User login
Chasing the dragon
CASE: Confusion after discharge
Mr. G, age 37, is transferred to our medical center from a local hospital for treatment of altered mental status. His wife reports that 1 month ago he had been admitted to a different hospital for a heroin overdose. His urine toxicology screen then was positive for benzodiazepines, cocaine, and opioids. Mr. G’s 2-week stay was complicated by respiratory arrest, intubation, and mechanical ventilation. He also developed hypotension, acute renal failure, and aspiration pneumonia, but recovered.
His wife says 2 weeks after Mr. G was discharged home, she noticed he was becoming increasingly confused and forgetful. Initially she observed dificulty with short-term memory. He was involved in a motor vehicle accident far from home while reporting to a job he no longer held. She found him confused and watering the lawn in the rain. After she discovered him talking on the phone with no one on the line, she brought him to the emergency room (ER).
His urine toxicology screen was negative. Routine examination of cerebrospinal fluid and tests for glucose, protein, lactate, lactate dehydrogenase, red blood cell count, white blood cell count with differential, syphilis serology, Gram’s stain, and bacterial culture were negative.
Brain MRI showed diffuse new white matter signal abnormality superior to the tentorium of the cerebellum, suggestive of low-grade white matter ischemia or inflammation. Mr. G’s mental status did not improve in the ER, and he was transferred to our facility.
The authors’ observations
Based on abnormal brain imaging findings, we initially suspect a type of white matter disorder ( Table 1 ).1 We attempt to conduct a thorough history.
Table 1
Differential diagnoses: Types of white matter disorders
| Category | Example |
|---|---|
| Genetic | Metachromatic leukodystrophy |
| Demyelinative | Multiple sclerosis |
| Infectious | AIDS dementia complex |
| Inflammatory | Systemic lupus erythematosus |
| Toxic | Toluene leukoencephalopathy |
| Metabolic | Vitamin B12 deficiency |
| Vascular | Binswanger’s disease* |
| Traumatic | Traumatic brain injury |
| Neoplastic | Gliomatosis cerebri |
| Hydrocephalic | Normal pressure hydrocephalus |
| *Degenerative dementia caused by thinning of subcortical white matter of the brain | |
| AIDS: acquired immune deficiency syndrome | |
| Source: Reference 1 | |
HISTORY: Missing information
Attempts to obtain collateral information are largely unsuccessful. Mr. G denies having a history of medical or psychiatric illness. He is vague about substance use but may have a history of opioid and cocaine dependence and alcohol abuse. He says he takes no prescribed or over-the-counter medications and has no known drug allergies.
Mr. G’s wife provides limited additional information. She married Mr. G 6 months ago; before that, he was in jail for 3.5 years for unclear reasons. He is unemployed, and the couple has no children.
Mr. G’s wife reports that Mr. G’s father had a history of diabetes mellitus and dialysis and died in his 40s from “Staph infection of the brain.” Mr. G is estranged from his mother. He has no family history of neurologic or psychiatric illness.
Mr. G’s wife denies that her husband has had recent fever, chills, weight loss, nausea, vomiting, diarrhea, or skin rash. He has no history of alcohol withdrawal symptoms, seizures, headache, diplopia, vertigo, hearing loss, swallowing difficulty, focal weakness, or sensory or speech changes. She did not notice personality or behavior changes in her husband before his recent confusion.
The authors’ observations
During our interview, Mr. G maintains minimal eye contact. His speech is minimal with impaired fluency; he responds to questions with 1- or 2-word answers. He describes his mood as “fine” but exhibits an incongruent and constricted affect, alternately laughing and crying. We are unable to assess his thought process and content because Mr. G is emotionally labile and unable to respond rationally to many of our questions. Mr. G’s insight and judgment are poor. His hygiene, grooming, and teeth also are poor, and he is wearing diapers for bowel/bladder incontinence.
Mr. G scores 9/30 on the Mini-Mental State Exam (MMSE), indicating severe cognitive impairment. He is not oriented to place or time, and cannot:
- spell “world” backwards
- subtract serial 7s from 100
- repeat the phrase “no ifs, ands, or buts”
- name 5 U.S. cities
- write any words
- copy a figure of intersecting pentagons.
Neurologic exam reveals apathy, inattention, impaired executive function, and generalized hyperreflexia with bilateral unsustained ankle clonus and Babinski’s sign. In addition, Mr. G has a snout reflex, bilateral hand and foot grasping, and bilateral palmomental reflexes but no mydriasis or nasal septum perforation. Repeat MRI shows the same white matter changes.
Based on Mr. G’s history and brain imaging findings, we suspect that he is suffering from toxic leukoencephalopathy. He meets these diagnostic criteria:
- documented exposure to a toxin
- neurobehavioral deficits
- neuroradiologic abnormalities.2
Toxic leukoencephalopathy can be caused by environmental exposure, radiation, chemotherapy, or substance abuse.3 Because Mr. G has a history of substance abuse, we believe his symptoms developed as a result of heroin vapor inhalation.
‘Chasing the dragon’
Inhaling heroin vapor is known by drug users as “chasing the dragon.” Users place a small amount of heroin powder on aluminum foil, which they heat from below with a flame. The heroin liquidizes and emits a white vapor, which users inhale.3
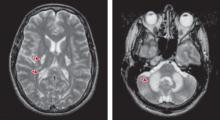
This method of heroin use can result in a form of toxic spongiform leukoencephalopathy. Brain imaging shows widespread white matter hyperintensities involving both supra and infratentorial compartments that are considered highly specific for this type of leukoencephalopathy ( Figure ). These hyperintensities are most commonly found in the:
- posterior cerebral and cerebellar white matter
- cerebellar peduncles
- splenium of the corpus callosum
- posterior limb of the internal capsule.4
Involvement of the cerebellum and posterior limb of the internal capsule while sparing the anterior limb helps to distinguish heroin vapor inhalation from other causes of toxic leukoencephalopathy.3 Extensive damage to the white matter is believed to be caused by a contaminant that is activated when heroin is heated.4,5
Toxic leukoencephalopathy has not been observed in heroin users who snort or inject the drug. Despite the prevalence of heroin abuse, fewer than 100 cases of leukoencephalopathy associated with heroin vapor inhalation have been reported as of 2000, the most recent year for which data are available.6 Patients with this form of leukoencephalopathy typically progress through 3 clinical stages: initial, intermediate, and terminal ( Table 2 ).4
Figure: White matter changes in a patient who inhaled heroin vapor
Photos: © Frank Gaillard/Radiopaedia.org
Seen on brain MRI as ultra-white patches, white matter hyperintensities (WMHs) are areas of increased signal intensity that indicate injury to the axons. In this typical patient (not Mr. G) who developed toxic leukoencephalopathy from heroin vapor inhalation, WMHs are evident in supra and infratentorial compartments, with characteristic involvement of the posterior limb of the internal capsule and cerebellum. Table 2
Stages of heroin vapor inhalation leukoencephalopathy
| Stage | Features |
|---|---|
| Initial | Soft (pseudobulbar) speech, cerebellar ataxia, motor restlessness, apathy/bradyphrenia |
| Intermediate | Pyramidal tract lesions, pseudobulbar reflexes, spastic paresis, tremor/myoclonic jerks, choreoathetoid movements |
| Terminal | Stretching spasms, hypotonic paresis, akinetic mutism, central pyrexia, death |
| Source: Reference 4 | |
TREATMENT Stimulant medication
We prescribe methylphenidate, 2.5 mg bid, to which Mr. G responds well. His cognition and mood improve, he is more goal-directed in his responses, and his MMSE score increases to 13/30. Mr. G eventually is able to converse minimally, and he confirms that he had heated heroin on a piece of foil and inhaled the vapors through his mouth.
