User login
Bolus Insulin Prescribing Recommendations for Patients With Type 2 Diabetes Mellitus (FULL)
Individuals with type 2 diabetes mellitus (T2DM) spend between 5 and 10 years with elevated hemoglobin A1c (HbA1c) before initiation of insulin.1 Once the basal insulin is initiated, the patient can go years with only adjustment of the basal insulin, resulting in over-basalization. In general, the total daily dose (TDD) of insulin should be composed of about 50% basal “background” insulin and 50% bolus “meal” insulin. When the fasting glucose readings are on target but HbA1c is still above the mutually set goal range, postprandial readings need to be evaluated.
This article focuses on initiating and titrating bolus insulin in nonpregnant patients with T2DM. Before initiation of bolus insulin, it is important for the patient to be actively engaged with a diabetes educator for diabetes self-management education and support (DSME/S), including the understanding of the correct use of insulin, carbohydrate counting, and increasing physical activities. Ensuring the correct technique of insulin administration and self-monitoring of blood glucose (SMBG) is critical. A knowledge deficit of carbohydrate information can lead to uncontrolled blood glucose (BG). The authors have encountered numerous times when patients were drinking sugary beverages or consuming large amounts of “healthy” food without realizing the carbohydrate content. Therefore, treatment in concert with a registered dietitian and certified diabetes educator is highly recommended.
Initiation of Bolus Insulin
There are 3 options of postprandial coverage with bolus insulin when a patient is taking basal insulin: basal plus, basal-bolus, or premix insulin. A possible fourth option for postprandial coverage is to add glucagon-like peptide-1 receptor agonist (GLP-1 RA), an injectable noninsulin antihyperglycemic agent, which has shown noninferior efficacy to adding bolus insulin and a favorable effect on weight with less risk of hypoglycemia.2-5 Although it can be expensive, combining GLP-1 RA to basal insulin results in lowering HbA1c of 0.66 up to 1.74% (or lowering mmol/mol of 7 up to 19) from the baseline.6 However, adding bolus insulin may be the only option to avoid glucotoxicity and prevent further diabetes complications when the HbA1c level is well above the goal range. It is usually recommended to discontinue sulfonylurea when bolus insulin is added due to the β-cell exhaustion with advancing natural history of diabetes.7,8
Method 1: Basal Plus
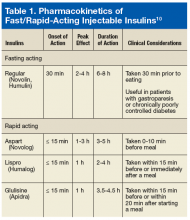
The health care provider (HCP) needs to consider whether the patient is over-basalized when the HbAlc and postprandial BG readings are still not at goal despite careful titration of basal insulin dose to > 0.5 U/kg/d.9 This is the time to discuss with the patient the coverage of mealtime glucose excursions. In the basal plus regimen, the prescribing provider may add 1 bolus insulin injection for the meal with the highest amount of carbohydrates or add 2 bolus injections for the most and second most meals with carbohydrates. Multiple types of bolus insulin are available in the current U.S. market (Table 1).
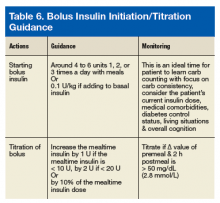
There are 2 ways to add bolus insulin: fixed and flexible. In the fixed regimen, the patient will take the same amount of bolus insulin regardless of premeal BG readings and carbohydrate content of the food. The authors recommend adding bolus insulin of about 4 to 6 units once or twice a day with meals, depending on the number of meals a day, carbohydrate content of the meal, current and desired degree of diabetes control, and physical activities. Another way to calculate a bolus insulin dose is to start at 0.1 U/kg if adding to the basal insulin.10 Flexible regimen allows various bolus doses based on premeal BG, carbohydrate intake, and activities. Information on this regimen, will be discussed more later.
Patient Cases
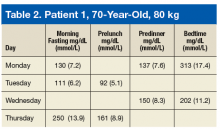
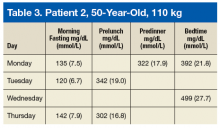
Tables 2 and 3 describe 2 patient cases. For example, patient 1 weighs 80 kg. If the prescribing HCP and patient decide to add only 1 bolus to the largest carbohydrate meal at dinnertime, then the patient may take 8 units (80 kg × 0.1 U/kg/meal = 8 units for meal). The patient’s current insulin dose, medical comorbidities, current diabetes control status, living situations, and overall cognition also should be considered.
Imagine patient 1 is taking 14 units daily of a long-acting insulin (LAI). If the patient is taking a fairly low dose of LAI, has multiple comorbidities, recent BG log/HgAlc, and lives alone but demonstrates good cognition to follow instructions, the prescriber may consider adding the bolus insulin of 4 units for dinner; thus, the bolus dose is about one-third of total basal insulin dose. However, if the patient is on 40 units of LAI and has symptoms of hyperglycemia, 6 to 8 units for the dinner is reasonable. An important point to convey to this patient is to make sure there is carbohydrate consistency. The patient’s premeal BG was 137 mg/dL (7.6 mmol/L) on Monday, but it rose significantly to 313 mg/dL (17.4 mmol/L) after dinner. On Wednesday, the patient’s premeal BG prior to dinner was 150 mg/dL (8.3 mmol/L); it rose to 202 mg/dL (11.2 mmol/L) after dinner, which is high but not as high as on Monday.
Multiple factors may affect this variability; for example, on Monday the patient may have consumed more than the usual amount of carbohydrates for dinner, forgot to take oral medication for dinner, or missed his/her usual after-dinner walk. Or simply, the patient may have eaten a lot less than the usual amount of carbohydrates, walked the neighborhood, or vacuumed the entire house after dinner on Wednesday. Thus, it is imperative to carefully assess the patient’s lifestyle and recommend carbohydrate consistency at each meal.
Method 2: Basal-Bolus
When basal plus is insufficient to get the HbA1c and BG readings to goal, taking bolus insulin for all main meals containing carbohydrates must be considered. This is often called basal-bolus, multiple daily injections, or intensive insulin therapy.
In order to understand the concept of basal-bolus, HCPs should consider normal physiology. The pancreas releases a constant amount of insulin, aka background insulin, to cover glucose produced by the liver to the cells between meals. In addition, a burst of insulin, aka bolus insulin, to meet the blood glucose elevation from food to maintain homeostasis. In patients with T2DM, the relative amount produced by the pancreas is insufficient to meet the demand due to pancreatic exhaustion or insulin resistance. This necessitates the need to replace background and bolus insulin.7
The ideal final total bolus insulin amount (the sum of all meal bolus doses) should be about half the basal dosing. Calculation of starting bolus dosing can be done as in the basal plus regimen, either 4 to 6 units per meal or 0.1 U/kg/d.10 Alternatively, if the patient is on 60 units of long-acting analog and BGs are well above goal range, the prescriber could consider about 20 units of bolus dose (60 U divided by 3 meals) if the patient eats 3 routine meals a day with at least 30 g of carbohydrates, and physical activity levels are fairly consistent. If the patient eats the most carbohydrates at lunchtime, consider more bolus at lunch (ie, 18 U of bolus for breakfast and dinner and 24 U of bolus for lunch coverage).
