User login
Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
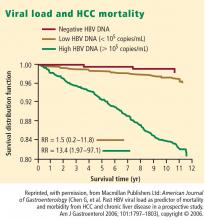
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.
Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.

Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.

Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
KEY POINTS
- A high viral load is a significant predictor of the development of hepatocellular carcinoma in patients aged 30 years or older with chronic HBV infection.
- The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of alanine aminotransferase.
- Continuous antiviral therapy to suppress viral load dramatically reduces the risk of complications from HBV infection and reduces the rate of disease progression, as long as patients maintain a therapeutic response.
Strategies for managing coinfection with hepatitis B virus and HIV
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
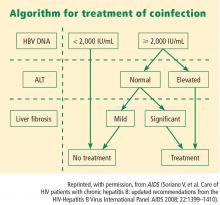
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.
TREATMENT OPTIONS
The potential therapies for HBV in the coinfected patient are the same as those for the patient infected with HBV alone (see “Hepatitis B treatment: Current best practices, avoiding resistance”), with the addition of tenofovir and emtricitabine in combination.
Interferon
Early studies of interferon for the treatment of chronic HBV infection included many patients who were also HIV positive. These early studies revealed a lower rate of HBeAg seroconversion in HIV-positive patients compared with HIV-negative patients. Di Martino et al11 found that approximately half (26 of 50) of HIV-negative patients treated with interferon seroconverted at 6 years, compared with only 4 of 26 interferon-treated patients with chronic HBV who were coinfected with HIV. Based on results such as these, interferon therapy in the HBV/HIV-coinfected patient should be limited to patients who are likely to seroconvert: ie, those who are female and younger than 40 years with high ALT levels, low serum HBV DNA levels, and active liver histology (a subgroup that is more likely to undergo spontaneous seroconversion than other HBV-infected groups).
Standard or pegylated interferon is a treatment option for coinfected patients who do not yet require HAART, especially patients who have high ALT levels, low viral loads, and positive HBeAg status without liver decompensation.12
Nucleoside and nucleotide analogues
The nucleoside and nucleotide analogues used for HBV therapy have different degrees of effectiveness against HIV polymerase, but none can be used as monotherapy because of the risk of inducing HIV resistance. Lamivudine and tenofovir are used as part of the standard cocktail for the treatment of HIV (see “Case: HIV/HBV with resistance to tenofovir”). Entecavir is now recognized as a partial inhibitor of HIV replication. Both lamivudine and entecavir induce the YMDD mutation, an indication of resistance to therapy, in HIV polymerase. Tenofovir also may select for resistance mutations in HIV polymerase.
At dosages used for the treatment of HBV infection, adefovir has weak activity against HIV, and therefore HIV would not be under significant selective pressure to develop resistance mutations. In HIV polymerase, the mutations that confer resistance to adefovir also confer resistance to tenofovir, and therefore use of adefovir may induce tenofovir resistance.
Telbivudine has not been studied in HIV-infected patients, but its resistance profile is similar to that of lamivudine.
Treating both infections
When both HBV and HIV infections require treatment, HAART is necessary for HIV.12 The treatment strategy for coinfection is to use standard therapy for HIV, selecting two agents that are effective against HBV infection.
The need to avoid antiviral resistance complicates the selection of active agents. Resistance to HIV therapy limits the choices for treatment of HBV infection. The immediate aim of therapy, an undetectable level of HBV DNA, eliminates the use of less potent agents. The best choice for therapy is the most potent agent that can be used, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.
Antiviral resistance
For the coinfected patient who develops resistance to lamivudine, the recommendation is to treat with tenofovir plus entecavir (the preferable choice because of absence of cross-reactivity between the two agents) or tenofovir plus lamivudine or emtricitabine. There is some evidence that lamivudine resistance predisposes to entecavir resistance, but the studies that generated these results were conducted in patients who had very high baseline viral loads13; the effectiveness of entecavir in patients with low baseline viral loads is unknown. Presumably, when entecavir is used in combination with another potent nucleoside analogue in coinfected patients, the sensitivity of HBV will be more durable than when entecavir is used as monotherapy.
Long-term monitoring
Long-term monitoring for the coinfected patient is similar to that for the patient infected with HBV only. HBV DNA levels should be monitored every 3 months for signs of resistance until levels have plateaued or become undetectable. Once the HBV DNA level is stable or undetectable, the monitoring interval can be extended. Ultrasonographic screening for hepatocellular carcinoma should be conducted every 6 months. Patients with cirrhosis should be screened for esophageal varices.

