User login
Does anal cancer screening reduce morbidity and mortality in men who have sex with men?
IT’S UNCLEAR whether anal cancer screening benefits men who have sex with men because high-quality studies on this subject are lacking. In the absence of high-quality data, anal pap smears aren’t recommended for routine screening of men who have sex with men (strength of recommendation: C, expert opinion).
Evidence summary
The National Cancer Institute reports an annual anal cancer incidence of 1.6 per 100,000 and as of November of last year, expected that 5820 men and women would receive the diagnosis in 2011.1 The 5-year survival rate is 64.9%. For men who have sex with men, the incidence ranges from 35 to 100 per 100,000, with a higher incidence in HIV-positive men.2
Men who have sex with men also have a higher prevalence of human papillomavirus (HPV) than the general population.3,4 HPV is the most common cause of anal squamous intraepithelial lesions. In theory, screening for anal cancer may reduce morbidity and mortality by identifying and treating anal cancer precursors, much as screening has done for cervical cancer.
Small studies suggest that screening may be effective
One study has demonstrated that anal pap smears are potentially effective as a screening tool for detecting anal intraepithelial neoplasia.5 The study was limited by small sample size and failure to address patient-centered outcomes, however. It included only 395 subjects, most of whom (54%) were HIV positive. Additional studies evaluated 265 HIV-positive men and 658 men, of whom 407 were HIV positive, with similar findings.6,7
But a larger study shows no impact
The largest study to date, which included 5083 HIV-positive patients (contributing 13,411 patient-years), didn’t demonstrate a decrease in invasive anal carcinoma during the screening period.8 The difference in HPV prevalence between HIV-positive and HIV-negative men who have sex with men (96% vs 58.9%; P<.001) limits the ability to generalize the conclusions of this study to all men who have sex with men.9
Recommendations
No consensus guidelines exist on screening for anal cancer in men who have sex with men, regardless of HIV status.
The New York State Department of Health recommends baseline cytology and annual anal cancer screening for all HIV-positive men who have sex with men.
Based on the high prevalence of HPV in the HIV-positive population, some experts suggest anal cancer screening for HIV-positive men who have sex with men.10
1. National Cancer Institute. SEER stat fact sheets: anal cancer. November 10, 2011. Available at: http://seer.cancer.gov/statfacts/html/anus.html. Accessed February 24, 2012.
2. Altekruse SF, Kosary CL, Krapcho M, et al. eds. SEER cancer statistics review, 1975-2007. Available at: http://seer.cancer.gov/csr/1975_2007. Accessed February 24, 2012.
3. Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE Study. J Infect Dis. 2004;190:2070-2076.
4. Chin-Hong PV, Berry JM, Cheng SC, et al. Comparison of patient- and clinician-collected anal cytology samples to screen for human papillomavirus-associated anal intraepithelial neoplasia in men who have sex with men. Ann Intern Med. 2008;149:300-306.
5. Nathan M, Singh N, Garrett N, et al. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010;24:373-379.
6. Scott H, Khoury J, Moore BA, et al. Routine anal cytology screening for anal squamous intraepithelial lesions in an urban HIV clinic. Sex Transm Dis. 2008;35:197-202.
7. Palefsky JM, Holly EA, Hogeboom CJ, et al. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:415-422.
8. Mathews C, Caperna J, Cachay ER, et al. Early impact and performance characteristics of an established anal dysplasia screening program: program evaluation considerations. Open AIDS J. 2007;1:11-20.
9. Gao L, Zhou F, Li X, et al. Anal HPV infection in HIV-positive men who have sex with men from China. PLoS ONE. 2010;5:e15256.-
10. Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337-2345.
IT’S UNCLEAR whether anal cancer screening benefits men who have sex with men because high-quality studies on this subject are lacking. In the absence of high-quality data, anal pap smears aren’t recommended for routine screening of men who have sex with men (strength of recommendation: C, expert opinion).
Evidence summary
The National Cancer Institute reports an annual anal cancer incidence of 1.6 per 100,000 and as of November of last year, expected that 5820 men and women would receive the diagnosis in 2011.1 The 5-year survival rate is 64.9%. For men who have sex with men, the incidence ranges from 35 to 100 per 100,000, with a higher incidence in HIV-positive men.2
Men who have sex with men also have a higher prevalence of human papillomavirus (HPV) than the general population.3,4 HPV is the most common cause of anal squamous intraepithelial lesions. In theory, screening for anal cancer may reduce morbidity and mortality by identifying and treating anal cancer precursors, much as screening has done for cervical cancer.
Small studies suggest that screening may be effective
One study has demonstrated that anal pap smears are potentially effective as a screening tool for detecting anal intraepithelial neoplasia.5 The study was limited by small sample size and failure to address patient-centered outcomes, however. It included only 395 subjects, most of whom (54%) were HIV positive. Additional studies evaluated 265 HIV-positive men and 658 men, of whom 407 were HIV positive, with similar findings.6,7
But a larger study shows no impact
The largest study to date, which included 5083 HIV-positive patients (contributing 13,411 patient-years), didn’t demonstrate a decrease in invasive anal carcinoma during the screening period.8 The difference in HPV prevalence between HIV-positive and HIV-negative men who have sex with men (96% vs 58.9%; P<.001) limits the ability to generalize the conclusions of this study to all men who have sex with men.9
Recommendations
No consensus guidelines exist on screening for anal cancer in men who have sex with men, regardless of HIV status.
The New York State Department of Health recommends baseline cytology and annual anal cancer screening for all HIV-positive men who have sex with men.
Based on the high prevalence of HPV in the HIV-positive population, some experts suggest anal cancer screening for HIV-positive men who have sex with men.10
IT’S UNCLEAR whether anal cancer screening benefits men who have sex with men because high-quality studies on this subject are lacking. In the absence of high-quality data, anal pap smears aren’t recommended for routine screening of men who have sex with men (strength of recommendation: C, expert opinion).
Evidence summary
The National Cancer Institute reports an annual anal cancer incidence of 1.6 per 100,000 and as of November of last year, expected that 5820 men and women would receive the diagnosis in 2011.1 The 5-year survival rate is 64.9%. For men who have sex with men, the incidence ranges from 35 to 100 per 100,000, with a higher incidence in HIV-positive men.2
Men who have sex with men also have a higher prevalence of human papillomavirus (HPV) than the general population.3,4 HPV is the most common cause of anal squamous intraepithelial lesions. In theory, screening for anal cancer may reduce morbidity and mortality by identifying and treating anal cancer precursors, much as screening has done for cervical cancer.
Small studies suggest that screening may be effective
One study has demonstrated that anal pap smears are potentially effective as a screening tool for detecting anal intraepithelial neoplasia.5 The study was limited by small sample size and failure to address patient-centered outcomes, however. It included only 395 subjects, most of whom (54%) were HIV positive. Additional studies evaluated 265 HIV-positive men and 658 men, of whom 407 were HIV positive, with similar findings.6,7
But a larger study shows no impact
The largest study to date, which included 5083 HIV-positive patients (contributing 13,411 patient-years), didn’t demonstrate a decrease in invasive anal carcinoma during the screening period.8 The difference in HPV prevalence between HIV-positive and HIV-negative men who have sex with men (96% vs 58.9%; P<.001) limits the ability to generalize the conclusions of this study to all men who have sex with men.9
Recommendations
No consensus guidelines exist on screening for anal cancer in men who have sex with men, regardless of HIV status.
The New York State Department of Health recommends baseline cytology and annual anal cancer screening for all HIV-positive men who have sex with men.
Based on the high prevalence of HPV in the HIV-positive population, some experts suggest anal cancer screening for HIV-positive men who have sex with men.10
1. National Cancer Institute. SEER stat fact sheets: anal cancer. November 10, 2011. Available at: http://seer.cancer.gov/statfacts/html/anus.html. Accessed February 24, 2012.
2. Altekruse SF, Kosary CL, Krapcho M, et al. eds. SEER cancer statistics review, 1975-2007. Available at: http://seer.cancer.gov/csr/1975_2007. Accessed February 24, 2012.
3. Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE Study. J Infect Dis. 2004;190:2070-2076.
4. Chin-Hong PV, Berry JM, Cheng SC, et al. Comparison of patient- and clinician-collected anal cytology samples to screen for human papillomavirus-associated anal intraepithelial neoplasia in men who have sex with men. Ann Intern Med. 2008;149:300-306.
5. Nathan M, Singh N, Garrett N, et al. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010;24:373-379.
6. Scott H, Khoury J, Moore BA, et al. Routine anal cytology screening for anal squamous intraepithelial lesions in an urban HIV clinic. Sex Transm Dis. 2008;35:197-202.
7. Palefsky JM, Holly EA, Hogeboom CJ, et al. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:415-422.
8. Mathews C, Caperna J, Cachay ER, et al. Early impact and performance characteristics of an established anal dysplasia screening program: program evaluation considerations. Open AIDS J. 2007;1:11-20.
9. Gao L, Zhou F, Li X, et al. Anal HPV infection in HIV-positive men who have sex with men from China. PLoS ONE. 2010;5:e15256.-
10. Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337-2345.
1. National Cancer Institute. SEER stat fact sheets: anal cancer. November 10, 2011. Available at: http://seer.cancer.gov/statfacts/html/anus.html. Accessed February 24, 2012.
2. Altekruse SF, Kosary CL, Krapcho M, et al. eds. SEER cancer statistics review, 1975-2007. Available at: http://seer.cancer.gov/csr/1975_2007. Accessed February 24, 2012.
3. Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE Study. J Infect Dis. 2004;190:2070-2076.
4. Chin-Hong PV, Berry JM, Cheng SC, et al. Comparison of patient- and clinician-collected anal cytology samples to screen for human papillomavirus-associated anal intraepithelial neoplasia in men who have sex with men. Ann Intern Med. 2008;149:300-306.
5. Nathan M, Singh N, Garrett N, et al. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010;24:373-379.
6. Scott H, Khoury J, Moore BA, et al. Routine anal cytology screening for anal squamous intraepithelial lesions in an urban HIV clinic. Sex Transm Dis. 2008;35:197-202.
7. Palefsky JM, Holly EA, Hogeboom CJ, et al. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:415-422.
8. Mathews C, Caperna J, Cachay ER, et al. Early impact and performance characteristics of an established anal dysplasia screening program: program evaluation considerations. Open AIDS J. 2007;1:11-20.
