disease despite single-drug therapy, or more broadly, with increasing evidence that combination therapy may preserve the glomerular filtration rate.13,20 This article suggests one way to apply the several available renin-angiotensin-aldosterone inhibitors, keeping in mind extensive interindividual variations, uncertain responses, and the absence of a linear evidence-based strategy known to be broadly successful.
INITIAL CONSIDERATION: WHAT IS THE BLOOD PRESSURE GOAL?
Determining the blood pressure goal for a patient may not be as straightforward as usually assumed. Typically, advisories suggest a discrete goal; for example, the Seventh Joint National Committee22 recommended a systolic blood pressure of 130 mm Hg or lower for patients with chronic kidney disease or diabetes. However, if we weigh the risks and benefits, we find that the situation is more nuanced. The blood pressure goal should vary among patients, depending on age, amount of proteinuria, whether the patient can tolerate the lowered blood pressure, and whether lowering the blood pressure to this goal stabilizes kidney function.
Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) study demonstrated a benefit of setting the goal mean arterial pressure to less than 92 mm Hg (about 125 mm Hg systolic) regardless of proteinuria.23 In addition, a meta-analysis suggested that nondiabetic proteinuric patients benefit from even lower systolic blood pressures (110–119 mm Hg).19
In older patients
However, in the MDRD study, the goal of approximately 125 mm Hg systolic pertained only to patients no older than 60 years.23 The goal was increased to about 130 mm Hg for patients 61 to 70 years old. In addition, major clinical studies of chronic kidney disease have excluded patients older than 70 years.2–7,23
Therapy for chronic kidney disease in this older age group is essentially unstudied, and we should be cautious about extrapolating results of aggressive blood pressure-lowering (and renin-angiotensin-aldosterone inhibition) from younger patients to older patients, who may have extensive vascular disease.24,25
For patients older than 70 years, guidance is perhaps best provided by the Systolic Hypertension in the Elderly Program (SHEP), which found that lowering systolic blood pressure to an average of 143 mm Hg reduced the incidence of stroke and cardiovascular disease.26 The SHEP study does not establish the optimal blood pressure goal for preventing progressive chronic kidney disease (or even cardiovascular disease) in the older age group. However, this is the lowest systolic pressure yet shown to be generally safe and associated with any improved outcome for these patients.
Additional studies are needed to evaluate whether this blood pressure level provides the best outcomes in patients with chronic kidney disease, or whether even lower blood pressures in the elderly are safe and will further improve either renal or cardiovascular outcomes.
In younger patients
In contrast, younger patients without diabetes or vascular disease may, in theory, be candidates for even lower blood pressure. No major study of chronic kidney disease isolated patients from about 20 to 40 years old for analysis, precluding direct evidence-based guidelines for this cohort at this time.
However, some of these patients may have had premorbid systolic blood pressures of 90 to 110 mm Hg, so systolic pressures of 110 to 120 mm Hg would be “hypertensive” by 10 to 30 mm Hg for them. It is possible that some patients in this cohort will tolerate a systolic pressure lower than 110 mm Hg, and that the lower blood pressure may provide additional long-term renal protection for them. This notion is theoretical, however, and has not been verified by clinical studies.
No one pressure fits all
In summary, an initial target systolic pressure for proteinuric patients, based on available evidence, might be less than 130 mm Hg for patients 61 to 70 years old,23 less than 125 mm Hg for patients younger than 61 years,23 and perhaps as low as 110 to 119 mm Hg for non-diabetic patients.19 Caution is advised against targeting systolic blood pressure less than 140 mm Hg for patients older than 70 years.
These are only initial goals and should be reevaluated as treatment progresses. The achieved blood pressure must be clinically tolerated—symptoms of tissue hypoperfusion indicate that the blood pressure is too low for the patient. In addition, the blood pressure goal (like the proteinuria goal) is only a surrogate end point, and if kidney function declines even though the surrogate end points are attained, then those end points should be reevaluated.
Tailoring blood pressure goals to the individual patient dovetails with the recent suggestion that blood pressure should not be perceived as a rigid dichotomy of “hypertension” vs “normal.”27 There is, in general, a continuous correlation between blood pressure, beginning at low levels, and the risk of cardiorenal disease, and choosing an optimal blood pressure goal for an individual patient requires an ongoing assessment of benefits, risks, and side effects.
STARTING ANTIHYPERTENSIVE THERAPY
The question of which antihypertensive drug to try first is moot in chronic kidney disease because almost all patients need multiple medicines to reach their blood pressure goals.
The Seventh Joint National Committee recommended an ACE inhibitor for initial therapy in hypertensive patients with chronic kidney disease,22 although an ARB is a reasonable first choice for those with type 2 diabetes.5,6
Diuretics potentiate the effects of ACE inhibitors and ARBs and are generally prescribed concomitantly or as the second choice.
A beta-blocker may be recommended as a third medicine (when needed), to provide a complementary class of antihypertensive, to address the high incidence of concomitant coronary artery disease and systolic dysfunction, and because of evidence that sympathetic excess contributes to the hypertension and progression of chronic kidney disease.28,29 The National Kidney Foundation30 suggests that the dose of beta-blocker be increased if the heart rate is greater than 84.
INTENSIFYING RENIN-ANGIOTENSIN-ALDOSTERONE INHIBITION: WHICH DRUGS, AND WHEN?
When hypertension and proteinuria persist despite the use of an ACE inhibitor or an ARB, additional inhibition of the renin-angiotensin-aldosterone system is generally recommended to lower both the blood pressure and the protein excretion. Increasing the dose of ACE inhibitor or ARB,31–34 combining an ACE inhibitor and an ARB,20 or adding an aldosterone receptor antagonist to either an ACE inhibitor or an ARB11–13 have all been shown to reduce proteinuria (as a surrogate end point), and several studies have, importantly, found that these combinations preserve kidney function over time.13,20
However, lacking long-term studies that compare these options, we cannot insist upon specific treatment choices or sequences in these situations.
An approach based on serum potassium and volume status
For example, if a patient has obvious signs of volume excess (eg, edema, jugular venous distention, rales) and the serum potassium concentration is less than about 5.0 or 5.5 mEq/L, then an aldosterone receptor antagonist may logically be added or increased in dose.
Aldosterone is more than a kidney hormone
Increasing the diuretic or renin-angiotensin-aldosterone inhibition
For patients who have obvious signs of volume excess and a serum potassium level greater than 5.0 mEq/L, the dosage of kaliuretic (potassium-excreting) diuretic (usually a loop diuretic in chronic kidney disease) can be increased. Although kaliuretic diuretics do not specifically lower proteinuria, they will help control volume and blood pressure and, by lowering the serum potassium level, facilitate the subsequent augmention of renin-angiotensin-aldosterone inhibition.
When a hypertensive patient does not seem to have excess volume or tachycardia and the serum potassium level is less than about 5.5 mEq/L, then additional renin-angiotensin-aldosterone inhibition is indicated.16 This may be accomplished either by increasing the ACE inhibitor or the ARB to its maximal antihypertensive dose or by starting combination therapy.
Starting a calcium channel blocker
When the serum potassium level is higher than about 5.5 mEq/L, further inhibition of the renin-angiotensin-aldosterone system is contraindicated, and a nondihydropyridine calcium channel blocker can be added for its anti-hypertensive and antiproteinuric effects.16,36
When nondihydropyridine calcium channel blockers are contraindicated due to their antiinotropic effect, an attractive alternative may be to cautiously increase the dose of kaliuretic diuretics. Given the high prevalence of (often covert) volume excess in chronic kidney disease, empiric diuresis may lower blood pressure, particularly in patients already receiving several vasodilators.37 Moreover, as mentioned, by reducing serum potassium, kaliuretic diuretics help allow for a subsequent increase in renin-angiotensin-aldosterone inhibition.