Patients need to separate the time between the bolus doses, usually a minimum of 4 hours apart, to avoid insulin stacking, which is a common reason for hypoglycemia. Insulin stacking occurs when additional quick or rapid insulin is injected when the previous insulin is still in the body or when there is insulin on board.8,11 Typically, bolus analogs stay in the body for about 4 to 6 hours, thus necessitating separation of the doses at least 4 hours apart. Patients sometimes inject more bolus insulin after high postprandial readings, which can result in insulin stacking. In some cases, the patient may misunderstand and take mealtime insulin at a scheduled time instead of at the time of the meal.
Injecting bolus insulin for every snack must be avoided to prevent a vicious cycle: Postprandial hyperglycemia –> extra bolus insulin, resulting in insulin stacking –> hypoglycemia –> overtreatment with food –> hyperglycemia –> extra bolus insulin, resulting in insulin stacking –> and so on. Whenever there are readings in the hypoglycemic and hyperglycemic range, address hypoglycemia first because hyperglycemia often is due to overtreatment of hypoglycemia.
Method 3: Premix or Split-Mix (Patient-Mix) Insulin
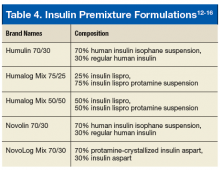
Postprandial BG excursions can be minimized by changing basal insulin to premix or split-mix (patient-mix) insulin that has a mixture of mealtime and intermediate action insulin (Table 4).12-16 The use of premixed insulin is a viable option due to its ease of use and for those who have restrictions based on the complexity of the basal-bolus regimen.7 If a patient has routine meals and prefers not to carry around insulin for lunch, the schedule of premix insulin taken at breakfast and dinner is ideal.
Some caveats for safe prescribing should be understood. A recent summary of premixed insulin regimens noted that they seem to have a similar efficacy and safety profile compared with regimens that include basal insulin with or without mealtime insulin; however, cost and patient adherence are improved.17 It is important to monitor insulin-naïve patients for hypoglycemia and reduced efficacy when used twice daily compared with basal plus 3-times daily prandial insulin in patients needing insulin intensification.17
A randomized trial noted that hypoglycemia rates were twice as high with premixed insulin compared with basal-bolus insulin.18 This study also noted that the premixed insulin group experienced the highest dropout rate, partly due to hypoglycemia. A regimen of basal insulin with the option to add a single prandial insulin injection at the main meal was as effective in reducing HbA1c with less hypoglycemia. The premixed insulin is convenient but does not allow a separate correction of either mealtime or intermediate-acting insulin doses. If the premixed dose needs to be adjusted due to fasting hyperglycemia > 180 mg/dL(10.0 mmol/L), the TDD can be increased by 10%.2
In contrast, a split-mix (patient-mix) insulin regimen allows for the ability to vary the amount/ratio of combinations and adjustment of bolus and intermediate insulin doses. The disadvantages of split-mix insulin include the inconvenience of manually mixing of insulin and the potential for dosing errors. The patient needs to be taught additional steps on how to mix both insulins. Ensure the correct mixing order to maintain insulin potency; regular first, then neutral protamine Hagedorn (NPH). An HCP should remember the RN acronym if the patient is combining regular insulin and NPH. If there is doubt about the patient’s insulin injection technique, HCPs should ask the patient to demonstrate how to correctly pull up a dose of normal saline and inject it during a clinic visit. The only basal insulin that can be physically mixed with quick or rapid insulin is NPH. It should never be mixed with long-acting analogs. The patient should not even use the same syringe to draw up bolus analog insulin and inject it and then use the same syringe to draw up long-acting analog insulin.
One caveat to a fixed regimen (same amount of insulin dose) is that providers often expect that the patient will eat a consistent amount of carbohydrates at each meal and premeal glucose readings are fairly stable. Oftentimes, this is not true. If a patient took a bolus dose of 8 units of rapid-acting insulin and ate a 6 oz steak, 3 oz baked potato, steamed broccoli at a dinner; and no bread, the after dinner BG might register 145 mg/dL (8.1 mmol/L). Then, the next day for dinner, if he or she took the same amount of 8 units of rapid-acting insulin and ate 1 cup of spaghetti, ½ cup of spaghetti meat sauce, and 2 slices of garlic bread, the after dinner reading might be 322 mg/dL (17.9 mmol/L). The patient’s BG was higher on the second day because of the higher carbohydrate content of the meal. If the rapid-acting insulin was increased to 12 units of bolus based on the high carbohydrate meal and the patient ate a lower carbohydrate meal, hypoglycemia could ensue. Thus, it is important to work with the patient regarding the consumption of a consistent amount of carbohydrates and refer to a registered dietitian for carbohydrate consistency.
For the flexible regimen, the prescriber may consider using an insulin to carbohydrate (IC) ratio and sensitivity factor (SF), also called sliding scale or correction factor. The IC ratio represents how much insulin is needed to cover consumed carbohydrates. For instance, if the patient uses IC ratio of 1:15, 1 unit of bolus insulin will cover 15 g of carbohydrates. If the patient eats a meal with 60 g of carbohydrates and is using IC ratio of 1:15, the patient will inject 4 units of bolus insulin. Sensitivity factor represents how much BG will be lowered in mg/dL by taking 1 unit of bolus insulin. For example, if the patient uses SF of 1:50, 1 unit of bolus insulin will lower BG by 50 mg/dL (2.8 mmol/L). When the desired (target) BG reading is 100 mg/dL (5.6 mmol/L) and the patient’s current BG is 200 mg/dL (11.1 mmol/L), the patient will divide 100 mg/dL (5.6 mmol/L) by 50 (derived from SF of 1:50). The net result is 2 units of bolus insulin are needed to lower BG by 100 mg/dL (5.6 mmol/L). If the premeal BG is 200 mg/dL (11.1 mmol/L) and 60 g of carbohydrates are eaten, then the patient will need a total of 6 units (4 U for carbohydrate and 2 U for high BG) bolus before the meal. For additional information, readers are encouraged to read the articles by Petznick and by Joslin Diabetes Center for IC and SF.19,20
Bolus Insulin Titration
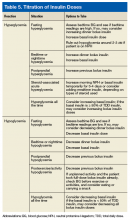
When the difference in BG readings before and 2 hours after a meal, called the Δ value, is > 50 mg/dL (2.8 mmol/L), the bolus insulin may need to be adjusted after ensuring the patient is ingesting consistent carbohydrates and performs the usual amount of activities around mealtime. For example, if the premeal reading was 130 mg/dL (7.2 mmol/L) but the 2-hour postprandial reading is > 180 mg/dL (10.0 mmol/L), the prescriber can increase the mealtime insulin by 1 unit if the mealtime insulin is < 10 units, by 2 units if < 20 units, or by 10% of the mealtime insulin dose. If the premeal BG is < 80 mg/dL (4.4 mmol/L) and the drop in BG is > Δ value of 50 mg/dL (2.8 mmol/L), the prescriber can decrease the mealtime insulin using the same calculation. Monitoring BG and titration recommendations are shown in Table 5. When adjusting the bolus insulin dose, it is best to make adjustments gradually rather than making several changes at once.