9. Gao L, Zhou F, Li X, et al. Anal HPV infection in HIV-positive men who have sex with men from China. PLoS ONE. 2010;5:e15256.-
10. Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337-2345.
Evidence-based answers from the Family Physicians Inquiries Network
Can probiotics safely prevent recurrent vaginitis?
YES, using vaginal suppositories or eating yogurt with Lactobacillus may reduce recurrences of bacterial vaginosis (BV) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results).
Neither suppositories nor yogurt containing Lactobacillus are likely to prevent recurrences of vulvovaginal candidiasis (VVC) (SOR: B, RCTs with conflicting results).
Probiotic suppositories and yogurt don’t appear to have significant adverse effects (SOR: A, RCTs).
Evidence summary
A double-blind RCT found that probiotic vaginal suppositories reduce the incidence of recurrent BV. Investigators randomized 120 Chinese women, 18 to 55 years of age with a history of 2 or more episodes of BV in the previous year, to use suppositories containing either probiotics (Lactobacillus rhamnosus,L acidophilus, and Streptococcus thermophilus, total of 8×109 colony-forming units [cfu]) or placebo.1 All the women used suppositories daily for a week, stopped for a week, and then used them for another week.
Fewer women who used probiotic suppositories had recurrences of BV on examination during the following 2 months than women who used placebo (16% vs 45%; P<.001; number needed to treat [NNT]=3.4), and fewer reported recurrences in telephone interviews 2 to 11 months after treatment (11% vs 28%; P<.05; NNT=5.8). Interviewers recorded two-thirds fewer complaints of discharge and malodor among women who used probiotics than among women who used placebo (P<.05 for both comparisons).
But another RCT finds no effect on recurrent BV or VVC
Another RCT treated 95 women 18 to 45 years of age with clindamycin ovules (for BV) or clotrimazole suppositories (for VVC) and, after 5 days, randomized them to use probiotic suppositories (Lactobacillus species, 108-1010 cfu) or placebo for 5 more days.2
Probiotic suppositories after treatment didn’t reduce clinician-diagnosed recurrences of either BV or VVC compared with placebo (7% vs 17% after 2-3 days; 22% vs 29% after the first menstrual cycle; P=not significant for both). Probiotics did reduce self-reported malodorous discharge, however (P=.03). Probiotics didn’t produce adverse effects.
Probiotic yogurt decreases recurrent BV but not VVC in an RCT
An RCT that randomized 46 women, 20 to 39 years of age with a history of 4 or more episodes of BV or VVC in the previous year, to eat L acidophilus-enriched yogurt (108 cfu) or pasteurized yogurt daily for 2 months found that consuming probiotic-containing yogurt reduced the incidence of recurrent BV but not VVC.3
Women who ate L acidophilus yogurt had fewer episodes of clinician-diagnosed BV at 1 month than women who ate pasteurized yogurt (24% vs 53%; P<.05) and also at 2 months (4% vs 36%; P<.05). However, they didn’t have significantly fewer episodes of VVC (43% vs 37% at 1 month, 21% vs 29% at 2 months; P=not significant for both). Investigators reported no adverse effects.
Small, flawed trial finds fewer episodes of VVC with yogurt
An unblinded crossover trial found that daily consumption of probiotic yogurt reduced VVC recurrences in women with a history of the infection. Investigators randomized 33 women 24 to 50 years of age to eat either 8 ounces a day of yogurt (with L acidophilus, 108 cfu) or a yogurt-free diet.4 After 6 months, the groups switched. Investigators saw all patients monthly.
Women who ate yogurt had fewer episodes of VVC than women who didn’t (0.4 vs 2.5 over 6 months; P<.001) and reported no adverse effects. The study was flawed by small size and high attrition rates (only 13 women completed the trial).
Recommendations
The World Health Organization says some clinical evidence suggests that oral and vaginal administration of lactobacilli can eradicate asymptomatic and symptomatic BV. Supporting evidence for prevention of recurrent BV or VVC by probiotics is limited.5
A literature review by the Natural Standard Research Collaboration states that insufficient evidence exists to recommend probiotics for treating or preventing bacterial vaginosis and that preventing or treating vaginal yeast infections with probiotics hasn’t been adequately studied.6
1. Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1-120.e6.
2. Ehrstrom S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691-699.
3. Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med. 1996;5:593-596.
4. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. October 1-4, 2001. Cordoba, Argentina.
6. National Standard Research Collaboration. Unclear if probiotics effective for bacterial vaginosis. October 2009. Available at: http://www.naturalstandard.com/news/news20091028.asp. Accessed September 1, 2011.
YES, using vaginal suppositories or eating yogurt with Lactobacillus may reduce recurrences of bacterial vaginosis (BV) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results).
Neither suppositories nor yogurt containing Lactobacillus are likely to prevent recurrences of vulvovaginal candidiasis (VVC) (SOR: B, RCTs with conflicting results).
Probiotic suppositories and yogurt don’t appear to have significant adverse effects (SOR: A, RCTs).
Evidence summary
A double-blind RCT found that probiotic vaginal suppositories reduce the incidence of recurrent BV. Investigators randomized 120 Chinese women, 18 to 55 years of age with a history of 2 or more episodes of BV in the previous year, to use suppositories containing either probiotics (Lactobacillus rhamnosus,L acidophilus, and Streptococcus thermophilus, total of 8×109 colony-forming units [cfu]) or placebo.1 All the women used suppositories daily for a week, stopped for a week, and then used them for another week.
Fewer women who used probiotic suppositories had recurrences of BV on examination during the following 2 months than women who used placebo (16% vs 45%; P<.001; number needed to treat [NNT]=3.4), and fewer reported recurrences in telephone interviews 2 to 11 months after treatment (11% vs 28%; P<.05; NNT=5.8). Interviewers recorded two-thirds fewer complaints of discharge and malodor among women who used probiotics than among women who used placebo (P<.05 for both comparisons).
But another RCT finds no effect on recurrent BV or VVC
Another RCT treated 95 women 18 to 45 years of age with clindamycin ovules (for BV) or clotrimazole suppositories (for VVC) and, after 5 days, randomized them to use probiotic suppositories (Lactobacillus species, 108-1010 cfu) or placebo for 5 more days.2
Probiotic suppositories after treatment didn’t reduce clinician-diagnosed recurrences of either BV or VVC compared with placebo (7% vs 17% after 2-3 days; 22% vs 29% after the first menstrual cycle; P=not significant for both). Probiotics did reduce self-reported malodorous discharge, however (P=.03). Probiotics didn’t produce adverse effects.
Probiotic yogurt decreases recurrent BV but not VVC in an RCT
An RCT that randomized 46 women, 20 to 39 years of age with a history of 4 or more episodes of BV or VVC in the previous year, to eat L acidophilus-enriched yogurt (108 cfu) or pasteurized yogurt daily for 2 months found that consuming probiotic-containing yogurt reduced the incidence of recurrent BV but not VVC.3
Women who ate L acidophilus yogurt had fewer episodes of clinician-diagnosed BV at 1 month than women who ate pasteurized yogurt (24% vs 53%; P<.05) and also at 2 months (4% vs 36%; P<.05). However, they didn’t have significantly fewer episodes of VVC (43% vs 37% at 1 month, 21% vs 29% at 2 months; P=not significant for both). Investigators reported no adverse effects.
Small, flawed trial finds fewer episodes of VVC with yogurt
An unblinded crossover trial found that daily consumption of probiotic yogurt reduced VVC recurrences in women with a history of the infection. Investigators randomized 33 women 24 to 50 years of age to eat either 8 ounces a day of yogurt (with L acidophilus, 108 cfu) or a yogurt-free diet.4 After 6 months, the groups switched. Investigators saw all patients monthly.
Women who ate yogurt had fewer episodes of VVC than women who didn’t (0.4 vs 2.5 over 6 months; P<.001) and reported no adverse effects. The study was flawed by small size and high attrition rates (only 13 women completed the trial).
Recommendations
The World Health Organization says some clinical evidence suggests that oral and vaginal administration of lactobacilli can eradicate asymptomatic and symptomatic BV. Supporting evidence for prevention of recurrent BV or VVC by probiotics is limited.5
A literature review by the Natural Standard Research Collaboration states that insufficient evidence exists to recommend probiotics for treating or preventing bacterial vaginosis and that preventing or treating vaginal yeast infections with probiotics hasn’t been adequately studied.6
YES, using vaginal suppositories or eating yogurt with Lactobacillus may reduce recurrences of bacterial vaginosis (BV) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results).
Neither suppositories nor yogurt containing Lactobacillus are likely to prevent recurrences of vulvovaginal candidiasis (VVC) (SOR: B, RCTs with conflicting results).
Probiotic suppositories and yogurt don’t appear to have significant adverse effects (SOR: A, RCTs).
Evidence summary
A double-blind RCT found that probiotic vaginal suppositories reduce the incidence of recurrent BV. Investigators randomized 120 Chinese women, 18 to 55 years of age with a history of 2 or more episodes of BV in the previous year, to use suppositories containing either probiotics (Lactobacillus rhamnosus,L acidophilus, and Streptococcus thermophilus, total of 8×109 colony-forming units [cfu]) or placebo.1 All the women used suppositories daily for a week, stopped for a week, and then used them for another week.
Fewer women who used probiotic suppositories had recurrences of BV on examination during the following 2 months than women who used placebo (16% vs 45%; P<.001; number needed to treat [NNT]=3.4), and fewer reported recurrences in telephone interviews 2 to 11 months after treatment (11% vs 28%; P<.05; NNT=5.8). Interviewers recorded two-thirds fewer complaints of discharge and malodor among women who used probiotics than among women who used placebo (P<.05 for both comparisons).
But another RCT finds no effect on recurrent BV or VVC
Another RCT treated 95 women 18 to 45 years of age with clindamycin ovules (for BV) or clotrimazole suppositories (for VVC) and, after 5 days, randomized them to use probiotic suppositories (Lactobacillus species, 108-1010 cfu) or placebo for 5 more days.2
Probiotic suppositories after treatment didn’t reduce clinician-diagnosed recurrences of either BV or VVC compared with placebo (7% vs 17% after 2-3 days; 22% vs 29% after the first menstrual cycle; P=not significant for both). Probiotics did reduce self-reported malodorous discharge, however (P=.03). Probiotics didn’t produce adverse effects.
