User login
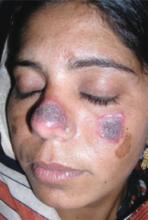
Dark brown scaly plaques on face and ears
A 27-year-old woman, otherwise healthy, presented for evaluation of a mildly pruritic eruption of plaques on her face and ears. The eruption started as a few small, scaly red papules on her cheeks and nose about 3 months earlier. The papules slowly expanded to form well-demarcated, rounded, dark brown plaques covered with superficial scale. Similar lesions appeared on the concha of both ears. The older lesions started to regress after 2 months. Complete regression was followed by residual scarring and hyperpigmentation.
Physical examination revealed multiple well-defined, erythematous, hyperpigmented, round plaques covered with adherent scale affecting her nose, cheeks, and the concha of both ears (FIGURE 1). The mucosae and the rest of her skin and adnexa were unaffected. She had no personal or family history of similar skin findings or autoimmune disorders. She also had no history of any drug intake prior to the eruption. Her routine blood tests and urinalysis results were unremarkable, and the serological analysis for antinuclear antibodies had negative results.
A punch biopsy was taken from one of the lesions for histopathology. The epidermal histopathological findings were remarkable for hyperkeratosis, follicular plugging, pigment incontinence, and vacuolization of the basal cell layer. There was a predominantly lymphocytic infiltrate of the subepidermal and perivascular dermal areas.
FIGURE 1
Facial plaques
What is your diagnosis?
How would you treat this patient?
Diagnosis: Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is an autoimmune inflammatory disorder of the skin that often leads to scarring and alopecia. It may be localized to sun-exposed areas such as the face, ears, and scalp but occasionally is much more extensive, involving the trunk and extremities. Most patients are otherwise healthy, and DLE may be the only clinical finding.
Although its prevalence is not known, DLE is not uncommon. It affects females twice as often as males, and patients fall between the ages of 25 to 45 years, with no racial predilection. While about 15% to 20% of patients with systemic lupus erythematosus (SLE) manifest DLE lesions, only about 5% to 10% of patients with DLE go on to develop SLE.1
Like SLE, DLE is believed to be an autoimmune disorder. Unlike SLE, however, DLE patients do not have similar serologic abnormalities. Skin trauma and ultraviolet light exposure have been reported to induce or exacerbate the lesions of DLE. Sex hormones may also play a role: exacerbation may occur during pregnancy, during menstrual or premenstrual periods, or while taking oral contraceptives. Drugs such as procainamide, hydralazine, isoniazid, diphenylhydantoin, methyldopa, penicillamine, guanides, and lithium may also precipitate DLE lesions.2
DLE usually begins as dull red macules with adherent scales on sun-exposed areas. If the overlying scales are removed, an undersurface of horny plugs is revealed. These plugs fill the follicles and resemble carpet tacks or a cat’s tongue (langue au chat). The macules slowly expand to form large plaques.
Usually only a mild pruritus and tenderness is seen with DLE lesions. As the lesions progress, the scale may thicken and pigmentary changes become evident. The lesions heal with atrophy, scarring, telangiectasias, and changes in pigmentation. Morphological appearance of older lesions may vary from erythematous plaques to hyperkeratotic, dark gray plaques with centrally depressed scars.3 Scalp involvement in DLE results in more sclerotic and depressed scars with subsequent scarring alopecia.4
Differential diagnosis is wide. The differential diagnosis of DLE is extensive and includes actinic keratosis, dermatomyositis, granuloma annulare, granuloma faciale, keratoacanthoma, lichen planus, subacute cutaneous lupus erythematosus, psoriasis, rosacea, sarcoidosis, squamous cell carcinoma, syphilis, and nongenital warts.