SUMMARY
HBV in the setting of HIV is more aggressive than in a patient infected with HBV only, and treatment must be comparably aggressive and carefully selected. The primary goal of HBV treatment in a coinfected patient is the same as in a patient with HBV infection only: reduction of viral load to undetectable levels. Treatment decisions are based on viral load, ALT level, findings on liver biopsy, the need for HAART, and the drug’s resistance profile. None of the nucleoside or nucleotide analogues can be used as monotherapy in the coinfected patient because of the risk of inducing resistance to HIV therapy. When the patient requires HAART, then the general recommendation is to select a combination of two drugs that have activity against HIV. If resistance develops, the preferred strategy is treatment with tenofovir plus entecavir. Monitoring includes measurement of HBV DNA levels every 3 months and ultrasonographic screening for hepatocellular carcinoma every 6 months.
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–S9.
- Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22:1399–1410.
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5:374–382.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev 2007; 9:25–39.
- Hoffman CJ, Thio CL. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis 2007; 7:402–409.
- Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(suppl 1):S65–S70.
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18:2285–2293.
- Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US-Canadian multicenter study. J Hepatol 2007; 47:527–537.
- Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926.
- Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS 2005; 19:2117–2125.
- Di Martino V, Thevenot T, Colin J-F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002; 123:1812–1822.
- Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol 2008; 23:699–706.
- Sherman M, Yurdaydin C, Sollano J, et al; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130:2039–2049.
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.
TREATMENT OPTIONS
The potential therapies for HBV in the coinfected patient are the same as those for the patient infected with HBV alone (see “Hepatitis B treatment: Current best practices, avoiding resistance”), with the addition of tenofovir and emtricitabine in combination.
Interferon
Early studies of interferon for the treatment of chronic HBV infection included many patients who were also HIV positive. These early studies revealed a lower rate of HBeAg seroconversion in HIV-positive patients compared with HIV-negative patients. Di Martino et al11 found that approximately half (26 of 50) of HIV-negative patients treated with interferon seroconverted at 6 years, compared with only 4 of 26 interferon-treated patients with chronic HBV who were coinfected with HIV. Based on results such as these, interferon therapy in the HBV/HIV-coinfected patient should be limited to patients who are likely to seroconvert: ie, those who are female and younger than 40 years with high ALT levels, low serum HBV DNA levels, and active liver histology (a subgroup that is more likely to undergo spontaneous seroconversion than other HBV-infected groups).
Standard or pegylated interferon is a treatment option for coinfected patients who do not yet require HAART, especially patients who have high ALT levels, low viral loads, and positive HBeAg status without liver decompensation.12
Nucleoside and nucleotide analogues
The nucleoside and nucleotide analogues used for HBV therapy have different degrees of effectiveness against HIV polymerase, but none can be used as monotherapy because of the risk of inducing HIV resistance. Lamivudine and tenofovir are used as part of the standard cocktail for the treatment of HIV (see “Case: HIV/HBV with resistance to tenofovir”). Entecavir is now recognized as a partial inhibitor of HIV replication. Both lamivudine and entecavir induce the YMDD mutation, an indication of resistance to therapy, in HIV polymerase. Tenofovir also may select for resistance mutations in HIV polymerase.
At dosages used for the treatment of HBV infection, adefovir has weak activity against HIV, and therefore HIV would not be under significant selective pressure to develop resistance mutations. In HIV polymerase, the mutations that confer resistance to adefovir also confer resistance to tenofovir, and therefore use of adefovir may induce tenofovir resistance.
Telbivudine has not been studied in HIV-infected patients, but its resistance profile is similar to that of lamivudine.

