User login
Extensive Erosions and Ulcerations on the Trunk and Extremities in a Neonate
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
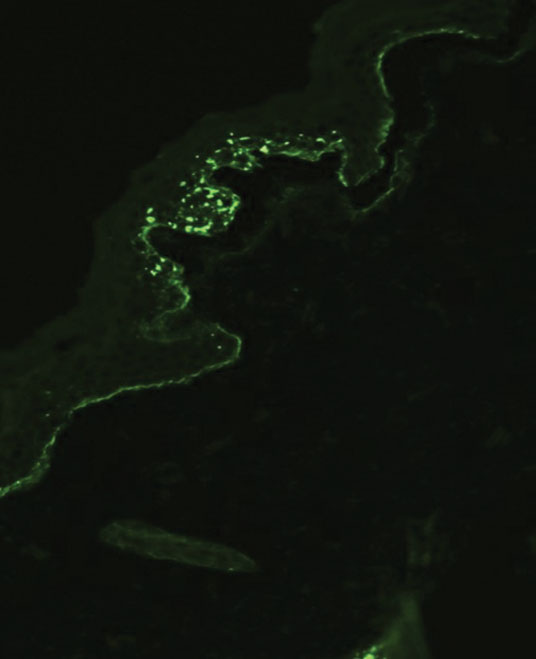
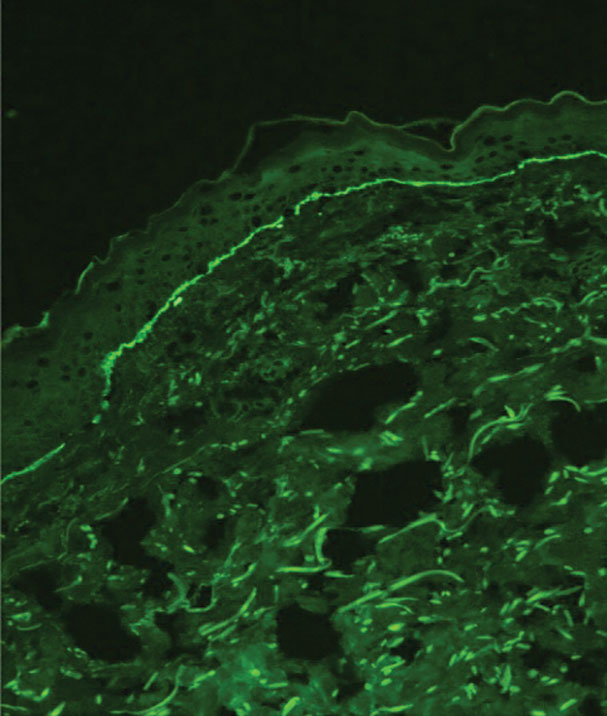
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5
Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).
Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12
When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
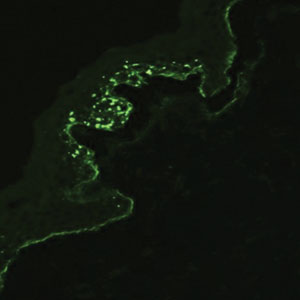
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5

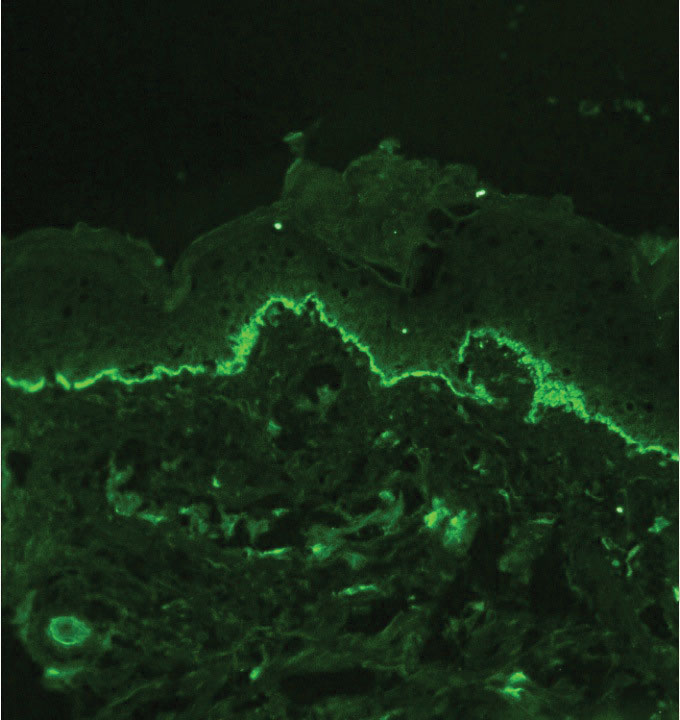
Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).

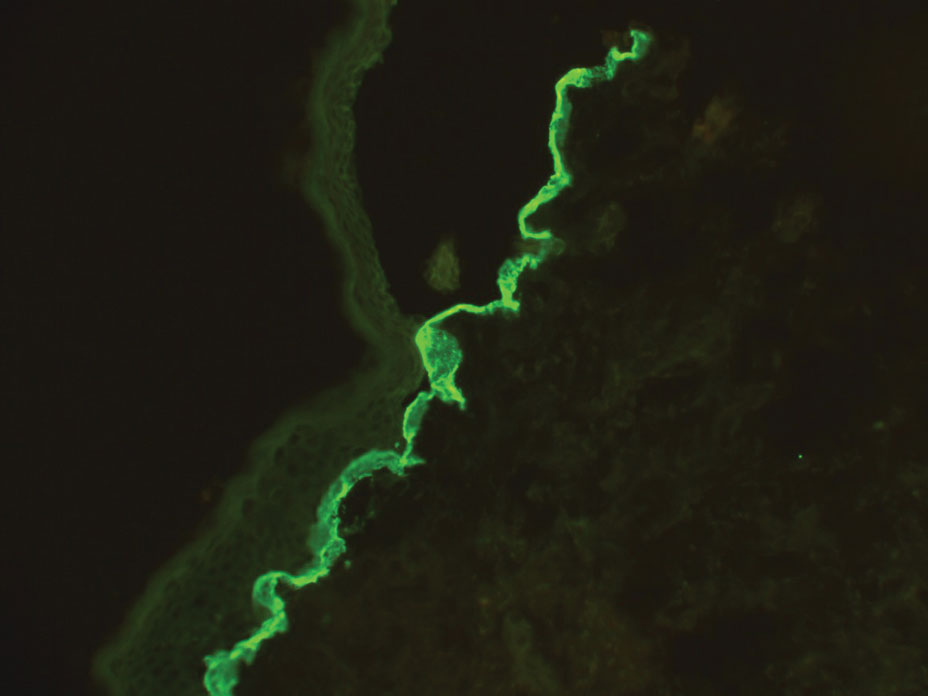
Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12

When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5

Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).

Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12

When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
A neonate was born with extensive erosions and ulcerations on the trunk and extremities. The eroded areas had a beefy red appearance. A biopsy taken from a small blister was stained for type VII collagen by indirect immunofluorescence.