Mr. G reports on the day of discharge that he still has cravings for illicit drugs and plans to continue using them. He is not interested in chemical dependency treatment.
The authors’ observations
Little published data exist on treating toxic leukoencephalopathy. Treatment mainly is supportive, although some researchers have suggested a role for coenzyme Q and vitamin supplements.3
Some studies have found methylphenidate beneficial in treating cognitive slowing in cancer patients.1,7 The extent of Mr. G’s cognitive impairment—which was severe—and evidence supporting stimulant medication prompted us to prescribe a low-dose methylphenidate trial, even though we were well aware of its abuse potential. Mr. G improved after starting methylphenidate, but unfortunately he was lost to follow-up.
Related resource
- Filley CM, Kleinschmidt-Demasters BK. Toxic leukoencephalopathy. N Engl J Med. 2001;345(6):425-432.
Drug brand name
- Methylphenidate • Ritalin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Filley CM. Neurobehavioral aspects of cerebral white matter disorders. Psychiatr Clin North Am. 2005;28(3):685-700.
2. Jordan JD, Lloyd T, Pardo-Villamizar C. Case 16: Chasing the dragon. Medscape General Medicine. 2007;9(2):14.-Available at: http://www.medscape.com/viewarticle/554308. Accessed December 7, 2009.
3. Keogh CF, Andrews GT, Spacey SD, et al. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon.” Am J Roentgen. 2003;180:847-850.
4. Hagel J, Andrews G, Vertinsky T, et al. “Chasing the dragon”—imaging of heroin inhalation leukoencephalopathy. Can Assoc Radiol J. 2005;56(4):199-203.
5. Wolters EC, van Wijngaarden GK, Stam FC, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet. 1982;2:1233-1237.
6. Hill MD, Cooper PW, Perry JR. Chasing the dragon—neurological toxicity associated with inhalation of heroin vapor: case report. CMAJ. 2000;162:236-238.
7. Weitzner MA, Meyers CA, Valentine AD. Methylphenidate in the treatment of neurobehavioral slowing associated with cancer and cancer treatment. J Neuropsychiatry Clin Neurosci. 1995;7:347-350.
CASE: Confusion after discharge
Mr. G, age 37, is transferred to our medical center from a local hospital for treatment of altered mental status. His wife reports that 1 month ago he had been admitted to a different hospital for a heroin overdose. His urine toxicology screen then was positive for benzodiazepines, cocaine, and opioids. Mr. G’s 2-week stay was complicated by respiratory arrest, intubation, and mechanical ventilation. He also developed hypotension, acute renal failure, and aspiration pneumonia, but recovered.
His wife says 2 weeks after Mr. G was discharged home, she noticed he was becoming increasingly confused and forgetful. Initially she observed dificulty with short-term memory. He was involved in a motor vehicle accident far from home while reporting to a job he no longer held. She found him confused and watering the lawn in the rain. After she discovered him talking on the phone with no one on the line, she brought him to the emergency room (ER).
His urine toxicology screen was negative. Routine examination of cerebrospinal fluid and tests for glucose, protein, lactate, lactate dehydrogenase, red blood cell count, white blood cell count with differential, syphilis serology, Gram’s stain, and bacterial culture were negative.
Brain MRI showed diffuse new white matter signal abnormality superior to the tentorium of the cerebellum, suggestive of low-grade white matter ischemia or inflammation. Mr. G’s mental status did not improve in the ER, and he was transferred to our facility.
The authors’ observations
Based on abnormal brain imaging findings, we initially suspect a type of white matter disorder ( Table 1 ).1 We attempt to conduct a thorough history.
Table 1
Differential diagnoses: Types of white matter disorders
| Category | Example |
|---|---|
| Genetic | Metachromatic leukodystrophy |
| Demyelinative | Multiple sclerosis |
| Infectious | AIDS dementia complex |
| Inflammatory | Systemic lupus erythematosus |
| Toxic | Toluene leukoencephalopathy |
| Metabolic | Vitamin B12 deficiency |
| Vascular | Binswanger’s disease* |
| Traumatic | Traumatic brain injury |
| Neoplastic | Gliomatosis cerebri |
| Hydrocephalic | Normal pressure hydrocephalus |
| *Degenerative dementia caused by thinning of subcortical white matter of the brain | |
| AIDS: acquired immune deficiency syndrome | |
| Source: Reference 1 | |
HISTORY: Missing information
Attempts to obtain collateral information are largely unsuccessful. Mr. G denies having a history of medical or psychiatric illness. He is vague about substance use but may have a history of opioid and cocaine dependence and alcohol abuse. He says he takes no prescribed or over-the-counter medications and has no known drug allergies.
Mr. G’s wife provides limited additional information. She married Mr. G 6 months ago; before that, he was in jail for 3.5 years for unclear reasons. He is unemployed, and the couple has no children.
Mr. G’s wife reports that Mr. G’s father had a history of diabetes mellitus and dialysis and died in his 40s from “Staph infection of the brain.” Mr. G is estranged from his mother. He has no family history of neurologic or psychiatric illness.
Mr. G’s wife denies that her husband has had recent fever, chills, weight loss, nausea, vomiting, diarrhea, or skin rash. He has no history of alcohol withdrawal symptoms, seizures, headache, diplopia, vertigo, hearing loss, swallowing difficulty, focal weakness, or sensory or speech changes. She did not notice personality or behavior changes in her husband before his recent confusion.
The authors’ observations
During our interview, Mr. G maintains minimal eye contact. His speech is minimal with impaired fluency; he responds to questions with 1- or 2-word answers. He describes his mood as “fine” but exhibits an incongruent and constricted affect, alternately laughing and crying. We are unable to assess his thought process and content because Mr. G is emotionally labile and unable to respond rationally to many of our questions. Mr. G’s insight and judgment are poor. His hygiene, grooming, and teeth also are poor, and he is wearing diapers for bowel/bladder incontinence.
Mr. G scores 9/30 on the Mini-Mental State Exam (MMSE), indicating severe cognitive impairment. He is not oriented to place or time, and cannot:
- spell “world” backwards
- subtract serial 7s from 100
- repeat the phrase “no ifs, ands, or buts”
- name 5 U.S. cities
- write any words
- copy a figure of intersecting pentagons.
Neurologic exam reveals apathy, inattention, impaired executive function, and generalized hyperreflexia with bilateral unsustained ankle clonus and Babinski’s sign. In addition, Mr. G has a snout reflex, bilateral hand and foot grasping, and bilateral palmomental reflexes but no mydriasis or nasal septum perforation. Repeat MRI shows the same white matter changes.
Based on Mr. G’s history and brain imaging findings, we suspect that he is suffering from toxic leukoencephalopathy. He meets these diagnostic criteria:
- documented exposure to a toxin
- neurobehavioral deficits
- neuroradiologic abnormalities.2
Toxic leukoencephalopathy can be caused by environmental exposure, radiation, chemotherapy, or substance abuse.3 Because Mr. G has a history of substance abuse, we believe his symptoms developed as a result of heroin vapor inhalation.