The 15/15 rule needs to be followed in cases involving hypoglycemia.21 When the BG is ≤ 70 mg/dL (3.9 mmol/L) and the patient is conscious and able to eat or drink, it is recommended they eat 15 g (30 g if BG is below 50) of carbohydrates then repeat BG check every 15 minutes until the BG is in the target range.22,23 If the patient is unconscious, providers should administer glucagon (if available), place the patient in a lateral position to avoid aspiration, and call 911. If hyperkalemia is an issue in chronic kidney disease, patients should consume apple juice rather than orange juice due to its lower potassium content. If the patient is taking α glucosidase inhibitors (AGI) like acarbose or miglitol, only pure glucose like glucose tablets needs to be given to treat hypoglycemia instead of regular soda or candy, as the AGI will slow absorption of other types of carbohydrates.24,25 After the severe hypoglycemic episode, it is imperative to assess for the cause and explore ways to prevent subsequent hypoglycemia. Providers also should advise the patient to wear medical emergency identification.
Conclusion
Click here to read the digital edition.
1. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540.
2. American Association of Clinical Endocrinologists. AACE/ACE comprehensive type 2 diabetes management algorithm 2017. https://www.aace.com/publications/algorithm. Accessed August 24, 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4-S5.
4. Diamant M, Nauck MA, Shaginian R, et al; 4B Study Group. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763-2773.
5. Rosenstock J, Fonseca VA, Gross JL, et al; Harmony 6 Study Group. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317-2325.
6. Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care. 2013;36(suppl 2):S226-S232.
7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38(1):140-149.
8. McCulloch DK. Insulin therapy in type 2 diabetes mellitus. http://www.uptodate.com/contents/insulin-therapy-in-type-2-diabetes-mellitus?source=machineLearning&search=insulin+on+board&selectedTitle=3%7E150§ionRank=5&anchor=H24#H1331866. Updated July, 21, 2017. Accessed August 24, 2017.
9. Guthrie D, Guthrie R. Management of Diabetes Mellitus: A Guide to the Pattern Approach. 6th ed. New York, NY: Springer Publishing Co; 2008.
10. Presswala L, Shubrook J. What to do after basal insulin: 3 Tx strategies for type 2 diabetes. J Fam Pract. 2015;64(4):214-220.
11. McCulloch DK. Management of blood glucose in adults with type 1 diabetes mellitus. http://www.uptodate.com/contents/management-of-blood-glucose-in-adults -with-type-1-diabetes-mellitus?source=machineLearning&search=insulin+injection+frequency&selectedTitle=7%7E150§ionRank=1&anchor=H17221321#H17221321. Updated July 21, 2017. Accessed August 24, 2017.
12. Humulin 70/30. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
13. Humalog Mix 75/25. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
14. Humalog Mix 50/50. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
15. Novolin 70/30. [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2016.
16. Novolog Mix 70/30. [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2017.
17. Tsapas A, Karagiannis T, Bekiari E. Premixed insulin regimens for type 2 diabetes. Endocrine. 2016;51(3):387-389.
18. Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16(5):396-402.
19. Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84(2):183-190.
20. Joslin Diabetes Center. Dosing insulin. http://www.joslin.org/info/dosing-insulin.html. Accessed September 8, 2017.
21. National Institutes of Health. 15-15 rule. https://medlineplus.gov/ency/imagepages/19815.htm. Updated April 15, 2017. Accessed September 3, 2017.
22. University of Michigan Comprehensive Diabetes Center. Diabetes: low blood sugar. http://www.med.umich.edu/1libr/MEND/Diabetes-Hypoglycemia.pdf. Revised July 2017. Accessed September 3, 2017.
23. Diabetes Research Institute, University of Miami. Low blood sugar: hypoglycemia. http://www.diabetesresearch.org/document.doc?id=275. Accessed August 29, 2017.24. Cavanaugh KL. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes. 2007;25(3):90-97.
25. National Institute of Diabetes and Digestive and Kidney Diseases. Low blood glucose (hypoglycemia). http://www.niddk.nih.gov/health-information/health-topics/Diabetes/hypoglycemia/Pages/index.aspx. Accessed August 29, 2017.
26. Deakin TA, McShane CE,Cade JE, Williams R. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2): CD003417.
Individuals with type 2 diabetes mellitus (T2DM) spend between 5 and 10 years with elevated hemoglobin A1c (HbA1c) before initiation of insulin.1 Once the basal insulin is initiated, the patient can go years with only adjustment of the basal insulin, resulting in over-basalization. In general, the total daily dose (TDD) of insulin should be composed of about 50% basal “background” insulin and 50% bolus “meal” insulin. When the fasting glucose readings are on target but HbA1c is still above the mutually set goal range, postprandial readings need to be evaluated.
This article focuses on initiating and titrating bolus insulin in nonpregnant patients with T2DM. Before initiation of bolus insulin, it is important for the patient to be actively engaged with a diabetes educator for diabetes self-management education and support (DSME/S), including the understanding of the correct use of insulin, carbohydrate counting, and increasing physical activities. Ensuring the correct technique of insulin administration and self-monitoring of blood glucose (SMBG) is critical. A knowledge deficit of carbohydrate information can lead to uncontrolled blood glucose (BG). The authors have encountered numerous times when patients were drinking sugary beverages or consuming large amounts of “healthy” food without realizing the carbohydrate content. Therefore, treatment in concert with a registered dietitian and certified diabetes educator is highly recommended.