Histopathology
Histopathological findings include hyperkeratosis, parakeratosis, follicular plugging, telangiectasias, and atrophy of the epidermis. Liquefaction or hydropic degeneration of the basal layer leads to pigmentary incontinence. A perivascular and perifollicular mononuclear inflammatory cell infiltrate is present in the superficial and deep dermis.5,6
Direct immunofluorescence demonstrates immunoglobulins and complement deposits at the dermoepidermal junction.5 This test was not recommended for this patient, as the diagnosis of DLE had already been confirmed on clinical and histopathologic grounds.
Workup: Exam, biopsy
In addition to a routine history and physical examination, the workup for DLE should include a complete blood count, antinuclear antibody levels, anti-Ro, anti-La, hepatic and renal function tests, and urinalysis. Consider a diagnosis of SLE by using the American College of Rheumatology criteria. A biopsy for histopathology of a fresh lesion or a biopsy for immunofluorescence of an old lesion can confirm the diagnosis.
Therapeutic options are varied
Effective early therapies for DLE are available, but patients who do not respond appropriately may end up with deep scars, alopecia, and pigmentary changes that are considerably disfiguring, especially for dark-skinned people. Therefore, the goal of treatment is not only to improve the appearance of the skin by minimizing the scarring and preventing further lesions, but also to prevent future complications.
Avoiding sunlight. The primary therapeutic approach is to educate patients regarding exposure to sunlight. Sun-protective measures include the use of high-SPF sunscreen lotions and protective clothing, such as baseball caps without vent holes and wide-brimmed hats.7
Medication options. Current treatment options for DLE include antimalarial agents such as chloroquine, topical and intralesional glucocorticoids, and thalidomide. The use of potent topical steroids may prevent significant scarring and deformity, especially of the face. Common side effects include steroid withdrawal syndrome, perioral dermatitis, steroid acne, and rosacea. All of these side effects can be treated and result in less long-term deformity than untreated DLE (FIGURE 2).
Systemic agents. Widespread disease may require you use systemic agents, such as antimalarials. Chloroquine has long been considered the gold standard in the treatment of DLE.8 Due to the frequency of ocular side effects with chloroquine, hydroxychloroquine is by far the most widely used agent. Hydroxychloroquine at a dose of 6.5 mg/kg/d for 3 months may lead to resolution of lesions for many patients.7 In resistant cases, higher dosages (eg, 400 mg/d) or combinations (eg, hydroxychloroquine 200 mg/d plus quinacrine 50 to 100 mg/d) may be required for months or even years. An ophthalmological evaluation is advisable before starting antimalarial treatment, and you should repeat it at 4- to 6-month intervals during treatment.9 Systemic corticosteroids may be needed to obtain timely initial control, especially for widespread and disfiguring lesions.
When this therapy is inadequate, other treatments for DLE include azathioprine, retinoids, and dapsone. Recent reports confirm the efficacy of thalidomide for cutaneous lupus erythematosus in dosages ranging from 50 to 200 mg/d.10 Twice-daily application of tacrolimus 0.01% has also been shown to be effective in a few clinical trials.11
Surgery. Surgical intervention is useful to remove scarred lesions, and laser therapy has also been effective, especially for lesions with prominent telangiectasias.12
Patient education. Patient education plays an important role in the treatment of DLE. Advising patients on how to avoid the sun and instructing them in the proper use of sunscreens is extremely important. Smoking cessation has also been shown to be beneficial.13
Patients with DLE generally have a favorable prognosis with regards to morbidity and mortality. Because DLE is usually self-limited, the course is most often benign; therefore, early recognition and adequate therapy may prevent clinical complications. Many patients with DLE go on to develop destructive or deforming scarring or pigmentary disturbances,14 especially in the spring and summer months when the sun is the strongest.
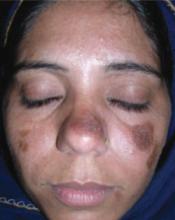
FIGURE 2
After treatment
This patient’s outcome
Our patient was treated with topical fluocinolone acetonide 0.025% ointment and oral hydroxychloroquine 200 mg/d for 1 month. The patient responded well to the treatment and the skin lesion regressed perceptibly (FIGURE 2). The treatment was nevertheless continued for another 5 months, and resulted in complete regression of the lesions, with minimal residual scarring.
