User login
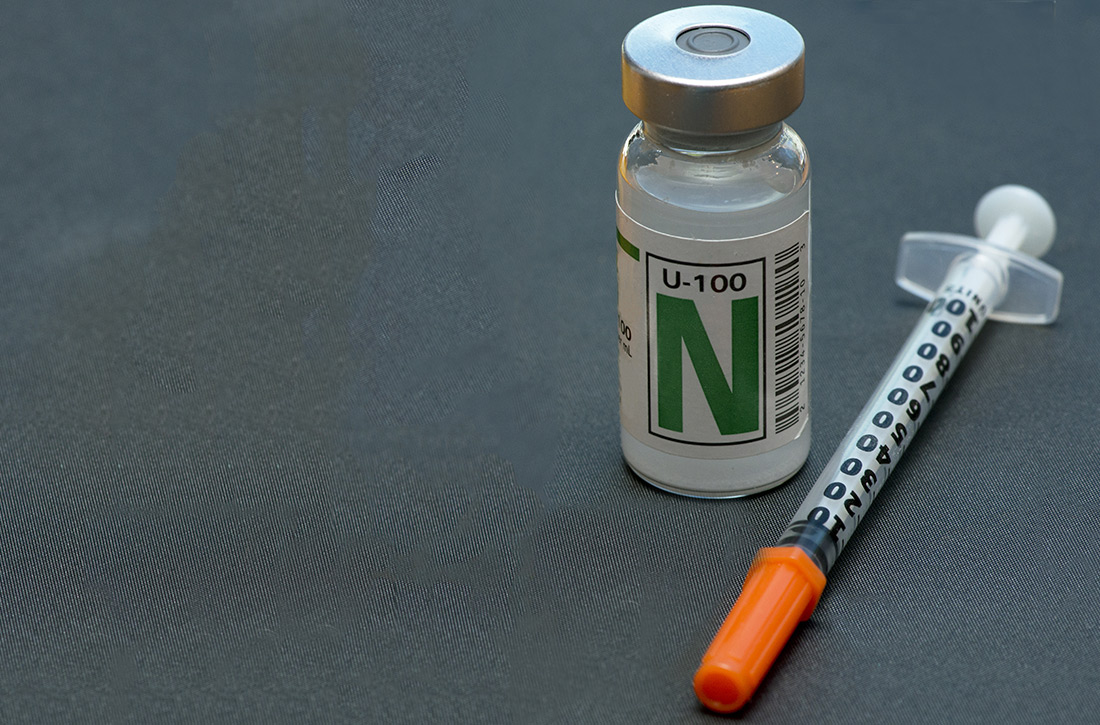
NPH insulin: It remains a good option
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
PRACTICE CHANGER
Consider NPH insulin for patients who require initiation of long-acting insulin therapy because it is as safe as, and more cost-effective than, basal insulin analogs.
STRENGTH OF RECOMMENDATION
B: Based on a single, large, retrospective, observational study.
Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.1