Probiotic yogurt decreases recurrent BV but not VVC in an RCT
An RCT that randomized 46 women, 20 to 39 years of age with a history of 4 or more episodes of BV or VVC in the previous year, to eat L acidophilus-enriched yogurt (108 cfu) or pasteurized yogurt daily for 2 months found that consuming probiotic-containing yogurt reduced the incidence of recurrent BV but not VVC.3
Women who ate L acidophilus yogurt had fewer episodes of clinician-diagnosed BV at 1 month than women who ate pasteurized yogurt (24% vs 53%; P<.05) and also at 2 months (4% vs 36%; P<.05). However, they didn’t have significantly fewer episodes of VVC (43% vs 37% at 1 month, 21% vs 29% at 2 months; P=not significant for both). Investigators reported no adverse effects.
Small, flawed trial finds fewer episodes of VVC with yogurt
An unblinded crossover trial found that daily consumption of probiotic yogurt reduced VVC recurrences in women with a history of the infection. Investigators randomized 33 women 24 to 50 years of age to eat either 8 ounces a day of yogurt (with L acidophilus, 108 cfu) or a yogurt-free diet.4 After 6 months, the groups switched. Investigators saw all patients monthly.
Women who ate yogurt had fewer episodes of VVC than women who didn’t (0.4 vs 2.5 over 6 months; P<.001) and reported no adverse effects. The study was flawed by small size and high attrition rates (only 13 women completed the trial).
Recommendations
The World Health Organization says some clinical evidence suggests that oral and vaginal administration of lactobacilli can eradicate asymptomatic and symptomatic BV. Supporting evidence for prevention of recurrent BV or VVC by probiotics is limited.5
A literature review by the Natural Standard Research Collaboration states that insufficient evidence exists to recommend probiotics for treating or preventing bacterial vaginosis and that preventing or treating vaginal yeast infections with probiotics hasn’t been adequately studied.6
1. Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1-120.e6.
2. Ehrstrom S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691-699.
3. Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med. 1996;5:593-596.
4. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. October 1-4, 2001. Cordoba, Argentina.
6. National Standard Research Collaboration. Unclear if probiotics effective for bacterial vaginosis. October 2009. Available at: http://www.naturalstandard.com/news/news20091028.asp. Accessed September 1, 2011.
1. Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1-120.e6.
2. Ehrstrom S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691-699.
3. Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med. 1996;5:593-596.
4. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. October 1-4, 2001. Cordoba, Argentina.
6. National Standard Research Collaboration. Unclear if probiotics effective for bacterial vaginosis. October 2009. Available at: http://www.naturalstandard.com/news/news20091028.asp. Accessed September 1, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
What drugs are effective for periodic limb movement disorder?
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
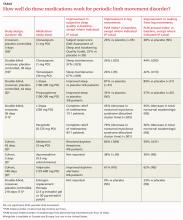
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
Evidence-based answers from the Family Physicians Inquiries Network
Medication vs radioablation for Graves’ disease: How do they compare?
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
Evidence-based answers from the Family Physicians Inquiries Network
How does smoking in the home affect children with asthma?
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
Evidence-based answers from the Family Physicians Inquiries Network
Is high-dose oral B12 a safe and effective alternative to a B12 injection?
YES. Both high-dose oral B12 and injected B12 raised low vitamin B12 levels and improved hematologic parameters and neurologic symptoms in short-term studies (3-4 months) predominantly involving patients with conditions associated with intestinal malabsorption (strength of recommendation: A, randomized controlled trials [RCTs]).
Both forms are well tolerated. Oral B12 is less expensive.
Evidence summary
Two open-label RCTs compared oral and intramuscular (IM) therapy for vitamin B12 deficiency.1,2 Both studies enrolled patients from hospital-based clinics—not primary care centers. Most patients (63 of 93 total) had conditions associated with intestinal malabsorption, including 7 patients with pernicious anemia and 3 with ileal resection. Both trials excluded patients with celiac and inflammatory bowel disease.
Oral therapy works as well as injections and costs less
One RCT compared the effects of oral B12 with IM therapy in 60 patients (mean age 62 years) with B12 deficiency and megaloblastic anemia.1 Investigators gave patients in each group equivalent doses of cobalamin: 1000 mcg daily for 10 days, weekly for 4 weeks, and then monthly to complete a 90-day course.
The mean hemoglobin increased significantly in both the oral and IM groups (from 8.4 to 13.8 g/dL, P<.001 for oral therapy; from 8.3 to 13.7 g/dL, P<.001 for IM therapy), as did mean serum B12 levels (from 73 to 214 pg/mL, P<.001, oral; and from 70 to 226 pg/mL, P<.001, IM). Neurologic symptoms (sensitive peripheral neuropathy, alteration of cognitive function, loss of sense of vibration) either cleared or improved markedly in both groups within one month (7 of 9 patients with oral therapy and 9 of 12 patients with IM treatment; P value not given).
Oral therapy cost less ($80 vs $220 per patient) and neither group reported adverse effects.1
B12 therapy changes hematologic parameters
The second RCT compared oral with IM B12 therapy in 33 patients (mean age 72 years) with newly diagnosed B12 deficiency.2 Investigators randomized patients to receive either oral cyanocobalamin (2000 mcg daily) for 120 days or IM cobalamin (1000 mcg) on Days 1, 3, 7, 10, 14, 21, 30, 60, and 90.
At 4 months, both groups had improved significantly from baseline in all metabolite measures and achieved a normal serum cobalamin level. The higher-dose oral therapy raised cobalamin levels more than IM therapy (from 93 to 1005 pg/mL, P<.0005 with oral therapy vs from 95 to 325 pg/mL, P<.0005 with IM treatment). Oral therapy increased cobalamin levels above 300 pg/mL in all patients; only half the patients treated with injections reached that level.
B12 therapy also significantly changed hematologic parameters from baseline even though the patients in the study didn’t have anemia. Mean corpuscular volume, for example, decreased from 100 to 90 fL with oral therapy and 102 to 97 fL with IM therapy (P<.005 for each). Neurologic symptoms (memory loss, paresthesias, ataxia) either cleared or improved markedly in all patients. Investigators compared all parameters against baseline values but didn’t directly compare oral with IM therapy. The trial didn’t assess safety outcomes.2
Recommendations
Canada’s British Columbia Medical Association and Ministry of Health recommend oral replacement of B12 (1000-2000 mcg/d) for most cases of vitamin B12 deficiency, including pernicious anemia. For patients with neurologic symptoms, they recommend an initial B12 injection (1000 mcg IM) followed by oral replacement.3
The US Centers for Disease Control and Prevention recommends either oral (1000 mcg daily) or parenteral B12 replacement. They advise giving parenteral therapy either subcutaneously (to reduce the burning sensation) or IM (1000 mcg per week for 8 weeks, then monthly for life).4
1. Bolaman Z, Kadikoylu G, Yukselen, et al. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center prospective, randomized, open-label study. Clin Ther. 2003;25:3124-3134.
2. Kuzminski AM, Del Giacco EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191-1198.
3. British Columbia Ministry of Health and the Guidelines and Protocols Advisory Committee. B12 deficiency: investigation and management of vitamin B12 and folate deficiency. Clinical Practice Guidelines and Protocols in British Columbia. December 15, 2006. Available at: www.bcguidelines.ca/gpac/guideline_b12.html. Accessed May 27, 2010.
4. Centers for Disease Control and Prevention Managing patients with evidence of vitamin B12 deficiency. June 29, 2009. Available at: http://cdc.gov/ncbddd/b12/patients.html. Accessed May 27, 2010.
YES. Both high-dose oral B12 and injected B12 raised low vitamin B12 levels and improved hematologic parameters and neurologic symptoms in short-term studies (3-4 months) predominantly involving patients with conditions associated with intestinal malabsorption (strength of recommendation: A, randomized controlled trials [RCTs]).
Both forms are well tolerated. Oral B12 is less expensive.
Evidence summary
Two open-label RCTs compared oral and intramuscular (IM) therapy for vitamin B12 deficiency.1,2 Both studies enrolled patients from hospital-based clinics—not primary care centers. Most patients (63 of 93 total) had conditions associated with intestinal malabsorption, including 7 patients with pernicious anemia and 3 with ileal resection. Both trials excluded patients with celiac and inflammatory bowel disease.
Oral therapy works as well as injections and costs less
One RCT compared the effects of oral B12 with IM therapy in 60 patients (mean age 62 years) with B12 deficiency and megaloblastic anemia.1 Investigators gave patients in each group equivalent doses of cobalamin: 1000 mcg daily for 10 days, weekly for 4 weeks, and then monthly to complete a 90-day course.
The mean hemoglobin increased significantly in both the oral and IM groups (from 8.4 to 13.8 g/dL, P<.001 for oral therapy; from 8.3 to 13.7 g/dL, P<.001 for IM therapy), as did mean serum B12 levels (from 73 to 214 pg/mL, P<.001, oral; and from 70 to 226 pg/mL, P<.001, IM). Neurologic symptoms (sensitive peripheral neuropathy, alteration of cognitive function, loss of sense of vibration) either cleared or improved markedly in both groups within one month (7 of 9 patients with oral therapy and 9 of 12 patients with IM treatment; P value not given).
Oral therapy cost less ($80 vs $220 per patient) and neither group reported adverse effects.1
B12 therapy changes hematologic parameters
The second RCT compared oral with IM B12 therapy in 33 patients (mean age 72 years) with newly diagnosed B12 deficiency.2 Investigators randomized patients to receive either oral cyanocobalamin (2000 mcg daily) for 120 days or IM cobalamin (1000 mcg) on Days 1, 3, 7, 10, 14, 21, 30, 60, and 90.
At 4 months, both groups had improved significantly from baseline in all metabolite measures and achieved a normal serum cobalamin level. The higher-dose oral therapy raised cobalamin levels more than IM therapy (from 93 to 1005 pg/mL, P<.0005 with oral therapy vs from 95 to 325 pg/mL, P<.0005 with IM treatment). Oral therapy increased cobalamin levels above 300 pg/mL in all patients; only half the patients treated with injections reached that level.