Initiation of Bolus Insulin
There are 3 options of postprandial coverage with bolus insulin when a patient is taking basal insulin: basal plus, basal-bolus, or premix insulin. A possible fourth option for postprandial coverage is to add glucagon-like peptide-1 receptor agonist (GLP-1 RA), an injectable noninsulin antihyperglycemic agent, which has shown noninferior efficacy to adding bolus insulin and a favorable effect on weight with less risk of hypoglycemia.2-5 Although it can be expensive, combining GLP-1 RA to basal insulin results in lowering HbA1c of 0.66 up to 1.74% (or lowering mmol/mol of 7 up to 19) from the baseline.6 However, adding bolus insulin may be the only option to avoid glucotoxicity and prevent further diabetes complications when the HbA1c level is well above the goal range. It is usually recommended to discontinue sulfonylurea when bolus insulin is added due to the β-cell exhaustion with advancing natural history of diabetes.7,8
Method 1: Basal Plus
The health care provider (HCP) needs to consider whether the patient is over-basalized when the HbAlc and postprandial BG readings are still not at goal despite careful titration of basal insulin dose to > 0.5 U/kg/d.9 This is the time to discuss with the patient the coverage of mealtime glucose excursions. In the basal plus regimen, the prescribing provider may add 1 bolus insulin injection for the meal with the highest amount of carbohydrates or add 2 bolus injections for the most and second most meals with carbohydrates. Multiple types of bolus insulin are available in the current U.S. market (Table 1).
There are 2 ways to add bolus insulin: fixed and flexible. In the fixed regimen, the patient will take the same amount of bolus insulin regardless of premeal BG readings and carbohydrate content of the food. The authors recommend adding bolus insulin of about 4 to 6 units once or twice a day with meals, depending on the number of meals a day, carbohydrate content of the meal, current and desired degree of diabetes control, and physical activities. Another way to calculate a bolus insulin dose is to start at 0.1 U/kg if adding to the basal insulin.10 Flexible regimen allows various bolus doses based on premeal BG, carbohydrate intake, and activities. Information on this regimen, will be discussed more later.
Patient Cases
Tables 2 and 3 describe 2 patient cases. For example, patient 1 weighs 80 kg. If the prescribing HCP and patient decide to add only 1 bolus to the largest carbohydrate meal at dinnertime, then the patient may take 8 units (80 kg × 0.1 U/kg/meal = 8 units for meal). The patient’s current insulin dose, medical comorbidities, current diabetes control status, living situations, and overall cognition also should be considered.
Imagine patient 1 is taking 14 units daily of a long-acting insulin (LAI). If the patient is taking a fairly low dose of LAI, has multiple comorbidities, recent BG log/HgAlc, and lives alone but demonstrates good cognition to follow instructions, the prescriber may consider adding the bolus insulin of 4 units for dinner; thus, the bolus dose is about one-third of total basal insulin dose. However, if the patient is on 40 units of LAI and has symptoms of hyperglycemia, 6 to 8 units for the dinner is reasonable. An important point to convey to this patient is to make sure there is carbohydrate consistency. The patient’s premeal BG was 137 mg/dL (7.6 mmol/L) on Monday, but it rose significantly to 313 mg/dL (17.4 mmol/L) after dinner. On Wednesday, the patient’s premeal BG prior to dinner was 150 mg/dL (8.3 mmol/L); it rose to 202 mg/dL (11.2 mmol/L) after dinner, which is high but not as high as on Monday.
Multiple factors may affect this variability; for example, on Monday the patient may have consumed more than the usual amount of carbohydrates for dinner, forgot to take oral medication for dinner, or missed his/her usual after-dinner walk. Or simply, the patient may have eaten a lot less than the usual amount of carbohydrates, walked the neighborhood, or vacuumed the entire house after dinner on Wednesday. Thus, it is imperative to carefully assess the patient’s lifestyle and recommend carbohydrate consistency at each meal.
Method 2: Basal-Bolus
When basal plus is insufficient to get the HbA1c and BG readings to goal, taking bolus insulin for all main meals containing carbohydrates must be considered. This is often called basal-bolus, multiple daily injections, or intensive insulin therapy.
In order to understand the concept of basal-bolus, HCPs should consider normal physiology. The pancreas releases a constant amount of insulin, aka background insulin, to cover glucose produced by the liver to the cells between meals. In addition, a burst of insulin, aka bolus insulin, to meet the blood glucose elevation from food to maintain homeostasis. In patients with T2DM, the relative amount produced by the pancreas is insufficient to meet the demand due to pancreatic exhaustion or insulin resistance. This necessitates the need to replace background and bolus insulin.7
The ideal final total bolus insulin amount (the sum of all meal bolus doses) should be about half the basal dosing. Calculation of starting bolus dosing can be done as in the basal plus regimen, either 4 to 6 units per meal or 0.1 U/kg/d.10 Alternatively, if the patient is on 60 units of long-acting analog and BGs are well above goal range, the prescriber could consider about 20 units of bolus dose (60 U divided by 3 meals) if the patient eats 3 routine meals a day with at least 30 g of carbohydrates, and physical activity levels are fairly consistent. If the patient eats the most carbohydrates at lunchtime, consider more bolus at lunch (ie, 18 U of bolus for breakfast and dinner and 24 U of bolus for lunch coverage).
Patients need to separate the time between the bolus doses, usually a minimum of 4 hours apart, to avoid insulin stacking, which is a common reason for hypoglycemia. Insulin stacking occurs when additional quick or rapid insulin is injected when the previous insulin is still in the body or when there is insulin on board.8,11 Typically, bolus analogs stay in the body for about 4 to 6 hours, thus necessitating separation of the doses at least 4 hours apart. Patients sometimes inject more bolus insulin after high postprandial readings, which can result in insulin stacking. In some cases, the patient may misunderstand and take mealtime insulin at a scheduled time instead of at the time of the meal.
Injecting bolus insulin for every snack must be avoided to prevent a vicious cycle: Postprandial hyperglycemia –> extra bolus insulin, resulting in insulin stacking –> hypoglycemia –> overtreatment with food –> hyperglycemia –> extra bolus insulin, resulting in insulin stacking –> and so on. Whenever there are readings in the hypoglycemic and hyperglycemic range, address hypoglycemia first because hyperglycemia often is due to overtreatment of hypoglycemia.
Method 3: Premix or Split-Mix (Patient-Mix) Insulin
Postprandial BG excursions can be minimized by changing basal insulin to premix or split-mix (patient-mix) insulin that has a mixture of mealtime and intermediate action insulin (Table 4).12-16 The use of premixed insulin is a viable option due to its ease of use and for those who have restrictions based on the complexity of the basal-bolus regimen.7 If a patient has routine meals and prefers not to carry around insulin for lunch, the schedule of premix insulin taken at breakfast and dinner is ideal.