‘Chasing the dragon’
Inhaling heroin vapor is known by drug users as “chasing the dragon.” Users place a small amount of heroin powder on aluminum foil, which they heat from below with a flame. The heroin liquidizes and emits a white vapor, which users inhale.3
This method of heroin use can result in a form of toxic spongiform leukoencephalopathy. Brain imaging shows widespread white matter hyperintensities involving both supra and infratentorial compartments that are considered highly specific for this type of leukoencephalopathy ( Figure ). These hyperintensities are most commonly found in the:
- posterior cerebral and cerebellar white matter
- cerebellar peduncles
- splenium of the corpus callosum
- posterior limb of the internal capsule.4
Involvement of the cerebellum and posterior limb of the internal capsule while sparing the anterior limb helps to distinguish heroin vapor inhalation from other causes of toxic leukoencephalopathy.3 Extensive damage to the white matter is believed to be caused by a contaminant that is activated when heroin is heated.4,5
Toxic leukoencephalopathy has not been observed in heroin users who snort or inject the drug. Despite the prevalence of heroin abuse, fewer than 100 cases of leukoencephalopathy associated with heroin vapor inhalation have been reported as of 2000, the most recent year for which data are available.6 Patients with this form of leukoencephalopathy typically progress through 3 clinical stages: initial, intermediate, and terminal ( Table 2 ).4
Figure: White matter changes in a patient who inhaled heroin vapor
Photos: © Frank Gaillard/Radiopaedia.org
Seen on brain MRI as ultra-white patches, white matter hyperintensities (WMHs) are areas of increased signal intensity that indicate injury to the axons. In this typical patient (not Mr. G) who developed toxic leukoencephalopathy from heroin vapor inhalation, WMHs are evident in supra and infratentorial compartments, with characteristic involvement of the posterior limb of the internal capsule and cerebellum. Table 2
Stages of heroin vapor inhalation leukoencephalopathy
| Stage | Features |
|---|---|
| Initial | Soft (pseudobulbar) speech, cerebellar ataxia, motor restlessness, apathy/bradyphrenia |
| Intermediate | Pyramidal tract lesions, pseudobulbar reflexes, spastic paresis, tremor/myoclonic jerks, choreoathetoid movements |
| Terminal | Stretching spasms, hypotonic paresis, akinetic mutism, central pyrexia, death |
| Source: Reference 4 | |
TREATMENT Stimulant medication
We prescribe methylphenidate, 2.5 mg bid, to which Mr. G responds well. His cognition and mood improve, he is more goal-directed in his responses, and his MMSE score increases to 13/30. Mr. G eventually is able to converse minimally, and he confirms that he had heated heroin on a piece of foil and inhaled the vapors through his mouth.
Mr. G reports on the day of discharge that he still has cravings for illicit drugs and plans to continue using them. He is not interested in chemical dependency treatment.
The authors’ observations
Little published data exist on treating toxic leukoencephalopathy. Treatment mainly is supportive, although some researchers have suggested a role for coenzyme Q and vitamin supplements.3
Some studies have found methylphenidate beneficial in treating cognitive slowing in cancer patients.1,7 The extent of Mr. G’s cognitive impairment—which was severe—and evidence supporting stimulant medication prompted us to prescribe a low-dose methylphenidate trial, even though we were well aware of its abuse potential. Mr. G improved after starting methylphenidate, but unfortunately he was lost to follow-up.
Related resource
- Filley CM, Kleinschmidt-Demasters BK. Toxic leukoencephalopathy. N Engl J Med. 2001;345(6):425-432.
Drug brand name
- Methylphenidate • Ritalin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Confusion after discharge
Mr. G, age 37, is transferred to our medical center from a local hospital for treatment of altered mental status. His wife reports that 1 month ago he had been admitted to a different hospital for a heroin overdose. His urine toxicology screen then was positive for benzodiazepines, cocaine, and opioids. Mr. G’s 2-week stay was complicated by respiratory arrest, intubation, and mechanical ventilation. He also developed hypotension, acute renal failure, and aspiration pneumonia, but recovered.
His wife says 2 weeks after Mr. G was discharged home, she noticed he was becoming increasingly confused and forgetful. Initially she observed dificulty with short-term memory. He was involved in a motor vehicle accident far from home while reporting to a job he no longer held. She found him confused and watering the lawn in the rain. After she discovered him talking on the phone with no one on the line, she brought him to the emergency room (ER).
His urine toxicology screen was negative. Routine examination of cerebrospinal fluid and tests for glucose, protein, lactate, lactate dehydrogenase, red blood cell count, white blood cell count with differential, syphilis serology, Gram’s stain, and bacterial culture were negative.
Brain MRI showed diffuse new white matter signal abnormality superior to the tentorium of the cerebellum, suggestive of low-grade white matter ischemia or inflammation. Mr. G’s mental status did not improve in the ER, and he was transferred to our facility.
The authors’ observations
Based on abnormal brain imaging findings, we initially suspect a type of white matter disorder ( Table 1 ).1 We attempt to conduct a thorough history.
Table 1
Differential diagnoses: Types of white matter disorders
| Category | Example |
|---|---|
| Genetic | Metachromatic leukodystrophy |
| Demyelinative | Multiple sclerosis |
| Infectious | AIDS dementia complex |
| Inflammatory | Systemic lupus erythematosus |
| Toxic | Toluene leukoencephalopathy |
| Metabolic | Vitamin B12 deficiency |
| Vascular | Binswanger’s disease* |
| Traumatic | Traumatic brain injury |
| Neoplastic | Gliomatosis cerebri |
| Hydrocephalic | Normal pressure hydrocephalus |
| *Degenerative dementia caused by thinning of subcortical white matter of the brain | |
| AIDS: acquired immune deficiency syndrome | |
| Source: Reference 1 | |
HISTORY: Missing information
Attempts to obtain collateral information are largely unsuccessful. Mr. G denies having a history of medical or psychiatric illness. He is vague about substance use but may have a history of opioid and cocaine dependence and alcohol abuse. He says he takes no prescribed or over-the-counter medications and has no known drug allergies.
Mr. G’s wife provides limited additional information. She married Mr. G 6 months ago; before that, he was in jail for 3.5 years for unclear reasons. He is unemployed, and the couple has no children.
Mr. G’s wife reports that Mr. G’s father had a history of diabetes mellitus and dialysis and died in his 40s from “Staph infection of the brain.” Mr. G is estranged from his mother. He has no family history of neurologic or psychiatric illness.
Mr. G’s wife denies that her husband has had recent fever, chills, weight loss, nausea, vomiting, diarrhea, or skin rash. He has no history of alcohol withdrawal symptoms, seizures, headache, diplopia, vertigo, hearing loss, swallowing difficulty, focal weakness, or sensory or speech changes. She did not notice personality or behavior changes in her husband before his recent confusion.
The authors’ observations
During our interview, Mr. G maintains minimal eye contact. His speech is minimal with impaired fluency; he responds to questions with 1- or 2-word answers. He describes his mood as “fine” but exhibits an incongruent and constricted affect, alternately laughing and crying. We are unable to assess his thought process and content because Mr. G is emotionally labile and unable to respond rationally to many of our questions. Mr. G’s insight and judgment are poor. His hygiene, grooming, and teeth also are poor, and he is wearing diapers for bowel/bladder incontinence.
Mr. G scores 9/30 on the Mini-Mental State Exam (MMSE), indicating severe cognitive impairment. He is not oriented to place or time, and cannot:
- spell “world” backwards
- subtract serial 7s from 100
- repeat the phrase “no ifs, ands, or buts”
- name 5 U.S. cities
- write any words
- copy a figure of intersecting pentagons.
Neurologic exam reveals apathy, inattention, impaired executive function, and generalized hyperreflexia with bilateral unsustained ankle clonus and Babinski’s sign. In addition, Mr. G has a snout reflex, bilateral hand and foot grasping, and bilateral palmomental reflexes but no mydriasis or nasal septum perforation. Repeat MRI shows the same white matter changes.