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Department of Dermatology, 450 Clarkson Avenue Box 46, Brooklyn, NY 11203. E-mail: amorkh@pol.net
1. Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. Clinical and laboratory findings in seventeen patients. J Am Acad Dermatol 1985;12(2 Pt 1):278-288.
2. Hess E. Drug-related lupus. N Engl J Med 1988;318:1460-1462.
3. Callen JP, Fowler JF, Kulick KB. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol 1985;13(5 Pt 1):748-755.
4. Wilson CL, Burge SM, Dean D, Dawber RP. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol 1992;126:307-314.
5. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002;20:373-385, v.
6. Biesla I, et al. Histopathologic findings in cutaneous lupus erythematosus. Arch Dermatol 1994;130:54-58.
7. Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin 1994;12:201-206.
8. Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc 1953;152:1428-1429.
9. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983;75:35-39.
10. Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol 2000;39:218-222.
11. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1171.
12. Nunez M, Boixeda P, Miralles ES, et al. Pulsed dye laser treatment of telangiectatic chronic erythema of cutaneous lupus erythematosus. Arch Dermatol 1996;132:354-355.
13. Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology 2005;211:118-122.
14. de Berker D, Dissaneyeka M, Burge S. The sequelae of chronic cutaneous lupus erythematosus. Lupus 1992;1:181-186.
A 27-year-old woman, otherwise healthy, presented for evaluation of a mildly pruritic eruption of plaques on her face and ears. The eruption started as a few small, scaly red papules on her cheeks and nose about 3 months earlier. The papules slowly expanded to form well-demarcated, rounded, dark brown plaques covered with superficial scale. Similar lesions appeared on the concha of both ears. The older lesions started to regress after 2 months. Complete regression was followed by residual scarring and hyperpigmentation.
Physical examination revealed multiple well-defined, erythematous, hyperpigmented, round plaques covered with adherent scale affecting her nose, cheeks, and the concha of both ears (FIGURE 1). The mucosae and the rest of her skin and adnexa were unaffected. She had no personal or family history of similar skin findings or autoimmune disorders. She also had no history of any drug intake prior to the eruption. Her routine blood tests and urinalysis results were unremarkable, and the serological analysis for antinuclear antibodies had negative results.
A punch biopsy was taken from one of the lesions for histopathology. The epidermal histopathological findings were remarkable for hyperkeratosis, follicular plugging, pigment incontinence, and vacuolization of the basal cell layer. There was a predominantly lymphocytic infiltrate of the subepidermal and perivascular dermal areas.
FIGURE 1
Facial plaques
What is your diagnosis?
How would you treat this patient?
Diagnosis: Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is an autoimmune inflammatory disorder of the skin that often leads to scarring and alopecia. It may be localized to sun-exposed areas such as the face, ears, and scalp but occasionally is much more extensive, involving the trunk and extremities. Most patients are otherwise healthy, and DLE may be the only clinical finding.
Although its prevalence is not known, DLE is not uncommon. It affects females twice as often as males, and patients fall between the ages of 25 to 45 years, with no racial predilection. While about 15% to 20% of patients with systemic lupus erythematosus (SLE) manifest DLE lesions, only about 5% to 10% of patients with DLE go on to develop SLE.1
Like SLE, DLE is believed to be an autoimmune disorder. Unlike SLE, however, DLE patients do not have similar serologic abnormalities. Skin trauma and ultraviolet light exposure have been reported to induce or exacerbate the lesions of DLE. Sex hormones may also play a role: exacerbation may occur during pregnancy, during menstrual or premenstrual periods, or while taking oral contraceptives. Drugs such as procainamide, hydralazine, isoniazid, diphenylhydantoin, methyldopa, penicillamine, guanides, and lithium may also precipitate DLE lesions.2
DLE usually begins as dull red macules with adherent scales on sun-exposed areas. If the overlying scales are removed, an undersurface of horny plugs is revealed. These plugs fill the follicles and resemble carpet tacks or a cat’s tongue (langue au chat). The macules slowly expand to form large plaques.