IF BLOOD PRESSURE IS NORMAL, BUT PROTEINURIA PERSISTS
Because lowering blood pressure does not necessarily reduce protein excretion, some patients achieve their blood pressure goal but still have excessive proteinuria. Proponents of the dual-goal approach suggest that these patients require further treatment modifications to reach the proteinuria goal and their optimal renal prognosis.
A cautious increase in renin-angiotensin-aldosterone inhibition is possible but is likely to be limited by low blood pressure. When applicable, any nonessential antihypertensive drug that does not specifically reduce proteinuria (ie, dihydropyridine calcium channel blockers and central and direct vasodilators) should first be discontinued. This allows additional renin-angiotensin-aldosterone inhibition to reduce proteinuria without causing hypotension.
In addition, “ultra-high” doses of these drugs—two or more times the maximal antihypertensive dose—appear to reduce proteinuria without further reducing blood pressure.31–34
Various combinations of an ACE inhibitor, an ARB, and an aldosterone receptor antagonist (and possibly a renin inhibitor) may also be prescribed, striving for more complete suppression of the renin-angiotensin-aldosterone system, with dose adjustments to prevent hypotension.
KEEPING SERUM POTASSIUM AT SAFE LEVELS
Intensive inhibition of the renin-angiotensin-aldosterone system, via higher doses or combination therapy, increases the risk of hyperkalemia. This risk must be addressed energetically to prevent a potentially life-threatening complication.
When prescribed by nephrologists in clinical studies, renin-angiotensin-aldosterone inhibition has proven safe, with minimal adverse events (including hyperkalemia), even with high doses,32–34 in stage 4 chronic kidney disease (ie, with a glomerular filtration rate of 15 to 29 mL/min/1.73m2, inclusively)7 and with combination therapy.11–13,20
However, the increased incidence of hyperkalemia reported with spironolactone in patients with congestive heart failure following publication of the Randomized Aldactone Evaluation Study38 suggests that safety in clinical studies should not be extrapolated to mean safety in routine, community use. Patients with chronic kidney disease should not be given high doses or combinations of these drugs unless the treating physician is experienced in the prevention and treatment of hyperkalemia; typically such therapy should be guided by a nephrologist.
When serum potassium levels exceed 5.6 mEq/L, renin-angiotensin-aldosterone inhibitors should be decreased in dose or discontinued.39 Ideally, the drug or drugs should be restarted (to provide the potential benefits of these classes of drugs) when hyperkalemia has resolved, but this requires not only resolution of hyperkalemia but also steps to prevent this serious problem from recurring. The serum potassium level should be checked frequently, particularly after any increase in renin-angiotensin-aldosterone inhibition.
Treating hyperkalemia
Potential treatments for hyperkalemia include dietary restriction, sodium bicarbonate,39 fludrocortisone (Florinef),40 kaliuretic diuretics, and sodium polystyrene sulfonate (Kayexalate). Nonsteroidal anti-inflammatory drugs should be avoided.
Dietary restriction should be particularly emphasized: if potassium intake is decreased to the same extent as renin-angiotensin-aldosterone inhibitors reduce its excretion, then the serum potassium level will remain acceptable. All dietary supplements whose contents are not precisely known should be proscribed. A list of high-potassium foods to avoid should be given with the initial prescription for the drug. If briefly reviewed at each visit, with feedback given based on measured serum potassium levels, dietary treatment is typically effective (personal observation).
Fludrocortisone is an option when dietary potassium restriction fails.
An increase in the dose of diuretic is typically required with fludrocortisone to prevent sodium retention. The combination of dietary potassium restriction, fludrocortisone (0.1 mg/day, 3–5 days a week), and furosemide (Lasix) allowed high doses of an ACE inhibitor or a combination of an ACE inhibitor and an ARB to be given in 132 patients with chronic kidney disease.40 Over several years, their mean peak potassium level was 4.87 mEq/L, and no instance of acute hyperkalemia requiried stopping the ACE inhibitor or ARB.
However, fludrocortisone is an aldosterone analogue with potentially long-term aldosterone-mediated injurious effects on heart and renal function, even though only low doses were required in the three-pronged approach to hyperkalemia.40 The long-term effect of a regimen of an ACE inhibitor plus an ARB plus fludrocortisone on cardiac and renal outcomes is unknown and of concern.
Therefore, fludrocortisone should probably be avoided in patients with systolic heart dysfunction and should be used cautiously in general. Its use might be limited to patients with proteinuric chronic kidney disease that progresses despite therapy, particularly when that progression is in the context of inability to give significant renin-angiotensin-aldosterone inhibition because of hyperkalemia.
MORE STUDY NEEDED
Chronic kidney disease treatment is becoming increasingly complex, with a lengthening list of potentially effective drugs, difficult-to-reach goals, and a less structured approach. This complexity is magnified by issues of potassium homeostasis and interindividual variations in response to renin-angiotensin-aldosterone inhibition.
More prospective studies are needed to confirm the benefits of targeting proteinuria along with blood pressure and the metrics of the goals in tandem, but, based on available information, the dual-goal approach has been recommended for proteinuric patients,10,15–18 and evidence is accumulating for greater renal protection from larger doses of renin-angiotensin-aldosterone inhibitors and from using these drugs in combination.
- US Renal Data System. Excerpts from the USRDS 2005 Annual Data Report. Am J Kidney Dis 2006; 47(suppl 1):S1–S286.
- Lewis E, Hunsicker L, Bain R, Rohde Rfor the Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993; 329:1456–1462.
- Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996; 334:939–945.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997; 349:1857–1863.
- Brenner B, Cooper M, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869.
- Lewis E, Hunsicker L, Clarke W, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–860.
- Hou F, Zhang X, Zhang G, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006; 354:131–140.
- De Zeeuw D, Remuzzi G, Parving H-H, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004; 65:2309–2320.
- Eijkelkamp W, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotension 2 Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007; 18:1540–1546.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol. 2006; 1:229–235.
- Chrysostomou A, Pedagogoa E, MacGregor L, Becker G. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin 2 receptor blocker. Clin J Am Soc Nephrol. 2006; 1:256–262.
- Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006; 70:536–542.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70:2116–2123.
- Azizi M, Menard J, Bissery A, Guyene T-T, Bura-Riviere A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combinations in sodium-replete normotensive individuals Clin J Am Soc Nephrol 2007; 2:947–955.
- Hebert L, Wilmer W, Falkenhain M, Ladson-Wofford S, Nahman S, Rovin B. Renoprotection: one or many therapies? Kidney Int 2001; 59:1211–1226.
- Shieppate A, Remuzzi G. The future of renoprotection: frustration and promises. Kidney Int. 2003; 64:1947–1955.
- Zandi-Nejad K, Brenner B. Strategies to retard the progression of chronic kidney disease. Med Clin North Am. 2005; 89:489–509.
- Ritz E, Dikow R. Hypertension and antihypertensive treatment of diabetic nephropathy. Nat Clinl Pract Nephrol. 2006; 2:562–567.
- Jafar T, Stark P, Schmid C, et al., for the AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann Intern Med. 2003; 139:244–252.
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin 2 receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomized controlled trial. Lancet. 2003; 361:117–124.
- Hou F, Xie D, Zhang X, et al. Renoprotection of optimal antiproteinuric doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007; 18:1889–1898.
- Chobanian AV, Bakris GL, Black HR. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572.
- Sarnak M, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005; 142:342–351.
- Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006; 69:2155–2161.
- Locatelli F, Pozzoni P. Chronic kidney disease in the elderly: is it really a premise for overwhelming renal failure? Kidney Int 2006; 69:2118–2120.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991; 265:3255–3264.
- Forman JP, Brenner BM. ‘Hypertension’ and ‘microalbuminuria’: the bell tolls for thee. Kidney Int. 2006; 69:22–28.
- Bakris G, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006; 70:1905–1913.
- UKPD Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective diabetes study group. BMJ. 1998; 317:713–720.
- Bakris G, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertensive and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000; 36:646–661.
- Navis G, Kramer A, de Jong P. High-dose ACE inhibition: can it improve renoprotection? Am J Kidney Dis 2002; 40:664–666.