B12 therapy also significantly changed hematologic parameters from baseline even though the patients in the study didn’t have anemia. Mean corpuscular volume, for example, decreased from 100 to 90 fL with oral therapy and 102 to 97 fL with IM therapy (P<.005 for each). Neurologic symptoms (memory loss, paresthesias, ataxia) either cleared or improved markedly in all patients. Investigators compared all parameters against baseline values but didn’t directly compare oral with IM therapy. The trial didn’t assess safety outcomes.2
Recommendations
Canada’s British Columbia Medical Association and Ministry of Health recommend oral replacement of B12 (1000-2000 mcg/d) for most cases of vitamin B12 deficiency, including pernicious anemia. For patients with neurologic symptoms, they recommend an initial B12 injection (1000 mcg IM) followed by oral replacement.3
The US Centers for Disease Control and Prevention recommends either oral (1000 mcg daily) or parenteral B12 replacement. They advise giving parenteral therapy either subcutaneously (to reduce the burning sensation) or IM (1000 mcg per week for 8 weeks, then monthly for life).4
YES. Both high-dose oral B12 and injected B12 raised low vitamin B12 levels and improved hematologic parameters and neurologic symptoms in short-term studies (3-4 months) predominantly involving patients with conditions associated with intestinal malabsorption (strength of recommendation: A, randomized controlled trials [RCTs]).
Both forms are well tolerated. Oral B12 is less expensive.
Evidence summary
Two open-label RCTs compared oral and intramuscular (IM) therapy for vitamin B12 deficiency.1,2 Both studies enrolled patients from hospital-based clinics—not primary care centers. Most patients (63 of 93 total) had conditions associated with intestinal malabsorption, including 7 patients with pernicious anemia and 3 with ileal resection. Both trials excluded patients with celiac and inflammatory bowel disease.
Oral therapy works as well as injections and costs less
One RCT compared the effects of oral B12 with IM therapy in 60 patients (mean age 62 years) with B12 deficiency and megaloblastic anemia.1 Investigators gave patients in each group equivalent doses of cobalamin: 1000 mcg daily for 10 days, weekly for 4 weeks, and then monthly to complete a 90-day course.
The mean hemoglobin increased significantly in both the oral and IM groups (from 8.4 to 13.8 g/dL, P<.001 for oral therapy; from 8.3 to 13.7 g/dL, P<.001 for IM therapy), as did mean serum B12 levels (from 73 to 214 pg/mL, P<.001, oral; and from 70 to 226 pg/mL, P<.001, IM). Neurologic symptoms (sensitive peripheral neuropathy, alteration of cognitive function, loss of sense of vibration) either cleared or improved markedly in both groups within one month (7 of 9 patients with oral therapy and 9 of 12 patients with IM treatment; P value not given).
Oral therapy cost less ($80 vs $220 per patient) and neither group reported adverse effects.1
B12 therapy changes hematologic parameters
The second RCT compared oral with IM B12 therapy in 33 patients (mean age 72 years) with newly diagnosed B12 deficiency.2 Investigators randomized patients to receive either oral cyanocobalamin (2000 mcg daily) for 120 days or IM cobalamin (1000 mcg) on Days 1, 3, 7, 10, 14, 21, 30, 60, and 90.
At 4 months, both groups had improved significantly from baseline in all metabolite measures and achieved a normal serum cobalamin level. The higher-dose oral therapy raised cobalamin levels more than IM therapy (from 93 to 1005 pg/mL, P<.0005 with oral therapy vs from 95 to 325 pg/mL, P<.0005 with IM treatment). Oral therapy increased cobalamin levels above 300 pg/mL in all patients; only half the patients treated with injections reached that level.
B12 therapy also significantly changed hematologic parameters from baseline even though the patients in the study didn’t have anemia. Mean corpuscular volume, for example, decreased from 100 to 90 fL with oral therapy and 102 to 97 fL with IM therapy (P<.005 for each). Neurologic symptoms (memory loss, paresthesias, ataxia) either cleared or improved markedly in all patients. Investigators compared all parameters against baseline values but didn’t directly compare oral with IM therapy. The trial didn’t assess safety outcomes.2
Recommendations
Canada’s British Columbia Medical Association and Ministry of Health recommend oral replacement of B12 (1000-2000 mcg/d) for most cases of vitamin B12 deficiency, including pernicious anemia. For patients with neurologic symptoms, they recommend an initial B12 injection (1000 mcg IM) followed by oral replacement.3
The US Centers for Disease Control and Prevention recommends either oral (1000 mcg daily) or parenteral B12 replacement. They advise giving parenteral therapy either subcutaneously (to reduce the burning sensation) or IM (1000 mcg per week for 8 weeks, then monthly for life).4
1. Bolaman Z, Kadikoylu G, Yukselen, et al. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center prospective, randomized, open-label study. Clin Ther. 2003;25:3124-3134.
2. Kuzminski AM, Del Giacco EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191-1198.
3. British Columbia Ministry of Health and the Guidelines and Protocols Advisory Committee. B12 deficiency: investigation and management of vitamin B12 and folate deficiency. Clinical Practice Guidelines and Protocols in British Columbia. December 15, 2006. Available at: www.bcguidelines.ca/gpac/guideline_b12.html. Accessed May 27, 2010.
4. Centers for Disease Control and Prevention Managing patients with evidence of vitamin B12 deficiency. June 29, 2009. Available at: http://cdc.gov/ncbddd/b12/patients.html. Accessed May 27, 2010.
1. Bolaman Z, Kadikoylu G, Yukselen, et al. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center prospective, randomized, open-label study. Clin Ther. 2003;25:3124-3134.
2. Kuzminski AM, Del Giacco EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191-1198.
3. British Columbia Ministry of Health and the Guidelines and Protocols Advisory Committee. B12 deficiency: investigation and management of vitamin B12 and folate deficiency. Clinical Practice Guidelines and Protocols in British Columbia. December 15, 2006. Available at: www.bcguidelines.ca/gpac/guideline_b12.html. Accessed May 27, 2010.
4. Centers for Disease Control and Prevention Managing patients with evidence of vitamin B12 deficiency. June 29, 2009. Available at: http://cdc.gov/ncbddd/b12/patients.html. Accessed May 27, 2010.
Evidence-based answers from the Family Physicians Inquiries Network
Which drugs work best for early Parkinson’s disease?
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
What’s best for croup?
A SINGLE DOSE OF CORTICOSTEROIDS is the first-line treatment for croup, resulting in fewer return visits and hospital admissions, shorter lengths of stay in the emergency department (ED) or hospital, and less need for supplemental medication (strength of recommendation [SOR]: A, meta-analysis and randomized controlled trials [RCTs]). A 0.15 mg/kg dose of oral dexamethasone is as effective as larger doses (SOR: B, small RCTs).
Nebulized racemic or L-epinephrine reduces severity of symptoms in moderate-to-severe croup (SOR: C, limited-quality disease-oriented evidence).
The role of heliox in moderate to severe croup remains uncertain. Studies to date have been inadequate (SOR: C, limited-quality disease-oriented evidence).
Humidified air provides no demonstrable benefit in the acute setting (SOR: A, meta-analysis).
Evidence summary
Standard management for croup has included glucocorticoids, nebulized racemic epinephrine, humidified air, and, for patients with severe respiratory distress and impending respiratory failure, helium-oxygen mixtures.
Glucocorticoids have significant benefits
A 2011 Cochrane review of glucocorticoids in children with croup identified 38 RCTs with 4299 patients.1 Effective treatments included dexamethasone (oral, subcutaneous, intramuscular, nebulized), budesonide (inhaled), and prednisolone (oral). Meta-analysis revealed a significant decrease in the rate of return visits and (re)admissions for patients treated with glucocorticoids compared with placebo (relative risk=0.5; 95% confidence interval [CI], 0.3-0.7). Glucocorticoid-treated children spent less time in the ED or hospital (weighted mean difference=-12 hours; 95% CI, -5 to -19) and were less likely to need epinephrine (risk difference=10%; 95% CI, 1%-20%).
The standardized improvement in the Westley score (TABLE) for all glucocorticoid treatments compared with placebo was -1.2 (95% CI, -1.6 to -0.8) at 6 hours and -1.9 (95% CI, -2.4 to -1.3) at 12 hours. No statistically significant difference was found at 24 hours (-1.3; 95% CI, -2.7 to 0.2). The combined studies favored glucocorticoids over placebo with a number needed to treat of 5. Meta-regression analysis didn’t demonstrate superiority for any single glucocorticoid.
A single 0.15 mg/kg dose of oral dexamethasone proved as effective as higher doses of 0.3 to 0.6 mg/kg in 3 RCTs (N=100, 120, and 99).2-4
TABLE
Westley Croup Score5
| Score | ||||||
|---|---|---|---|---|---|---|
| Symptom | 0 | 1 | 2 | 3 | 4 | 5 |
| Level of consciousness | Normal, including sleep | ________ | __________ | __________ | __________ | Disoriented |
| Cyanosis | None | ________ | __________ | __________ | With agitation | At rest |
| Stridor | None | With agitation | At rest | __________ | __________ | __________ |
| Air entry | Normal | Decreased | Markedly decreased | __________ | __________ | __________ |
| Retractions | None | Mild | Moderate | Severe | __________ | __________ |
| Scoring: Mild croup=≤2; moderate croup=3-7; severe croup=≥8. | ||||||
Nebulized epinephrine improves moderate to severe croup
Three RCTs (N=54, 20, and 13) found that in moderate to severe croup, treatment with nebulized racemic epinephrine improved croup score within 10 to 30 minutes.5
A small RCT (31 children, 6 months to 6 years of age) demonstrated L-epinephrine [1:1000] to be as effective and well tolerated as racemic epinephrine in moderate to severe croup. Improvement in croup score and respiratory rate peaked at 30 minutes. The effect of epinephrine (racemic or L-form) didn’t last beyond 120 minutes.6
In a retrospective study of 50 children with croup who were given aerosolized racemic epinephrine and observed in the ED for 2 hours after treatment, 58% received steroids during observation and 34% were prescribed prednisolone at discharge. Only 1 child required a return visit within 48 hours.7
Effect of helium-oxygen mixtures isn’t clear
A 2010 Cochrane review identified 2 RCTs of heliox in acute croup. No significant differences in croup score changes were found when heliox was compared with 30% oxygen (n=15, mild to moderate croup) and 100% oxygen with prn nebulized racemic epinephrine (n=29, moderate to severe croup).8 Both studies were underpowered and had significant methodological limitations.
Humidified air shows no benefit
A Cochrane review of 3 RCTs comparing humidified air with room air in emergency settings (n=135) found no evidence of benefit in croup score, oxygen saturation, or pulse rate.9
Recommendations
The 2008 Alberta Medical Association guideline recommends that all children with croup be treated with 0.6 mg/kg oral dexamethasone.10 Children with mild croup can be discharged home without further observation. Children with moderate croup should be observed for at least 4 hours. Hospitalization should be considered for children who fail to show adequate improvement. The guideline advises giving both steroids and nebulized epinephrine to children with severe croup.