Based on Mr. G’s history and brain imaging findings, we suspect that he is suffering from toxic leukoencephalopathy. He meets these diagnostic criteria:
- documented exposure to a toxin
- neurobehavioral deficits
- neuroradiologic abnormalities.2
Toxic leukoencephalopathy can be caused by environmental exposure, radiation, chemotherapy, or substance abuse.3 Because Mr. G has a history of substance abuse, we believe his symptoms developed as a result of heroin vapor inhalation.
‘Chasing the dragon’
Inhaling heroin vapor is known by drug users as “chasing the dragon.” Users place a small amount of heroin powder on aluminum foil, which they heat from below with a flame. The heroin liquidizes and emits a white vapor, which users inhale.3
This method of heroin use can result in a form of toxic spongiform leukoencephalopathy. Brain imaging shows widespread white matter hyperintensities involving both supra and infratentorial compartments that are considered highly specific for this type of leukoencephalopathy ( Figure ). These hyperintensities are most commonly found in the:
- posterior cerebral and cerebellar white matter
- cerebellar peduncles
- splenium of the corpus callosum
- posterior limb of the internal capsule.4
Involvement of the cerebellum and posterior limb of the internal capsule while sparing the anterior limb helps to distinguish heroin vapor inhalation from other causes of toxic leukoencephalopathy.3 Extensive damage to the white matter is believed to be caused by a contaminant that is activated when heroin is heated.4,5
Toxic leukoencephalopathy has not been observed in heroin users who snort or inject the drug. Despite the prevalence of heroin abuse, fewer than 100 cases of leukoencephalopathy associated with heroin vapor inhalation have been reported as of 2000, the most recent year for which data are available.6 Patients with this form of leukoencephalopathy typically progress through 3 clinical stages: initial, intermediate, and terminal ( Table 2 ).4
Figure: White matter changes in a patient who inhaled heroin vapor
Photos: © Frank Gaillard/Radiopaedia.org
Seen on brain MRI as ultra-white patches, white matter hyperintensities (WMHs) are areas of increased signal intensity that indicate injury to the axons. In this typical patient (not Mr. G) who developed toxic leukoencephalopathy from heroin vapor inhalation, WMHs are evident in supra and infratentorial compartments, with characteristic involvement of the posterior limb of the internal capsule and cerebellum. Table 2
Stages of heroin vapor inhalation leukoencephalopathy
| Stage | Features |
|---|---|
| Initial | Soft (pseudobulbar) speech, cerebellar ataxia, motor restlessness, apathy/bradyphrenia |
| Intermediate | Pyramidal tract lesions, pseudobulbar reflexes, spastic paresis, tremor/myoclonic jerks, choreoathetoid movements |
| Terminal | Stretching spasms, hypotonic paresis, akinetic mutism, central pyrexia, death |
| Source: Reference 4 | |
TREATMENT Stimulant medication
We prescribe methylphenidate, 2.5 mg bid, to which Mr. G responds well. His cognition and mood improve, he is more goal-directed in his responses, and his MMSE score increases to 13/30. Mr. G eventually is able to converse minimally, and he confirms that he had heated heroin on a piece of foil and inhaled the vapors through his mouth.
Mr. G reports on the day of discharge that he still has cravings for illicit drugs and plans to continue using them. He is not interested in chemical dependency treatment.
The authors’ observations
Little published data exist on treating toxic leukoencephalopathy. Treatment mainly is supportive, although some researchers have suggested a role for coenzyme Q and vitamin supplements.3
Some studies have found methylphenidate beneficial in treating cognitive slowing in cancer patients.1,7 The extent of Mr. G’s cognitive impairment—which was severe—and evidence supporting stimulant medication prompted us to prescribe a low-dose methylphenidate trial, even though we were well aware of its abuse potential. Mr. G improved after starting methylphenidate, but unfortunately he was lost to follow-up.
Related resource
- Filley CM, Kleinschmidt-Demasters BK. Toxic leukoencephalopathy. N Engl J Med. 2001;345(6):425-432.
Drug brand name
- Methylphenidate • Ritalin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Filley CM. Neurobehavioral aspects of cerebral white matter disorders. Psychiatr Clin North Am. 2005;28(3):685-700.
2. Jordan JD, Lloyd T, Pardo-Villamizar C. Case 16: Chasing the dragon. Medscape General Medicine. 2007;9(2):14.-Available at: http://www.medscape.com/viewarticle/554308. Accessed December 7, 2009.
3. Keogh CF, Andrews GT, Spacey SD, et al. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon.” Am J Roentgen. 2003;180:847-850.
4. Hagel J, Andrews G, Vertinsky T, et al. “Chasing the dragon”—imaging of heroin inhalation leukoencephalopathy. Can Assoc Radiol J. 2005;56(4):199-203.
5. Wolters EC, van Wijngaarden GK, Stam FC, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet. 1982;2:1233-1237.
6. Hill MD, Cooper PW, Perry JR. Chasing the dragon—neurological toxicity associated with inhalation of heroin vapor: case report. CMAJ. 2000;162:236-238.
7. Weitzner MA, Meyers CA, Valentine AD. Methylphenidate in the treatment of neurobehavioral slowing associated with cancer and cancer treatment. J Neuropsychiatry Clin Neurosci. 1995;7:347-350.
1. Filley CM. Neurobehavioral aspects of cerebral white matter disorders. Psychiatr Clin North Am. 2005;28(3):685-700.
2. Jordan JD, Lloyd T, Pardo-Villamizar C. Case 16: Chasing the dragon. Medscape General Medicine. 2007;9(2):14.-Available at: http://www.medscape.com/viewarticle/554308. Accessed December 7, 2009.
3. Keogh CF, Andrews GT, Spacey SD, et al. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon.” Am J Roentgen. 2003;180:847-850.
4. Hagel J, Andrews G, Vertinsky T, et al. “Chasing the dragon”—imaging of heroin inhalation leukoencephalopathy. Can Assoc Radiol J. 2005;56(4):199-203.
5. Wolters EC, van Wijngaarden GK, Stam FC, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet. 1982;2:1233-1237.
6. Hill MD, Cooper PW, Perry JR. Chasing the dragon—neurological toxicity associated with inhalation of heroin vapor: case report. CMAJ. 2000;162:236-238.
7. Weitzner MA, Meyers CA, Valentine AD. Methylphenidate in the treatment of neurobehavioral slowing associated with cancer and cancer treatment. J Neuropsychiatry Clin Neurosci. 1995;7:347-350.
A mysterious loss of memory
Case: Worsening memory
Mrs. K, age 46, is being treated by a neurologist for stable relapsing-remitting multiple sclerosis (MS) and migraine headaches when she complains of worsening memory over the past 5 years. She reports having difficulty recalling details of recent events and conversations. She describes occasional word-finding difficulties and problems maintaining her train of thought. She forgets where she places things and has gotten lost while driving, even on familiar routes. Her husband reports she takes more time to process things in general.
Mrs. K’s cognitive decline has affected her daily life and ability to work. For 4 years, she has been an office assistant at a campground, where she takes phone reservations and keeps a site schedule. Formerly simple tasks—such as taking a phone number—have become increasingly difficult, and she cannot recall a list of 3 things to buy at the supermarket without writing them down.
