User login
Resection of infected sacrohysteropexy mesh
OR safety and efficiency: Measuring and monitoring all factors—including surgical volume
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1
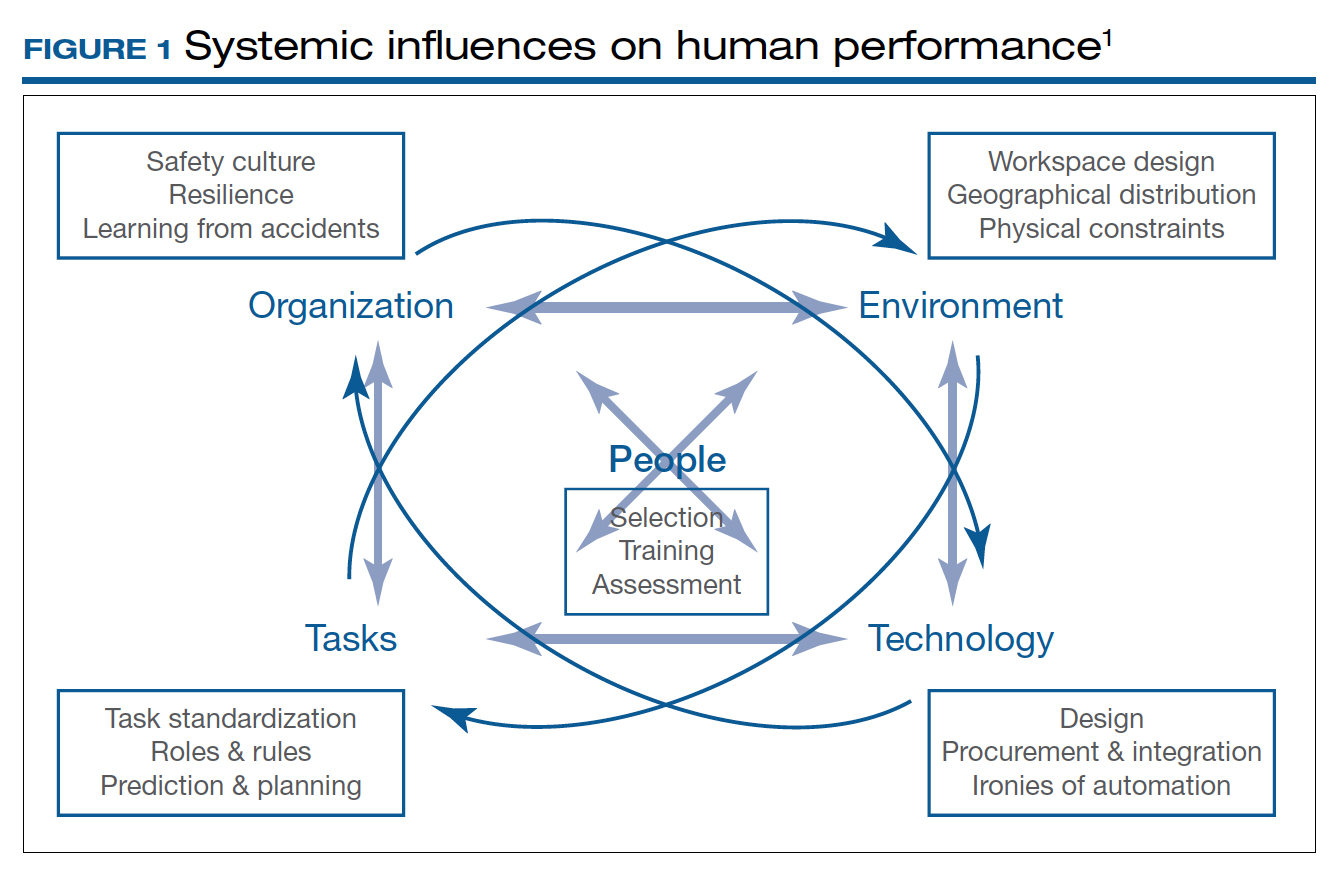
Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.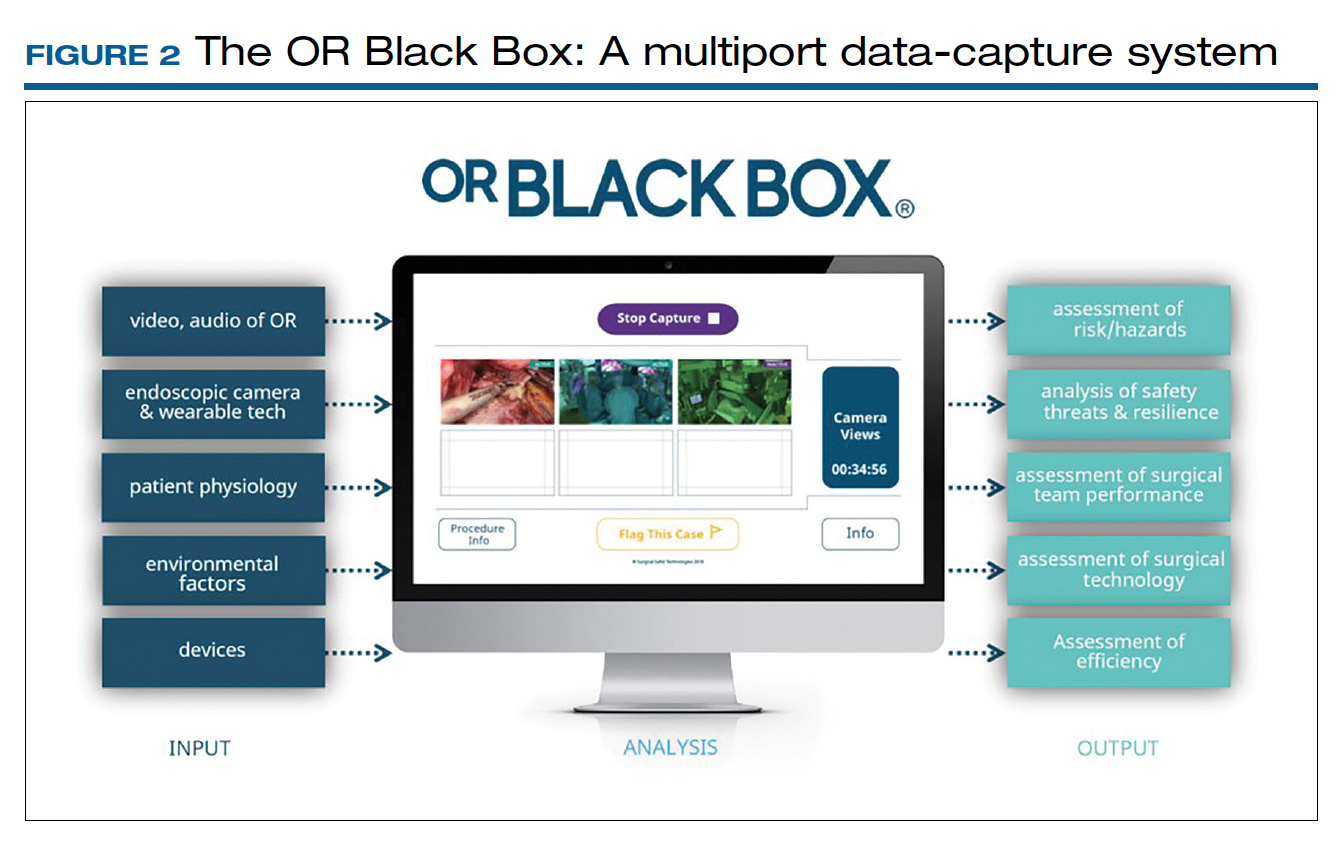
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
Society of Gynecologic Surgeons meeting champions training of future gynecologic surgeons
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
Circumferential urethral diverticulum: A surgical conundrum
Colpocleisis: A simple, effective, and underutilized procedure
CASE 1: Problematic prolapse, but no incontinence
An 81-year-old multiparous woman, who has a history of recurrent stage-III pelvic organ prolapse (POP), reports worsening discomfort that makes it difficult for her to care for her ailing husband. She also has “trouble” with bladder emptying and constipation, but denies any loss of urine. She has not had vaginal intercourse in more than a decade because of her husband’s medical condition.