Some caveats for safe prescribing should be understood. A recent summary of premixed insulin regimens noted that they seem to have a similar efficacy and safety profile compared with regimens that include basal insulin with or without mealtime insulin; however, cost and patient adherence are improved.17 It is important to monitor insulin-naïve patients for hypoglycemia and reduced efficacy when used twice daily compared with basal plus 3-times daily prandial insulin in patients needing insulin intensification.17
A randomized trial noted that hypoglycemia rates were twice as high with premixed insulin compared with basal-bolus insulin.18 This study also noted that the premixed insulin group experienced the highest dropout rate, partly due to hypoglycemia. A regimen of basal insulin with the option to add a single prandial insulin injection at the main meal was as effective in reducing HbA1c with less hypoglycemia. The premixed insulin is convenient but does not allow a separate correction of either mealtime or intermediate-acting insulin doses. If the premixed dose needs to be adjusted due to fasting hyperglycemia > 180 mg/dL(10.0 mmol/L), the TDD can be increased by 10%.2
In contrast, a split-mix (patient-mix) insulin regimen allows for the ability to vary the amount/ratio of combinations and adjustment of bolus and intermediate insulin doses. The disadvantages of split-mix insulin include the inconvenience of manually mixing of insulin and the potential for dosing errors. The patient needs to be taught additional steps on how to mix both insulins. Ensure the correct mixing order to maintain insulin potency; regular first, then neutral protamine Hagedorn (NPH). An HCP should remember the RN acronym if the patient is combining regular insulin and NPH. If there is doubt about the patient’s insulin injection technique, HCPs should ask the patient to demonstrate how to correctly pull up a dose of normal saline and inject it during a clinic visit. The only basal insulin that can be physically mixed with quick or rapid insulin is NPH. It should never be mixed with long-acting analogs. The patient should not even use the same syringe to draw up bolus analog insulin and inject it and then use the same syringe to draw up long-acting analog insulin.
One caveat to a fixed regimen (same amount of insulin dose) is that providers often expect that the patient will eat a consistent amount of carbohydrates at each meal and premeal glucose readings are fairly stable. Oftentimes, this is not true. If a patient took a bolus dose of 8 units of rapid-acting insulin and ate a 6 oz steak, 3 oz baked potato, steamed broccoli at a dinner; and no bread, the after dinner BG might register 145 mg/dL (8.1 mmol/L). Then, the next day for dinner, if he or she took the same amount of 8 units of rapid-acting insulin and ate 1 cup of spaghetti, ½ cup of spaghetti meat sauce, and 2 slices of garlic bread, the after dinner reading might be 322 mg/dL (17.9 mmol/L). The patient’s BG was higher on the second day because of the higher carbohydrate content of the meal. If the rapid-acting insulin was increased to 12 units of bolus based on the high carbohydrate meal and the patient ate a lower carbohydrate meal, hypoglycemia could ensue. Thus, it is important to work with the patient regarding the consumption of a consistent amount of carbohydrates and refer to a registered dietitian for carbohydrate consistency.
For the flexible regimen, the prescriber may consider using an insulin to carbohydrate (IC) ratio and sensitivity factor (SF), also called sliding scale or correction factor. The IC ratio represents how much insulin is needed to cover consumed carbohydrates. For instance, if the patient uses IC ratio of 1:15, 1 unit of bolus insulin will cover 15 g of carbohydrates. If the patient eats a meal with 60 g of carbohydrates and is using IC ratio of 1:15, the patient will inject 4 units of bolus insulin. Sensitivity factor represents how much BG will be lowered in mg/dL by taking 1 unit of bolus insulin. For example, if the patient uses SF of 1:50, 1 unit of bolus insulin will lower BG by 50 mg/dL (2.8 mmol/L). When the desired (target) BG reading is 100 mg/dL (5.6 mmol/L) and the patient’s current BG is 200 mg/dL (11.1 mmol/L), the patient will divide 100 mg/dL (5.6 mmol/L) by 50 (derived from SF of 1:50). The net result is 2 units of bolus insulin are needed to lower BG by 100 mg/dL (5.6 mmol/L). If the premeal BG is 200 mg/dL (11.1 mmol/L) and 60 g of carbohydrates are eaten, then the patient will need a total of 6 units (4 U for carbohydrate and 2 U for high BG) bolus before the meal. For additional information, readers are encouraged to read the articles by Petznick and by Joslin Diabetes Center for IC and SF.19,20
Bolus Insulin Titration
When the difference in BG readings before and 2 hours after a meal, called the Δ value, is > 50 mg/dL (2.8 mmol/L), the bolus insulin may need to be adjusted after ensuring the patient is ingesting consistent carbohydrates and performs the usual amount of activities around mealtime. For example, if the premeal reading was 130 mg/dL (7.2 mmol/L) but the 2-hour postprandial reading is > 180 mg/dL (10.0 mmol/L), the prescriber can increase the mealtime insulin by 1 unit if the mealtime insulin is < 10 units, by 2 units if < 20 units, or by 10% of the mealtime insulin dose. If the premeal BG is < 80 mg/dL (4.4 mmol/L) and the drop in BG is > Δ value of 50 mg/dL (2.8 mmol/L), the prescriber can decrease the mealtime insulin using the same calculation. Monitoring BG and titration recommendations are shown in Table 5. When adjusting the bolus insulin dose, it is best to make adjustments gradually rather than making several changes at once.
The 15/15 rule needs to be followed in cases involving hypoglycemia.21 When the BG is ≤ 70 mg/dL (3.9 mmol/L) and the patient is conscious and able to eat or drink, it is recommended they eat 15 g (30 g if BG is below 50) of carbohydrates then repeat BG check every 15 minutes until the BG is in the target range.22,23 If the patient is unconscious, providers should administer glucagon (if available), place the patient in a lateral position to avoid aspiration, and call 911. If hyperkalemia is an issue in chronic kidney disease, patients should consume apple juice rather than orange juice due to its lower potassium content. If the patient is taking α glucosidase inhibitors (AGI) like acarbose or miglitol, only pure glucose like glucose tablets needs to be given to treat hypoglycemia instead of regular soda or candy, as the AGI will slow absorption of other types of carbohydrates.24,25 After the severe hypoglycemic episode, it is imperative to assess for the cause and explore ways to prevent subsequent hypoglycemia. Providers also should advise the patient to wear medical emergency identification.
Conclusion
Click here to read the digital edition.
Individuals with type 2 diabetes mellitus (T2DM) spend between 5 and 10 years with elevated hemoglobin A1c (HbA1c) before initiation of insulin.1 Once the basal insulin is initiated, the patient can go years with only adjustment of the basal insulin, resulting in over-basalization. In general, the total daily dose (TDD) of insulin should be composed of about 50% basal “background” insulin and 50% bolus “meal” insulin. When the fasting glucose readings are on target but HbA1c is still above the mutually set goal range, postprandial readings need to be evaluated.
