User login
Pregnancy of unknown location: Evidence-based evaluation and management
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.
Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
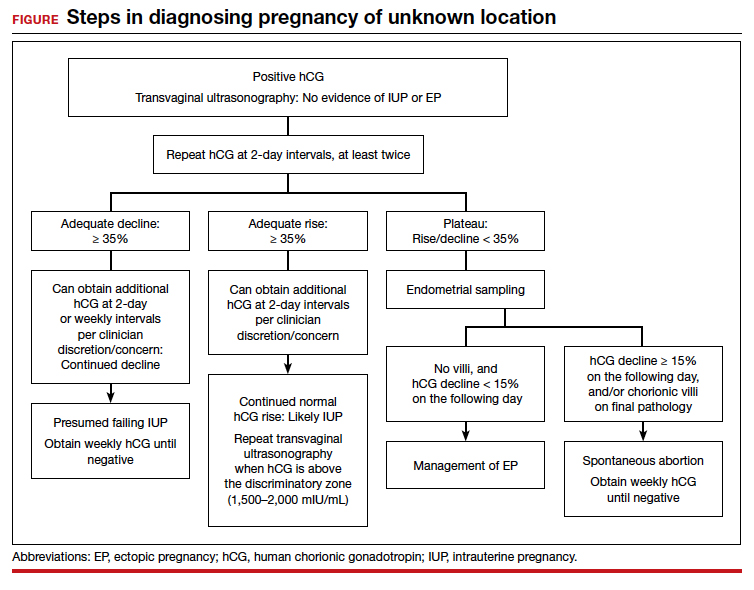
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.
Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.

Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.

Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.

Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.

Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
How ovarian reserve testing can (and cannot) address your patients’ fertility concerns
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.
A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
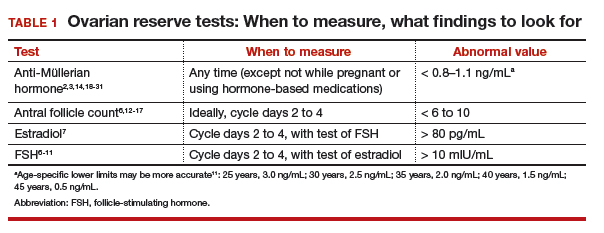
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7
Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.
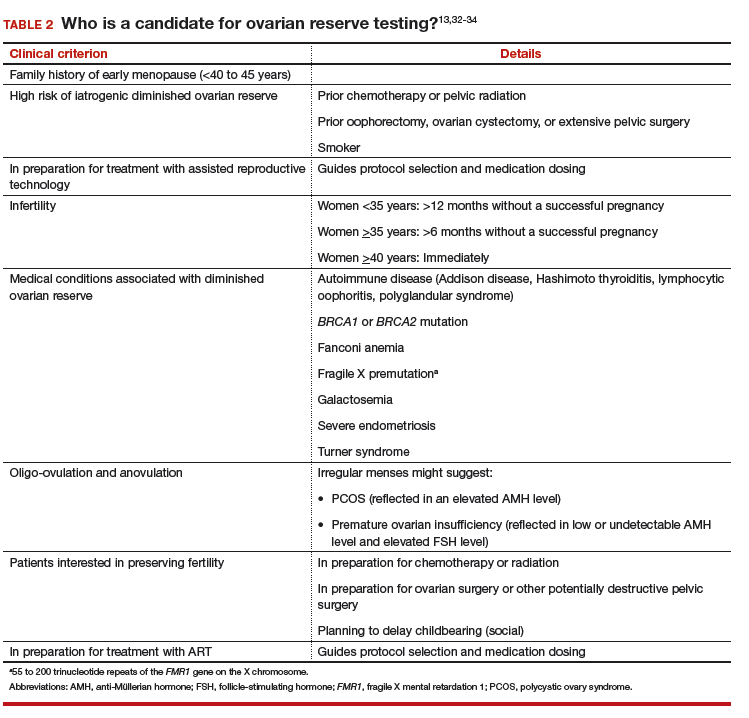
Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.

A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7

Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.

Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.

A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7

Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.

Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.