As a teenager, Mrs. K suffered migraines but did not seek treatment, and her headaches remitted for about 10 years. At age 29, she started to experience tunnel vision. Three years later she reported bilateral foot numbness and was diagnosed with MS. She responded well to interferon beta-1b but her migraines returned, occurring several times a week. Her migraines are successfully treated with topiramate, 75 mg/d, for prophylactic therapy and rizatriptan, 10 mg, as needed for abortive therapy. Her medication regimen also includes:
- eszopiclone, 2 mg/d, and amitriptyline, 10 mg/d, for insomnia
- butalbital/aspirin/caffeine, 50/325/40 mg, as needed for tension headaches
- fexofenadine, 12 mg/d, and budesonide, 32 mcg, 4 sprays/d, for allergy symptoms
- esomeprazole, 80 mg/d, and famotidine, 20 mg/d, as needed for dyspepsia
- propranolol, 120 mg/d, for hypertension
- levothyroxine, 75 mcg/d, for hypothyroidism
- conjugatedestrogens, 0.45 mg/d, for hypoestrogenemia
- alprazolam, 0.25 mg/d, aspirin, 81 mg/d, vitamin E, 800 IU/d, and a multivitamin.
The neurologist orders neuropsychological testing. Mrs. K demonstrates some depressive symptoms but is within normal limits across all aspects of neurocognition, including basic and complex attention, memory, bilateral motor functioning, expressive and receptive language, visuospatial/constructional function, and self-regulatory/executive functioning. The neurologist refers Mrs. K for psychiatric evaluation of her depressive symptoms.
The author’s observations
Many neuropsychiatric abnormalities may accompany MS (Table).1 These can be classified as cognitive dysfunction or disturbances in mood, affect, and behavior.
Although the cause of cognitive impairment in patients with MS is unclear, its extent and profound impact on functioning has become widely recognized over the past 20 years.2
An estimated 40% to 65% of patients with MS suffer from cognitive dysfunction.1,3 Testing indicates deficiencies most often in:
- attention
- information processing speed
- working memory
- verbal memory
- visuospatial function
- executive functions.4
- reduced social interactions
- increased sexual dysfunction
- greater difficulty with household tasks.6
Long-term interferon beta-1b treatment prevents MS relapses, but a recent study found that interferon beta-1b had a negative impact on patients’ mental health composite score and in most quality-of-life subscales over 2 years.8 Nevertheless, Mrs. K received interferon beta-1b therapy for at least 9 years without noticing cognitive decline.
Table
Neuropsychiatric conditions associated with MS
| Disorder | Prevalence |
|---|---|
| Major depression | Lifetime prevalence: 50% |
| Bipolar disorder | Estimated prevalence is 10%, twice that of the general population |
| Euphoria | 25% |
| Pseudobulbar affect | Pathological laughing or crying, emotional incontinence; affects 10% of patients |
| Psychosis | 2% to 3% vs 0.5% to 1% in general population |
| Cognitive impairment | 40% to 60% |
| Source: Reference 1 | |
EVALUATION: Dysthymia
Mrs. K reports memory problems as her chief complaint. She also complains of a depressed mood, irritability, distractibility, and insomnia since her memory problems began, and admits being readily tearful. Mrs. K has difficulty “turning off her thinking” at night, which leads to delayed sleep onset, but denies sleeplessness, racing thoughts, or feelings of euphoria.
Her thought process is coherent and goal-directed, and she denies having auditory or visual hallucinations or active or passive suicidal or homicidal ideation. She scores 29/30 on the Mini-Mental State Exam, but by interview she appears to have impaired remote memory. Mrs. K demonstrates unimpaired judgment and good insight.
The author’s observations
Mrs. K meets DSM-IV-TR criteria for dysthymic disorder and agrees to start mirtazapine, 15 mg at bedtime. I chose this antidepressant because Mrs. K continues to complain of difficulty falling asleep, and mirtazapine is known to significantly decrease sleep latency and increase total sleep time. Approximately one-half of patients with MS will experience depression.1,9 In a recent study of 245 MS patients followed in a neurology clinic, two-thirds of those who met criteria for major depressive disorder did not receive antidepressants.10
TREATMENT: A new strategy
After 2 more months Mrs. K’s mood is euthymic and she demonstrates a bright affect, but she experiences continued decline in short- and long-term memory and reports increasing frustration with simple tasks. The rest of her mental status exam is unremarkable. I instruct her to reduce the mirtazapine dosage to 15 mg/d.
At the next visit 10 weeks later, she again presents with a euthymic mood and a bright affect. She says she attempted to decrease mirtazapine but experienced increased irritability so she remained on the 30-mg dose, with a positive effect on her mood and reduced irritability. Unfortunately, her memory problems persist.
Approximately 2 years after Mrs. K’s first visit, I devise a new pharmacologic strategy. Mrs. K believes that she no longer is depressed and that her only problem is her inability to recall events. To address this, I decide to try memantine, which has been shown to cause modest improvement in clinical symptoms in severe stages of Alzheimer’s disease11 and also has been reported to be useful in the treatment of cognitive impairment in some bipolar disorder patients.12 I start memantine at 10 mg/d and titrate up to 20 mg/d in 3 months.
The author’s observations
Mrs. K’s substantial memory improvement while receiving memantine warrants considering the drug for patients with cognitive dysfunction attributable to MS. Memantine is an uncompetitive NMDA receptor antagonist that the FDA approved in 2003 to treat moderate-to-severe Alzheimer’s disease (Box).11,13 It is generally well tolerated and safe, with a low potential for drug-drug interactions. In clinical trials of patients receiving memantine for Alzheimer’s disease and vascular dementia, the most commonly reported side effects were dizziness, headache, constipation, and confusion.14
A recent trial of memantine therapy for MS at the University of Navarra was suspended for reversible mild-to-moderate neurologic side effects.15 A phase II/phase III double-blind placebo-controlled trial at the University of Oregon designed to determine whether memantine is an effective treatment for memory and cognitive problems associated with MS is recruiting participants.16
Memantine has been reported to successfully treat other MS symptoms. A 1997 retrospective study found that 11 patients with acquired pendular nystagmus (APN) secondary to MS experienced complete resolution of APN when given memantine.17
Memantine was FDA-approved in 2003 to treat moderate-to-severe Alzheimer’s disease dementia. The drug also has been used off-label to treat vascular dementia, dementia of Wernicke-Korsakoff syndrome, and acquired pendular nystagmus.11
Although the neurobiologic basis for memantine’s therapeutic activity in patients with dementia is not fully understood, it is thought to reduce glutamatergic excitotoxicity. The mechanism of action is voltage-dependent, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonism with low-to-moderate affinity and fast blocking/unblocking kinetics.12 Its kinetic profile is beneficial because it allows memantine to occupy the receptor for a sufficient time to prevent pathologic activation of glutamate receptors. However, it dissociates when the physiologic activation of glutamate receptors is necessary, thus preserving normal NMDA receptor activity required for learning and memory. By blocking the effects of abnormal glutamate activity, memantine may prevent abnormal neuronal cell death and cognitive dysfunction.
- Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699-723.
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Budesonide • Rhinocort
- Butalbital/Aspirin/Caffeine • Fiorinal
- Conjugated Estrogens • Premarin
- Esomeprazole • Nexium
- Eszopiclone • Lunesta
- Famotidine • Pepcid
- Fexofenadine • Allegra
- Interferon beta-1b • Betaseron
- Levothyroxine • Synthroid
- Memantine • Namenda
- Propranolol • Inderal
- Rizatriptan • Maxalt
- Topiramate • Topamax
Dr. Rao is a speaker for Forest Pharmaceuticals.
Acknowledgement
The author thanks Alexander M. Timchak, MS-IV, Stritch School of Medicine, Loyola University, Chicago, for his assistance with this article.
1. Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20(3):278-285.
2. Amato MP, Portaccio E, Zipoli V. Are there protective treatments for cognitive decline in MS? J Neurol Sci. 2006;245(1-2):183-186.
3. Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16(3):283-288.
4. Hoffmann S, Tittgemeyer M, von Cramon DY. Cognitive impairment in multiple sclerosis. Curr Opin Neurol. 2007;20(3):275-280.