This article focuses on initiating and titrating bolus insulin in nonpregnant patients with T2DM. Before initiation of bolus insulin, it is important for the patient to be actively engaged with a diabetes educator for diabetes self-management education and support (DSME/S), including the understanding of the correct use of insulin, carbohydrate counting, and increasing physical activities. Ensuring the correct technique of insulin administration and self-monitoring of blood glucose (SMBG) is critical. A knowledge deficit of carbohydrate information can lead to uncontrolled blood glucose (BG). The authors have encountered numerous times when patients were drinking sugary beverages or consuming large amounts of “healthy” food without realizing the carbohydrate content. Therefore, treatment in concert with a registered dietitian and certified diabetes educator is highly recommended.
Initiation of Bolus Insulin
There are 3 options of postprandial coverage with bolus insulin when a patient is taking basal insulin: basal plus, basal-bolus, or premix insulin. A possible fourth option for postprandial coverage is to add glucagon-like peptide-1 receptor agonist (GLP-1 RA), an injectable noninsulin antihyperglycemic agent, which has shown noninferior efficacy to adding bolus insulin and a favorable effect on weight with less risk of hypoglycemia.2-5 Although it can be expensive, combining GLP-1 RA to basal insulin results in lowering HbA1c of 0.66 up to 1.74% (or lowering mmol/mol of 7 up to 19) from the baseline.6 However, adding bolus insulin may be the only option to avoid glucotoxicity and prevent further diabetes complications when the HbA1c level is well above the goal range. It is usually recommended to discontinue sulfonylurea when bolus insulin is added due to the β-cell exhaustion with advancing natural history of diabetes.7,8
Method 1: Basal Plus
The health care provider (HCP) needs to consider whether the patient is over-basalized when the HbAlc and postprandial BG readings are still not at goal despite careful titration of basal insulin dose to > 0.5 U/kg/d.9 This is the time to discuss with the patient the coverage of mealtime glucose excursions. In the basal plus regimen, the prescribing provider may add 1 bolus insulin injection for the meal with the highest amount of carbohydrates or add 2 bolus injections for the most and second most meals with carbohydrates. Multiple types of bolus insulin are available in the current U.S. market (Table 1).
There are 2 ways to add bolus insulin: fixed and flexible. In the fixed regimen, the patient will take the same amount of bolus insulin regardless of premeal BG readings and carbohydrate content of the food. The authors recommend adding bolus insulin of about 4 to 6 units once or twice a day with meals, depending on the number of meals a day, carbohydrate content of the meal, current and desired degree of diabetes control, and physical activities. Another way to calculate a bolus insulin dose is to start at 0.1 U/kg if adding to the basal insulin.10 Flexible regimen allows various bolus doses based on premeal BG, carbohydrate intake, and activities. Information on this regimen, will be discussed more later.
Patient Cases
Tables 2 and 3 describe 2 patient cases. For example, patient 1 weighs 80 kg. If the prescribing HCP and patient decide to add only 1 bolus to the largest carbohydrate meal at dinnertime, then the patient may take 8 units (80 kg × 0.1 U/kg/meal = 8 units for meal). The patient’s current insulin dose, medical comorbidities, current diabetes control status, living situations, and overall cognition also should be considered.
Imagine patient 1 is taking 14 units daily of a long-acting insulin (LAI). If the patient is taking a fairly low dose of LAI, has multiple comorbidities, recent BG log/HgAlc, and lives alone but demonstrates good cognition to follow instructions, the prescriber may consider adding the bolus insulin of 4 units for dinner; thus, the bolus dose is about one-third of total basal insulin dose. However, if the patient is on 40 units of LAI and has symptoms of hyperglycemia, 6 to 8 units for the dinner is reasonable. An important point to convey to this patient is to make sure there is carbohydrate consistency. The patient’s premeal BG was 137 mg/dL (7.6 mmol/L) on Monday, but it rose significantly to 313 mg/dL (17.4 mmol/L) after dinner. On Wednesday, the patient’s premeal BG prior to dinner was 150 mg/dL (8.3 mmol/L); it rose to 202 mg/dL (11.2 mmol/L) after dinner, which is high but not as high as on Monday.
Multiple factors may affect this variability; for example, on Monday the patient may have consumed more than the usual amount of carbohydrates for dinner, forgot to take oral medication for dinner, or missed his/her usual after-dinner walk. Or simply, the patient may have eaten a lot less than the usual amount of carbohydrates, walked the neighborhood, or vacuumed the entire house after dinner on Wednesday. Thus, it is imperative to carefully assess the patient’s lifestyle and recommend carbohydrate consistency at each meal.
Method 2: Basal-Bolus
When basal plus is insufficient to get the HbA1c and BG readings to goal, taking bolus insulin for all main meals containing carbohydrates must be considered. This is often called basal-bolus, multiple daily injections, or intensive insulin therapy.
In order to understand the concept of basal-bolus, HCPs should consider normal physiology. The pancreas releases a constant amount of insulin, aka background insulin, to cover glucose produced by the liver to the cells between meals. In addition, a burst of insulin, aka bolus insulin, to meet the blood glucose elevation from food to maintain homeostasis. In patients with T2DM, the relative amount produced by the pancreas is insufficient to meet the demand due to pancreatic exhaustion or insulin resistance. This necessitates the need to replace background and bolus insulin.7
The ideal final total bolus insulin amount (the sum of all meal bolus doses) should be about half the basal dosing. Calculation of starting bolus dosing can be done as in the basal plus regimen, either 4 to 6 units per meal or 0.1 U/kg/d.10 Alternatively, if the patient is on 60 units of long-acting analog and BGs are well above goal range, the prescriber could consider about 20 units of bolus dose (60 U divided by 3 meals) if the patient eats 3 routine meals a day with at least 30 g of carbohydrates, and physical activity levels are fairly consistent. If the patient eats the most carbohydrates at lunchtime, consider more bolus at lunch (ie, 18 U of bolus for breakfast and dinner and 24 U of bolus for lunch coverage).
Patients need to separate the time between the bolus doses, usually a minimum of 4 hours apart, to avoid insulin stacking, which is a common reason for hypoglycemia. Insulin stacking occurs when additional quick or rapid insulin is injected when the previous insulin is still in the body or when there is insulin on board.8,11 Typically, bolus analogs stay in the body for about 4 to 6 hours, thus necessitating separation of the doses at least 4 hours apart. Patients sometimes inject more bolus insulin after high postprandial readings, which can result in insulin stacking. In some cases, the patient may misunderstand and take mealtime insulin at a scheduled time instead of at the time of the meal.
Injecting bolus insulin for every snack must be avoided to prevent a vicious cycle: Postprandial hyperglycemia –> extra bolus insulin, resulting in insulin stacking –> hypoglycemia –> overtreatment with food –> hyperglycemia –> extra bolus insulin, resulting in insulin stacking –> and so on. Whenever there are readings in the hypoglycemic and hyperglycemic range, address hypoglycemia first because hyperglycemia often is due to overtreatment of hypoglycemia.