- Rossing K, Schjoedt K, Jensin B, Boomsma F, Parving H-H. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int. 2005; 68:1190–1198.
- Schmieder R, Klingbeil A, Fleischman E, Veelken R, Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: a double-blind, randomized, prospective study. J Am Soc Nephrol. 2005; 16:3038–3045.
- Aranda P, Segura J, Ruilope L, et al. Long-term renoprotective effects of standard versus high doses of telmisartan in hypertensive nondiabetic nephropathies. Am J Kidney Dis. 2005; 46:1074–1079.
- Calhoun D. Aldosteronism and hypertension. Clin J Am Soc Nephrol. 2006; 1:1039–1045.
- Bakris G, Weir M, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004; 65:1991–2002.
- Hirsch S. A different approach to resistant hypertension. Cleve Clin J Med 2007: 74;449–456.
- Juurling D, Mamdani M, Lee D, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004; 351:543–551.
- Palmer B. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004; 351:585–592.
- Moskowitz D. From pharmacogenomics to improved patient outcomes: angiotensin 1-converting enzyme as an example. Diabetes Tech Ther. 2002; 4:519–532.
When angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor blockers (ARBs) were introduced, we hoped that these drugs would slow or stop the inexorable progression of chronic kidney disease. This hasn’t come to pass: the incidence of end-stage renal disease continued to increase throughout the 1990s, and although it may have finally reached a plateau, it remains unacceptably high.1 One reason may be that, used singly, drugs that block the renin-angiotensin-aldosterone system are only moderately successful, as approximately 20% to 40% of patients still reach unfavorable renal end points such as doubling of the serum creatinine level or dialysis.2–7
In view of these disappointing results, some experts are advocating a new strategy in which they advise that both blood pressure and urinary albumin excretion be lowered to specific goals. To achieve these goals, we will generally have to give higher doses of ACE inhibitors and ARBs alone or use a combination of these and other drugs that block the renin-angiotensin-aldosterone system at various sites.
This article describes how the dual-goal approach, with a focus on renin-angiotensin-aldosterone system inhibition, may be applied in the therapy of proteinuric chronic kidney disease. This appears to be a reasonable approach, based on current evidence, to address the epidemic of renal failure. However, further studies are needed to establish the effectiveness of this approach, and the risk of hyperkalemia following aggressive inhibition of the renin-angiotensin-aldosterone system poses a significant management problem.
ALBUMIN MAY BE TOXIC
While hypertension has long been associated with poor renal outcomes, urinary albumin has more recently been implicated by observational and experimental evidence as a tubular-interstitial toxin that may also accelerate the progression of renal disease.
For example, in both the Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) study8 and the Ramipril Efficacy in Nephropathy study,4 baseline proteinuria was almost linearly related to worse renal outcomes. In RENAAL, patients who excreted more than about 3 g of albumin per day had an 8.1-fold higher risk of progressing to end-stage renal disease.8 Moreover, the more that protein excretion could be reduced, the better the renal outcomes, down to a level of about 500 mg/day.8
Of importance, lowering blood pressure did not always decrease protein excretion—nearly 40% of patients had a dissociation between the two.9 In fact, prescribing a single ACE inhibitor or ARB while targeting only blood pressure has not predictably reduced protein excretion to 500 mg/day (the proposed goal).2–7
Although albumin has not been conclusively proven to be a renal toxin, targeting the reduction of proteinuria may also succeed if urinary albumin simply serves as a marker of the success of chronic kidney disease treatment and reflects prognosis.
A DUAL-GOAL APPROACH
In view of the observational and experimental evidence, many experts10,15–18 are advocating a dual-goal approach that stresses lowering both blood pressure and urinary protein (albumin) excretion. The recommended goal for systolic blood pressure is less than 120 to 125 mm Hg; the goal for proteinuria is less than 300 to 500 mg/24 hours,16,17,19 aiming to slow the decline in glomerular filtration rate to less than 2 mL/ min/year.11,20
The strategy of targeting both proteinuria and blood pressure has recently received further support. In a prospective randomized controlled study,21 nondiabetic patients with proteinuria received either an ACE inhibitor or an ARB. In one group, the dose was adjusted to lower the blood pressure to less than 130/80 mm/Hg; in the other group, the dose was adjusted to lower the blood pressure to 130/80 and to reduce protein excretion maximally. Only about half as many patients in the group with the dual-goal strategy reached the composite primary end point (doubling of serum creatinine, end-stage renal disease, or death) over a median of 3.7 years of follow-up, as compared with those treated by targeting the blood pressure alone.
In retrospect, the suboptimal success in the earlier landmark studies2–7 may have derived from the failure of ACE inhibitors and ARBs, used by themselves at moderate doses, to either lower the blood pressure to the recently advised goal (the actual results obtained varied from about 128 to about 145 mm Hg systolic) or, perhaps, to reduce proteinuria to the goal level.
Not all nephrologists currently pursue the stringent proteinuria goal of 500 mg per day—the targeted reduction of proteinuria requires further prospective evidence to support it. However, nephrologists do commonly follow the broad theme that antihypertensive therapy in proteinuric chronic kidney disease should accentuate medicines that protect the kidney beyond their antihypertensive effect (Table 1), and that proteinuria is an important metric that, at the very least, reflects the response to therapy and prognosis.
BLOCKING RENIN-ANGIOTENSIN- ALDOSTERONE MORE COMPLETELY
These issues may be addressed by more complete inhibition of the renin-angiotensin-aldosterone system, now achievable with the addition of aldosterone receptor antagonists and direct renin inhibitors to the ACE inhibitors and ARBs. Although we lack long-term studies of the relative efficacy of these medicines alone or in various combinations, the multistep sequence of the renin-angiotensin-aldosterone system allows for the possibility that more complete suppression via coordinated pharmacologic attention to multiple sites will yield beneficial results.
Combining an ACE inhibitor and an ARB
Even in the absence of ACE, angiotensin II is also produced by other kinases and therefore is not completely suppressed by an ACE inhibitor. For this and other reasons, there are theoretical advantages to adding an ARB to an ACE inhibitor.
In the Combination Treatment of Angiotensin 2 Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-Diabetic Renal Disease (COOPERATE) study,20 the combination of an ACE inhibitor and an ARB protected the kidneys better than either medicine alone, not only in terms of less protein in the urine but also in terms of significantly fewer patients progressing to the primary end points of doubling of serum creatinine or end-stage renal disease after 3 years of follow-up (11% of patients on combination therapy vs 23% on single therapy).
Aldosterone receptor antagonists or renin inhibitors plus ACE inhibitors and ARBs
Aldosterone escape is common during long-term therapy with ACE inhibitors and ARBs, and an aldosterone-receptor antagonist reduces proteinuria11–13 and stabilizes kidney function13 in a manner additive to that of ACE inhibitors and ARBs.
Direct renin inhibitors overcome the reactive rises in renin activity and in angiotensin II that complicate therapy with ACE inhibitors and ARBs, and they also reduce urinary aldosterone excretion.14
When to consider combination therapy
Inhibition of the renin-angiotensin-aldosterone system at multiple sites may be considered in cases of persistent hypertension or proteinuria, or of progression of chronic kidney disease despite single-drug therapy, or more broadly, with increasing evidence that combination therapy may preserve the glomerular filtration rate.13,20 This article suggests one way to apply the several available renin-angiotensin-aldosterone inhibitors, keeping in mind extensive interindividual variations, uncertain responses, and the absence of a linear evidence-based strategy known to be broadly successful.
INITIAL CONSIDERATION: WHAT IS THE BLOOD PRESSURE GOAL?
Determining the blood pressure goal for a patient may not be as straightforward as usually assumed. Typically, advisories suggest a discrete goal; for example, the Seventh Joint National Committee22 recommended a systolic blood pressure of 130 mm Hg or lower for patients with chronic kidney disease or diabetes. However, if we weigh the risks and benefits, we find that the situation is more nuanced. The blood pressure goal should vary among patients, depending on age, amount of proteinuria, whether the patient can tolerate the lowered blood pressure, and whether lowering the blood pressure to this goal stabilizes kidney function.
Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) study demonstrated a benefit of setting the goal mean arterial pressure to less than 92 mm Hg (about 125 mm Hg systolic) regardless of proteinuria.23 In addition, a meta-analysis suggested that nondiabetic proteinuric patients benefit from even lower systolic blood pressures (110–119 mm Hg).19
In older patients
However, in the MDRD study, the goal of approximately 125 mm Hg systolic pertained only to patients no older than 60 years.23 The goal was increased to about 130 mm Hg for patients 61 to 70 years old. In addition, major clinical studies of chronic kidney disease have excluded patients older than 70 years.2–7,23
Therapy for chronic kidney disease in this older age group is essentially unstudied, and we should be cautious about extrapolating results of aggressive blood pressure-lowering (and renin-angiotensin-aldosterone inhibition) from younger patients to older patients, who may have extensive vascular disease.24,25
For patients older than 70 years, guidance is perhaps best provided by the Systolic Hypertension in the Elderly Program (SHEP), which found that lowering systolic blood pressure to an average of 143 mm Hg reduced the incidence of stroke and cardiovascular disease.26 The SHEP study does not establish the optimal blood pressure goal for preventing progressive chronic kidney disease (or even cardiovascular disease) in the older age group. However, this is the lowest systolic pressure yet shown to be generally safe and associated with any improved outcome for these patients.
Additional studies are needed to evaluate whether this blood pressure level provides the best outcomes in patients with chronic kidney disease, or whether even lower blood pressures in the elderly are safe and will further improve either renal or cardiovascular outcomes.
In younger patients
In contrast, younger patients without diabetes or vascular disease may, in theory, be candidates for even lower blood pressure. No major study of chronic kidney disease isolated patients from about 20 to 40 years old for analysis, precluding direct evidence-based guidelines for this cohort at this time.
However, some of these patients may have had premorbid systolic blood pressures of 90 to 110 mm Hg, so systolic pressures of 110 to 120 mm Hg would be “hypertensive” by 10 to 30 mm Hg for them. It is possible that some patients in this cohort will tolerate a systolic pressure lower than 110 mm Hg, and that the lower blood pressure may provide additional long-term renal protection for them. This notion is theoretical, however, and has not been verified by clinical studies.
No one pressure fits all
In summary, an initial target systolic pressure for proteinuric patients, based on available evidence, might be less than 130 mm Hg for patients 61 to 70 years old,23 less than 125 mm Hg for patients younger than 61 years,23 and perhaps as low as 110 to 119 mm Hg for non-diabetic patients.19 Caution is advised against targeting systolic blood pressure less than 140 mm Hg for patients older than 70 years.
These are only initial goals and should be reevaluated as treatment progresses. The achieved blood pressure must be clinically tolerated—symptoms of tissue hypoperfusion indicate that the blood pressure is too low for the patient. In addition, the blood pressure goal (like the proteinuria goal) is only a surrogate end point, and if kidney function declines even though the surrogate end points are attained, then those end points should be reevaluated.
Tailoring blood pressure goals to the individual patient dovetails with the recent suggestion that blood pressure should not be perceived as a rigid dichotomy of “hypertension” vs “normal.”27 There is, in general, a continuous correlation between blood pressure, beginning at low levels, and the risk of cardiorenal disease, and choosing an optimal blood pressure goal for an individual patient requires an ongoing assessment of benefits, risks, and side effects.
STARTING ANTIHYPERTENSIVE THERAPY
The question of which antihypertensive drug to try first is moot in chronic kidney disease because almost all patients need multiple medicines to reach their blood pressure goals.
The Seventh Joint National Committee recommended an ACE inhibitor for initial therapy in hypertensive patients with chronic kidney disease,22 although an ARB is a reasonable first choice for those with type 2 diabetes.5,6
Diuretics potentiate the effects of ACE inhibitors and ARBs and are generally prescribed concomitantly or as the second choice.
A beta-blocker may be recommended as a third medicine (when needed), to provide a complementary class of antihypertensive, to address the high incidence of concomitant coronary artery disease and systolic dysfunction, and because of evidence that sympathetic excess contributes to the hypertension and progression of chronic kidney disease.28,29 The National Kidney Foundation30 suggests that the dose of beta-blocker be increased if the heart rate is greater than 84.
INTENSIFYING RENIN-ANGIOTENSIN-ALDOSTERONE INHIBITION: WHICH DRUGS, AND WHEN?
When hypertension and proteinuria persist despite the use of an ACE inhibitor or an ARB, additional inhibition of the renin-angiotensin-aldosterone system is generally recommended to lower both the blood pressure and the protein excretion. Increasing the dose of ACE inhibitor or ARB,31–34 combining an ACE inhibitor and an ARB,20 or adding an aldosterone receptor antagonist to either an ACE inhibitor or an ARB11–13 have all been shown to reduce proteinuria (as a surrogate end point), and several studies have, importantly, found that these combinations preserve kidney function over time.13,20
However, lacking long-term studies that compare these options, we cannot insist upon specific treatment choices or sequences in these situations.
An approach based on serum potassium and volume status
For example, if a patient has obvious signs of volume excess (eg, edema, jugular venous distention, rales) and the serum potassium concentration is less than about 5.0 or 5.5 mEq/L, then an aldosterone receptor antagonist may logically be added or increased in dose.
Aldosterone is more than a kidney hormone
Increasing the diuretic or renin-angiotensin-aldosterone inhibition
For patients who have obvious signs of volume excess and a serum potassium level greater than 5.0 mEq/L, the dosage of kaliuretic (potassium-excreting) diuretic (usually a loop diuretic in chronic kidney disease) can be increased. Although kaliuretic diuretics do not specifically lower proteinuria, they will help control volume and blood pressure and, by lowering the serum potassium level, facilitate the subsequent augmention of renin-angiotensin-aldosterone inhibition.
When a hypertensive patient does not seem to have excess volume or tachycardia and the serum potassium level is less than about 5.5 mEq/L, then additional renin-angiotensin-aldosterone inhibition is indicated.16 This may be accomplished either by increasing the ACE inhibitor or the ARB to its maximal antihypertensive dose or by starting combination therapy.
Starting a calcium channel blocker
When the serum potassium level is higher than about 5.5 mEq/L, further inhibition of the renin-angiotensin-aldosterone system is contraindicated, and a nondihydropyridine calcium channel blocker can be added for its anti-hypertensive and antiproteinuric effects.16,36
When nondihydropyridine calcium channel blockers are contraindicated due to their antiinotropic effect, an attractive alternative may be to cautiously increase the dose of kaliuretic diuretics. Given the high prevalence of (often covert) volume excess in chronic kidney disease, empiric diuresis may lower blood pressure, particularly in patients already receiving several vasodilators.37 Moreover, as mentioned, by reducing serum potassium, kaliuretic diuretics help allow for a subsequent increase in renin-angiotensin-aldosterone inhibition.
IF BLOOD PRESSURE IS NORMAL, BUT PROTEINURIA PERSISTS
Because lowering blood pressure does not necessarily reduce protein excretion, some patients achieve their blood pressure goal but still have excessive proteinuria. Proponents of the dual-goal approach suggest that these patients require further treatment modifications to reach the proteinuria goal and their optimal renal prognosis.
A cautious increase in renin-angiotensin-aldosterone inhibition is possible but is likely to be limited by low blood pressure. When applicable, any nonessential antihypertensive drug that does not specifically reduce proteinuria (ie, dihydropyridine calcium channel blockers and central and direct vasodilators) should first be discontinued. This allows additional renin-angiotensin-aldosterone inhibition to reduce proteinuria without causing hypotension.
In addition, “ultra-high” doses of these drugs—two or more times the maximal antihypertensive dose—appear to reduce proteinuria without further reducing blood pressure.31–34
Various combinations of an ACE inhibitor, an ARB, and an aldosterone receptor antagonist (and possibly a renin inhibitor) may also be prescribed, striving for more complete suppression of the renin-angiotensin-aldosterone system, with dose adjustments to prevent hypotension.