5. Pierson SH, Griffith N. Treatment of cognitive impairment in multiple sclerosis. Behav Neurol. 2006;17(1):53-67.
6. Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: natural history, pathophysiology and management. CNS Drugs. 2002;16(7):445-455.
7. Martin R, Kuzniecky R, Ho S, et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52(2):321-327.
8. Simone IL, Ceccarelli A, Tortorella C, et al. Influence of Interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual Life Outcomes. 2006;4:96.-
9. Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.
10. Mohr DC, Hart SL, Fonareva I, et al. Treatment of depression for patients with multiple sclerosis in neurology clinics. Mult Scler. 2006;12(2):204-208.
11. Kumar S. Memantine: pharmacological properties and clinical uses. Neurol India. 2004;52(3):307-309.
12. Teng CT, Demetrio FN. Memantine may acutely improve cognition and have a mood stabilizing effect in treatment-resistant bipolar disorder. Rev Bras Psiquiatr. 2006;28(3):252-4.
13. Danysz W, Parsons CG, Mobius HJ, et al. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease: a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res. 2000;2(2-3):85-97.
14. Namenda [package insert] St. Louis, MO: Forest Pharmaceuticals; 2007.
15. Memantine therapy for multiple sclerosis (NCT00638833) Available at: http://www.clinicaltrials.gov/ct2/show/NCT00638833. Accessed February 17, 2009.
16. Trial of memantine for cognitive impairment in multiple sclerosis (NCT00300716) Available at: http://www.clinicaltrials.gov/ct2/show/NCT00300716. Accessed February 17, 2009.
17. Starck M, Albrecht H, Pöllmann W, et al. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol. 1997;244(1):9-16.
Case: Worsening memory
Mrs. K, age 46, is being treated by a neurologist for stable relapsing-remitting multiple sclerosis (MS) and migraine headaches when she complains of worsening memory over the past 5 years. She reports having difficulty recalling details of recent events and conversations. She describes occasional word-finding difficulties and problems maintaining her train of thought. She forgets where she places things and has gotten lost while driving, even on familiar routes. Her husband reports she takes more time to process things in general.
Mrs. K’s cognitive decline has affected her daily life and ability to work. For 4 years, she has been an office assistant at a campground, where she takes phone reservations and keeps a site schedule. Formerly simple tasks—such as taking a phone number—have become increasingly difficult, and she cannot recall a list of 3 things to buy at the supermarket without writing them down.
As a teenager, Mrs. K suffered migraines but did not seek treatment, and her headaches remitted for about 10 years. At age 29, she started to experience tunnel vision. Three years later she reported bilateral foot numbness and was diagnosed with MS. She responded well to interferon beta-1b but her migraines returned, occurring several times a week. Her migraines are successfully treated with topiramate, 75 mg/d, for prophylactic therapy and rizatriptan, 10 mg, as needed for abortive therapy. Her medication regimen also includes:
- eszopiclone, 2 mg/d, and amitriptyline, 10 mg/d, for insomnia
- butalbital/aspirin/caffeine, 50/325/40 mg, as needed for tension headaches
- fexofenadine, 12 mg/d, and budesonide, 32 mcg, 4 sprays/d, for allergy symptoms
- esomeprazole, 80 mg/d, and famotidine, 20 mg/d, as needed for dyspepsia
- propranolol, 120 mg/d, for hypertension
- levothyroxine, 75 mcg/d, for hypothyroidism
- conjugatedestrogens, 0.45 mg/d, for hypoestrogenemia
- alprazolam, 0.25 mg/d, aspirin, 81 mg/d, vitamin E, 800 IU/d, and a multivitamin.
The neurologist orders neuropsychological testing. Mrs. K demonstrates some depressive symptoms but is within normal limits across all aspects of neurocognition, including basic and complex attention, memory, bilateral motor functioning, expressive and receptive language, visuospatial/constructional function, and self-regulatory/executive functioning. The neurologist refers Mrs. K for psychiatric evaluation of her depressive symptoms.
The author’s observations
Many neuropsychiatric abnormalities may accompany MS (Table).1 These can be classified as cognitive dysfunction or disturbances in mood, affect, and behavior.
Although the cause of cognitive impairment in patients with MS is unclear, its extent and profound impact on functioning has become widely recognized over the past 20 years.2
An estimated 40% to 65% of patients with MS suffer from cognitive dysfunction.1,3 Testing indicates deficiencies most often in:
- attention
- information processing speed
- working memory
- verbal memory
- visuospatial function
- executive functions.4
- reduced social interactions
- increased sexual dysfunction
- greater difficulty with household tasks.6
Long-term interferon beta-1b treatment prevents MS relapses, but a recent study found that interferon beta-1b had a negative impact on patients’ mental health composite score and in most quality-of-life subscales over 2 years.8 Nevertheless, Mrs. K received interferon beta-1b therapy for at least 9 years without noticing cognitive decline.
Table
Neuropsychiatric conditions associated with MS
| Disorder | Prevalence |
|---|---|
| Major depression | Lifetime prevalence: 50% |
| Bipolar disorder | Estimated prevalence is 10%, twice that of the general population |
| Euphoria | 25% |
| Pseudobulbar affect | Pathological laughing or crying, emotional incontinence; affects 10% of patients |
| Psychosis | 2% to 3% vs 0.5% to 1% in general population |
| Cognitive impairment | 40% to 60% |
| Source: Reference 1 | |
EVALUATION: Dysthymia
Mrs. K reports memory problems as her chief complaint. She also complains of a depressed mood, irritability, distractibility, and insomnia since her memory problems began, and admits being readily tearful. Mrs. K has difficulty “turning off her thinking” at night, which leads to delayed sleep onset, but denies sleeplessness, racing thoughts, or feelings of euphoria.
Her thought process is coherent and goal-directed, and she denies having auditory or visual hallucinations or active or passive suicidal or homicidal ideation. She scores 29/30 on the Mini-Mental State Exam, but by interview she appears to have impaired remote memory. Mrs. K demonstrates unimpaired judgment and good insight.
The author’s observations
Mrs. K meets DSM-IV-TR criteria for dysthymic disorder and agrees to start mirtazapine, 15 mg at bedtime. I chose this antidepressant because Mrs. K continues to complain of difficulty falling asleep, and mirtazapine is known to significantly decrease sleep latency and increase total sleep time. Approximately one-half of patients with MS will experience depression.1,9 In a recent study of 245 MS patients followed in a neurology clinic, two-thirds of those who met criteria for major depressive disorder did not receive antidepressants.10
TREATMENT: A new strategy
After 2 more months Mrs. K’s mood is euthymic and she demonstrates a bright affect, but she experiences continued decline in short- and long-term memory and reports increasing frustration with simple tasks. The rest of her mental status exam is unremarkable. I instruct her to reduce the mirtazapine dosage to 15 mg/d.
At the next visit 10 weeks later, she again presents with a euthymic mood and a bright affect. She says she attempted to decrease mirtazapine but experienced increased irritability so she remained on the 30-mg dose, with a positive effect on her mood and reduced irritability. Unfortunately, her memory problems persist.
Approximately 2 years after Mrs. K’s first visit, I devise a new pharmacologic strategy. Mrs. K believes that she no longer is depressed and that her only problem is her inability to recall events. To address this, I decide to try memantine, which has been shown to cause modest improvement in clinical symptoms in severe stages of Alzheimer’s disease11 and also has been reported to be useful in the treatment of cognitive impairment in some bipolar disorder patients.12 I start memantine at 10 mg/d and titrate up to 20 mg/d in 3 months.