Usually only a mild pruritus and tenderness is seen with DLE lesions. As the lesions progress, the scale may thicken and pigmentary changes become evident. The lesions heal with atrophy, scarring, telangiectasias, and changes in pigmentation. Morphological appearance of older lesions may vary from erythematous plaques to hyperkeratotic, dark gray plaques with centrally depressed scars.3 Scalp involvement in DLE results in more sclerotic and depressed scars with subsequent scarring alopecia.4
Differential diagnosis is wide. The differential diagnosis of DLE is extensive and includes actinic keratosis, dermatomyositis, granuloma annulare, granuloma faciale, keratoacanthoma, lichen planus, subacute cutaneous lupus erythematosus, psoriasis, rosacea, sarcoidosis, squamous cell carcinoma, syphilis, and nongenital warts.
Histopathology
Histopathological findings include hyperkeratosis, parakeratosis, follicular plugging, telangiectasias, and atrophy of the epidermis. Liquefaction or hydropic degeneration of the basal layer leads to pigmentary incontinence. A perivascular and perifollicular mononuclear inflammatory cell infiltrate is present in the superficial and deep dermis.5,6
Direct immunofluorescence demonstrates immunoglobulins and complement deposits at the dermoepidermal junction.5 This test was not recommended for this patient, as the diagnosis of DLE had already been confirmed on clinical and histopathologic grounds.
Workup: Exam, biopsy
In addition to a routine history and physical examination, the workup for DLE should include a complete blood count, antinuclear antibody levels, anti-Ro, anti-La, hepatic and renal function tests, and urinalysis. Consider a diagnosis of SLE by using the American College of Rheumatology criteria. A biopsy for histopathology of a fresh lesion or a biopsy for immunofluorescence of an old lesion can confirm the diagnosis.
Therapeutic options are varied
Effective early therapies for DLE are available, but patients who do not respond appropriately may end up with deep scars, alopecia, and pigmentary changes that are considerably disfiguring, especially for dark-skinned people. Therefore, the goal of treatment is not only to improve the appearance of the skin by minimizing the scarring and preventing further lesions, but also to prevent future complications.
Avoiding sunlight. The primary therapeutic approach is to educate patients regarding exposure to sunlight. Sun-protective measures include the use of high-SPF sunscreen lotions and protective clothing, such as baseball caps without vent holes and wide-brimmed hats.7
Medication options. Current treatment options for DLE include antimalarial agents such as chloroquine, topical and intralesional glucocorticoids, and thalidomide. The use of potent topical steroids may prevent significant scarring and deformity, especially of the face. Common side effects include steroid withdrawal syndrome, perioral dermatitis, steroid acne, and rosacea. All of these side effects can be treated and result in less long-term deformity than untreated DLE (FIGURE 2).
Systemic agents. Widespread disease may require you use systemic agents, such as antimalarials. Chloroquine has long been considered the gold standard in the treatment of DLE.8 Due to the frequency of ocular side effects with chloroquine, hydroxychloroquine is by far the most widely used agent. Hydroxychloroquine at a dose of 6.5 mg/kg/d for 3 months may lead to resolution of lesions for many patients.7 In resistant cases, higher dosages (eg, 400 mg/d) or combinations (eg, hydroxychloroquine 200 mg/d plus quinacrine 50 to 100 mg/d) may be required for months or even years. An ophthalmological evaluation is advisable before starting antimalarial treatment, and you should repeat it at 4- to 6-month intervals during treatment.9 Systemic corticosteroids may be needed to obtain timely initial control, especially for widespread and disfiguring lesions.