KEEPING SERUM POTASSIUM AT SAFE LEVELS
Intensive inhibition of the renin-angiotensin-aldosterone system, via higher doses or combination therapy, increases the risk of hyperkalemia. This risk must be addressed energetically to prevent a potentially life-threatening complication.
When prescribed by nephrologists in clinical studies, renin-angiotensin-aldosterone inhibition has proven safe, with minimal adverse events (including hyperkalemia), even with high doses,32–34 in stage 4 chronic kidney disease (ie, with a glomerular filtration rate of 15 to 29 mL/min/1.73m2, inclusively)7 and with combination therapy.11–13,20
However, the increased incidence of hyperkalemia reported with spironolactone in patients with congestive heart failure following publication of the Randomized Aldactone Evaluation Study38 suggests that safety in clinical studies should not be extrapolated to mean safety in routine, community use. Patients with chronic kidney disease should not be given high doses or combinations of these drugs unless the treating physician is experienced in the prevention and treatment of hyperkalemia; typically such therapy should be guided by a nephrologist.
When serum potassium levels exceed 5.6 mEq/L, renin-angiotensin-aldosterone inhibitors should be decreased in dose or discontinued.39 Ideally, the drug or drugs should be restarted (to provide the potential benefits of these classes of drugs) when hyperkalemia has resolved, but this requires not only resolution of hyperkalemia but also steps to prevent this serious problem from recurring. The serum potassium level should be checked frequently, particularly after any increase in renin-angiotensin-aldosterone inhibition.
Treating hyperkalemia
Potential treatments for hyperkalemia include dietary restriction, sodium bicarbonate,39 fludrocortisone (Florinef),40 kaliuretic diuretics, and sodium polystyrene sulfonate (Kayexalate). Nonsteroidal anti-inflammatory drugs should be avoided.
Dietary restriction should be particularly emphasized: if potassium intake is decreased to the same extent as renin-angiotensin-aldosterone inhibitors reduce its excretion, then the serum potassium level will remain acceptable. All dietary supplements whose contents are not precisely known should be proscribed. A list of high-potassium foods to avoid should be given with the initial prescription for the drug. If briefly reviewed at each visit, with feedback given based on measured serum potassium levels, dietary treatment is typically effective (personal observation).
Fludrocortisone is an option when dietary potassium restriction fails.
An increase in the dose of diuretic is typically required with fludrocortisone to prevent sodium retention. The combination of dietary potassium restriction, fludrocortisone (0.1 mg/day, 3–5 days a week), and furosemide (Lasix) allowed high doses of an ACE inhibitor or a combination of an ACE inhibitor and an ARB to be given in 132 patients with chronic kidney disease.40 Over several years, their mean peak potassium level was 4.87 mEq/L, and no instance of acute hyperkalemia requiried stopping the ACE inhibitor or ARB.
However, fludrocortisone is an aldosterone analogue with potentially long-term aldosterone-mediated injurious effects on heart and renal function, even though only low doses were required in the three-pronged approach to hyperkalemia.40 The long-term effect of a regimen of an ACE inhibitor plus an ARB plus fludrocortisone on cardiac and renal outcomes is unknown and of concern.
Therefore, fludrocortisone should probably be avoided in patients with systolic heart dysfunction and should be used cautiously in general. Its use might be limited to patients with proteinuric chronic kidney disease that progresses despite therapy, particularly when that progression is in the context of inability to give significant renin-angiotensin-aldosterone inhibition because of hyperkalemia.
MORE STUDY NEEDED
Chronic kidney disease treatment is becoming increasingly complex, with a lengthening list of potentially effective drugs, difficult-to-reach goals, and a less structured approach. This complexity is magnified by issues of potassium homeostasis and interindividual variations in response to renin-angiotensin-aldosterone inhibition.
More prospective studies are needed to confirm the benefits of targeting proteinuria along with blood pressure and the metrics of the goals in tandem, but, based on available information, the dual-goal approach has been recommended for proteinuric patients,10,15–18 and evidence is accumulating for greater renal protection from larger doses of renin-angiotensin-aldosterone inhibitors and from using these drugs in combination.
When angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor blockers (ARBs) were introduced, we hoped that these drugs would slow or stop the inexorable progression of chronic kidney disease. This hasn’t come to pass: the incidence of end-stage renal disease continued to increase throughout the 1990s, and although it may have finally reached a plateau, it remains unacceptably high.1 One reason may be that, used singly, drugs that block the renin-angiotensin-aldosterone system are only moderately successful, as approximately 20% to 40% of patients still reach unfavorable renal end points such as doubling of the serum creatinine level or dialysis.2–7
In view of these disappointing results, some experts are advocating a new strategy in which they advise that both blood pressure and urinary albumin excretion be lowered to specific goals. To achieve these goals, we will generally have to give higher doses of ACE inhibitors and ARBs alone or use a combination of these and other drugs that block the renin-angiotensin-aldosterone system at various sites.
This article describes how the dual-goal approach, with a focus on renin-angiotensin-aldosterone system inhibition, may be applied in the therapy of proteinuric chronic kidney disease. This appears to be a reasonable approach, based on current evidence, to address the epidemic of renal failure. However, further studies are needed to establish the effectiveness of this approach, and the risk of hyperkalemia following aggressive inhibition of the renin-angiotensin-aldosterone system poses a significant management problem.
ALBUMIN MAY BE TOXIC
While hypertension has long been associated with poor renal outcomes, urinary albumin has more recently been implicated by observational and experimental evidence as a tubular-interstitial toxin that may also accelerate the progression of renal disease.
For example, in both the Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) study8 and the Ramipril Efficacy in Nephropathy study,4 baseline proteinuria was almost linearly related to worse renal outcomes. In RENAAL, patients who excreted more than about 3 g of albumin per day had an 8.1-fold higher risk of progressing to end-stage renal disease.8 Moreover, the more that protein excretion could be reduced, the better the renal outcomes, down to a level of about 500 mg/day.8
Of importance, lowering blood pressure did not always decrease protein excretion—nearly 40% of patients had a dissociation between the two.9 In fact, prescribing a single ACE inhibitor or ARB while targeting only blood pressure has not predictably reduced protein excretion to 500 mg/day (the proposed goal).2–7
Although albumin has not been conclusively proven to be a renal toxin, targeting the reduction of proteinuria may also succeed if urinary albumin simply serves as a marker of the success of chronic kidney disease treatment and reflects prognosis.
A DUAL-GOAL APPROACH
In view of the observational and experimental evidence, many experts10,15–18 are advocating a dual-goal approach that stresses lowering both blood pressure and urinary protein (albumin) excretion. The recommended goal for systolic blood pressure is less than 120 to 125 mm Hg; the goal for proteinuria is less than 300 to 500 mg/24 hours,16,17,19 aiming to slow the decline in glomerular filtration rate to less than 2 mL/ min/year.11,20
The strategy of targeting both proteinuria and blood pressure has recently received further support. In a prospective randomized controlled study,21 nondiabetic patients with proteinuria received either an ACE inhibitor or an ARB. In one group, the dose was adjusted to lower the blood pressure to less than 130/80 mm/Hg; in the other group, the dose was adjusted to lower the blood pressure to 130/80 and to reduce protein excretion maximally. Only about half as many patients in the group with the dual-goal strategy reached the composite primary end point (doubling of serum creatinine, end-stage renal disease, or death) over a median of 3.7 years of follow-up, as compared with those treated by targeting the blood pressure alone.
In retrospect, the suboptimal success in the earlier landmark studies2–7 may have derived from the failure of ACE inhibitors and ARBs, used by themselves at moderate doses, to either lower the blood pressure to the recently advised goal (the actual results obtained varied from about 128 to about 145 mm Hg systolic) or, perhaps, to reduce proteinuria to the goal level.