Aside from health issues—she suffers from obesity, coronary artery disease, hypertension, and diabetes—the patient is content with her marriage of 58 years.
Urodynamic testing fails to demonstrate detrusor overactivity, stress urinary incontinence, or intrinsic sphincteric deficiency. A cough stress test is repeated after reduction of her prolapse using a large cotton swab, and confirms the findings of the urodynamic tests.
Is reconstructive surgery appropriate for this patient?
Traditional reconstructive surgical procedures for treating POP fail in as many as 30% of patients, and new approaches—some involving grafts—are proposed every day, often without much data behind them.1
Regardless of the approach, reconstructive surgery is a lengthy procedure that subjects patients who are already medically compromised to significant risk, including bleeding, infection, and fluid shifts. Delayed return to normal activity may be especially costly among elderly women because of the risk of venous thromboembolism.
Because of the high failure rate, slow recovery, and risk of complications, reconstructive surgery may not be as appropriate as colpocleisis for the woman described above. Colpocleisis—suturing the inside walls of the vagina together—has an efficacy rate exceeding 90%.2 This relatively simple operation has been around for almost two centuries and has a good track record, but is often overlooked when counseling a patient about her options.
Any frail, elderly woman who has stage-III or -IV POP who does not desire to preserve coital ability is a candidate for colpocleisis (TABLE). Advantages include:
- a short operating time
- few complications
- amenability of local anesthesia
- short hospitalization
- speedy recovery
- high success rate
- low rate of regret.2-5
Because it precludes coital activity, however, colpocleisis may cause problems with self-image. It also may lead to de novo or worsening urinary incontinence and complicate or delay the diagnosis of cervical and endometrial pathology.
This article explores these issues through a case-based discussion of colpocleisis, including a detailed description of surgical technique.
TABLE
Requirements for colpocleisis
Both of the following must be present
|
Plus at least one of the following
|
Colpocleisis, as noted, entails suturing the inside walls of the vagina together. It is controversial because of its impact on coital activity. With careful patient selection, however, colpocleisis is considered a valid option for frail and elderly women who have POP and do not desire or foresee the possibility of future vaginal intercourse. Such women may represent a surprising percentage of the elderly population. A community-based survey found that 78% of married women 70 to 79 years old are not sexually active,6 and a study from The Netherlands found a prevalence of symptomatic POP of 11.4% among white women 45 to 85 years old.7
The fundamental reason for choosing an obliterative procedure such as colpocleisis over total pelvic reconstruction is to treat the prolapse with the least invasive technique in the shortest time. Hysterectomy, which often adds 30 to 80 minutes to the procedure, should therefore be performed only in patients who have a suspicious finding upon initial evaluation. For the same reason, partial colpocleisis—performed using the LeFort technique with limited dissection—has become the most popular obliterative approach. We try to avoid a total colpocleisis procedure—also known as colpectomy—in which the entire vaginal epithelium is stripped, because it is feasible only when the uterus is already absent or scheduled to be removed concomitantly.
(Note: The term vaginectomy should be reserved for gynecologic oncology procedures performed to remove vaginal cancer. Vaginectomy entails full-thickness excision of the vaginal walls, including the fibromuscular layer, as opposed to excision of the epithelial layer only, as in colpocleisis. In this article, we present the LeFort method, a partial colpocleisis technique, because we believe it is more easily adapted by the general gynecologist.8)
CASE 1 RESOLVED
After detailed counseling, which includes family members, the patient opts to undergo colpocleisis. The procedure takes 45 minutes. She is discharged on postoperative Day 1, and reports substantially improved quality of life.
CASE 2: Recurrent prolapse and problems with a pessary
A 72-year-old multiparous, widowed woman experiences recurrent stage-III isolated apical prolapse. She has already undergone two reconstructive procedures, and was discouraged from undergoing a third because of her chronic obstructive lung disease. She tried to use a Gellhorn-type pessary, which required a doctor’s intervention to insert and remove. Frustrated by the many office visits involved in having the pessary checked, she now demands surgical therapy. Another gynecologist has offered to repair the prolapse using mesh, but the patient has concerns about the safety and efficacy of the procedure because it is a relatively new approach.
In addition to the recurrent prolapse, she loses urine with stress and urge. She often has a postvoid residual volume >100 cc; urodynamic assessment confirms mixed urinary incontinence. The patient does not foresee any change in her social status (unmarried, sexually inactive).
Is colpocleisis a reasonable option?
Although the pessary is a helpful conservative alternative for women who are either unable or unwilling to undergo complex surgical pelvic repair and is considered first-line treatment by a majority of urogynecologists, it sometimes becomes more difficult to maintain than the patient is willing to tolerate.9 When a woman cannot remove and reinsert the device herself, the pessary requires a lifelong commitment to doctor’s visits every 2 or 3 months. This commitment is especially problematic for patients who become unable to drive or who lack social support.
Maintenance of the pessary becomes more frustrating as the patient becomes more dependent. Many gynecologists have seen a patient who developed a serious complication such as vesicovaginal or rectovaginal fistula because of a neglected pessary.10
In Case 2, the patient appears to be a potential candidate for colpocleisis, given her age and single status. Although pelvic floor repair appears to be safe in older women, any perioperative complication in a patient 70 years of age or older doubles the risk of discharge to a care facility.11,12 Women who have already undergone several surgeries or who have advanced medical problems such as coronary artery disease or cancer should be counseled thoroughly about the safety and efficacy of colpocleisis.
As for self-image, colpocleisis eliminates prolapse and reduces the genital hiatus. If the patient understands that colpocleisis is obliterative for the vagina but may improve the external appearance of the genital area, she may be more accepting of the procedure. One recent prospective, multicenter study found that only 2% of women thought their body looked worse 1 year after colpocleisis; 60% thought their body looked better.5
When reviewing treatment options, inform the patient that the pessary is a palliative option, whereas surgical therapy aims to be definitive.
CASE 2 RESOLVED
After comprehensive counseling, the patient elects to undergo colpocleisis, along with placement of a midurethral sling. She is discharged 1 day after surgery, and reports substantially improved urinary function, including bladder emptying, and quality of life. She says she would recommend the procedure to any woman who has a similar condition.
CASE 3: Pessary-related complications, incontinence, and underlying medical conditions
A 92-year-old multiparous widow, whose stage-IV uterovaginal prolapse has been managed by a pessary, develops vaginal ulcers in both anterior and posterior walls. After removal of the pessary and 4 weeks of treatment with vaginal estrogen, a smaller pessary is inserted, but she again develops ulcers and bleeding.
The patient’s medical condition is complicated by hypertension and generalized arthritis. She has urodynamically confirmed mixed urinary incontinence. She lives with her daughter and does not want to be placed in a nursing home.
What treatment options should you offer to her?
Because of this patient’s advanced age, poor health, and pessary-related problems, she is an ideal candidate for colpocleisis, provided she consents to the procedure after thorough counseling about its benefits and limitations.
Preoperative concerns
A thorough history, physical examination, and normal Pap test are necessary. If a suspicious pelvic mass or uterine bleeding is present, transvaginal ultrasonography (US) is crucial. In-office endometrial sampling also is necessary in any woman who has unexplained vaginal bleeding. More invasive procedures such as dilatation and curettage and hysteroscopy are needed only when the biopsy is inadequate or endometrial thickness exceeds 4 mm on transvaginal US.13
All elderly women who have high-risk medical problems must be cleared for surgery, with the necessary cardiac and pulmonary workup completed before the procedure.