The author’s observations
Mrs. K’s substantial memory improvement while receiving memantine warrants considering the drug for patients with cognitive dysfunction attributable to MS. Memantine is an uncompetitive NMDA receptor antagonist that the FDA approved in 2003 to treat moderate-to-severe Alzheimer’s disease (Box).11,13 It is generally well tolerated and safe, with a low potential for drug-drug interactions. In clinical trials of patients receiving memantine for Alzheimer’s disease and vascular dementia, the most commonly reported side effects were dizziness, headache, constipation, and confusion.14
A recent trial of memantine therapy for MS at the University of Navarra was suspended for reversible mild-to-moderate neurologic side effects.15 A phase II/phase III double-blind placebo-controlled trial at the University of Oregon designed to determine whether memantine is an effective treatment for memory and cognitive problems associated with MS is recruiting participants.16
Memantine has been reported to successfully treat other MS symptoms. A 1997 retrospective study found that 11 patients with acquired pendular nystagmus (APN) secondary to MS experienced complete resolution of APN when given memantine.17
Memantine was FDA-approved in 2003 to treat moderate-to-severe Alzheimer’s disease dementia. The drug also has been used off-label to treat vascular dementia, dementia of Wernicke-Korsakoff syndrome, and acquired pendular nystagmus.11
Although the neurobiologic basis for memantine’s therapeutic activity in patients with dementia is not fully understood, it is thought to reduce glutamatergic excitotoxicity. The mechanism of action is voltage-dependent, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonism with low-to-moderate affinity and fast blocking/unblocking kinetics.12 Its kinetic profile is beneficial because it allows memantine to occupy the receptor for a sufficient time to prevent pathologic activation of glutamate receptors. However, it dissociates when the physiologic activation of glutamate receptors is necessary, thus preserving normal NMDA receptor activity required for learning and memory. By blocking the effects of abnormal glutamate activity, memantine may prevent abnormal neuronal cell death and cognitive dysfunction.
- Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699-723.
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Budesonide • Rhinocort
- Butalbital/Aspirin/Caffeine • Fiorinal
- Conjugated Estrogens • Premarin
- Esomeprazole • Nexium
- Eszopiclone • Lunesta
- Famotidine • Pepcid
- Fexofenadine • Allegra
- Interferon beta-1b • Betaseron
- Levothyroxine • Synthroid
- Memantine • Namenda
- Propranolol • Inderal
- Rizatriptan • Maxalt
- Topiramate • Topamax
Dr. Rao is a speaker for Forest Pharmaceuticals.
Acknowledgement
The author thanks Alexander M. Timchak, MS-IV, Stritch School of Medicine, Loyola University, Chicago, for his assistance with this article.
Case: Worsening memory
Mrs. K, age 46, is being treated by a neurologist for stable relapsing-remitting multiple sclerosis (MS) and migraine headaches when she complains of worsening memory over the past 5 years. She reports having difficulty recalling details of recent events and conversations. She describes occasional word-finding difficulties and problems maintaining her train of thought. She forgets where she places things and has gotten lost while driving, even on familiar routes. Her husband reports she takes more time to process things in general.
Mrs. K’s cognitive decline has affected her daily life and ability to work. For 4 years, she has been an office assistant at a campground, where she takes phone reservations and keeps a site schedule. Formerly simple tasks—such as taking a phone number—have become increasingly difficult, and she cannot recall a list of 3 things to buy at the supermarket without writing them down.
As a teenager, Mrs. K suffered migraines but did not seek treatment, and her headaches remitted for about 10 years. At age 29, she started to experience tunnel vision. Three years later she reported bilateral foot numbness and was diagnosed with MS. She responded well to interferon beta-1b but her migraines returned, occurring several times a week. Her migraines are successfully treated with topiramate, 75 mg/d, for prophylactic therapy and rizatriptan, 10 mg, as needed for abortive therapy. Her medication regimen also includes:
- eszopiclone, 2 mg/d, and amitriptyline, 10 mg/d, for insomnia
- butalbital/aspirin/caffeine, 50/325/40 mg, as needed for tension headaches
- fexofenadine, 12 mg/d, and budesonide, 32 mcg, 4 sprays/d, for allergy symptoms
- esomeprazole, 80 mg/d, and famotidine, 20 mg/d, as needed for dyspepsia
- propranolol, 120 mg/d, for hypertension
- levothyroxine, 75 mcg/d, for hypothyroidism
- conjugatedestrogens, 0.45 mg/d, for hypoestrogenemia
- alprazolam, 0.25 mg/d, aspirin, 81 mg/d, vitamin E, 800 IU/d, and a multivitamin.
The neurologist orders neuropsychological testing. Mrs. K demonstrates some depressive symptoms but is within normal limits across all aspects of neurocognition, including basic and complex attention, memory, bilateral motor functioning, expressive and receptive language, visuospatial/constructional function, and self-regulatory/executive functioning. The neurologist refers Mrs. K for psychiatric evaluation of her depressive symptoms.
The author’s observations
Many neuropsychiatric abnormalities may accompany MS (Table).1 These can be classified as cognitive dysfunction or disturbances in mood, affect, and behavior.
Although the cause of cognitive impairment in patients with MS is unclear, its extent and profound impact on functioning has become widely recognized over the past 20 years.2
An estimated 40% to 65% of patients with MS suffer from cognitive dysfunction.1,3 Testing indicates deficiencies most often in:
- attention
- information processing speed
- working memory
- verbal memory
- visuospatial function
- executive functions.4
- reduced social interactions
- increased sexual dysfunction
- greater difficulty with household tasks.6
Long-term interferon beta-1b treatment prevents MS relapses, but a recent study found that interferon beta-1b had a negative impact on patients’ mental health composite score and in most quality-of-life subscales over 2 years.8 Nevertheless, Mrs. K received interferon beta-1b therapy for at least 9 years without noticing cognitive decline.
Table
Neuropsychiatric conditions associated with MS
| Disorder | Prevalence |
|---|---|
| Major depression | Lifetime prevalence: 50% |
| Bipolar disorder | Estimated prevalence is 10%, twice that of the general population |
| Euphoria | 25% |
| Pseudobulbar affect | Pathological laughing or crying, emotional incontinence; affects 10% of patients |
| Psychosis | 2% to 3% vs 0.5% to 1% in general population |
| Cognitive impairment | 40% to 60% |
| Source: Reference 1 | |
EVALUATION: Dysthymia
Mrs. K reports memory problems as her chief complaint. She also complains of a depressed mood, irritability, distractibility, and insomnia since her memory problems began, and admits being readily tearful. Mrs. K has difficulty “turning off her thinking” at night, which leads to delayed sleep onset, but denies sleeplessness, racing thoughts, or feelings of euphoria.
Her thought process is coherent and goal-directed, and she denies having auditory or visual hallucinations or active or passive suicidal or homicidal ideation. She scores 29/30 on the Mini-Mental State Exam, but by interview she appears to have impaired remote memory. Mrs. K demonstrates unimpaired judgment and good insight.
The author’s observations
Mrs. K meets DSM-IV-TR criteria for dysthymic disorder and agrees to start mirtazapine, 15 mg at bedtime. I chose this antidepressant because Mrs. K continues to complain of difficulty falling asleep, and mirtazapine is known to significantly decrease sleep latency and increase total sleep time. Approximately one-half of patients with MS will experience depression.1,9 In a recent study of 245 MS patients followed in a neurology clinic, two-thirds of those who met criteria for major depressive disorder did not receive antidepressants.10
TREATMENT: A new strategy
After 2 more months Mrs. K’s mood is euthymic and she demonstrates a bright affect, but she experiences continued decline in short- and long-term memory and reports increasing frustration with simple tasks. The rest of her mental status exam is unremarkable. I instruct her to reduce the mirtazapine dosage to 15 mg/d.