Not all nephrologists currently pursue the stringent proteinuria goal of 500 mg per day—the targeted reduction of proteinuria requires further prospective evidence to support it. However, nephrologists do commonly follow the broad theme that antihypertensive therapy in proteinuric chronic kidney disease should accentuate medicines that protect the kidney beyond their antihypertensive effect (Table 1), and that proteinuria is an important metric that, at the very least, reflects the response to therapy and prognosis.
BLOCKING RENIN-ANGIOTENSIN- ALDOSTERONE MORE COMPLETELY
These issues may be addressed by more complete inhibition of the renin-angiotensin-aldosterone system, now achievable with the addition of aldosterone receptor antagonists and direct renin inhibitors to the ACE inhibitors and ARBs. Although we lack long-term studies of the relative efficacy of these medicines alone or in various combinations, the multistep sequence of the renin-angiotensin-aldosterone system allows for the possibility that more complete suppression via coordinated pharmacologic attention to multiple sites will yield beneficial results.
Combining an ACE inhibitor and an ARB
Even in the absence of ACE, angiotensin II is also produced by other kinases and therefore is not completely suppressed by an ACE inhibitor. For this and other reasons, there are theoretical advantages to adding an ARB to an ACE inhibitor.
In the Combination Treatment of Angiotensin 2 Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-Diabetic Renal Disease (COOPERATE) study,20 the combination of an ACE inhibitor and an ARB protected the kidneys better than either medicine alone, not only in terms of less protein in the urine but also in terms of significantly fewer patients progressing to the primary end points of doubling of serum creatinine or end-stage renal disease after 3 years of follow-up (11% of patients on combination therapy vs 23% on single therapy).
Aldosterone receptor antagonists or renin inhibitors plus ACE inhibitors and ARBs
Aldosterone escape is common during long-term therapy with ACE inhibitors and ARBs, and an aldosterone-receptor antagonist reduces proteinuria11–13 and stabilizes kidney function13 in a manner additive to that of ACE inhibitors and ARBs.
Direct renin inhibitors overcome the reactive rises in renin activity and in angiotensin II that complicate therapy with ACE inhibitors and ARBs, and they also reduce urinary aldosterone excretion.14
When to consider combination therapy
Inhibition of the renin-angiotensin-aldosterone system at multiple sites may be considered in cases of persistent hypertension or proteinuria, or of progression of chronic kidney disease despite single-drug therapy, or more broadly, with increasing evidence that combination therapy may preserve the glomerular filtration rate.13,20 This article suggests one way to apply the several available renin-angiotensin-aldosterone inhibitors, keeping in mind extensive interindividual variations, uncertain responses, and the absence of a linear evidence-based strategy known to be broadly successful.
INITIAL CONSIDERATION: WHAT IS THE BLOOD PRESSURE GOAL?
Determining the blood pressure goal for a patient may not be as straightforward as usually assumed. Typically, advisories suggest a discrete goal; for example, the Seventh Joint National Committee22 recommended a systolic blood pressure of 130 mm Hg or lower for patients with chronic kidney disease or diabetes. However, if we weigh the risks and benefits, we find that the situation is more nuanced. The blood pressure goal should vary among patients, depending on age, amount of proteinuria, whether the patient can tolerate the lowered blood pressure, and whether lowering the blood pressure to this goal stabilizes kidney function.
Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) study demonstrated a benefit of setting the goal mean arterial pressure to less than 92 mm Hg (about 125 mm Hg systolic) regardless of proteinuria.23 In addition, a meta-analysis suggested that nondiabetic proteinuric patients benefit from even lower systolic blood pressures (110–119 mm Hg).19
In older patients
However, in the MDRD study, the goal of approximately 125 mm Hg systolic pertained only to patients no older than 60 years.23 The goal was increased to about 130 mm Hg for patients 61 to 70 years old. In addition, major clinical studies of chronic kidney disease have excluded patients older than 70 years.2–7,23
Therapy for chronic kidney disease in this older age group is essentially unstudied, and we should be cautious about extrapolating results of aggressive blood pressure-lowering (and renin-angiotensin-aldosterone inhibition) from younger patients to older patients, who may have extensive vascular disease.24,25
For patients older than 70 years, guidance is perhaps best provided by the Systolic Hypertension in the Elderly Program (SHEP), which found that lowering systolic blood pressure to an average of 143 mm Hg reduced the incidence of stroke and cardiovascular disease.26 The SHEP study does not establish the optimal blood pressure goal for preventing progressive chronic kidney disease (or even cardiovascular disease) in the older age group. However, this is the lowest systolic pressure yet shown to be generally safe and associated with any improved outcome for these patients.
Additional studies are needed to evaluate whether this blood pressure level provides the best outcomes in patients with chronic kidney disease, or whether even lower blood pressures in the elderly are safe and will further improve either renal or cardiovascular outcomes.
In younger patients
In contrast, younger patients without diabetes or vascular disease may, in theory, be candidates for even lower blood pressure. No major study of chronic kidney disease isolated patients from about 20 to 40 years old for analysis, precluding direct evidence-based guidelines for this cohort at this time.
However, some of these patients may have had premorbid systolic blood pressures of 90 to 110 mm Hg, so systolic pressures of 110 to 120 mm Hg would be “hypertensive” by 10 to 30 mm Hg for them. It is possible that some patients in this cohort will tolerate a systolic pressure lower than 110 mm Hg, and that the lower blood pressure may provide additional long-term renal protection for them. This notion is theoretical, however, and has not been verified by clinical studies.
No one pressure fits all
In summary, an initial target systolic pressure for proteinuric patients, based on available evidence, might be less than 130 mm Hg for patients 61 to 70 years old,23 less than 125 mm Hg for patients younger than 61 years,23 and perhaps as low as 110 to 119 mm Hg for non-diabetic patients.19 Caution is advised against targeting systolic blood pressure less than 140 mm Hg for patients older than 70 years.
These are only initial goals and should be reevaluated as treatment progresses. The achieved blood pressure must be clinically tolerated—symptoms of tissue hypoperfusion indicate that the blood pressure is too low for the patient. In addition, the blood pressure goal (like the proteinuria goal) is only a surrogate end point, and if kidney function declines even though the surrogate end points are attained, then those end points should be reevaluated.
Tailoring blood pressure goals to the individual patient dovetails with the recent suggestion that blood pressure should not be perceived as a rigid dichotomy of “hypertension” vs “normal.”27 There is, in general, a continuous correlation between blood pressure, beginning at low levels, and the risk of cardiorenal disease, and choosing an optimal blood pressure goal for an individual patient requires an ongoing assessment of benefits, risks, and side effects.
STARTING ANTIHYPERTENSIVE THERAPY
The question of which antihypertensive drug to try first is moot in chronic kidney disease because almost all patients need multiple medicines to reach their blood pressure goals.
The Seventh Joint National Committee recommended an ACE inhibitor for initial therapy in hypertensive patients with chronic kidney disease,22 although an ARB is a reasonable first choice for those with type 2 diabetes.5,6
Diuretics potentiate the effects of ACE inhibitors and ARBs and are generally prescribed concomitantly or as the second choice.
A beta-blocker may be recommended as a third medicine (when needed), to provide a complementary class of antihypertensive, to address the high incidence of concomitant coronary artery disease and systolic dysfunction, and because of evidence that sympathetic excess contributes to the hypertension and progression of chronic kidney disease.28,29 The National Kidney Foundation30 suggests that the dose of beta-blocker be increased if the heart rate is greater than 84.
INTENSIFYING RENIN-ANGIOTENSIN-ALDOSTERONE INHIBITION: WHICH DRUGS, AND WHEN?
When hypertension and proteinuria persist despite the use of an ACE inhibitor or an ARB, additional inhibition of the renin-angiotensin-aldosterone system is generally recommended to lower both the blood pressure and the protein excretion. Increasing the dose of ACE inhibitor or ARB,31–34 combining an ACE inhibitor and an ARB,20 or adding an aldosterone receptor antagonist to either an ACE inhibitor or an ARB11–13 have all been shown to reduce proteinuria (as a surrogate end point), and several studies have, importantly, found that these combinations preserve kidney function over time.13,20
However, lacking long-term studies that compare these options, we cannot insist upon specific treatment choices or sequences in these situations.