When this therapy is inadequate, other treatments for DLE include azathioprine, retinoids, and dapsone. Recent reports confirm the efficacy of thalidomide for cutaneous lupus erythematosus in dosages ranging from 50 to 200 mg/d.10 Twice-daily application of tacrolimus 0.01% has also been shown to be effective in a few clinical trials.11
Surgery. Surgical intervention is useful to remove scarred lesions, and laser therapy has also been effective, especially for lesions with prominent telangiectasias.12
Patient education. Patient education plays an important role in the treatment of DLE. Advising patients on how to avoid the sun and instructing them in the proper use of sunscreens is extremely important. Smoking cessation has also been shown to be beneficial.13
Patients with DLE generally have a favorable prognosis with regards to morbidity and mortality. Because DLE is usually self-limited, the course is most often benign; therefore, early recognition and adequate therapy may prevent clinical complications. Many patients with DLE go on to develop destructive or deforming scarring or pigmentary disturbances,14 especially in the spring and summer months when the sun is the strongest.
FIGURE 2
After treatment
This patient’s outcome
Our patient was treated with topical fluocinolone acetonide 0.025% ointment and oral hydroxychloroquine 200 mg/d for 1 month. The patient responded well to the treatment and the skin lesion regressed perceptibly (FIGURE 2). The treatment was nevertheless continued for another 5 months, and resulted in complete regression of the lesions, with minimal residual scarring.
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Department of Dermatology, 450 Clarkson Avenue Box 46, Brooklyn, NY 11203. E-mail: amorkh@pol.net
A 27-year-old woman, otherwise healthy, presented for evaluation of a mildly pruritic eruption of plaques on her face and ears. The eruption started as a few small, scaly red papules on her cheeks and nose about 3 months earlier. The papules slowly expanded to form well-demarcated, rounded, dark brown plaques covered with superficial scale. Similar lesions appeared on the concha of both ears. The older lesions started to regress after 2 months. Complete regression was followed by residual scarring and hyperpigmentation.
Physical examination revealed multiple well-defined, erythematous, hyperpigmented, round plaques covered with adherent scale affecting her nose, cheeks, and the concha of both ears (FIGURE 1). The mucosae and the rest of her skin and adnexa were unaffected. She had no personal or family history of similar skin findings or autoimmune disorders. She also had no history of any drug intake prior to the eruption. Her routine blood tests and urinalysis results were unremarkable, and the serological analysis for antinuclear antibodies had negative results.
A punch biopsy was taken from one of the lesions for histopathology. The epidermal histopathological findings were remarkable for hyperkeratosis, follicular plugging, pigment incontinence, and vacuolization of the basal cell layer. There was a predominantly lymphocytic infiltrate of the subepidermal and perivascular dermal areas.
FIGURE 1
Facial plaques
What is your diagnosis?
How would you treat this patient?
Diagnosis: Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is an autoimmune inflammatory disorder of the skin that often leads to scarring and alopecia. It may be localized to sun-exposed areas such as the face, ears, and scalp but occasionally is much more extensive, involving the trunk and extremities. Most patients are otherwise healthy, and DLE may be the only clinical finding.
Although its prevalence is not known, DLE is not uncommon. It affects females twice as often as males, and patients fall between the ages of 25 to 45 years, with no racial predilection. While about 15% to 20% of patients with systemic lupus erythematosus (SLE) manifest DLE lesions, only about 5% to 10% of patients with DLE go on to develop SLE.1
Like SLE, DLE is believed to be an autoimmune disorder. Unlike SLE, however, DLE patients do not have similar serologic abnormalities. Skin trauma and ultraviolet light exposure have been reported to induce or exacerbate the lesions of DLE. Sex hormones may also play a role: exacerbation may occur during pregnancy, during menstrual or premenstrual periods, or while taking oral contraceptives. Drugs such as procainamide, hydralazine, isoniazid, diphenylhydantoin, methyldopa, penicillamine, guanides, and lithium may also precipitate DLE lesions.2
DLE usually begins as dull red macules with adherent scales on sun-exposed areas. If the overlying scales are removed, an undersurface of horny plugs is revealed. These plugs fill the follicles and resemble carpet tacks or a cat’s tongue (langue au chat). The macules slowly expand to form large plaques.