Because colpocleisis is an extraperitoneal procedure, we have adapted use of over-the-counter enema products on the day before surgery in lieu of mechanical bowel preparation, which may lead to dehydration in very elderly women.
Coordinated consultation between the surgeon and anesthesiologist is necessary to determine the type of anesthesia to be used. Sedation and local anesthesia can be adequate for extremely high-risk women.14,15 Antibiotic prophylaxis is conventional for all patients.
Surgical technique
The LeFort method involves denudation and approximation of the midportions of the anterior and posterior vaginal walls.8 This operation creates a longitudinal vaginal septum with bilateral channels on each side, which serve as conduits for any secretion or bleeding from the apical vagina (FIGURE 1A AND B). Aggressive perineorraphy is also needed to shorten the genital hiatus. The following description incorporates perineorraphy into the LeFort technique.
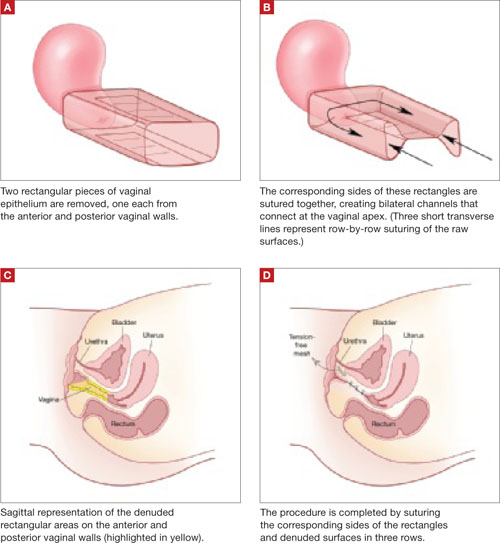
FIGURE 1 Principles of LeFort colpocleisis
The depiction here is not anatomically precise: The vagina is illustrated as a rectangular prism to clarify the relationship between tissues.
Patient positioning
Place the patient in the dorsal lithotomy position, using stirrups to support the entire leg up to the knee. Let the patient’s buttocks overhang the edge of the table by 1 to 2 inches. A slight Trendelenburg position is imperative, especially when operating on the anterior compartment of the vagina. The bladder should be only partially emptied because the leakage of urine from the bladder makes it easier to identify inadvertent cystotomy. Infiltration of local anesthetic solution to develop the surgical planes is acceptable.
Initiating the procedure
Remove a rectangular piece of vaginal epithelium from the anterior vaginal wall, beginning 2 to 3 cm distal to the vaginal apex (or cervix, if the uterus is present) and ending immediately proximal to the urethrovesical junction to leave space for midurethral sling placement. Remove a similarly sized piece of epithelium from the posterior vaginal wall. This posterior rectangle is an almost geometric projection of the anterior rectangle, but is somewhat longer (2 to 3 cm) (FIGURE 1).
When removing the vaginal epithelium, it may be helpful to use the skills developed for anterior and posterior colporraphy. Our operation begins with a 5- to 6-cm transverse incision at the anterior vaginal apex, which creates the proximal side of the anterior rectangle described above (FIGURE 2A).
As you develop the plane between the epithelium and fibromuscular layer, make a midline sagittal incision and extend it to the urethrovesical junction (FIGURE 2B). Dissect the epithelium off the fibromuscular layer approximately 3 cm bilaterally, then make a transverse incision at the urethrovesical junction. Finally, remove the anterior rectangle in two pieces by cutting along the lateral sides (FIGURE 2C AND D). Remove the posterior rectangle using the same technique, but also excise a triangular piece of skin from the posterior fourchette for the perineorraphy portion of the procedure (FIGURE 2E).
FIGURE 2 LeFort technique, step by step
Begin with a 5–6 cm transverse incision at the anterior vaginal apex.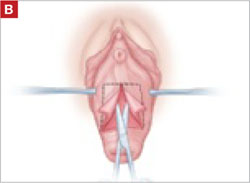
Dissect the epithelium off the fibromuscular layer, with a midline sagittal incision extending to the urethrovesical junction.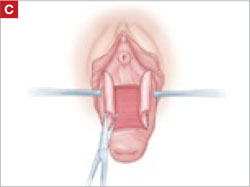
After dissection is completed, make a transverse incision at the urethrovesical junction, and remove the anterior rectangle in two pieces by cutting along the lateral sides.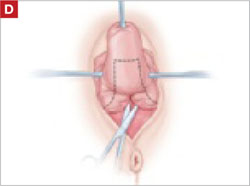
Denude the posterior rectangle using the same technique. In addition, excise a triangular piece of skin from the perineum.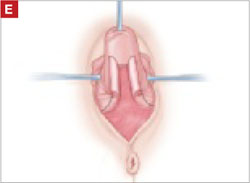
The posterior rectangle is ready for removal.
Suturing
Suture the apical sides of the anterior and posterior rectangles together using a continuous running technique (FIGURE 2F). Then approximate the lateral sides bilaterally using continuous sutures.
To ensure adherence of the anterior and posterior rectangles, stitch the raw surfaces together in three rows (FIGURE 2G). Do not include the distal 2 cm of the posterior vagina because you will need to leave room for perineorraphy.
Using several sutures, reapproximate the torn perineal fibromuscular structures in the midline to perform perineorraphy (FIGURE 2H). Close the distal vagina, beginning at the midpoint of the anterior transverse side, which lies at the urethrovesical junction (FIGURE 2I). Continue this suture on the posterior vagina and then the perineal body, sagittally, creating a small invagination in the distal vagina (FIGURE 2J).
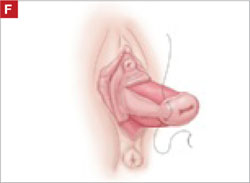
FIGURE 2 LeFort technique, step by step
Suture all but the distal sides of the rectangles between the anterior and posterior vaginal walls.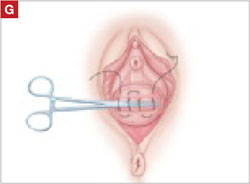
Also stitch together the raw surfaces in three rows in an imbricating fashion.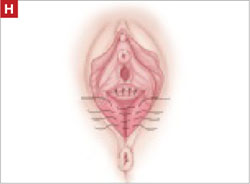
Perform perineorraphy.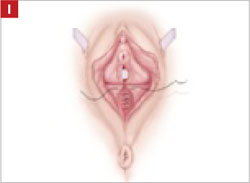
Close the distal vagina, starting at the midpoint of the anterior transverse side. If indicated, place a midurethral sling.
Final appearance.
Sling procedure
We place a midurethral sling as part of most colpocleisis operations. It is best to do this after the colpocleisis but before the perineorraphy.
In our cases, cystoscopy with simultaneous intravenous indigo carmine injection is standard before perineorraphy, even when a sling procedure is not planned. This safeguard ensures ureteral patency, which can be compromised (although rarely) in these procedures. Cutting and replacement of one of the sutures that approximate the raw tissues typically resolve the problem.16
Special considerations
Here are additional key points about colpocleisis, based on our experience:
- If an ulcer lies within the area designated to be denuded, some debridement to freshen up the surface will suffice. An ulcer is not an indication to deviate from the standard procedure.
- A modification developed by Goodall and Power may allow coitus by removing only a triangular piece of epithelium from each wall, leaving more room for the channels.17
- We have been unable to find any report of uterine or cervical cancer after colpocleisis, despite a MEDLINE search of the literature in English. Even so, the lateral channels created by the LeFort procedure allow any bleeding to escape the vagina, and may therefore enable recognition of malignancy. When noninvasive imaging techniques such as US or magnetic resonance are inadequate, vaginoscopy and hysteroscopy may be accomplished via these channels.