Treating both infections
When both HBV and HIV infections require treatment, HAART is necessary for HIV.12 The treatment strategy for coinfection is to use standard therapy for HIV, selecting two agents that are effective against HBV infection.
The need to avoid antiviral resistance complicates the selection of active agents. Resistance to HIV therapy limits the choices for treatment of HBV infection. The immediate aim of therapy, an undetectable level of HBV DNA, eliminates the use of less potent agents. The best choice for therapy is the most potent agent that can be used, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.
Antiviral resistance
For the coinfected patient who develops resistance to lamivudine, the recommendation is to treat with tenofovir plus entecavir (the preferable choice because of absence of cross-reactivity between the two agents) or tenofovir plus lamivudine or emtricitabine. There is some evidence that lamivudine resistance predisposes to entecavir resistance, but the studies that generated these results were conducted in patients who had very high baseline viral loads13; the effectiveness of entecavir in patients with low baseline viral loads is unknown. Presumably, when entecavir is used in combination with another potent nucleoside analogue in coinfected patients, the sensitivity of HBV will be more durable than when entecavir is used as monotherapy.
Long-term monitoring
Long-term monitoring for the coinfected patient is similar to that for the patient infected with HBV only. HBV DNA levels should be monitored every 3 months for signs of resistance until levels have plateaued or become undetectable. Once the HBV DNA level is stable or undetectable, the monitoring interval can be extended. Ultrasonographic screening for hepatocellular carcinoma should be conducted every 6 months. Patients with cirrhosis should be screened for esophageal varices.

SUMMARY
HBV in the setting of HIV is more aggressive than in a patient infected with HBV only, and treatment must be comparably aggressive and carefully selected. The primary goal of HBV treatment in a coinfected patient is the same as in a patient with HBV infection only: reduction of viral load to undetectable levels. Treatment decisions are based on viral load, ALT level, findings on liver biopsy, the need for HAART, and the drug’s resistance profile. None of the nucleoside or nucleotide analogues can be used as monotherapy in the coinfected patient because of the risk of inducing resistance to HIV therapy. When the patient requires HAART, then the general recommendation is to select a combination of two drugs that have activity against HIV. If resistance develops, the preferred strategy is treatment with tenofovir plus entecavir. Monitoring includes measurement of HBV DNA levels every 3 months and ultrasonographic screening for hepatocellular carcinoma every 6 months.
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.
TREATMENT OPTIONS
The potential therapies for HBV in the coinfected patient are the same as those for the patient infected with HBV alone (see “Hepatitis B treatment: Current best practices, avoiding resistance”), with the addition of tenofovir and emtricitabine in combination.
Interferon
Early studies of interferon for the treatment of chronic HBV infection included many patients who were also HIV positive. These early studies revealed a lower rate of HBeAg seroconversion in HIV-positive patients compared with HIV-negative patients. Di Martino et al11 found that approximately half (26 of 50) of HIV-negative patients treated with interferon seroconverted at 6 years, compared with only 4 of 26 interferon-treated patients with chronic HBV who were coinfected with HIV. Based on results such as these, interferon therapy in the HBV/HIV-coinfected patient should be limited to patients who are likely to seroconvert: ie, those who are female and younger than 40 years with high ALT levels, low serum HBV DNA levels, and active liver histology (a subgroup that is more likely to undergo spontaneous seroconversion than other HBV-infected groups).
Standard or pegylated interferon is a treatment option for coinfected patients who do not yet require HAART, especially patients who have high ALT levels, low viral loads, and positive HBeAg status without liver decompensation.12
Nucleoside and nucleotide analogues
The nucleoside and nucleotide analogues used for HBV therapy have different degrees of effectiveness against HIV polymerase, but none can be used as monotherapy because of the risk of inducing HIV resistance. Lamivudine and tenofovir are used as part of the standard cocktail for the treatment of HIV (see “Case: HIV/HBV with resistance to tenofovir”). Entecavir is now recognized as a partial inhibitor of HIV replication. Both lamivudine and entecavir induce the YMDD mutation, an indication of resistance to therapy, in HIV polymerase. Tenofovir also may select for resistance mutations in HIV polymerase.
At dosages used for the treatment of HBV infection, adefovir has weak activity against HIV, and therefore HIV would not be under significant selective pressure to develop resistance mutations. In HIV polymerase, the mutations that confer resistance to adefovir also confer resistance to tenofovir, and therefore use of adefovir may induce tenofovir resistance.
Telbivudine has not been studied in HIV-infected patients, but its resistance profile is similar to that of lamivudine.