Usually only a mild pruritus and tenderness is seen with DLE lesions. As the lesions progress, the scale may thicken and pigmentary changes become evident. The lesions heal with atrophy, scarring, telangiectasias, and changes in pigmentation. Morphological appearance of older lesions may vary from erythematous plaques to hyperkeratotic, dark gray plaques with centrally depressed scars.3 Scalp involvement in DLE results in more sclerotic and depressed scars with subsequent scarring alopecia.4
Differential diagnosis is wide. The differential diagnosis of DLE is extensive and includes actinic keratosis, dermatomyositis, granuloma annulare, granuloma faciale, keratoacanthoma, lichen planus, subacute cutaneous lupus erythematosus, psoriasis, rosacea, sarcoidosis, squamous cell carcinoma, syphilis, and nongenital warts.
Histopathology
Histopathological findings include hyperkeratosis, parakeratosis, follicular plugging, telangiectasias, and atrophy of the epidermis. Liquefaction or hydropic degeneration of the basal layer leads to pigmentary incontinence. A perivascular and perifollicular mononuclear inflammatory cell infiltrate is present in the superficial and deep dermis.5,6
Direct immunofluorescence demonstrates immunoglobulins and complement deposits at the dermoepidermal junction.5 This test was not recommended for this patient, as the diagnosis of DLE had already been confirmed on clinical and histopathologic grounds.
Workup: Exam, biopsy
In addition to a routine history and physical examination, the workup for DLE should include a complete blood count, antinuclear antibody levels, anti-Ro, anti-La, hepatic and renal function tests, and urinalysis. Consider a diagnosis of SLE by using the American College of Rheumatology criteria. A biopsy for histopathology of a fresh lesion or a biopsy for immunofluorescence of an old lesion can confirm the diagnosis.
Therapeutic options are varied
Effective early therapies for DLE are available, but patients who do not respond appropriately may end up with deep scars, alopecia, and pigmentary changes that are considerably disfiguring, especially for dark-skinned people. Therefore, the goal of treatment is not only to improve the appearance of the skin by minimizing the scarring and preventing further lesions, but also to prevent future complications.
Avoiding sunlight. The primary therapeutic approach is to educate patients regarding exposure to sunlight. Sun-protective measures include the use of high-SPF sunscreen lotions and protective clothing, such as baseball caps without vent holes and wide-brimmed hats.7
Medication options. Current treatment options for DLE include antimalarial agents such as chloroquine, topical and intralesional glucocorticoids, and thalidomide. The use of potent topical steroids may prevent significant scarring and deformity, especially of the face. Common side effects include steroid withdrawal syndrome, perioral dermatitis, steroid acne, and rosacea. All of these side effects can be treated and result in less long-term deformity than untreated DLE (FIGURE 2).
Systemic agents. Widespread disease may require you use systemic agents, such as antimalarials. Chloroquine has long been considered the gold standard in the treatment of DLE.8 Due to the frequency of ocular side effects with chloroquine, hydroxychloroquine is by far the most widely used agent. Hydroxychloroquine at a dose of 6.5 mg/kg/d for 3 months may lead to resolution of lesions for many patients.7 In resistant cases, higher dosages (eg, 400 mg/d) or combinations (eg, hydroxychloroquine 200 mg/d plus quinacrine 50 to 100 mg/d) may be required for months or even years. An ophthalmological evaluation is advisable before starting antimalarial treatment, and you should repeat it at 4- to 6-month intervals during treatment.9 Systemic corticosteroids may be needed to obtain timely initial control, especially for widespread and disfiguring lesions.