- When colpocleisis is performed in a hysterectomized woman, no lateral channel is necessary. Therefore, it is appropriate to do total colpocleisis.18,19
- When a patient with POP has a rectovaginal or vesicovaginal fistula caused by a neglected pessary, the addition of LeFort colpocleisis to the fistula repair may provide an effective treatment for both problems.10
Surgical outcomes
Success rate
Evidence concerning colpocleisis comes from case series, some of which are more than 30 years old. Although the definition of success is not clear in some series, the reported success rate has always exceeded 90% over the past three decades.2,18-22 Moreover, some of these reports involve as many as 30 years of follow-up.
Perioperative complication
In a recent review of the literature, the procedure-related mortality rate was 0.025%.2 When the authors focused only on studies published since 1980, major complications due to the patient’s underlying cardiovascular and pulmonary condition were seen in 2% of cases. Major surgical complications such as pyelonephritis and bleeding requiring transfusion occurred in 4% of cases, and less severe complications occurred in 15%.
In a study that included women who underwent concomitant vaginal hysterectomy, hysterectomy prolonged the surgery by 52 minutes, with a 5% rate of laparotomy as a result of intraoperative bleeding.22
In our series of 40 colpocleisis cases, we noted no instance in which a patient regretted the procedure.18 Others have also reported a low rate of regret—the highest being 9%.3-5,19-21
Using validated questionnaires, FitzGerald and colleagues found significant improvement in mental and physical quality of life, as well as urinary, colorectal, and bulge-related pelvic floor symptoms, 1 year after colpocleisis.5
De novo or worsening urinary incontinence is one of the drawbacks of colpocleisis. However, the same risk is present in approximately 40% of women who undergo surgical reconstructive procedures for POP without a continence operation.23 Because preoperative urinary retention is common in women who have POP, the decision to add a potentially harmful continence procedure is complicated in colpocleisis candidates. A small case series reported that the success rate ranged from 90% to 94% in women who underwent a midurethral tension-free sling procedure for the treatment of urinary incontinence at the time of colpocleisis.5
Preoperative urodynamic studies to detect urethral intrinsic deficiency and detrusor dysfunction are prudent, and detailed counseling of the patient about urinary control is vital. We perform a midurethral sling procedure in most of our colpocleisis cases, and have had pleasing results.
CASE 3 RESOLVED
The patient decides to undergo partial colpocleisis using the LeFort procedure, along with placement of a midurethral sling, for a total operative time of 75 minutes. She is discharged 1 day later and reports substantial improvement in urinary function and quality of life.
1. Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1503.
2. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Weber A. For the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271.
3. Wheeler TL, Jr, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. Am J Obstet Gynecol. 2005;193:2067-2070.
4. Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
5. FitzGerald MP, Richter HE, Bradley CS, et al. For the Pelvic Floor Disorders Network. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1603-1609.
6. Patel D, Gillespie B, Foxman B. Sexual behavior of older women: results of a random-digit-dialing survey of 2,000 women in the United States. Sex Transm Dis. 2003;30:216-220.
7. Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1-184.e7.
8. Berlin F. Three cases of complete prolapsus uteri operated upon according to the method of Leon LeFort. Am J Obstet Gynecol. 1881;14:866-868.
9. Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 Pt 1):931-935.
10. Esin S, Harmanli OH. Large vesicovaginal fistula in women with pelvic organ prolapse: the role of colpocleisis revisited. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1711-1713.
11. Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111-2118.
12. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96:583-589.
13. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;113(2 Pt 1):462-464.
14. Moore RD, Miklos JR. Colpocleisis and tension-free vaginal tape sling for severe uterine and vaginal prolapse and stress urinary incontinence under local anesthesia. J Am Assoc Gynecol Laparosc. 2003;10:276-280.
15. Buchsbaum GM, Albushies DT, Schoenecker E, Duecy EE, Glantz JC. Local anesthesia with sedation for vaginal reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:211-214.
16. Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1478-1485.
17. Goodall JR, Power RMH. A modification of the Le Fort operation for increasing its scope. Am J Obstet Gynecol. 1937;34:968-976.
18. Harmanli OH, Dandolu V, Chatwani AJ, Grody MT. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
19. DeLancey JOL, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1232.
20. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
21. Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of Le Fort’s operation. Obstet Gynecol. 1973;42:415-420.
22. Von Pechmann WS, Mutone MD, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
23. Albo ME, Richter HE, Brubaker L, et al. For Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
CASE 1: Problematic prolapse, but no incontinence
An 81-year-old multiparous woman, who has a history of recurrent stage-III pelvic organ prolapse (POP), reports worsening discomfort that makes it difficult for her to care for her ailing husband. She also has “trouble” with bladder emptying and constipation, but denies any loss of urine. She has not had vaginal intercourse in more than a decade because of her husband’s medical condition.
Aside from health issues—she suffers from obesity, coronary artery disease, hypertension, and diabetes—the patient is content with her marriage of 58 years.
Urodynamic testing fails to demonstrate detrusor overactivity, stress urinary incontinence, or intrinsic sphincteric deficiency. A cough stress test is repeated after reduction of her prolapse using a large cotton swab, and confirms the findings of the urodynamic tests.
Is reconstructive surgery appropriate for this patient?
Traditional reconstructive surgical procedures for treating POP fail in as many as 30% of patients, and new approaches—some involving grafts—are proposed every day, often without much data behind them.1
Regardless of the approach, reconstructive surgery is a lengthy procedure that subjects patients who are already medically compromised to significant risk, including bleeding, infection, and fluid shifts. Delayed return to normal activity may be especially costly among elderly women because of the risk of venous thromboembolism.
Because of the high failure rate, slow recovery, and risk of complications, reconstructive surgery may not be as appropriate as colpocleisis for the woman described above. Colpocleisis—suturing the inside walls of the vagina together—has an efficacy rate exceeding 90%.2 This relatively simple operation has been around for almost two centuries and has a good track record, but is often overlooked when counseling a patient about her options.
Any frail, elderly woman who has stage-III or -IV POP who does not desire to preserve coital ability is a candidate for colpocleisis (TABLE). Advantages include:
- a short operating time
- few complications
- amenability of local anesthesia
- short hospitalization
- speedy recovery
- high success rate
- low rate of regret.2-5
Because it precludes coital activity, however, colpocleisis may cause problems with self-image. It also may lead to de novo or worsening urinary incontinence and complicate or delay the diagnosis of cervical and endometrial pathology.
This article explores these issues through a case-based discussion of colpocleisis, including a detailed description of surgical technique.
TABLE
Requirements for colpocleisis
Both of the following must be present
|
Plus at least one of the following
|
Colpocleisis, as noted, entails suturing the inside walls of the vagina together. It is controversial because of its impact on coital activity. With careful patient selection, however, colpocleisis is considered a valid option for frail and elderly women who have POP and do not desire or foresee the possibility of future vaginal intercourse. Such women may represent a surprising percentage of the elderly population. A community-based survey found that 78% of married women 70 to 79 years old are not sexually active,6 and a study from The Netherlands found a prevalence of symptomatic POP of 11.4% among white women 45 to 85 years old.7
The fundamental reason for choosing an obliterative procedure such as colpocleisis over total pelvic reconstruction is to treat the prolapse with the least invasive technique in the shortest time. Hysterectomy, which often adds 30 to 80 minutes to the procedure, should therefore be performed only in patients who have a suspicious finding upon initial evaluation. For the same reason, partial colpocleisis—performed using the LeFort technique with limited dissection—has become the most popular obliterative approach. We try to avoid a total colpocleisis procedure—also known as colpectomy—in which the entire vaginal epithelium is stripped, because it is feasible only when the uterus is already absent or scheduled to be removed concomitantly.
(Note: The term vaginectomy should be reserved for gynecologic oncology procedures performed to remove vaginal cancer. Vaginectomy entails full-thickness excision of the vaginal walls, including the fibromuscular layer, as opposed to excision of the epithelial layer only, as in colpocleisis. In this article, we present the LeFort method, a partial colpocleisis technique, because we believe it is more easily adapted by the general gynecologist.8)
CASE 1 RESOLVED
After detailed counseling, which includes family members, the patient opts to undergo colpocleisis. The procedure takes 45 minutes. She is discharged on postoperative Day 1, and reports substantially improved quality of life.
