User login
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
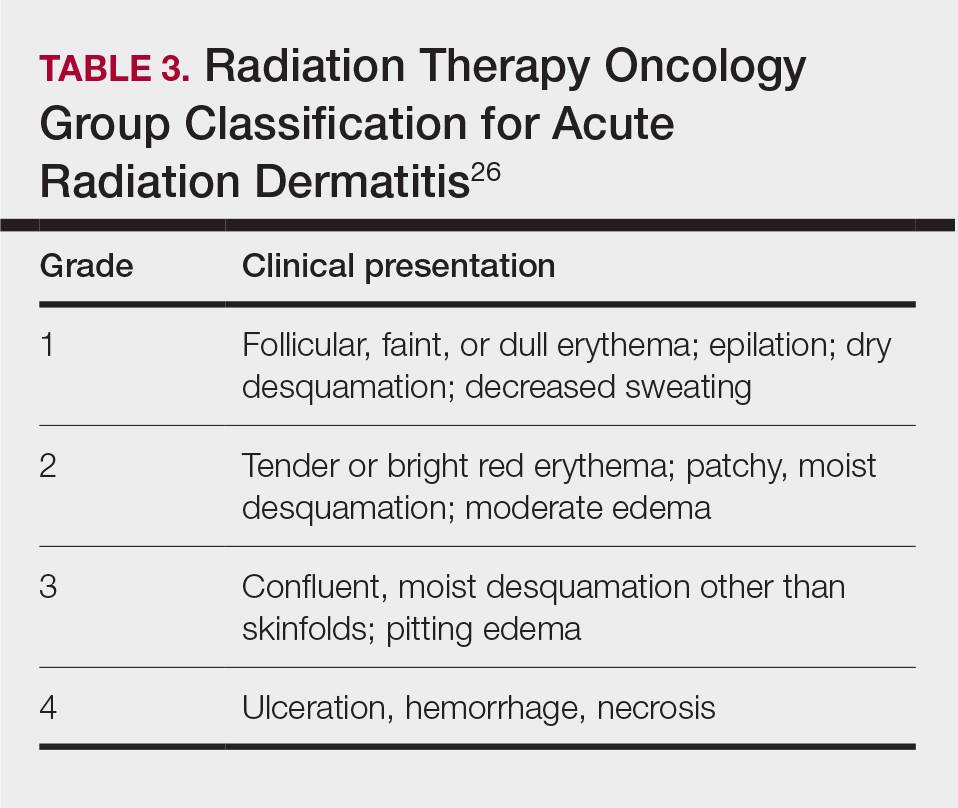
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).
Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27

Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
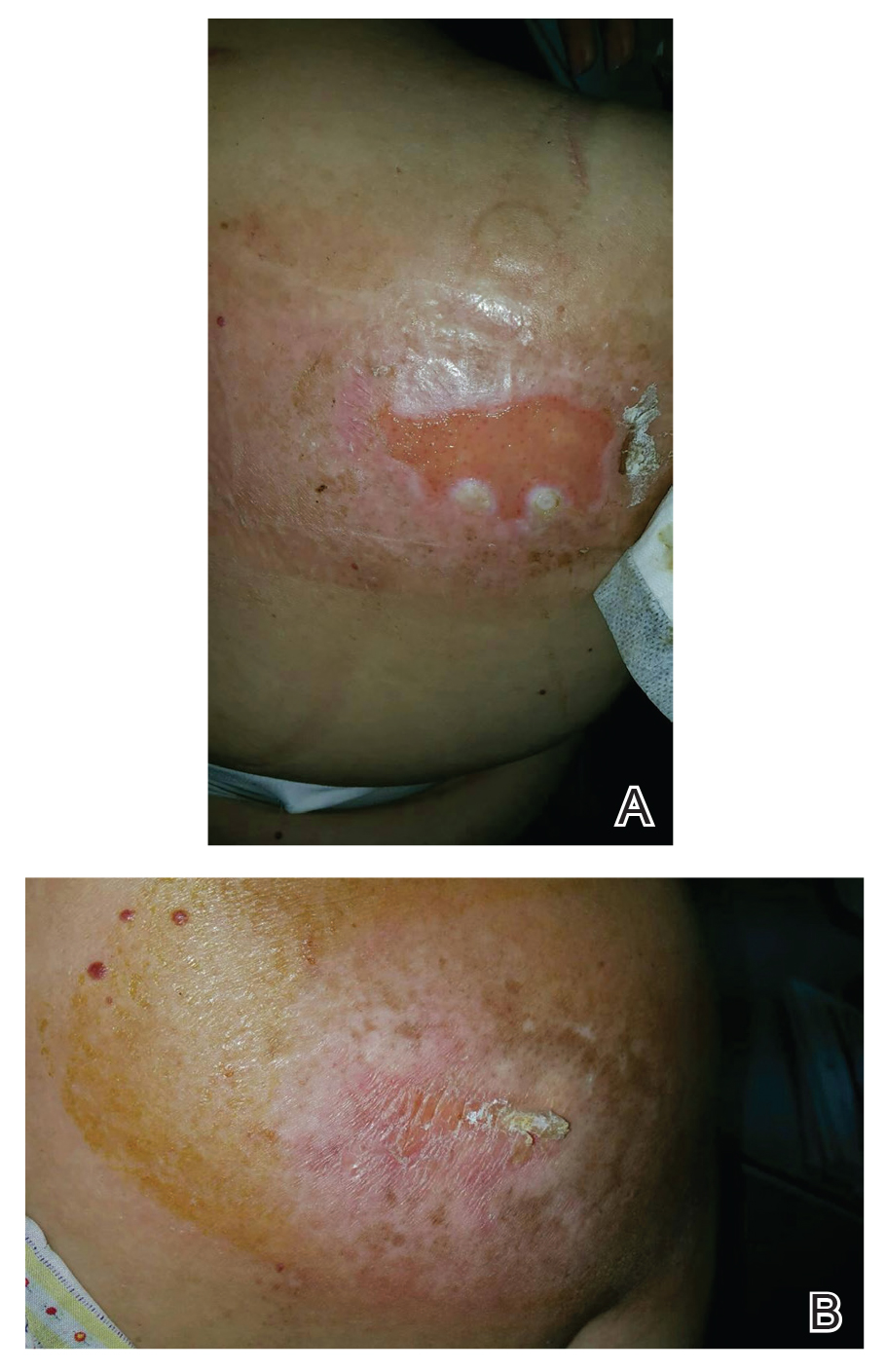
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45
Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
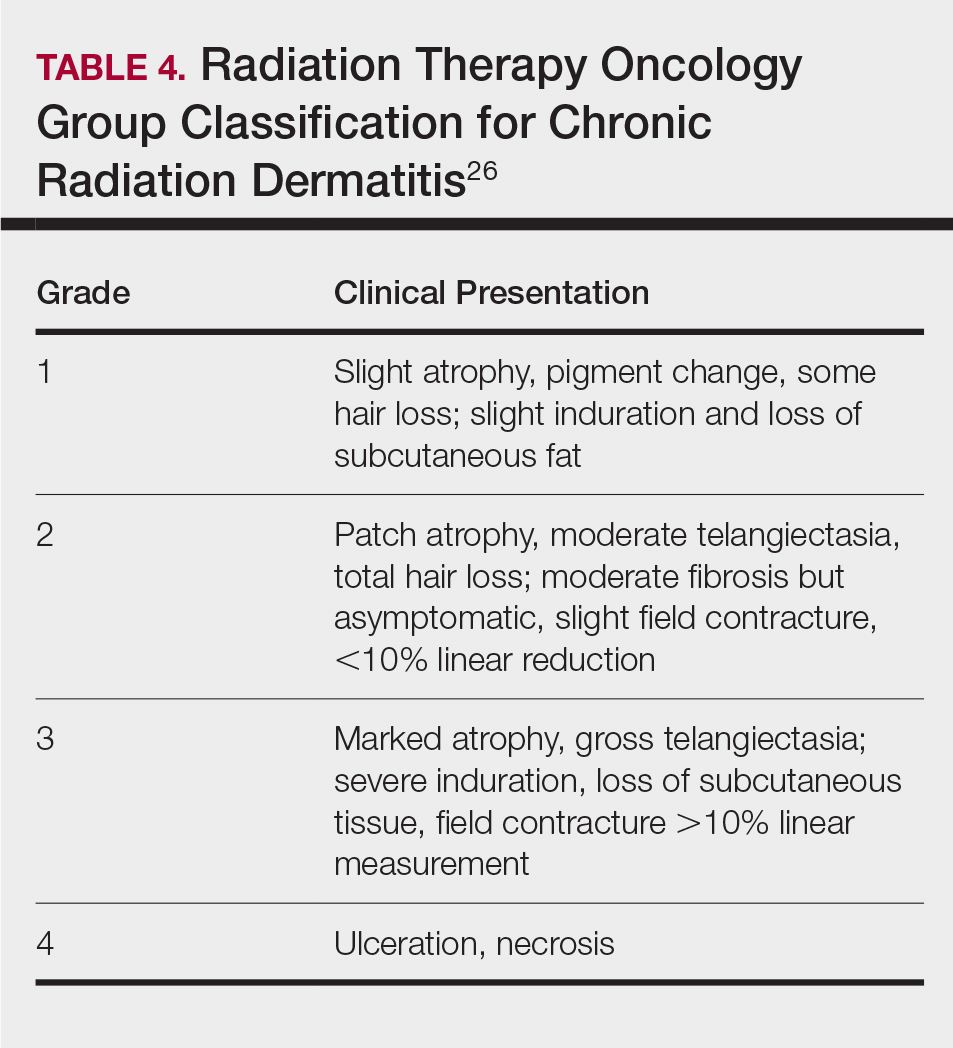
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
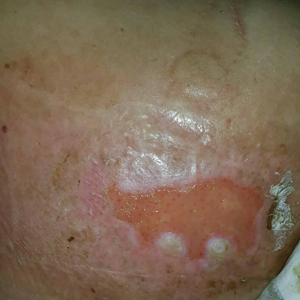
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
PRACTICE POINTS
- Fluoroscopy-induced chronic radiation dermatitis poses diagnostic challenges, as patients often are unable to associate a history of fluoroscopic procedures with the development of skin lesions.
- Scapular and subscapular lesions as well as those on the anterolateral chest and mid back should prompt clinicians to inquire about the patient’s history of fluoroscopic procedures.
- Because lesions can remain refractory to treatment, longterm monitoring is necessary if they are not excised.
Pericardial Effusion
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.