Method 3: Premix or Split-Mix (Patient-Mix) Insulin
Postprandial BG excursions can be minimized by changing basal insulin to premix or split-mix (patient-mix) insulin that has a mixture of mealtime and intermediate action insulin (Table 4).12-16 The use of premixed insulin is a viable option due to its ease of use and for those who have restrictions based on the complexity of the basal-bolus regimen.7 If a patient has routine meals and prefers not to carry around insulin for lunch, the schedule of premix insulin taken at breakfast and dinner is ideal.
Some caveats for safe prescribing should be understood. A recent summary of premixed insulin regimens noted that they seem to have a similar efficacy and safety profile compared with regimens that include basal insulin with or without mealtime insulin; however, cost and patient adherence are improved.17 It is important to monitor insulin-naïve patients for hypoglycemia and reduced efficacy when used twice daily compared with basal plus 3-times daily prandial insulin in patients needing insulin intensification.17
A randomized trial noted that hypoglycemia rates were twice as high with premixed insulin compared with basal-bolus insulin.18 This study also noted that the premixed insulin group experienced the highest dropout rate, partly due to hypoglycemia. A regimen of basal insulin with the option to add a single prandial insulin injection at the main meal was as effective in reducing HbA1c with less hypoglycemia. The premixed insulin is convenient but does not allow a separate correction of either mealtime or intermediate-acting insulin doses. If the premixed dose needs to be adjusted due to fasting hyperglycemia > 180 mg/dL(10.0 mmol/L), the TDD can be increased by 10%.2
In contrast, a split-mix (patient-mix) insulin regimen allows for the ability to vary the amount/ratio of combinations and adjustment of bolus and intermediate insulin doses. The disadvantages of split-mix insulin include the inconvenience of manually mixing of insulin and the potential for dosing errors. The patient needs to be taught additional steps on how to mix both insulins. Ensure the correct mixing order to maintain insulin potency; regular first, then neutral protamine Hagedorn (NPH). An HCP should remember the RN acronym if the patient is combining regular insulin and NPH. If there is doubt about the patient’s insulin injection technique, HCPs should ask the patient to demonstrate how to correctly pull up a dose of normal saline and inject it during a clinic visit. The only basal insulin that can be physically mixed with quick or rapid insulin is NPH. It should never be mixed with long-acting analogs. The patient should not even use the same syringe to draw up bolus analog insulin and inject it and then use the same syringe to draw up long-acting analog insulin.
One caveat to a fixed regimen (same amount of insulin dose) is that providers often expect that the patient will eat a consistent amount of carbohydrates at each meal and premeal glucose readings are fairly stable. Oftentimes, this is not true. If a patient took a bolus dose of 8 units of rapid-acting insulin and ate a 6 oz steak, 3 oz baked potato, steamed broccoli at a dinner; and no bread, the after dinner BG might register 145 mg/dL (8.1 mmol/L). Then, the next day for dinner, if he or she took the same amount of 8 units of rapid-acting insulin and ate 1 cup of spaghetti, ½ cup of spaghetti meat sauce, and 2 slices of garlic bread, the after dinner reading might be 322 mg/dL (17.9 mmol/L). The patient’s BG was higher on the second day because of the higher carbohydrate content of the meal. If the rapid-acting insulin was increased to 12 units of bolus based on the high carbohydrate meal and the patient ate a lower carbohydrate meal, hypoglycemia could ensue. Thus, it is important to work with the patient regarding the consumption of a consistent amount of carbohydrates and refer to a registered dietitian for carbohydrate consistency.
For the flexible regimen, the prescriber may consider using an insulin to carbohydrate (IC) ratio and sensitivity factor (SF), also called sliding scale or correction factor. The IC ratio represents how much insulin is needed to cover consumed carbohydrates. For instance, if the patient uses IC ratio of 1:15, 1 unit of bolus insulin will cover 15 g of carbohydrates. If the patient eats a meal with 60 g of carbohydrates and is using IC ratio of 1:15, the patient will inject 4 units of bolus insulin. Sensitivity factor represents how much BG will be lowered in mg/dL by taking 1 unit of bolus insulin. For example, if the patient uses SF of 1:50, 1 unit of bolus insulin will lower BG by 50 mg/dL (2.8 mmol/L). When the desired (target) BG reading is 100 mg/dL (5.6 mmol/L) and the patient’s current BG is 200 mg/dL (11.1 mmol/L), the patient will divide 100 mg/dL (5.6 mmol/L) by 50 (derived from SF of 1:50). The net result is 2 units of bolus insulin are needed to lower BG by 100 mg/dL (5.6 mmol/L). If the premeal BG is 200 mg/dL (11.1 mmol/L) and 60 g of carbohydrates are eaten, then the patient will need a total of 6 units (4 U for carbohydrate and 2 U for high BG) bolus before the meal. For additional information, readers are encouraged to read the articles by Petznick and by Joslin Diabetes Center for IC and SF.19,20
Bolus Insulin Titration
When the difference in BG readings before and 2 hours after a meal, called the Δ value, is > 50 mg/dL (2.8 mmol/L), the bolus insulin may need to be adjusted after ensuring the patient is ingesting consistent carbohydrates and performs the usual amount of activities around mealtime. For example, if the premeal reading was 130 mg/dL (7.2 mmol/L) but the 2-hour postprandial reading is > 180 mg/dL (10.0 mmol/L), the prescriber can increase the mealtime insulin by 1 unit if the mealtime insulin is < 10 units, by 2 units if < 20 units, or by 10% of the mealtime insulin dose. If the premeal BG is < 80 mg/dL (4.4 mmol/L) and the drop in BG is > Δ value of 50 mg/dL (2.8 mmol/L), the prescriber can decrease the mealtime insulin using the same calculation. Monitoring BG and titration recommendations are shown in Table 5. When adjusting the bolus insulin dose, it is best to make adjustments gradually rather than making several changes at once.
The 15/15 rule needs to be followed in cases involving hypoglycemia.21 When the BG is ≤ 70 mg/dL (3.9 mmol/L) and the patient is conscious and able to eat or drink, it is recommended they eat 15 g (30 g if BG is below 50) of carbohydrates then repeat BG check every 15 minutes until the BG is in the target range.22,23 If the patient is unconscious, providers should administer glucagon (if available), place the patient in a lateral position to avoid aspiration, and call 911. If hyperkalemia is an issue in chronic kidney disease, patients should consume apple juice rather than orange juice due to its lower potassium content. If the patient is taking α glucosidase inhibitors (AGI) like acarbose or miglitol, only pure glucose like glucose tablets needs to be given to treat hypoglycemia instead of regular soda or candy, as the AGI will slow absorption of other types of carbohydrates.24,25 After the severe hypoglycemic episode, it is imperative to assess for the cause and explore ways to prevent subsequent hypoglycemia. Providers also should advise the patient to wear medical emergency identification.