An approach based on serum potassium and volume status
For example, if a patient has obvious signs of volume excess (eg, edema, jugular venous distention, rales) and the serum potassium concentration is less than about 5.0 or 5.5 mEq/L, then an aldosterone receptor antagonist may logically be added or increased in dose.
Aldosterone is more than a kidney hormone
Increasing the diuretic or renin-angiotensin-aldosterone inhibition
For patients who have obvious signs of volume excess and a serum potassium level greater than 5.0 mEq/L, the dosage of kaliuretic (potassium-excreting) diuretic (usually a loop diuretic in chronic kidney disease) can be increased. Although kaliuretic diuretics do not specifically lower proteinuria, they will help control volume and blood pressure and, by lowering the serum potassium level, facilitate the subsequent augmention of renin-angiotensin-aldosterone inhibition.
When a hypertensive patient does not seem to have excess volume or tachycardia and the serum potassium level is less than about 5.5 mEq/L, then additional renin-angiotensin-aldosterone inhibition is indicated.16 This may be accomplished either by increasing the ACE inhibitor or the ARB to its maximal antihypertensive dose or by starting combination therapy.
Starting a calcium channel blocker
When the serum potassium level is higher than about 5.5 mEq/L, further inhibition of the renin-angiotensin-aldosterone system is contraindicated, and a nondihydropyridine calcium channel blocker can be added for its anti-hypertensive and antiproteinuric effects.16,36
When nondihydropyridine calcium channel blockers are contraindicated due to their antiinotropic effect, an attractive alternative may be to cautiously increase the dose of kaliuretic diuretics. Given the high prevalence of (often covert) volume excess in chronic kidney disease, empiric diuresis may lower blood pressure, particularly in patients already receiving several vasodilators.37 Moreover, as mentioned, by reducing serum potassium, kaliuretic diuretics help allow for a subsequent increase in renin-angiotensin-aldosterone inhibition.
IF BLOOD PRESSURE IS NORMAL, BUT PROTEINURIA PERSISTS
Because lowering blood pressure does not necessarily reduce protein excretion, some patients achieve their blood pressure goal but still have excessive proteinuria. Proponents of the dual-goal approach suggest that these patients require further treatment modifications to reach the proteinuria goal and their optimal renal prognosis.
A cautious increase in renin-angiotensin-aldosterone inhibition is possible but is likely to be limited by low blood pressure. When applicable, any nonessential antihypertensive drug that does not specifically reduce proteinuria (ie, dihydropyridine calcium channel blockers and central and direct vasodilators) should first be discontinued. This allows additional renin-angiotensin-aldosterone inhibition to reduce proteinuria without causing hypotension.
In addition, “ultra-high” doses of these drugs—two or more times the maximal antihypertensive dose—appear to reduce proteinuria without further reducing blood pressure.31–34
Various combinations of an ACE inhibitor, an ARB, and an aldosterone receptor antagonist (and possibly a renin inhibitor) may also be prescribed, striving for more complete suppression of the renin-angiotensin-aldosterone system, with dose adjustments to prevent hypotension.
KEEPING SERUM POTASSIUM AT SAFE LEVELS
Intensive inhibition of the renin-angiotensin-aldosterone system, via higher doses or combination therapy, increases the risk of hyperkalemia. This risk must be addressed energetically to prevent a potentially life-threatening complication.
When prescribed by nephrologists in clinical studies, renin-angiotensin-aldosterone inhibition has proven safe, with minimal adverse events (including hyperkalemia), even with high doses,32–34 in stage 4 chronic kidney disease (ie, with a glomerular filtration rate of 15 to 29 mL/min/1.73m2, inclusively)7 and with combination therapy.11–13,20
However, the increased incidence of hyperkalemia reported with spironolactone in patients with congestive heart failure following publication of the Randomized Aldactone Evaluation Study38 suggests that safety in clinical studies should not be extrapolated to mean safety in routine, community use. Patients with chronic kidney disease should not be given high doses or combinations of these drugs unless the treating physician is experienced in the prevention and treatment of hyperkalemia; typically such therapy should be guided by a nephrologist.
When serum potassium levels exceed 5.6 mEq/L, renin-angiotensin-aldosterone inhibitors should be decreased in dose or discontinued.39 Ideally, the drug or drugs should be restarted (to provide the potential benefits of these classes of drugs) when hyperkalemia has resolved, but this requires not only resolution of hyperkalemia but also steps to prevent this serious problem from recurring. The serum potassium level should be checked frequently, particularly after any increase in renin-angiotensin-aldosterone inhibition.
Treating hyperkalemia
Potential treatments for hyperkalemia include dietary restriction, sodium bicarbonate,39 fludrocortisone (Florinef),40 kaliuretic diuretics, and sodium polystyrene sulfonate (Kayexalate). Nonsteroidal anti-inflammatory drugs should be avoided.
Dietary restriction should be particularly emphasized: if potassium intake is decreased to the same extent as renin-angiotensin-aldosterone inhibitors reduce its excretion, then the serum potassium level will remain acceptable. All dietary supplements whose contents are not precisely known should be proscribed. A list of high-potassium foods to avoid should be given with the initial prescription for the drug. If briefly reviewed at each visit, with feedback given based on measured serum potassium levels, dietary treatment is typically effective (personal observation).
Fludrocortisone is an option when dietary potassium restriction fails.
An increase in the dose of diuretic is typically required with fludrocortisone to prevent sodium retention. The combination of dietary potassium restriction, fludrocortisone (0.1 mg/day, 3–5 days a week), and furosemide (Lasix) allowed high doses of an ACE inhibitor or a combination of an ACE inhibitor and an ARB to be given in 132 patients with chronic kidney disease.40 Over several years, their mean peak potassium level was 4.87 mEq/L, and no instance of acute hyperkalemia requiried stopping the ACE inhibitor or ARB.
However, fludrocortisone is an aldosterone analogue with potentially long-term aldosterone-mediated injurious effects on heart and renal function, even though only low doses were required in the three-pronged approach to hyperkalemia.40 The long-term effect of a regimen of an ACE inhibitor plus an ARB plus fludrocortisone on cardiac and renal outcomes is unknown and of concern.
Therefore, fludrocortisone should probably be avoided in patients with systolic heart dysfunction and should be used cautiously in general. Its use might be limited to patients with proteinuric chronic kidney disease that progresses despite therapy, particularly when that progression is in the context of inability to give significant renin-angiotensin-aldosterone inhibition because of hyperkalemia.
MORE STUDY NEEDED
Chronic kidney disease treatment is becoming increasingly complex, with a lengthening list of potentially effective drugs, difficult-to-reach goals, and a less structured approach. This complexity is magnified by issues of potassium homeostasis and interindividual variations in response to renin-angiotensin-aldosterone inhibition.
More prospective studies are needed to confirm the benefits of targeting proteinuria along with blood pressure and the metrics of the goals in tandem, but, based on available information, the dual-goal approach has been recommended for proteinuric patients,10,15–18 and evidence is accumulating for greater renal protection from larger doses of renin-angiotensin-aldosterone inhibitors and from using these drugs in combination.
- US Renal Data System. Excerpts from the USRDS 2005 Annual Data Report. Am J Kidney Dis 2006; 47(suppl 1):S1–S286.
- Lewis E, Hunsicker L, Bain R, Rohde Rfor the Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993; 329:1456–1462.
- Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996; 334:939–945.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997; 349:1857–1863.
- Brenner B, Cooper M, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869.
- Lewis E, Hunsicker L, Clarke W, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–860.
- Hou F, Zhang X, Zhang G, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006; 354:131–140.
- De Zeeuw D, Remuzzi G, Parving H-H, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004; 65:2309–2320.
- Eijkelkamp W, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotension 2 Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007; 18:1540–1546.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol. 2006; 1:229–235.