CASE 2: Recurrent prolapse and problems with a pessary
A 72-year-old multiparous, widowed woman experiences recurrent stage-III isolated apical prolapse. She has already undergone two reconstructive procedures, and was discouraged from undergoing a third because of her chronic obstructive lung disease. She tried to use a Gellhorn-type pessary, which required a doctor’s intervention to insert and remove. Frustrated by the many office visits involved in having the pessary checked, she now demands surgical therapy. Another gynecologist has offered to repair the prolapse using mesh, but the patient has concerns about the safety and efficacy of the procedure because it is a relatively new approach.
In addition to the recurrent prolapse, she loses urine with stress and urge. She often has a postvoid residual volume >100 cc; urodynamic assessment confirms mixed urinary incontinence. The patient does not foresee any change in her social status (unmarried, sexually inactive).
Is colpocleisis a reasonable option?
Although the pessary is a helpful conservative alternative for women who are either unable or unwilling to undergo complex surgical pelvic repair and is considered first-line treatment by a majority of urogynecologists, it sometimes becomes more difficult to maintain than the patient is willing to tolerate.9 When a woman cannot remove and reinsert the device herself, the pessary requires a lifelong commitment to doctor’s visits every 2 or 3 months. This commitment is especially problematic for patients who become unable to drive or who lack social support.
Maintenance of the pessary becomes more frustrating as the patient becomes more dependent. Many gynecologists have seen a patient who developed a serious complication such as vesicovaginal or rectovaginal fistula because of a neglected pessary.10
In Case 2, the patient appears to be a potential candidate for colpocleisis, given her age and single status. Although pelvic floor repair appears to be safe in older women, any perioperative complication in a patient 70 years of age or older doubles the risk of discharge to a care facility.11,12 Women who have already undergone several surgeries or who have advanced medical problems such as coronary artery disease or cancer should be counseled thoroughly about the safety and efficacy of colpocleisis.
As for self-image, colpocleisis eliminates prolapse and reduces the genital hiatus. If the patient understands that colpocleisis is obliterative for the vagina but may improve the external appearance of the genital area, she may be more accepting of the procedure. One recent prospective, multicenter study found that only 2% of women thought their body looked worse 1 year after colpocleisis; 60% thought their body looked better.5
When reviewing treatment options, inform the patient that the pessary is a palliative option, whereas surgical therapy aims to be definitive.
CASE 2 RESOLVED
After comprehensive counseling, the patient elects to undergo colpocleisis, along with placement of a midurethral sling. She is discharged 1 day after surgery, and reports substantially improved urinary function, including bladder emptying, and quality of life. She says she would recommend the procedure to any woman who has a similar condition.
CASE 3: Pessary-related complications, incontinence, and underlying medical conditions
A 92-year-old multiparous widow, whose stage-IV uterovaginal prolapse has been managed by a pessary, develops vaginal ulcers in both anterior and posterior walls. After removal of the pessary and 4 weeks of treatment with vaginal estrogen, a smaller pessary is inserted, but she again develops ulcers and bleeding.
The patient’s medical condition is complicated by hypertension and generalized arthritis. She has urodynamically confirmed mixed urinary incontinence. She lives with her daughter and does not want to be placed in a nursing home.
What treatment options should you offer to her?
Because of this patient’s advanced age, poor health, and pessary-related problems, she is an ideal candidate for colpocleisis, provided she consents to the procedure after thorough counseling about its benefits and limitations.
Preoperative concerns
A thorough history, physical examination, and normal Pap test are necessary. If a suspicious pelvic mass or uterine bleeding is present, transvaginal ultrasonography (US) is crucial. In-office endometrial sampling also is necessary in any woman who has unexplained vaginal bleeding. More invasive procedures such as dilatation and curettage and hysteroscopy are needed only when the biopsy is inadequate or endometrial thickness exceeds 4 mm on transvaginal US.13
All elderly women who have high-risk medical problems must be cleared for surgery, with the necessary cardiac and pulmonary workup completed before the procedure.
Because colpocleisis is an extraperitoneal procedure, we have adapted use of over-the-counter enema products on the day before surgery in lieu of mechanical bowel preparation, which may lead to dehydration in very elderly women.
Coordinated consultation between the surgeon and anesthesiologist is necessary to determine the type of anesthesia to be used. Sedation and local anesthesia can be adequate for extremely high-risk women.14,15 Antibiotic prophylaxis is conventional for all patients.
Surgical technique
The LeFort method involves denudation and approximation of the midportions of the anterior and posterior vaginal walls.8 This operation creates a longitudinal vaginal septum with bilateral channels on each side, which serve as conduits for any secretion or bleeding from the apical vagina (FIGURE 1A AND B). Aggressive perineorraphy is also needed to shorten the genital hiatus. The following description incorporates perineorraphy into the LeFort technique.

FIGURE 1 Principles of LeFort colpocleisis
The depiction here is not anatomically precise: The vagina is illustrated as a rectangular prism to clarify the relationship between tissues.
Patient positioning
Place the patient in the dorsal lithotomy position, using stirrups to support the entire leg up to the knee. Let the patient’s buttocks overhang the edge of the table by 1 to 2 inches. A slight Trendelenburg position is imperative, especially when operating on the anterior compartment of the vagina. The bladder should be only partially emptied because the leakage of urine from the bladder makes it easier to identify inadvertent cystotomy. Infiltration of local anesthetic solution to develop the surgical planes is acceptable.
Initiating the procedure
Remove a rectangular piece of vaginal epithelium from the anterior vaginal wall, beginning 2 to 3 cm distal to the vaginal apex (or cervix, if the uterus is present) and ending immediately proximal to the urethrovesical junction to leave space for midurethral sling placement. Remove a similarly sized piece of epithelium from the posterior vaginal wall. This posterior rectangle is an almost geometric projection of the anterior rectangle, but is somewhat longer (2 to 3 cm) (FIGURE 1).
When removing the vaginal epithelium, it may be helpful to use the skills developed for anterior and posterior colporraphy. Our operation begins with a 5- to 6-cm transverse incision at the anterior vaginal apex, which creates the proximal side of the anterior rectangle described above (FIGURE 2A).
As you develop the plane between the epithelium and fibromuscular layer, make a midline sagittal incision and extend it to the urethrovesical junction (FIGURE 2B). Dissect the epithelium off the fibromuscular layer approximately 3 cm bilaterally, then make a transverse incision at the urethrovesical junction. Finally, remove the anterior rectangle in two pieces by cutting along the lateral sides (FIGURE 2C AND D). Remove the posterior rectangle using the same technique, but also excise a triangular piece of skin from the posterior fourchette for the perineorraphy portion of the procedure (FIGURE 2E).

FIGURE 2 LeFort technique, step by step
Begin with a 5–6 cm transverse incision at the anterior vaginal apex.
Dissect the epithelium off the fibromuscular layer, with a midline sagittal incision extending to the urethrovesical junction.
After dissection is completed, make a transverse incision at the urethrovesical junction, and remove the anterior rectangle in two pieces by cutting along the lateral sides.
Denude the posterior rectangle using the same technique. In addition, excise a triangular piece of skin from the perineum.
The posterior rectangle is ready for removal.
Suturing
Suture the apical sides of the anterior and posterior rectangles together using a continuous running technique (FIGURE 2F). Then approximate the lateral sides bilaterally using continuous sutures.
To ensure adherence of the anterior and posterior rectangles, stitch the raw surfaces together in three rows (FIGURE 2G). Do not include the distal 2 cm of the posterior vagina because you will need to leave room for perineorraphy.
Using several sutures, reapproximate the torn perineal fibromuscular structures in the midline to perform perineorraphy (FIGURE 2H). Close the distal vagina, beginning at the midpoint of the anterior transverse side, which lies at the urethrovesical junction (FIGURE 2I). Continue this suture on the posterior vagina and then the perineal body, sagittally, creating a small invagination in the distal vagina (FIGURE 2J).