Conclusion
Click here to read the digital edition.
1. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540.
2. American Association of Clinical Endocrinologists. AACE/ACE comprehensive type 2 diabetes management algorithm 2017. https://www.aace.com/publications/algorithm. Accessed August 24, 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4-S5.
4. Diamant M, Nauck MA, Shaginian R, et al; 4B Study Group. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763-2773.
5. Rosenstock J, Fonseca VA, Gross JL, et al; Harmony 6 Study Group. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317-2325.
6. Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care. 2013;36(suppl 2):S226-S232.
7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38(1):140-149.
8. McCulloch DK. Insulin therapy in type 2 diabetes mellitus. http://www.uptodate.com/contents/insulin-therapy-in-type-2-diabetes-mellitus?source=machineLearning&search=insulin+on+board&selectedTitle=3%7E150§ionRank=5&anchor=H24#H1331866. Updated July, 21, 2017. Accessed August 24, 2017.
9. Guthrie D, Guthrie R. Management of Diabetes Mellitus: A Guide to the Pattern Approach. 6th ed. New York, NY: Springer Publishing Co; 2008.
10. Presswala L, Shubrook J. What to do after basal insulin: 3 Tx strategies for type 2 diabetes. J Fam Pract. 2015;64(4):214-220.
11. McCulloch DK. Management of blood glucose in adults with type 1 diabetes mellitus. http://www.uptodate.com/contents/management-of-blood-glucose-in-adults -with-type-1-diabetes-mellitus?source=machineLearning&search=insulin+injection+frequency&selectedTitle=7%7E150§ionRank=1&anchor=H17221321#H17221321. Updated July 21, 2017. Accessed August 24, 2017.
12. Humulin 70/30. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
13. Humalog Mix 75/25. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
14. Humalog Mix 50/50. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
15. Novolin 70/30. [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2016.
16. Novolog Mix 70/30. [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2017.
17. Tsapas A, Karagiannis T, Bekiari E. Premixed insulin regimens for type 2 diabetes. Endocrine. 2016;51(3):387-389.
18. Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16(5):396-402.
19. Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84(2):183-190.
20. Joslin Diabetes Center. Dosing insulin. http://www.joslin.org/info/dosing-insulin.html. Accessed September 8, 2017.
21. National Institutes of Health. 15-15 rule. https://medlineplus.gov/ency/imagepages/19815.htm. Updated April 15, 2017. Accessed September 3, 2017.
22. University of Michigan Comprehensive Diabetes Center. Diabetes: low blood sugar. http://www.med.umich.edu/1libr/MEND/Diabetes-Hypoglycemia.pdf. Revised July 2017. Accessed September 3, 2017.
23. Diabetes Research Institute, University of Miami. Low blood sugar: hypoglycemia. http://www.diabetesresearch.org/document.doc?id=275. Accessed August 29, 2017.24. Cavanaugh KL. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes. 2007;25(3):90-97.
25. National Institute of Diabetes and Digestive and Kidney Diseases. Low blood glucose (hypoglycemia). http://www.niddk.nih.gov/health-information/health-topics/Diabetes/hypoglycemia/Pages/index.aspx. Accessed August 29, 2017.
26. Deakin TA, McShane CE,Cade JE, Williams R. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2): CD003417.
1. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540.
2. American Association of Clinical Endocrinologists. AACE/ACE comprehensive type 2 diabetes management algorithm 2017. https://www.aace.com/publications/algorithm. Accessed August 24, 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4-S5.
4. Diamant M, Nauck MA, Shaginian R, et al; 4B Study Group. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763-2773.
5. Rosenstock J, Fonseca VA, Gross JL, et al; Harmony 6 Study Group. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317-2325.
6. Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care. 2013;36(suppl 2):S226-S232.
7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38(1):140-149.
8. McCulloch DK. Insulin therapy in type 2 diabetes mellitus. http://www.uptodate.com/contents/insulin-therapy-in-type-2-diabetes-mellitus?source=machineLearning&search=insulin+on+board&selectedTitle=3%7E150§ionRank=5&anchor=H24#H1331866. Updated July, 21, 2017. Accessed August 24, 2017.
9. Guthrie D, Guthrie R. Management of Diabetes Mellitus: A Guide to the Pattern Approach. 6th ed. New York, NY: Springer Publishing Co; 2008.
10. Presswala L, Shubrook J. What to do after basal insulin: 3 Tx strategies for type 2 diabetes. J Fam Pract. 2015;64(4):214-220.
11. McCulloch DK. Management of blood glucose in adults with type 1 diabetes mellitus. http://www.uptodate.com/contents/management-of-blood-glucose-in-adults -with-type-1-diabetes-mellitus?source=machineLearning&search=insulin+injection+frequency&selectedTitle=7%7E150§ionRank=1&anchor=H17221321#H17221321. Updated July 21, 2017. Accessed August 24, 2017.
12. Humulin 70/30. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
13. Humalog Mix 75/25. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
14. Humalog Mix 50/50. [package insert]. Indianapolis, IN: Eli Lilly and Co; 2017.
15. Novolin 70/30. [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2016.
16. Novolog Mix 70/30. [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2017.
17. Tsapas A, Karagiannis T, Bekiari E. Premixed insulin regimens for type 2 diabetes. Endocrine. 2016;51(3):387-389.
18. Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16(5):396-402.
19. Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84(2):183-190.
20. Joslin Diabetes Center. Dosing insulin. http://www.joslin.org/info/dosing-insulin.html. Accessed September 8, 2017.
21. National Institutes of Health. 15-15 rule. https://medlineplus.gov/ency/imagepages/19815.htm. Updated April 15, 2017. Accessed September 3, 2017.
22. University of Michigan Comprehensive Diabetes Center. Diabetes: low blood sugar. http://www.med.umich.edu/1libr/MEND/Diabetes-Hypoglycemia.pdf. Revised July 2017. Accessed September 3, 2017.
23. Diabetes Research Institute, University of Miami. Low blood sugar: hypoglycemia. http://www.diabetesresearch.org/document.doc?id=275. Accessed August 29, 2017.24. Cavanaugh KL. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes. 2007;25(3):90-97.
25. National Institute of Diabetes and Digestive and Kidney Diseases. Low blood glucose (hypoglycemia). http://www.niddk.nih.gov/health-information/health-topics/Diabetes/hypoglycemia/Pages/index.aspx. Accessed August 29, 2017.
26. Deakin TA, McShane CE,Cade JE, Williams R. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2): CD003417.