- Chrysostomou A, Pedagogoa E, MacGregor L, Becker G. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin 2 receptor blocker. Clin J Am Soc Nephrol. 2006; 1:256–262.
- Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006; 70:536–542.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70:2116–2123.
- Azizi M, Menard J, Bissery A, Guyene T-T, Bura-Riviere A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combinations in sodium-replete normotensive individuals Clin J Am Soc Nephrol 2007; 2:947–955.
- Hebert L, Wilmer W, Falkenhain M, Ladson-Wofford S, Nahman S, Rovin B. Renoprotection: one or many therapies? Kidney Int 2001; 59:1211–1226.
- Shieppate A, Remuzzi G. The future of renoprotection: frustration and promises. Kidney Int. 2003; 64:1947–1955.
- Zandi-Nejad K, Brenner B. Strategies to retard the progression of chronic kidney disease. Med Clin North Am. 2005; 89:489–509.
- Ritz E, Dikow R. Hypertension and antihypertensive treatment of diabetic nephropathy. Nat Clinl Pract Nephrol. 2006; 2:562–567.
- Jafar T, Stark P, Schmid C, et al., for the AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann Intern Med. 2003; 139:244–252.
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin 2 receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomized controlled trial. Lancet. 2003; 361:117–124.
- Hou F, Xie D, Zhang X, et al. Renoprotection of optimal antiproteinuric doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007; 18:1889–1898.
- Chobanian AV, Bakris GL, Black HR. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572.
- Sarnak M, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005; 142:342–351.
- Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006; 69:2155–2161.
- Locatelli F, Pozzoni P. Chronic kidney disease in the elderly: is it really a premise for overwhelming renal failure? Kidney Int 2006; 69:2118–2120.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991; 265:3255–3264.
- Forman JP, Brenner BM. ‘Hypertension’ and ‘microalbuminuria’: the bell tolls for thee. Kidney Int. 2006; 69:22–28.
- Bakris G, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006; 70:1905–1913.
- UKPD Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective diabetes study group. BMJ. 1998; 317:713–720.
- Bakris G, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertensive and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000; 36:646–661.
- Navis G, Kramer A, de Jong P. High-dose ACE inhibition: can it improve renoprotection? Am J Kidney Dis 2002; 40:664–666.
- Rossing K, Schjoedt K, Jensin B, Boomsma F, Parving H-H. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int. 2005; 68:1190–1198.
- Schmieder R, Klingbeil A, Fleischman E, Veelken R, Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: a double-blind, randomized, prospective study. J Am Soc Nephrol. 2005; 16:3038–3045.
- Aranda P, Segura J, Ruilope L, et al. Long-term renoprotective effects of standard versus high doses of telmisartan in hypertensive nondiabetic nephropathies. Am J Kidney Dis. 2005; 46:1074–1079.
- Calhoun D. Aldosteronism and hypertension. Clin J Am Soc Nephrol. 2006; 1:1039–1045.
- Bakris G, Weir M, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004; 65:1991–2002.
- Hirsch S. A different approach to resistant hypertension. Cleve Clin J Med 2007: 74;449–456.
- Juurling D, Mamdani M, Lee D, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004; 351:543–551.
- Palmer B. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004; 351:585–592.
- Moskowitz D. From pharmacogenomics to improved patient outcomes: angiotensin 1-converting enzyme as an example. Diabetes Tech Ther. 2002; 4:519–532.
- US Renal Data System. Excerpts from the USRDS 2005 Annual Data Report. Am J Kidney Dis 2006; 47(suppl 1):S1–S286.
- Lewis E, Hunsicker L, Bain R, Rohde Rfor the Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993; 329:1456–1462.
- Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996; 334:939–945.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997; 349:1857–1863.
- Brenner B, Cooper M, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869.
- Lewis E, Hunsicker L, Clarke W, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–860.
- Hou F, Zhang X, Zhang G, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006; 354:131–140.
- De Zeeuw D, Remuzzi G, Parving H-H, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004; 65:2309–2320.
- Eijkelkamp W, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotension 2 Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007; 18:1540–1546.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol. 2006; 1:229–235.
- Chrysostomou A, Pedagogoa E, MacGregor L, Becker G. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin 2 receptor blocker. Clin J Am Soc Nephrol. 2006; 1:256–262.
- Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006; 70:536–542.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70:2116–2123.
- Azizi M, Menard J, Bissery A, Guyene T-T, Bura-Riviere A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combinations in sodium-replete normotensive individuals Clin J Am Soc Nephrol 2007; 2:947–955.
- Hebert L, Wilmer W, Falkenhain M, Ladson-Wofford S, Nahman S, Rovin B. Renoprotection: one or many therapies? Kidney Int 2001; 59:1211–1226.
- Shieppate A, Remuzzi G. The future of renoprotection: frustration and promises. Kidney Int. 2003; 64:1947–1955.
- Zandi-Nejad K, Brenner B. Strategies to retard the progression of chronic kidney disease. Med Clin North Am. 2005; 89:489–509.
- Ritz E, Dikow R. Hypertension and antihypertensive treatment of diabetic nephropathy. Nat Clinl Pract Nephrol. 2006; 2:562–567.
- Jafar T, Stark P, Schmid C, et al., for the AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann Intern Med. 2003; 139:244–252.
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin 2 receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomized controlled trial. Lancet. 2003; 361:117–124.
- Hou F, Xie D, Zhang X, et al. Renoprotection of optimal antiproteinuric doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007; 18:1889–1898.
- Chobanian AV, Bakris GL, Black HR. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572.
- Sarnak M, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005; 142:342–351.
- Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006; 69:2155–2161.
- Locatelli F, Pozzoni P. Chronic kidney disease in the elderly: is it really a premise for overwhelming renal failure? Kidney Int 2006; 69:2118–2120.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991; 265:3255–3264.
- Forman JP, Brenner BM. ‘Hypertension’ and ‘microalbuminuria’: the bell tolls for thee. Kidney Int. 2006; 69:22–28.
- Bakris G, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006; 70:1905–1913.
- UKPD Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective diabetes study group. BMJ. 1998; 317:713–720.
- Bakris G, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertensive and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000; 36:646–661.
- Navis G, Kramer A, de Jong P. High-dose ACE inhibition: can it improve renoprotection? Am J Kidney Dis 2002; 40:664–666.
- Rossing K, Schjoedt K, Jensin B, Boomsma F, Parving H-H. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int. 2005; 68:1190–1198.
- Schmieder R, Klingbeil A, Fleischman E, Veelken R, Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: a double-blind, randomized, prospective study. J Am Soc Nephrol. 2005; 16:3038–3045.
- Aranda P, Segura J, Ruilope L, et al. Long-term renoprotective effects of standard versus high doses of telmisartan in hypertensive nondiabetic nephropathies. Am J Kidney Dis. 2005; 46:1074–1079.
- Calhoun D. Aldosteronism and hypertension. Clin J Am Soc Nephrol. 2006; 1:1039–1045.
- Bakris G, Weir M, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004; 65:1991–2002.
- Hirsch S. A different approach to resistant hypertension. Cleve Clin J Med 2007: 74;449–456.
- Juurling D, Mamdani M, Lee D, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004; 351:543–551.
- Palmer B. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004; 351:585–592.
- Moskowitz D. From pharmacogenomics to improved patient outcomes: angiotensin 1-converting enzyme as an example. Diabetes Tech Ther. 2002; 4:519–532.
KEY POINTS
- Evidence is emerging that urinary albumin is toxic to the kidney.
- Lowering both blood pressure and urinary albumin excretion, as a means to prevent progressive renal disease, appears to require aggressive inhibition of the renin-angiotensin-aldosterone system, often with several complementary drugs, ie, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, aldosterone receptor antagonists, and possibly, direct renin inhibitors.
- Volume status and potassium levels may help suggest which of several available drugs could be added at different times.
- Serum potassium levels must be managed aggressively when using renin-angiotensin-aldosterone inhibitors in combination.