The Advanced Pediatric Life Support (APLS) course of the American Academy of Pediatrics and the American College of Emergency Physicians recommends treatment with corticosteroids.11 For severe croup, the APLS advocates racemic or L-epinephrine, followed by observation for 3 or 4 hours and hospital admission in the event of inadequate response or recurrence of severe distress.
1. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.-
2. Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double-blind placebo-controlled clinical trial. BMJ. 1996;313:140-142.
3. Geelhoed GC, Macdonald WBG. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362-368.
4. Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup. Emerg Med Australas. 2007;19:51-58.
5. Johnson D. Croup. BMJ Clin Evid [monograph online]. London: BMJ Publishing Group; Updated March 2009. Available at: http://clinicalevidence.com. Accessed July 12, 2010.
6. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics. 1992;89:302-306.
7. Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181-183.
8. Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2):CD006822.-
9. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;(3):CD002870.-
10. Alberta Medical Association. Guideline for the diagnosis and management of croup. 2008 update. Alberta Medical Association. Available at: www.topalbertadoctors.org. Accessed May 11, 2011.
11. American Academy of Pediatrics and American College of Emergency Physicians APLS: The pediatric emergency medicine Resource (American Academy of Pediatrics). 4th ed, rev. Boston, Mass: Jones and Bartlett Publishers; 2007:61–64.
A SINGLE DOSE OF CORTICOSTEROIDS is the first-line treatment for croup, resulting in fewer return visits and hospital admissions, shorter lengths of stay in the emergency department (ED) or hospital, and less need for supplemental medication (strength of recommendation [SOR]: A, meta-analysis and randomized controlled trials [RCTs]). A 0.15 mg/kg dose of oral dexamethasone is as effective as larger doses (SOR: B, small RCTs).
Nebulized racemic or L-epinephrine reduces severity of symptoms in moderate-to-severe croup (SOR: C, limited-quality disease-oriented evidence).
The role of heliox in moderate to severe croup remains uncertain. Studies to date have been inadequate (SOR: C, limited-quality disease-oriented evidence).
Humidified air provides no demonstrable benefit in the acute setting (SOR: A, meta-analysis).
Evidence summary
Standard management for croup has included glucocorticoids, nebulized racemic epinephrine, humidified air, and, for patients with severe respiratory distress and impending respiratory failure, helium-oxygen mixtures.
Glucocorticoids have significant benefits
A 2011 Cochrane review of glucocorticoids in children with croup identified 38 RCTs with 4299 patients.1 Effective treatments included dexamethasone (oral, subcutaneous, intramuscular, nebulized), budesonide (inhaled), and prednisolone (oral). Meta-analysis revealed a significant decrease in the rate of return visits and (re)admissions for patients treated with glucocorticoids compared with placebo (relative risk=0.5; 95% confidence interval [CI], 0.3-0.7). Glucocorticoid-treated children spent less time in the ED or hospital (weighted mean difference=-12 hours; 95% CI, -5 to -19) and were less likely to need epinephrine (risk difference=10%; 95% CI, 1%-20%).
The standardized improvement in the Westley score (TABLE) for all glucocorticoid treatments compared with placebo was -1.2 (95% CI, -1.6 to -0.8) at 6 hours and -1.9 (95% CI, -2.4 to -1.3) at 12 hours. No statistically significant difference was found at 24 hours (-1.3; 95% CI, -2.7 to 0.2). The combined studies favored glucocorticoids over placebo with a number needed to treat of 5. Meta-regression analysis didn’t demonstrate superiority for any single glucocorticoid.
A single 0.15 mg/kg dose of oral dexamethasone proved as effective as higher doses of 0.3 to 0.6 mg/kg in 3 RCTs (N=100, 120, and 99).2-4
TABLE
Westley Croup Score5
| Score | ||||||
|---|---|---|---|---|---|---|
| Symptom | 0 | 1 | 2 | 3 | 4 | 5 |
| Level of consciousness | Normal, including sleep | ________ | __________ | __________ | __________ | Disoriented |
| Cyanosis | None | ________ | __________ | __________ | With agitation | At rest |
| Stridor | None | With agitation | At rest | __________ | __________ | __________ |
| Air entry | Normal | Decreased | Markedly decreased | __________ | __________ | __________ |
| Retractions | None | Mild | Moderate | Severe | __________ | __________ |
| Scoring: Mild croup=≤2; moderate croup=3-7; severe croup=≥8. | ||||||
Nebulized epinephrine improves moderate to severe croup
Three RCTs (N=54, 20, and 13) found that in moderate to severe croup, treatment with nebulized racemic epinephrine improved croup score within 10 to 30 minutes.5
A small RCT (31 children, 6 months to 6 years of age) demonstrated L-epinephrine [1:1000] to be as effective and well tolerated as racemic epinephrine in moderate to severe croup. Improvement in croup score and respiratory rate peaked at 30 minutes. The effect of epinephrine (racemic or L-form) didn’t last beyond 120 minutes.6
In a retrospective study of 50 children with croup who were given aerosolized racemic epinephrine and observed in the ED for 2 hours after treatment, 58% received steroids during observation and 34% were prescribed prednisolone at discharge. Only 1 child required a return visit within 48 hours.7
Effect of helium-oxygen mixtures isn’t clear
A 2010 Cochrane review identified 2 RCTs of heliox in acute croup. No significant differences in croup score changes were found when heliox was compared with 30% oxygen (n=15, mild to moderate croup) and 100% oxygen with prn nebulized racemic epinephrine (n=29, moderate to severe croup).8 Both studies were underpowered and had significant methodological limitations.
Humidified air shows no benefit
A Cochrane review of 3 RCTs comparing humidified air with room air in emergency settings (n=135) found no evidence of benefit in croup score, oxygen saturation, or pulse rate.9
Recommendations
The 2008 Alberta Medical Association guideline recommends that all children with croup be treated with 0.6 mg/kg oral dexamethasone.10 Children with mild croup can be discharged home without further observation. Children with moderate croup should be observed for at least 4 hours. Hospitalization should be considered for children who fail to show adequate improvement. The guideline advises giving both steroids and nebulized epinephrine to children with severe croup.
The Advanced Pediatric Life Support (APLS) course of the American Academy of Pediatrics and the American College of Emergency Physicians recommends treatment with corticosteroids.11 For severe croup, the APLS advocates racemic or L-epinephrine, followed by observation for 3 or 4 hours and hospital admission in the event of inadequate response or recurrence of severe distress.
A SINGLE DOSE OF CORTICOSTEROIDS is the first-line treatment for croup, resulting in fewer return visits and hospital admissions, shorter lengths of stay in the emergency department (ED) or hospital, and less need for supplemental medication (strength of recommendation [SOR]: A, meta-analysis and randomized controlled trials [RCTs]). A 0.15 mg/kg dose of oral dexamethasone is as effective as larger doses (SOR: B, small RCTs).
Nebulized racemic or L-epinephrine reduces severity of symptoms in moderate-to-severe croup (SOR: C, limited-quality disease-oriented evidence).
The role of heliox in moderate to severe croup remains uncertain. Studies to date have been inadequate (SOR: C, limited-quality disease-oriented evidence).
Humidified air provides no demonstrable benefit in the acute setting (SOR: A, meta-analysis).
Evidence summary
Standard management for croup has included glucocorticoids, nebulized racemic epinephrine, humidified air, and, for patients with severe respiratory distress and impending respiratory failure, helium-oxygen mixtures.
Glucocorticoids have significant benefits
A 2011 Cochrane review of glucocorticoids in children with croup identified 38 RCTs with 4299 patients.1 Effective treatments included dexamethasone (oral, subcutaneous, intramuscular, nebulized), budesonide (inhaled), and prednisolone (oral). Meta-analysis revealed a significant decrease in the rate of return visits and (re)admissions for patients treated with glucocorticoids compared with placebo (relative risk=0.5; 95% confidence interval [CI], 0.3-0.7). Glucocorticoid-treated children spent less time in the ED or hospital (weighted mean difference=-12 hours; 95% CI, -5 to -19) and were less likely to need epinephrine (risk difference=10%; 95% CI, 1%-20%).
The standardized improvement in the Westley score (TABLE) for all glucocorticoid treatments compared with placebo was -1.2 (95% CI, -1.6 to -0.8) at 6 hours and -1.9 (95% CI, -2.4 to -1.3) at 12 hours. No statistically significant difference was found at 24 hours (-1.3; 95% CI, -2.7 to 0.2). The combined studies favored glucocorticoids over placebo with a number needed to treat of 5. Meta-regression analysis didn’t demonstrate superiority for any single glucocorticoid.
A single 0.15 mg/kg dose of oral dexamethasone proved as effective as higher doses of 0.3 to 0.6 mg/kg in 3 RCTs (N=100, 120, and 99).2-4
TABLE
Westley Croup Score5
| Score | ||||||
|---|---|---|---|---|---|---|
| Symptom | 0 | 1 | 2 | 3 | 4 | 5 |
| Level of consciousness | Normal, including sleep | ________ | __________ | __________ | __________ | Disoriented |
| Cyanosis | None | ________ | __________ | __________ | With agitation | At rest |
| Stridor | None | With agitation | At rest | __________ | __________ | __________ |
| Air entry | Normal | Decreased | Markedly decreased | __________ | __________ | __________ |
| Retractions | None | Mild | Moderate | Severe | __________ | __________ |
| Scoring: Mild croup=≤2; moderate croup=3-7; severe croup=≥8. | ||||||
Nebulized epinephrine improves moderate to severe croup
Three RCTs (N=54, 20, and 13) found that in moderate to severe croup, treatment with nebulized racemic epinephrine improved croup score within 10 to 30 minutes.5
A small RCT (31 children, 6 months to 6 years of age) demonstrated L-epinephrine [1:1000] to be as effective and well tolerated as racemic epinephrine in moderate to severe croup. Improvement in croup score and respiratory rate peaked at 30 minutes. The effect of epinephrine (racemic or L-form) didn’t last beyond 120 minutes.6
In a retrospective study of 50 children with croup who were given aerosolized racemic epinephrine and observed in the ED for 2 hours after treatment, 58% received steroids during observation and 34% were prescribed prednisolone at discharge. Only 1 child required a return visit within 48 hours.7
Effect of helium-oxygen mixtures isn’t clear
A 2010 Cochrane review identified 2 RCTs of heliox in acute croup. No significant differences in croup score changes were found when heliox was compared with 30% oxygen (n=15, mild to moderate croup) and 100% oxygen with prn nebulized racemic epinephrine (n=29, moderate to severe croup).8 Both studies were underpowered and had significant methodological limitations.