FIGURE 2 LeFort technique, step by step
Suture all but the distal sides of the rectangles between the anterior and posterior vaginal walls.
Also stitch together the raw surfaces in three rows in an imbricating fashion.
Perform perineorraphy.
Close the distal vagina, starting at the midpoint of the anterior transverse side. If indicated, place a midurethral sling.
Final appearance.
Sling procedure
We place a midurethral sling as part of most colpocleisis operations. It is best to do this after the colpocleisis but before the perineorraphy.
In our cases, cystoscopy with simultaneous intravenous indigo carmine injection is standard before perineorraphy, even when a sling procedure is not planned. This safeguard ensures ureteral patency, which can be compromised (although rarely) in these procedures. Cutting and replacement of one of the sutures that approximate the raw tissues typically resolve the problem.16
Special considerations
Here are additional key points about colpocleisis, based on our experience:
- If an ulcer lies within the area designated to be denuded, some debridement to freshen up the surface will suffice. An ulcer is not an indication to deviate from the standard procedure.
- A modification developed by Goodall and Power may allow coitus by removing only a triangular piece of epithelium from each wall, leaving more room for the channels.17
- We have been unable to find any report of uterine or cervical cancer after colpocleisis, despite a MEDLINE search of the literature in English. Even so, the lateral channels created by the LeFort procedure allow any bleeding to escape the vagina, and may therefore enable recognition of malignancy. When noninvasive imaging techniques such as US or magnetic resonance are inadequate, vaginoscopy and hysteroscopy may be accomplished via these channels.
- When colpocleisis is performed in a hysterectomized woman, no lateral channel is necessary. Therefore, it is appropriate to do total colpocleisis.18,19
- When a patient with POP has a rectovaginal or vesicovaginal fistula caused by a neglected pessary, the addition of LeFort colpocleisis to the fistula repair may provide an effective treatment for both problems.10
Surgical outcomes
Success rate
Evidence concerning colpocleisis comes from case series, some of which are more than 30 years old. Although the definition of success is not clear in some series, the reported success rate has always exceeded 90% over the past three decades.2,18-22 Moreover, some of these reports involve as many as 30 years of follow-up.
Perioperative complication
In a recent review of the literature, the procedure-related mortality rate was 0.025%.2 When the authors focused only on studies published since 1980, major complications due to the patient’s underlying cardiovascular and pulmonary condition were seen in 2% of cases. Major surgical complications such as pyelonephritis and bleeding requiring transfusion occurred in 4% of cases, and less severe complications occurred in 15%.
In a study that included women who underwent concomitant vaginal hysterectomy, hysterectomy prolonged the surgery by 52 minutes, with a 5% rate of laparotomy as a result of intraoperative bleeding.22
In our series of 40 colpocleisis cases, we noted no instance in which a patient regretted the procedure.18 Others have also reported a low rate of regret—the highest being 9%.3-5,19-21
Using validated questionnaires, FitzGerald and colleagues found significant improvement in mental and physical quality of life, as well as urinary, colorectal, and bulge-related pelvic floor symptoms, 1 year after colpocleisis.5
De novo or worsening urinary incontinence is one of the drawbacks of colpocleisis. However, the same risk is present in approximately 40% of women who undergo surgical reconstructive procedures for POP without a continence operation.23 Because preoperative urinary retention is common in women who have POP, the decision to add a potentially harmful continence procedure is complicated in colpocleisis candidates. A small case series reported that the success rate ranged from 90% to 94% in women who underwent a midurethral tension-free sling procedure for the treatment of urinary incontinence at the time of colpocleisis.5
Preoperative urodynamic studies to detect urethral intrinsic deficiency and detrusor dysfunction are prudent, and detailed counseling of the patient about urinary control is vital. We perform a midurethral sling procedure in most of our colpocleisis cases, and have had pleasing results.
CASE 3 RESOLVED
The patient decides to undergo partial colpocleisis using the LeFort procedure, along with placement of a midurethral sling, for a total operative time of 75 minutes. She is discharged 1 day later and reports substantial improvement in urinary function and quality of life.
CASE 1: Problematic prolapse, but no incontinence
An 81-year-old multiparous woman, who has a history of recurrent stage-III pelvic organ prolapse (POP), reports worsening discomfort that makes it difficult for her to care for her ailing husband. She also has “trouble” with bladder emptying and constipation, but denies any loss of urine. She has not had vaginal intercourse in more than a decade because of her husband’s medical condition.
Aside from health issues—she suffers from obesity, coronary artery disease, hypertension, and diabetes—the patient is content with her marriage of 58 years.
Urodynamic testing fails to demonstrate detrusor overactivity, stress urinary incontinence, or intrinsic sphincteric deficiency. A cough stress test is repeated after reduction of her prolapse using a large cotton swab, and confirms the findings of the urodynamic tests.
Is reconstructive surgery appropriate for this patient?
Traditional reconstructive surgical procedures for treating POP fail in as many as 30% of patients, and new approaches—some involving grafts—are proposed every day, often without much data behind them.1
Regardless of the approach, reconstructive surgery is a lengthy procedure that subjects patients who are already medically compromised to significant risk, including bleeding, infection, and fluid shifts. Delayed return to normal activity may be especially costly among elderly women because of the risk of venous thromboembolism.
Because of the high failure rate, slow recovery, and risk of complications, reconstructive surgery may not be as appropriate as colpocleisis for the woman described above. Colpocleisis—suturing the inside walls of the vagina together—has an efficacy rate exceeding 90%.2 This relatively simple operation has been around for almost two centuries and has a good track record, but is often overlooked when counseling a patient about her options.
Any frail, elderly woman who has stage-III or -IV POP who does not desire to preserve coital ability is a candidate for colpocleisis (TABLE). Advantages include:
- a short operating time
- few complications
- amenability of local anesthesia
- short hospitalization
- speedy recovery
- high success rate
- low rate of regret.2-5
Because it precludes coital activity, however, colpocleisis may cause problems with self-image. It also may lead to de novo or worsening urinary incontinence and complicate or delay the diagnosis of cervical and endometrial pathology.
This article explores these issues through a case-based discussion of colpocleisis, including a detailed description of surgical technique.
TABLE
Requirements for colpocleisis
Both of the following must be present
|
Plus at least one of the following
|
Colpocleisis, as noted, entails suturing the inside walls of the vagina together. It is controversial because of its impact on coital activity. With careful patient selection, however, colpocleisis is considered a valid option for frail and elderly women who have POP and do not desire or foresee the possibility of future vaginal intercourse. Such women may represent a surprising percentage of the elderly population. A community-based survey found that 78% of married women 70 to 79 years old are not sexually active,6 and a study from The Netherlands found a prevalence of symptomatic POP of 11.4% among white women 45 to 85 years old.7
The fundamental reason for choosing an obliterative procedure such as colpocleisis over total pelvic reconstruction is to treat the prolapse with the least invasive technique in the shortest time. Hysterectomy, which often adds 30 to 80 minutes to the procedure, should therefore be performed only in patients who have a suspicious finding upon initial evaluation. For the same reason, partial colpocleisis—performed using the LeFort technique with limited dissection—has become the most popular obliterative approach. We try to avoid a total colpocleisis procedure—also known as colpectomy—in which the entire vaginal epithelium is stripped, because it is feasible only when the uterus is already absent or scheduled to be removed concomitantly.
(Note: The term vaginectomy should be reserved for gynecologic oncology procedures performed to remove vaginal cancer. Vaginectomy entails full-thickness excision of the vaginal walls, including the fibromuscular layer, as opposed to excision of the epithelial layer only, as in colpocleisis. In this article, we present the LeFort method, a partial colpocleisis technique, because we believe it is more easily adapted by the general gynecologist.8)
CASE 1 RESOLVED
After detailed counseling, which includes family members, the patient opts to undergo colpocleisis. The procedure takes 45 minutes. She is discharged on postoperative Day 1, and reports substantially improved quality of life.