Treating both infections
When both HBV and HIV infections require treatment, HAART is necessary for HIV.12 The treatment strategy for coinfection is to use standard therapy for HIV, selecting two agents that are effective against HBV infection.
The need to avoid antiviral resistance complicates the selection of active agents. Resistance to HIV therapy limits the choices for treatment of HBV infection. The immediate aim of therapy, an undetectable level of HBV DNA, eliminates the use of less potent agents. The best choice for therapy is the most potent agent that can be used, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.
Antiviral resistance
For the coinfected patient who develops resistance to lamivudine, the recommendation is to treat with tenofovir plus entecavir (the preferable choice because of absence of cross-reactivity between the two agents) or tenofovir plus lamivudine or emtricitabine. There is some evidence that lamivudine resistance predisposes to entecavir resistance, but the studies that generated these results were conducted in patients who had very high baseline viral loads13; the effectiveness of entecavir in patients with low baseline viral loads is unknown. Presumably, when entecavir is used in combination with another potent nucleoside analogue in coinfected patients, the sensitivity of HBV will be more durable than when entecavir is used as monotherapy.
Long-term monitoring
Long-term monitoring for the coinfected patient is similar to that for the patient infected with HBV only. HBV DNA levels should be monitored every 3 months for signs of resistance until levels have plateaued or become undetectable. Once the HBV DNA level is stable or undetectable, the monitoring interval can be extended. Ultrasonographic screening for hepatocellular carcinoma should be conducted every 6 months. Patients with cirrhosis should be screened for esophageal varices.

SUMMARY
HBV in the setting of HIV is more aggressive than in a patient infected with HBV only, and treatment must be comparably aggressive and carefully selected. The primary goal of HBV treatment in a coinfected patient is the same as in a patient with HBV infection only: reduction of viral load to undetectable levels. Treatment decisions are based on viral load, ALT level, findings on liver biopsy, the need for HAART, and the drug’s resistance profile. None of the nucleoside or nucleotide analogues can be used as monotherapy in the coinfected patient because of the risk of inducing resistance to HIV therapy. When the patient requires HAART, then the general recommendation is to select a combination of two drugs that have activity against HIV. If resistance develops, the preferred strategy is treatment with tenofovir plus entecavir. Monitoring includes measurement of HBV DNA levels every 3 months and ultrasonographic screening for hepatocellular carcinoma every 6 months.
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–S9.
- Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22:1399–1410.
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5:374–382.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev 2007; 9:25–39.
- Hoffman CJ, Thio CL. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis 2007; 7:402–409.
- Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(suppl 1):S65–S70.
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18:2285–2293.
- Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US-Canadian multicenter study. J Hepatol 2007; 47:527–537.
- Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926.
- Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS 2005; 19:2117–2125.
- Di Martino V, Thevenot T, Colin J-F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002; 123:1812–1822.
- Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol 2008; 23:699–706.
- Sherman M, Yurdaydin C, Sollano J, et al; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130:2039–2049.
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–S9.
- Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22:1399–1410.
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5:374–382.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev 2007; 9:25–39.
- Hoffman CJ, Thio CL. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis 2007; 7:402–409.
- Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(suppl 1):S65–S70.
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18:2285–2293.
- Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US-Canadian multicenter study. J Hepatol 2007; 47:527–537.
- Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926.
- Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS 2005; 19:2117–2125.
- Di Martino V, Thevenot T, Colin J-F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002; 123:1812–1822.
- Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol 2008; 23:699–706.
- Sherman M, Yurdaydin C, Sollano J, et al; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130:2039–2049.
KEY POINTS
- Patients with HBV/HIV coinfection are at relatively high risk of frequent HBV activation, progression to cirrhosis, and death from liver-related causes.
- If the patient does not yet require HAART but requires treatment for HBV, this is itself an indication for HAART, since monotherapy for HBV is associated with development of resistance to HIV therapy.
- Nucleoside and nucleotide analogues should not be used as monotherapy in the HBV/HIV-coinfected patient because of the risk of inducing HIV resistance.