When this therapy is inadequate, other treatments for DLE include azathioprine, retinoids, and dapsone. Recent reports confirm the efficacy of thalidomide for cutaneous lupus erythematosus in dosages ranging from 50 to 200 mg/d.10 Twice-daily application of tacrolimus 0.01% has also been shown to be effective in a few clinical trials.11
Surgery. Surgical intervention is useful to remove scarred lesions, and laser therapy has also been effective, especially for lesions with prominent telangiectasias.12
Patient education. Patient education plays an important role in the treatment of DLE. Advising patients on how to avoid the sun and instructing them in the proper use of sunscreens is extremely important. Smoking cessation has also been shown to be beneficial.13
Patients with DLE generally have a favorable prognosis with regards to morbidity and mortality. Because DLE is usually self-limited, the course is most often benign; therefore, early recognition and adequate therapy may prevent clinical complications. Many patients with DLE go on to develop destructive or deforming scarring or pigmentary disturbances,14 especially in the spring and summer months when the sun is the strongest.
FIGURE 2
After treatment
This patient’s outcome
Our patient was treated with topical fluocinolone acetonide 0.025% ointment and oral hydroxychloroquine 200 mg/d for 1 month. The patient responded well to the treatment and the skin lesion regressed perceptibly (FIGURE 2). The treatment was nevertheless continued for another 5 months, and resulted in complete regression of the lesions, with minimal residual scarring.
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Department of Dermatology, 450 Clarkson Avenue Box 46, Brooklyn, NY 11203. E-mail: amorkh@pol.net
1. Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. Clinical and laboratory findings in seventeen patients. J Am Acad Dermatol 1985;12(2 Pt 1):278-288.
2. Hess E. Drug-related lupus. N Engl J Med 1988;318:1460-1462.
3. Callen JP, Fowler JF, Kulick KB. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol 1985;13(5 Pt 1):748-755.
4. Wilson CL, Burge SM, Dean D, Dawber RP. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol 1992;126:307-314.
5. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002;20:373-385, v.
6. Biesla I, et al. Histopathologic findings in cutaneous lupus erythematosus. Arch Dermatol 1994;130:54-58.
7. Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin 1994;12:201-206.
8. Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc 1953;152:1428-1429.
9. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983;75:35-39.
10. Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol 2000;39:218-222.
11. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1171.
12. Nunez M, Boixeda P, Miralles ES, et al. Pulsed dye laser treatment of telangiectatic chronic erythema of cutaneous lupus erythematosus. Arch Dermatol 1996;132:354-355.
13. Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology 2005;211:118-122.
14. de Berker D, Dissaneyeka M, Burge S. The sequelae of chronic cutaneous lupus erythematosus. Lupus 1992;1:181-186.
1. Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. Clinical and laboratory findings in seventeen patients. J Am Acad Dermatol 1985;12(2 Pt 1):278-288.
2. Hess E. Drug-related lupus. N Engl J Med 1988;318:1460-1462.
3. Callen JP, Fowler JF, Kulick KB. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol 1985;13(5 Pt 1):748-755.
4. Wilson CL, Burge SM, Dean D, Dawber RP. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol 1992;126:307-314.
5. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002;20:373-385, v.
6. Biesla I, et al. Histopathologic findings in cutaneous lupus erythematosus. Arch Dermatol 1994;130:54-58.
7. Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin 1994;12:201-206.
8. Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc 1953;152:1428-1429.
9. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983;75:35-39.
10. Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol 2000;39:218-222.
11. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1171.
12. Nunez M, Boixeda P, Miralles ES, et al. Pulsed dye laser treatment of telangiectatic chronic erythema of cutaneous lupus erythematosus. Arch Dermatol 1996;132:354-355.
13. Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology 2005;211:118-122.
14. de Berker D, Dissaneyeka M, Burge S. The sequelae of chronic cutaneous lupus erythematosus. Lupus 1992;1:181-186.
Pits on the soles of the feet
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
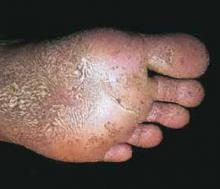
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: amorkh@pol.net
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: amorkh@pol.net
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: amorkh@pol.net
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.