CASE 2: Recurrent prolapse and problems with a pessary
A 72-year-old multiparous, widowed woman experiences recurrent stage-III isolated apical prolapse. She has already undergone two reconstructive procedures, and was discouraged from undergoing a third because of her chronic obstructive lung disease. She tried to use a Gellhorn-type pessary, which required a doctor’s intervention to insert and remove. Frustrated by the many office visits involved in having the pessary checked, she now demands surgical therapy. Another gynecologist has offered to repair the prolapse using mesh, but the patient has concerns about the safety and efficacy of the procedure because it is a relatively new approach.
In addition to the recurrent prolapse, she loses urine with stress and urge. She often has a postvoid residual volume >100 cc; urodynamic assessment confirms mixed urinary incontinence. The patient does not foresee any change in her social status (unmarried, sexually inactive).
Is colpocleisis a reasonable option?
Although the pessary is a helpful conservative alternative for women who are either unable or unwilling to undergo complex surgical pelvic repair and is considered first-line treatment by a majority of urogynecologists, it sometimes becomes more difficult to maintain than the patient is willing to tolerate.9 When a woman cannot remove and reinsert the device herself, the pessary requires a lifelong commitment to doctor’s visits every 2 or 3 months. This commitment is especially problematic for patients who become unable to drive or who lack social support.
Maintenance of the pessary becomes more frustrating as the patient becomes more dependent. Many gynecologists have seen a patient who developed a serious complication such as vesicovaginal or rectovaginal fistula because of a neglected pessary.10
In Case 2, the patient appears to be a potential candidate for colpocleisis, given her age and single status. Although pelvic floor repair appears to be safe in older women, any perioperative complication in a patient 70 years of age or older doubles the risk of discharge to a care facility.11,12 Women who have already undergone several surgeries or who have advanced medical problems such as coronary artery disease or cancer should be counseled thoroughly about the safety and efficacy of colpocleisis.
As for self-image, colpocleisis eliminates prolapse and reduces the genital hiatus. If the patient understands that colpocleisis is obliterative for the vagina but may improve the external appearance of the genital area, she may be more accepting of the procedure. One recent prospective, multicenter study found that only 2% of women thought their body looked worse 1 year after colpocleisis; 60% thought their body looked better.5
When reviewing treatment options, inform the patient that the pessary is a palliative option, whereas surgical therapy aims to be definitive.
CASE 2 RESOLVED
After comprehensive counseling, the patient elects to undergo colpocleisis, along with placement of a midurethral sling. She is discharged 1 day after surgery, and reports substantially improved urinary function, including bladder emptying, and quality of life. She says she would recommend the procedure to any woman who has a similar condition.
CASE 3: Pessary-related complications, incontinence, and underlying medical conditions
A 92-year-old multiparous widow, whose stage-IV uterovaginal prolapse has been managed by a pessary, develops vaginal ulcers in both anterior and posterior walls. After removal of the pessary and 4 weeks of treatment with vaginal estrogen, a smaller pessary is inserted, but she again develops ulcers and bleeding.
The patient’s medical condition is complicated by hypertension and generalized arthritis. She has urodynamically confirmed mixed urinary incontinence. She lives with her daughter and does not want to be placed in a nursing home.
What treatment options should you offer to her?
Because of this patient’s advanced age, poor health, and pessary-related problems, she is an ideal candidate for colpocleisis, provided she consents to the procedure after thorough counseling about its benefits and limitations.
Preoperative concerns
A thorough history, physical examination, and normal Pap test are necessary. If a suspicious pelvic mass or uterine bleeding is present, transvaginal ultrasonography (US) is crucial. In-office endometrial sampling also is necessary in any woman who has unexplained vaginal bleeding. More invasive procedures such as dilatation and curettage and hysteroscopy are needed only when the biopsy is inadequate or endometrial thickness exceeds 4 mm on transvaginal US.13
All elderly women who have high-risk medical problems must be cleared for surgery, with the necessary cardiac and pulmonary workup completed before the procedure.
Because colpocleisis is an extraperitoneal procedure, we have adapted use of over-the-counter enema products on the day before surgery in lieu of mechanical bowel preparation, which may lead to dehydration in very elderly women.
Coordinated consultation between the surgeon and anesthesiologist is necessary to determine the type of anesthesia to be used. Sedation and local anesthesia can be adequate for extremely high-risk women.14,15 Antibiotic prophylaxis is conventional for all patients.
Surgical technique
The LeFort method involves denudation and approximation of the midportions of the anterior and posterior vaginal walls.8 This operation creates a longitudinal vaginal septum with bilateral channels on each side, which serve as conduits for any secretion or bleeding from the apical vagina (FIGURE 1A AND B). Aggressive perineorraphy is also needed to shorten the genital hiatus. The following description incorporates perineorraphy into the LeFort technique.

FIGURE 1 Principles of LeFort colpocleisis
The depiction here is not anatomically precise: The vagina is illustrated as a rectangular prism to clarify the relationship between tissues.
Patient positioning
Place the patient in the dorsal lithotomy position, using stirrups to support the entire leg up to the knee. Let the patient’s buttocks overhang the edge of the table by 1 to 2 inches. A slight Trendelenburg position is imperative, especially when operating on the anterior compartment of the vagina. The bladder should be only partially emptied because the leakage of urine from the bladder makes it easier to identify inadvertent cystotomy. Infiltration of local anesthetic solution to develop the surgical planes is acceptable.
Initiating the procedure
Remove a rectangular piece of vaginal epithelium from the anterior vaginal wall, beginning 2 to 3 cm distal to the vaginal apex (or cervix, if the uterus is present) and ending immediately proximal to the urethrovesical junction to leave space for midurethral sling placement. Remove a similarly sized piece of epithelium from the posterior vaginal wall. This posterior rectangle is an almost geometric projection of the anterior rectangle, but is somewhat longer (2 to 3 cm) (FIGURE 1).
When removing the vaginal epithelium, it may be helpful to use the skills developed for anterior and posterior colporraphy. Our operation begins with a 5- to 6-cm transverse incision at the anterior vaginal apex, which creates the proximal side of the anterior rectangle described above (FIGURE 2A).
As you develop the plane between the epithelium and fibromuscular layer, make a midline sagittal incision and extend it to the urethrovesical junction (FIGURE 2B). Dissect the epithelium off the fibromuscular layer approximately 3 cm bilaterally, then make a transverse incision at the urethrovesical junction. Finally, remove the anterior rectangle in two pieces by cutting along the lateral sides (FIGURE 2C AND D). Remove the posterior rectangle using the same technique, but also excise a triangular piece of skin from the posterior fourchette for the perineorraphy portion of the procedure (FIGURE 2E).

FIGURE 2 LeFort technique, step by step
Begin with a 5–6 cm transverse incision at the anterior vaginal apex.
Dissect the epithelium off the fibromuscular layer, with a midline sagittal incision extending to the urethrovesical junction.
After dissection is completed, make a transverse incision at the urethrovesical junction, and remove the anterior rectangle in two pieces by cutting along the lateral sides.
Denude the posterior rectangle using the same technique. In addition, excise a triangular piece of skin from the perineum.
The posterior rectangle is ready for removal.
Suturing
Suture the apical sides of the anterior and posterior rectangles together using a continuous running technique (FIGURE 2F). Then approximate the lateral sides bilaterally using continuous sutures.
To ensure adherence of the anterior and posterior rectangles, stitch the raw surfaces together in three rows (FIGURE 2G). Do not include the distal 2 cm of the posterior vagina because you will need to leave room for perineorraphy.
Using several sutures, reapproximate the torn perineal fibromuscular structures in the midline to perform perineorraphy (FIGURE 2H). Close the distal vagina, beginning at the midpoint of the anterior transverse side, which lies at the urethrovesical junction (FIGURE 2I). Continue this suture on the posterior vagina and then the perineal body, sagittally, creating a small invagination in the distal vagina (FIGURE 2J).