Humidified air shows no benefit
A Cochrane review of 3 RCTs comparing humidified air with room air in emergency settings (n=135) found no evidence of benefit in croup score, oxygen saturation, or pulse rate.9
Recommendations
The 2008 Alberta Medical Association guideline recommends that all children with croup be treated with 0.6 mg/kg oral dexamethasone.10 Children with mild croup can be discharged home without further observation. Children with moderate croup should be observed for at least 4 hours. Hospitalization should be considered for children who fail to show adequate improvement. The guideline advises giving both steroids and nebulized epinephrine to children with severe croup.
The Advanced Pediatric Life Support (APLS) course of the American Academy of Pediatrics and the American College of Emergency Physicians recommends treatment with corticosteroids.11 For severe croup, the APLS advocates racemic or L-epinephrine, followed by observation for 3 or 4 hours and hospital admission in the event of inadequate response or recurrence of severe distress.
1. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.-
2. Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double-blind placebo-controlled clinical trial. BMJ. 1996;313:140-142.
3. Geelhoed GC, Macdonald WBG. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362-368.
4. Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup. Emerg Med Australas. 2007;19:51-58.
5. Johnson D. Croup. BMJ Clin Evid [monograph online]. London: BMJ Publishing Group; Updated March 2009. Available at: http://clinicalevidence.com. Accessed July 12, 2010.
6. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics. 1992;89:302-306.
7. Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181-183.
8. Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2):CD006822.-
9. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;(3):CD002870.-
10. Alberta Medical Association. Guideline for the diagnosis and management of croup. 2008 update. Alberta Medical Association. Available at: www.topalbertadoctors.org. Accessed May 11, 2011.
11. American Academy of Pediatrics and American College of Emergency Physicians APLS: The pediatric emergency medicine Resource (American Academy of Pediatrics). 4th ed, rev. Boston, Mass: Jones and Bartlett Publishers; 2007:61–64.
1. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.-
2. Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double-blind placebo-controlled clinical trial. BMJ. 1996;313:140-142.
3. Geelhoed GC, Macdonald WBG. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362-368.
4. Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup. Emerg Med Australas. 2007;19:51-58.
5. Johnson D. Croup. BMJ Clin Evid [monograph online]. London: BMJ Publishing Group; Updated March 2009. Available at: http://clinicalevidence.com. Accessed July 12, 2010.
6. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics. 1992;89:302-306.
7. Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181-183.
8. Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2):CD006822.-
9. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;(3):CD002870.-
10. Alberta Medical Association. Guideline for the diagnosis and management of croup. 2008 update. Alberta Medical Association. Available at: www.topalbertadoctors.org. Accessed May 11, 2011.
11. American Academy of Pediatrics and American College of Emergency Physicians APLS: The pediatric emergency medicine Resource (American Academy of Pediatrics). 4th ed, rev. Boston, Mass: Jones and Bartlett Publishers; 2007:61–64.
Evidence-based answers from the Family Physicians Inquiries Network
Does brief physician counseling promote weight loss?
IN SOME CASES, it may. While physician counseling alone isn’t more effective for weight loss than usual care (strength of recommendation [SOR]: A, larger randomized controlled trials [RCTs]), counseling (adults) as part of a multidisciplinary intervention may promote modest (2-3 kg) weight loss over 1 year (SOR: B, a single RCT).
Evidence summary
The TABLE summarizes the results of 6 RCTs that evaluated physician counseling for weight loss. The largest RCT, which included patients with elevated serum low-density lipoprotein levels (>75th percentile), randomized participants to 3 groups: physician counseling plus office support (dietary assessment tools, counseling algorithms, and in-office prompts), physician counseling alone, or usual care.1
Patients who received physician counseling with office support lost 2.3 kg (P<.001 vs usual care), whereas patients who received physician counseling alone lost 1.0 kg and patients who received usual care didn’t lose any weight.
TABLE
The effectiveness of weight loss counseling by physicians: What the RCTs reveal
| Number and characteristics of patients | Duration of intervention | Study design | Weight change |
|---|---|---|---|
| 1162 adults from internal medicine clinics (mean BMI=29 kg/m2)1 | 12 mo | 3 arms:
|
|
| 310 adult Hispanic patients with type 2 diabetes (mean BMI=35 kg/m2)2 | 12 mo | Physician counseling vs usual care | –0.1 kg vs +0.6 kg gain; P=.23 |
| 144 adult African American women (mean BMI=39 kg/m2)3 | 6 mo | Physician counseling vs usual care | –1.5 kg vs -0.6 kg at 9 mo; P=.01 0 kg net loss in both groups at 12- and 18-month follow-up |
| 96 Italian adults (mean BMI=25 kg/m2)4 | 5-6 mo | Physician counseling vs usual care | Men: BMI decrease from 30.3 to 29.5 kg/m2 vs increase from 31.9 to 32.4 kg/m2; P<.05 Women: BMI decrease from 30.6 to 30.2 kg/m2 vs increase from 30.7 to 31.0 kg/m2; P<.05 |
| 91 children (3-7 years of age) either overweight or with obese parents5 | 6 mo | 3 arms:
| No significant weight loss in any group |
| 30 Israeli adults with hypertension (mean BMI=34 kg/m2)6 | 6 mo | Resident physician counseling vs usual care | –0.9 kg vs +1.3 kg at 6 mo; P value not given No difference between groups at 12-mo follow-up |
| BMI, body mass index. | |||
Other large studies show mixed results
The second largest RCT randomized participants from community health centers in Colorado to receive either physician counseling (in which physicians reviewed nutritional and physical activity goals generated by a computer in response to a survey) or usual care (patient handouts alone).2 Although the physician-counseled group didn’t lose more total weight, more people in this group had lost 2.7 kg or more at the 12-month follow-up (32% vs 19% for usual care; P=.006).
The third largest RCT assigned low-income women from primary care clinics in Louisiana to either a 6-month tailored weight loss intervention or usual care.3 The intervention included monthly 15-minute visits with physician counseling about weight loss, fat intake, physical activity, barriers to weight loss, and weight loss maintenance. Women who received counseling lost 1.5 kg at the 9-month follow-up compared with a loss of 0.6 kg for women who received usual care. Both groups showed no net loss at the 12- and 18-month follow-up.
Counseling with follow-up leads to drop in BMI
Physician counseling in an Italian RCT included a 1-minute, patient-centered assessment of readiness for change, 2 to 5 minutes of exercise counseling by a physician for patients in active and maintenance stages, and phone or mail follow-up at 2 to 3 weeks.4 The reported decreases in body mass index (BMI) in the counseling group would translate to 2.4 and 1.0 kg of weight loss for men and women of average height, respectively.
No significant weight loss in a pediatric study
An RCT of children brought to a pediatric clinic for well-child visits recruited children who had either a BMI in the 85th to 95th percentile or obese parents (BMI ≥30 kg/m2).5 Parents were randomized to intensive counseling, minimal counseling, or usual care. The intensive intervention group participated in a 10- to 15-minute motivational interview with the pediatrician, followed by 2 45-minute sessions with a dietician at months 1 and 3 of the 6-month program; the minimal intervention group only participated in the motivational interview. No significant weight loss occurred in any of the 3 study groups.
The smallest RCT compared counseling by a family medicine resident with usual care in 30 adult patients.6 At 6 months, the counseling group had lost 0.9 kg compared with a gain of 1.3 kg in the usual care group, but follow-up at 12 months found no difference between the groups.
Recommendations
The US Preventive Services Task Force (USPSTF) says that intensive counseling (person-to-person meetings at least monthly, combined with diet, exercise, and behavioral interventions plus longer-term maintenance) can promote modest sustained weight loss and improve clinical outcomes.7 They recommend screening adults for obesity and offering intensive counseling and behavioral interventions for obese adults.
USPSTF notes, however, that evidence is insufficient to recommend for or against low- or moderate-intensity counseling and behavioral interventions in obese or overweight adults because the trials showed mixed results, typically had small sample sizes and high dropout rates, and reported average weight change rather than frequency of response.8
1. Ockene IS, Hebert JR, Ockene JK, et al. Effect of physician-delivered nutrition counseling training and an office-support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH). Arch Intern Med. 1999;159:725-731.
2. Christian JG, Bessen DH, Byers TE, et al. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168:141-146.
3. Martin PD, Dutton GR, Rhode PC, et al. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity. 2008;16:2462-2467.
4. Bolognesi M, Nigg CR, Massarini M, et al. Reducing obesity indicators through brief physical activity counseling (PACE) in Italian primary care settings. Ann Behav Med. 2006;31:179-185.
5. Schwartz RP, Hamre R, Dietz WH, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495-501.
6. Cohen MD, D’Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Fam Med. 1991;23:25-28.
7. Mctigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139:933-949.
8. United States Preventive Services Task Force. Screening for obesity in adults. December 2003. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed August 27, 2010.
IN SOME CASES, it may. While physician counseling alone isn’t more effective for weight loss than usual care (strength of recommendation [SOR]: A, larger randomized controlled trials [RCTs]), counseling (adults) as part of a multidisciplinary intervention may promote modest (2-3 kg) weight loss over 1 year (SOR: B, a single RCT).
Evidence summary
The TABLE summarizes the results of 6 RCTs that evaluated physician counseling for weight loss. The largest RCT, which included patients with elevated serum low-density lipoprotein levels (>75th percentile), randomized participants to 3 groups: physician counseling plus office support (dietary assessment tools, counseling algorithms, and in-office prompts), physician counseling alone, or usual care.1
Patients who received physician counseling with office support lost 2.3 kg (P<.001 vs usual care), whereas patients who received physician counseling alone lost 1.0 kg and patients who received usual care didn’t lose any weight.