At the next visit 10 weeks later, she again presents with a euthymic mood and a bright affect. She says she attempted to decrease mirtazapine but experienced increased irritability so she remained on the 30-mg dose, with a positive effect on her mood and reduced irritability. Unfortunately, her memory problems persist.
Approximately 2 years after Mrs. K’s first visit, I devise a new pharmacologic strategy. Mrs. K believes that she no longer is depressed and that her only problem is her inability to recall events. To address this, I decide to try memantine, which has been shown to cause modest improvement in clinical symptoms in severe stages of Alzheimer’s disease11 and also has been reported to be useful in the treatment of cognitive impairment in some bipolar disorder patients.12 I start memantine at 10 mg/d and titrate up to 20 mg/d in 3 months.
The author’s observations
Mrs. K’s substantial memory improvement while receiving memantine warrants considering the drug for patients with cognitive dysfunction attributable to MS. Memantine is an uncompetitive NMDA receptor antagonist that the FDA approved in 2003 to treat moderate-to-severe Alzheimer’s disease (Box).11,13 It is generally well tolerated and safe, with a low potential for drug-drug interactions. In clinical trials of patients receiving memantine for Alzheimer’s disease and vascular dementia, the most commonly reported side effects were dizziness, headache, constipation, and confusion.14
A recent trial of memantine therapy for MS at the University of Navarra was suspended for reversible mild-to-moderate neurologic side effects.15 A phase II/phase III double-blind placebo-controlled trial at the University of Oregon designed to determine whether memantine is an effective treatment for memory and cognitive problems associated with MS is recruiting participants.16
Memantine has been reported to successfully treat other MS symptoms. A 1997 retrospective study found that 11 patients with acquired pendular nystagmus (APN) secondary to MS experienced complete resolution of APN when given memantine.17
Memantine was FDA-approved in 2003 to treat moderate-to-severe Alzheimer’s disease dementia. The drug also has been used off-label to treat vascular dementia, dementia of Wernicke-Korsakoff syndrome, and acquired pendular nystagmus.11
Although the neurobiologic basis for memantine’s therapeutic activity in patients with dementia is not fully understood, it is thought to reduce glutamatergic excitotoxicity. The mechanism of action is voltage-dependent, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonism with low-to-moderate affinity and fast blocking/unblocking kinetics.12 Its kinetic profile is beneficial because it allows memantine to occupy the receptor for a sufficient time to prevent pathologic activation of glutamate receptors. However, it dissociates when the physiologic activation of glutamate receptors is necessary, thus preserving normal NMDA receptor activity required for learning and memory. By blocking the effects of abnormal glutamate activity, memantine may prevent abnormal neuronal cell death and cognitive dysfunction.
- Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699-723.
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Budesonide • Rhinocort
- Butalbital/Aspirin/Caffeine • Fiorinal
- Conjugated Estrogens • Premarin
- Esomeprazole • Nexium
- Eszopiclone • Lunesta
- Famotidine • Pepcid
- Fexofenadine • Allegra
- Interferon beta-1b • Betaseron
- Levothyroxine • Synthroid
- Memantine • Namenda
- Propranolol • Inderal
- Rizatriptan • Maxalt
- Topiramate • Topamax
Dr. Rao is a speaker for Forest Pharmaceuticals.
Acknowledgement
The author thanks Alexander M. Timchak, MS-IV, Stritch School of Medicine, Loyola University, Chicago, for his assistance with this article.
1. Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20(3):278-285.
2. Amato MP, Portaccio E, Zipoli V. Are there protective treatments for cognitive decline in MS? J Neurol Sci. 2006;245(1-2):183-186.
3. Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16(3):283-288.
4. Hoffmann S, Tittgemeyer M, von Cramon DY. Cognitive impairment in multiple sclerosis. Curr Opin Neurol. 2007;20(3):275-280.
5. Pierson SH, Griffith N. Treatment of cognitive impairment in multiple sclerosis. Behav Neurol. 2006;17(1):53-67.
6. Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: natural history, pathophysiology and management. CNS Drugs. 2002;16(7):445-455.
7. Martin R, Kuzniecky R, Ho S, et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52(2):321-327.
8. Simone IL, Ceccarelli A, Tortorella C, et al. Influence of Interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual Life Outcomes. 2006;4:96.-
9. Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.
10. Mohr DC, Hart SL, Fonareva I, et al. Treatment of depression for patients with multiple sclerosis in neurology clinics. Mult Scler. 2006;12(2):204-208.
11. Kumar S. Memantine: pharmacological properties and clinical uses. Neurol India. 2004;52(3):307-309.
12. Teng CT, Demetrio FN. Memantine may acutely improve cognition and have a mood stabilizing effect in treatment-resistant bipolar disorder. Rev Bras Psiquiatr. 2006;28(3):252-4.
13. Danysz W, Parsons CG, Mobius HJ, et al. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease: a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res. 2000;2(2-3):85-97.
14. Namenda [package insert] St. Louis, MO: Forest Pharmaceuticals; 2007.
15. Memantine therapy for multiple sclerosis (NCT00638833) Available at: http://www.clinicaltrials.gov/ct2/show/NCT00638833. Accessed February 17, 2009.
16. Trial of memantine for cognitive impairment in multiple sclerosis (NCT00300716) Available at: http://www.clinicaltrials.gov/ct2/show/NCT00300716. Accessed February 17, 2009.
17. Starck M, Albrecht H, Pöllmann W, et al. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol. 1997;244(1):9-16.
1. Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20(3):278-285.
2. Amato MP, Portaccio E, Zipoli V. Are there protective treatments for cognitive decline in MS? J Neurol Sci. 2006;245(1-2):183-186.
3. Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16(3):283-288.
4. Hoffmann S, Tittgemeyer M, von Cramon DY. Cognitive impairment in multiple sclerosis. Curr Opin Neurol. 2007;20(3):275-280.
5. Pierson SH, Griffith N. Treatment of cognitive impairment in multiple sclerosis. Behav Neurol. 2006;17(1):53-67.
6. Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: natural history, pathophysiology and management. CNS Drugs. 2002;16(7):445-455.
7. Martin R, Kuzniecky R, Ho S, et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52(2):321-327.
8. Simone IL, Ceccarelli A, Tortorella C, et al. Influence of Interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual Life Outcomes. 2006;4:96.-
9. Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.
10. Mohr DC, Hart SL, Fonareva I, et al. Treatment of depression for patients with multiple sclerosis in neurology clinics. Mult Scler. 2006;12(2):204-208.
11. Kumar S. Memantine: pharmacological properties and clinical uses. Neurol India. 2004;52(3):307-309.
12. Teng CT, Demetrio FN. Memantine may acutely improve cognition and have a mood stabilizing effect in treatment-resistant bipolar disorder. Rev Bras Psiquiatr. 2006;28(3):252-4.
13. Danysz W, Parsons CG, Mobius HJ, et al. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease: a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res. 2000;2(2-3):85-97.
14. Namenda [package insert] St. Louis, MO: Forest Pharmaceuticals; 2007.
15. Memantine therapy for multiple sclerosis (NCT00638833) Available at: http://www.clinicaltrials.gov/ct2/show/NCT00638833. Accessed February 17, 2009.
16. Trial of memantine for cognitive impairment in multiple sclerosis (NCT00300716) Available at: http://www.clinicaltrials.gov/ct2/show/NCT00300716. Accessed February 17, 2009.
17. Starck M, Albrecht H, Pöllmann W, et al. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol. 1997;244(1):9-16.