FIGURE 2 LeFort technique, step by step
Suture all but the distal sides of the rectangles between the anterior and posterior vaginal walls.
Also stitch together the raw surfaces in three rows in an imbricating fashion.
Perform perineorraphy.
Close the distal vagina, starting at the midpoint of the anterior transverse side. If indicated, place a midurethral sling.
Final appearance.
Sling procedure
We place a midurethral sling as part of most colpocleisis operations. It is best to do this after the colpocleisis but before the perineorraphy.
In our cases, cystoscopy with simultaneous intravenous indigo carmine injection is standard before perineorraphy, even when a sling procedure is not planned. This safeguard ensures ureteral patency, which can be compromised (although rarely) in these procedures. Cutting and replacement of one of the sutures that approximate the raw tissues typically resolve the problem.16
Special considerations
Here are additional key points about colpocleisis, based on our experience:
- If an ulcer lies within the area designated to be denuded, some debridement to freshen up the surface will suffice. An ulcer is not an indication to deviate from the standard procedure.
- A modification developed by Goodall and Power may allow coitus by removing only a triangular piece of epithelium from each wall, leaving more room for the channels.17
- We have been unable to find any report of uterine or cervical cancer after colpocleisis, despite a MEDLINE search of the literature in English. Even so, the lateral channels created by the LeFort procedure allow any bleeding to escape the vagina, and may therefore enable recognition of malignancy. When noninvasive imaging techniques such as US or magnetic resonance are inadequate, vaginoscopy and hysteroscopy may be accomplished via these channels.
- When colpocleisis is performed in a hysterectomized woman, no lateral channel is necessary. Therefore, it is appropriate to do total colpocleisis.18,19
- When a patient with POP has a rectovaginal or vesicovaginal fistula caused by a neglected pessary, the addition of LeFort colpocleisis to the fistula repair may provide an effective treatment for both problems.10
Surgical outcomes
Success rate
Evidence concerning colpocleisis comes from case series, some of which are more than 30 years old. Although the definition of success is not clear in some series, the reported success rate has always exceeded 90% over the past three decades.2,18-22 Moreover, some of these reports involve as many as 30 years of follow-up.
Perioperative complication
In a recent review of the literature, the procedure-related mortality rate was 0.025%.2 When the authors focused only on studies published since 1980, major complications due to the patient’s underlying cardiovascular and pulmonary condition were seen in 2% of cases. Major surgical complications such as pyelonephritis and bleeding requiring transfusion occurred in 4% of cases, and less severe complications occurred in 15%.
In a study that included women who underwent concomitant vaginal hysterectomy, hysterectomy prolonged the surgery by 52 minutes, with a 5% rate of laparotomy as a result of intraoperative bleeding.22
In our series of 40 colpocleisis cases, we noted no instance in which a patient regretted the procedure.18 Others have also reported a low rate of regret—the highest being 9%.3-5,19-21
Using validated questionnaires, FitzGerald and colleagues found significant improvement in mental and physical quality of life, as well as urinary, colorectal, and bulge-related pelvic floor symptoms, 1 year after colpocleisis.5
De novo or worsening urinary incontinence is one of the drawbacks of colpocleisis. However, the same risk is present in approximately 40% of women who undergo surgical reconstructive procedures for POP without a continence operation.23 Because preoperative urinary retention is common in women who have POP, the decision to add a potentially harmful continence procedure is complicated in colpocleisis candidates. A small case series reported that the success rate ranged from 90% to 94% in women who underwent a midurethral tension-free sling procedure for the treatment of urinary incontinence at the time of colpocleisis.5
Preoperative urodynamic studies to detect urethral intrinsic deficiency and detrusor dysfunction are prudent, and detailed counseling of the patient about urinary control is vital. We perform a midurethral sling procedure in most of our colpocleisis cases, and have had pleasing results.
CASE 3 RESOLVED
The patient decides to undergo partial colpocleisis using the LeFort procedure, along with placement of a midurethral sling, for a total operative time of 75 minutes. She is discharged 1 day later and reports substantial improvement in urinary function and quality of life.
1. Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1503.
2. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Weber A. For the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271.
3. Wheeler TL, Jr, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. Am J Obstet Gynecol. 2005;193:2067-2070.
4. Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
5. FitzGerald MP, Richter HE, Bradley CS, et al. For the Pelvic Floor Disorders Network. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1603-1609.
6. Patel D, Gillespie B, Foxman B. Sexual behavior of older women: results of a random-digit-dialing survey of 2,000 women in the United States. Sex Transm Dis. 2003;30:216-220.
7. Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1-184.e7.
8. Berlin F. Three cases of complete prolapsus uteri operated upon according to the method of Leon LeFort. Am J Obstet Gynecol. 1881;14:866-868.
9. Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 Pt 1):931-935.
10. Esin S, Harmanli OH. Large vesicovaginal fistula in women with pelvic organ prolapse: the role of colpocleisis revisited. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1711-1713.
11. Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111-2118.
12. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96:583-589.
13. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;113(2 Pt 1):462-464.
14. Moore RD, Miklos JR. Colpocleisis and tension-free vaginal tape sling for severe uterine and vaginal prolapse and stress urinary incontinence under local anesthesia. J Am Assoc Gynecol Laparosc. 2003;10:276-280.
15. Buchsbaum GM, Albushies DT, Schoenecker E, Duecy EE, Glantz JC. Local anesthesia with sedation for vaginal reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:211-214.
16. Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1478-1485.
17. Goodall JR, Power RMH. A modification of the Le Fort operation for increasing its scope. Am J Obstet Gynecol. 1937;34:968-976.
18. Harmanli OH, Dandolu V, Chatwani AJ, Grody MT. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
19. DeLancey JOL, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1232.
20. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
21. Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of Le Fort’s operation. Obstet Gynecol. 1973;42:415-420.
22. Von Pechmann WS, Mutone MD, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
23. Albo ME, Richter HE, Brubaker L, et al. For Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
1. Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1503.
2. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Weber A. For the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271.
3. Wheeler TL, Jr, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. Am J Obstet Gynecol. 2005;193:2067-2070.
4. Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
5. FitzGerald MP, Richter HE, Bradley CS, et al. For the Pelvic Floor Disorders Network. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1603-1609.
6. Patel D, Gillespie B, Foxman B. Sexual behavior of older women: results of a random-digit-dialing survey of 2,000 women in the United States. Sex Transm Dis. 2003;30:216-220.
7. Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1-184.e7.
8. Berlin F. Three cases of complete prolapsus uteri operated upon according to the method of Leon LeFort. Am J Obstet Gynecol. 1881;14:866-868.
9. Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 Pt 1):931-935.
10. Esin S, Harmanli OH. Large vesicovaginal fistula in women with pelvic organ prolapse: the role of colpocleisis revisited. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1711-1713.
11. Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111-2118.
12. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96:583-589.
13. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;113(2 Pt 1):462-464.
14. Moore RD, Miklos JR. Colpocleisis and tension-free vaginal tape sling for severe uterine and vaginal prolapse and stress urinary incontinence under local anesthesia. J Am Assoc Gynecol Laparosc. 2003;10:276-280.
15. Buchsbaum GM, Albushies DT, Schoenecker E, Duecy EE, Glantz JC. Local anesthesia with sedation for vaginal reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:211-214.
16. Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1478-1485.
17. Goodall JR, Power RMH. A modification of the Le Fort operation for increasing its scope. Am J Obstet Gynecol. 1937;34:968-976.
18. Harmanli OH, Dandolu V, Chatwani AJ, Grody MT. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
19. DeLancey JOL, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1232.
20. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
21. Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of Le Fort’s operation. Obstet Gynecol. 1973;42:415-420.
22. Von Pechmann WS, Mutone MD, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
23. Albo ME, Richter HE, Brubaker L, et al. For Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.