TABLE
The effectiveness of weight loss counseling by physicians: What the RCTs reveal
| Number and characteristics of patients | Duration of intervention | Study design | Weight change |
|---|---|---|---|
| 1162 adults from internal medicine clinics (mean BMI=29 kg/m2)1 | 12 mo | 3 arms:
|
|
| 310 adult Hispanic patients with type 2 diabetes (mean BMI=35 kg/m2)2 | 12 mo | Physician counseling vs usual care | –0.1 kg vs +0.6 kg gain; P=.23 |
| 144 adult African American women (mean BMI=39 kg/m2)3 | 6 mo | Physician counseling vs usual care | –1.5 kg vs -0.6 kg at 9 mo; P=.01 0 kg net loss in both groups at 12- and 18-month follow-up |
| 96 Italian adults (mean BMI=25 kg/m2)4 | 5-6 mo | Physician counseling vs usual care | Men: BMI decrease from 30.3 to 29.5 kg/m2 vs increase from 31.9 to 32.4 kg/m2; P<.05 Women: BMI decrease from 30.6 to 30.2 kg/m2 vs increase from 30.7 to 31.0 kg/m2; P<.05 |
| 91 children (3-7 years of age) either overweight or with obese parents5 | 6 mo | 3 arms:
| No significant weight loss in any group |
| 30 Israeli adults with hypertension (mean BMI=34 kg/m2)6 | 6 mo | Resident physician counseling vs usual care | –0.9 kg vs +1.3 kg at 6 mo; P value not given No difference between groups at 12-mo follow-up |
| BMI, body mass index. | |||
Other large studies show mixed results
The second largest RCT randomized participants from community health centers in Colorado to receive either physician counseling (in which physicians reviewed nutritional and physical activity goals generated by a computer in response to a survey) or usual care (patient handouts alone).2 Although the physician-counseled group didn’t lose more total weight, more people in this group had lost 2.7 kg or more at the 12-month follow-up (32% vs 19% for usual care; P=.006).
The third largest RCT assigned low-income women from primary care clinics in Louisiana to either a 6-month tailored weight loss intervention or usual care.3 The intervention included monthly 15-minute visits with physician counseling about weight loss, fat intake, physical activity, barriers to weight loss, and weight loss maintenance. Women who received counseling lost 1.5 kg at the 9-month follow-up compared with a loss of 0.6 kg for women who received usual care. Both groups showed no net loss at the 12- and 18-month follow-up.
Counseling with follow-up leads to drop in BMI
Physician counseling in an Italian RCT included a 1-minute, patient-centered assessment of readiness for change, 2 to 5 minutes of exercise counseling by a physician for patients in active and maintenance stages, and phone or mail follow-up at 2 to 3 weeks.4 The reported decreases in body mass index (BMI) in the counseling group would translate to 2.4 and 1.0 kg of weight loss for men and women of average height, respectively.
No significant weight loss in a pediatric study
An RCT of children brought to a pediatric clinic for well-child visits recruited children who had either a BMI in the 85th to 95th percentile or obese parents (BMI ≥30 kg/m2).5 Parents were randomized to intensive counseling, minimal counseling, or usual care. The intensive intervention group participated in a 10- to 15-minute motivational interview with the pediatrician, followed by 2 45-minute sessions with a dietician at months 1 and 3 of the 6-month program; the minimal intervention group only participated in the motivational interview. No significant weight loss occurred in any of the 3 study groups.
The smallest RCT compared counseling by a family medicine resident with usual care in 30 adult patients.6 At 6 months, the counseling group had lost 0.9 kg compared with a gain of 1.3 kg in the usual care group, but follow-up at 12 months found no difference between the groups.
Recommendations
The US Preventive Services Task Force (USPSTF) says that intensive counseling (person-to-person meetings at least monthly, combined with diet, exercise, and behavioral interventions plus longer-term maintenance) can promote modest sustained weight loss and improve clinical outcomes.7 They recommend screening adults for obesity and offering intensive counseling and behavioral interventions for obese adults.
USPSTF notes, however, that evidence is insufficient to recommend for or against low- or moderate-intensity counseling and behavioral interventions in obese or overweight adults because the trials showed mixed results, typically had small sample sizes and high dropout rates, and reported average weight change rather than frequency of response.8
IN SOME CASES, it may. While physician counseling alone isn’t more effective for weight loss than usual care (strength of recommendation [SOR]: A, larger randomized controlled trials [RCTs]), counseling (adults) as part of a multidisciplinary intervention may promote modest (2-3 kg) weight loss over 1 year (SOR: B, a single RCT).
Evidence summary
The TABLE summarizes the results of 6 RCTs that evaluated physician counseling for weight loss. The largest RCT, which included patients with elevated serum low-density lipoprotein levels (>75th percentile), randomized participants to 3 groups: physician counseling plus office support (dietary assessment tools, counseling algorithms, and in-office prompts), physician counseling alone, or usual care.1
Patients who received physician counseling with office support lost 2.3 kg (P<.001 vs usual care), whereas patients who received physician counseling alone lost 1.0 kg and patients who received usual care didn’t lose any weight.
TABLE
The effectiveness of weight loss counseling by physicians: What the RCTs reveal
| Number and characteristics of patients | Duration of intervention | Study design | Weight change |
|---|---|---|---|
| 1162 adults from internal medicine clinics (mean BMI=29 kg/m2)1 | 12 mo | 3 arms:
|
|
| 310 adult Hispanic patients with type 2 diabetes (mean BMI=35 kg/m2)2 | 12 mo | Physician counseling vs usual care | –0.1 kg vs +0.6 kg gain; P=.23 |
| 144 adult African American women (mean BMI=39 kg/m2)3 | 6 mo | Physician counseling vs usual care | –1.5 kg vs -0.6 kg at 9 mo; P=.01 0 kg net loss in both groups at 12- and 18-month follow-up |
| 96 Italian adults (mean BMI=25 kg/m2)4 | 5-6 mo | Physician counseling vs usual care | Men: BMI decrease from 30.3 to 29.5 kg/m2 vs increase from 31.9 to 32.4 kg/m2; P<.05 Women: BMI decrease from 30.6 to 30.2 kg/m2 vs increase from 30.7 to 31.0 kg/m2; P<.05 |
| 91 children (3-7 years of age) either overweight or with obese parents5 | 6 mo | 3 arms:
| No significant weight loss in any group |
| 30 Israeli adults with hypertension (mean BMI=34 kg/m2)6 | 6 mo | Resident physician counseling vs usual care | –0.9 kg vs +1.3 kg at 6 mo; P value not given No difference between groups at 12-mo follow-up |
| BMI, body mass index. | |||
Other large studies show mixed results
The second largest RCT randomized participants from community health centers in Colorado to receive either physician counseling (in which physicians reviewed nutritional and physical activity goals generated by a computer in response to a survey) or usual care (patient handouts alone).2 Although the physician-counseled group didn’t lose more total weight, more people in this group had lost 2.7 kg or more at the 12-month follow-up (32% vs 19% for usual care; P=.006).
The third largest RCT assigned low-income women from primary care clinics in Louisiana to either a 6-month tailored weight loss intervention or usual care.3 The intervention included monthly 15-minute visits with physician counseling about weight loss, fat intake, physical activity, barriers to weight loss, and weight loss maintenance. Women who received counseling lost 1.5 kg at the 9-month follow-up compared with a loss of 0.6 kg for women who received usual care. Both groups showed no net loss at the 12- and 18-month follow-up.
Counseling with follow-up leads to drop in BMI
Physician counseling in an Italian RCT included a 1-minute, patient-centered assessment of readiness for change, 2 to 5 minutes of exercise counseling by a physician for patients in active and maintenance stages, and phone or mail follow-up at 2 to 3 weeks.4 The reported decreases in body mass index (BMI) in the counseling group would translate to 2.4 and 1.0 kg of weight loss for men and women of average height, respectively.
No significant weight loss in a pediatric study
An RCT of children brought to a pediatric clinic for well-child visits recruited children who had either a BMI in the 85th to 95th percentile or obese parents (BMI ≥30 kg/m2).5 Parents were randomized to intensive counseling, minimal counseling, or usual care. The intensive intervention group participated in a 10- to 15-minute motivational interview with the pediatrician, followed by 2 45-minute sessions with a dietician at months 1 and 3 of the 6-month program; the minimal intervention group only participated in the motivational interview. No significant weight loss occurred in any of the 3 study groups.
The smallest RCT compared counseling by a family medicine resident with usual care in 30 adult patients.6 At 6 months, the counseling group had lost 0.9 kg compared with a gain of 1.3 kg in the usual care group, but follow-up at 12 months found no difference between the groups.
Recommendations
The US Preventive Services Task Force (USPSTF) says that intensive counseling (person-to-person meetings at least monthly, combined with diet, exercise, and behavioral interventions plus longer-term maintenance) can promote modest sustained weight loss and improve clinical outcomes.7 They recommend screening adults for obesity and offering intensive counseling and behavioral interventions for obese adults.
USPSTF notes, however, that evidence is insufficient to recommend for or against low- or moderate-intensity counseling and behavioral interventions in obese or overweight adults because the trials showed mixed results, typically had small sample sizes and high dropout rates, and reported average weight change rather than frequency of response.8
1. Ockene IS, Hebert JR, Ockene JK, et al. Effect of physician-delivered nutrition counseling training and an office-support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH). Arch Intern Med. 1999;159:725-731.
2. Christian JG, Bessen DH, Byers TE, et al. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168:141-146.
3. Martin PD, Dutton GR, Rhode PC, et al. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity. 2008;16:2462-2467.
4. Bolognesi M, Nigg CR, Massarini M, et al. Reducing obesity indicators through brief physical activity counseling (PACE) in Italian primary care settings. Ann Behav Med. 2006;31:179-185.
5. Schwartz RP, Hamre R, Dietz WH, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495-501.
6. Cohen MD, D’Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Fam Med. 1991;23:25-28.
7. Mctigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139:933-949.
8. United States Preventive Services Task Force. Screening for obesity in adults. December 2003. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed August 27, 2010.
1. Ockene IS, Hebert JR, Ockene JK, et al. Effect of physician-delivered nutrition counseling training and an office-support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH). Arch Intern Med. 1999;159:725-731.
2. Christian JG, Bessen DH, Byers TE, et al. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168:141-146.
3. Martin PD, Dutton GR, Rhode PC, et al. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity. 2008;16:2462-2467.
4. Bolognesi M, Nigg CR, Massarini M, et al. Reducing obesity indicators through brief physical activity counseling (PACE) in Italian primary care settings. Ann Behav Med. 2006;31:179-185.
5. Schwartz RP, Hamre R, Dietz WH, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495-501.
6. Cohen MD, D’Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Fam Med. 1991;23:25-28.
7. Mctigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139:933-949.
8. United States Preventive Services Task Force. Screening for obesity in adults. December 2003. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed August 27, 2010.
Evidence-based answers from the Family Physicians Inquiries Network
What is the long-term educational outlook for youngsters with ADHD?
CHILDREN AND ADOLESCENTS with attention deficit hyperactivity disorder (ADHD) complete fewer years of school, graduate from high school at a lower rate, and are less likely to enroll in graduate school. Older adolescents and young adults with ADHD tend to underperform in both educational and occupational settings (strength of recommendation: A, 2 prospective cohort studies and a case control study).
These findings are based solely on patients with ADHD who were referred to psychiatric clinics and therefore may reflect a more severe spectrum of ADHD effects.
Evidence summary
A prospective cohort study compared educational and employment outcomes among 91 middle-class white boys, 6 to 12 years of age, with ADHD who were referred to a psychiatric clinic with outcomes for 96 matched controls. Investigators used multiple educational achievement tests to evaluate participants when they enrolled in the study, then administered educational and occupational questionnaires 16 years later.
Boys with ADHD completed 2.5 fewer years of school than controls (P=.001). Although rates of employment for the 2 groups were the same at 90%, those with ADHD had a significantly poorer occupational ranking than controls using the Hollingshead and Redlich system, which rates occupations on 7-point scale, with 1 representing top-ranked occupations. Individuals with ADHD scored 4.4 points compared with 3.5 points for the control group (P<.001). However, by the end of the study, more individuals with ADHD owned and operated their own businesses compared with controls (18% vs 5%; P<.01).1
Fewer degrees but comparable employment rates
A similar prospective cohort study evaluated educational and occupational outcomes among 104 boys with ADHD and 106 controls. Investigators recruited boys 5 to 11 years of age from a psychiatric research clinic and followed them for a mean of 17 years using educational and occupational questionnaires.
Boys with ADHD completed 2 fewer years of school than controls (P=.0001), and more boys in the ADHD group failed to complete high school (25% vs 1%; P value not supplied). Fewer individuals with ADHD than controls obtained a bachelor’s degree (15% vs 50%; P<.001), and fewer enrolled in graduate school (3% vs 16%; P value not given). Employment was comparable in the 2 groups, however (92% vs 93%, P=.07).2
Less success in school and at work
Another prospective case-control study also found that people with ADHD achieved less educational and occupational success than controls. The study compared 224 subjects between 18 and 55 years of age with ADHD from a psychiatric referral clinic with 146 controls matched for age and intelligence quotient (IQ). Investigators correlated predicted educational achievement based on IQ in the controls with that observed in subjects with ADHD.
Five years later, subjects with ADHD didn’t perform as well as predicted. Fewer earned college degrees (29% vs 52%) or graduate degrees (20% vs 33%), and more earned no college or graduate school degrees (50% vs 16%) (P<.001 for comparison of observed compared with expected means using Wilcoxon matched pairs test). Similarly, fewer subjects with ADHD attained a level of 6 on the Hollingshead Socioeconomic Status Scale than controls (58% vs 80%; P<.001).3
Recommendations
We found no statements from national organizations about the long-term educational prognosis for children and adolescents with ADHD. However, the authors of the Multimodal Treatment Study of Children with ADHD have expressed the opinion that prognosis depends on initial presentation (including severity of symptoms and comorbid conduct disorders), intellect, social advantage, and response to treatment.4
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys: educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50:565-576.
2. Mannuzza S, Klein RG, Bessler A, et al. Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry. 1997;36:1222-1227.
3. Biederman J, Petty CR, Fried R, et al. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: a controlled study. J Clin Psychiatry. 2008;69:1217-1222.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500.
CHILDREN AND ADOLESCENTS with attention deficit hyperactivity disorder (ADHD) complete fewer years of school, graduate from high school at a lower rate, and are less likely to enroll in graduate school. Older adolescents and young adults with ADHD tend to underperform in both educational and occupational settings (strength of recommendation: A, 2 prospective cohort studies and a case control study).
These findings are based solely on patients with ADHD who were referred to psychiatric clinics and therefore may reflect a more severe spectrum of ADHD effects.
Evidence summary
A prospective cohort study compared educational and employment outcomes among 91 middle-class white boys, 6 to 12 years of age, with ADHD who were referred to a psychiatric clinic with outcomes for 96 matched controls. Investigators used multiple educational achievement tests to evaluate participants when they enrolled in the study, then administered educational and occupational questionnaires 16 years later.
Boys with ADHD completed 2.5 fewer years of school than controls (P=.001). Although rates of employment for the 2 groups were the same at 90%, those with ADHD had a significantly poorer occupational ranking than controls using the Hollingshead and Redlich system, which rates occupations on 7-point scale, with 1 representing top-ranked occupations. Individuals with ADHD scored 4.4 points compared with 3.5 points for the control group (P<.001). However, by the end of the study, more individuals with ADHD owned and operated their own businesses compared with controls (18% vs 5%; P<.01).1
Fewer degrees but comparable employment rates
A similar prospective cohort study evaluated educational and occupational outcomes among 104 boys with ADHD and 106 controls. Investigators recruited boys 5 to 11 years of age from a psychiatric research clinic and followed them for a mean of 17 years using educational and occupational questionnaires.
Boys with ADHD completed 2 fewer years of school than controls (P=.0001), and more boys in the ADHD group failed to complete high school (25% vs 1%; P value not supplied). Fewer individuals with ADHD than controls obtained a bachelor’s degree (15% vs 50%; P<.001), and fewer enrolled in graduate school (3% vs 16%; P value not given). Employment was comparable in the 2 groups, however (92% vs 93%, P=.07).2
Less success in school and at work
Another prospective case-control study also found that people with ADHD achieved less educational and occupational success than controls. The study compared 224 subjects between 18 and 55 years of age with ADHD from a psychiatric referral clinic with 146 controls matched for age and intelligence quotient (IQ). Investigators correlated predicted educational achievement based on IQ in the controls with that observed in subjects with ADHD.
Five years later, subjects with ADHD didn’t perform as well as predicted. Fewer earned college degrees (29% vs 52%) or graduate degrees (20% vs 33%), and more earned no college or graduate school degrees (50% vs 16%) (P<.001 for comparison of observed compared with expected means using Wilcoxon matched pairs test). Similarly, fewer subjects with ADHD attained a level of 6 on the Hollingshead Socioeconomic Status Scale than controls (58% vs 80%; P<.001).3
Recommendations
We found no statements from national organizations about the long-term educational prognosis for children and adolescents with ADHD. However, the authors of the Multimodal Treatment Study of Children with ADHD have expressed the opinion that prognosis depends on initial presentation (including severity of symptoms and comorbid conduct disorders), intellect, social advantage, and response to treatment.4
CHILDREN AND ADOLESCENTS with attention deficit hyperactivity disorder (ADHD) complete fewer years of school, graduate from high school at a lower rate, and are less likely to enroll in graduate school. Older adolescents and young adults with ADHD tend to underperform in both educational and occupational settings (strength of recommendation: A, 2 prospective cohort studies and a case control study).
These findings are based solely on patients with ADHD who were referred to psychiatric clinics and therefore may reflect a more severe spectrum of ADHD effects.
Evidence summary
A prospective cohort study compared educational and employment outcomes among 91 middle-class white boys, 6 to 12 years of age, with ADHD who were referred to a psychiatric clinic with outcomes for 96 matched controls. Investigators used multiple educational achievement tests to evaluate participants when they enrolled in the study, then administered educational and occupational questionnaires 16 years later.
Boys with ADHD completed 2.5 fewer years of school than controls (P=.001). Although rates of employment for the 2 groups were the same at 90%, those with ADHD had a significantly poorer occupational ranking than controls using the Hollingshead and Redlich system, which rates occupations on 7-point scale, with 1 representing top-ranked occupations. Individuals with ADHD scored 4.4 points compared with 3.5 points for the control group (P<.001). However, by the end of the study, more individuals with ADHD owned and operated their own businesses compared with controls (18% vs 5%; P<.01).1
Fewer degrees but comparable employment rates
A similar prospective cohort study evaluated educational and occupational outcomes among 104 boys with ADHD and 106 controls. Investigators recruited boys 5 to 11 years of age from a psychiatric research clinic and followed them for a mean of 17 years using educational and occupational questionnaires.
Boys with ADHD completed 2 fewer years of school than controls (P=.0001), and more boys in the ADHD group failed to complete high school (25% vs 1%; P value not supplied). Fewer individuals with ADHD than controls obtained a bachelor’s degree (15% vs 50%; P<.001), and fewer enrolled in graduate school (3% vs 16%; P value not given). Employment was comparable in the 2 groups, however (92% vs 93%, P=.07).2
Less success in school and at work
Another prospective case-control study also found that people with ADHD achieved less educational and occupational success than controls. The study compared 224 subjects between 18 and 55 years of age with ADHD from a psychiatric referral clinic with 146 controls matched for age and intelligence quotient (IQ). Investigators correlated predicted educational achievement based on IQ in the controls with that observed in subjects with ADHD.
Five years later, subjects with ADHD didn’t perform as well as predicted. Fewer earned college degrees (29% vs 52%) or graduate degrees (20% vs 33%), and more earned no college or graduate school degrees (50% vs 16%) (P<.001 for comparison of observed compared with expected means using Wilcoxon matched pairs test). Similarly, fewer subjects with ADHD attained a level of 6 on the Hollingshead Socioeconomic Status Scale than controls (58% vs 80%; P<.001).3
Recommendations
We found no statements from national organizations about the long-term educational prognosis for children and adolescents with ADHD. However, the authors of the Multimodal Treatment Study of Children with ADHD have expressed the opinion that prognosis depends on initial presentation (including severity of symptoms and comorbid conduct disorders), intellect, social advantage, and response to treatment.4
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys: educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50:565-576.
2. Mannuzza S, Klein RG, Bessler A, et al. Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry. 1997;36:1222-1227.
3. Biederman J, Petty CR, Fried R, et al. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: a controlled study. J Clin Psychiatry. 2008;69:1217-1222.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys: educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50:565-576.
2. Mannuzza S, Klein RG, Bessler A, et al. Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry. 1997;36:1222-1227.
3. Biederman J, Petty CR, Fried R, et al. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: a controlled study. J Clin Psychiatry. 2008;69:1217-1222.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500.
Evidence-based answers from the Family Physicians Inquiries Network