User login
Disseminated Erythematous-Violet Edematous Plaques and Necrotic Nodules
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

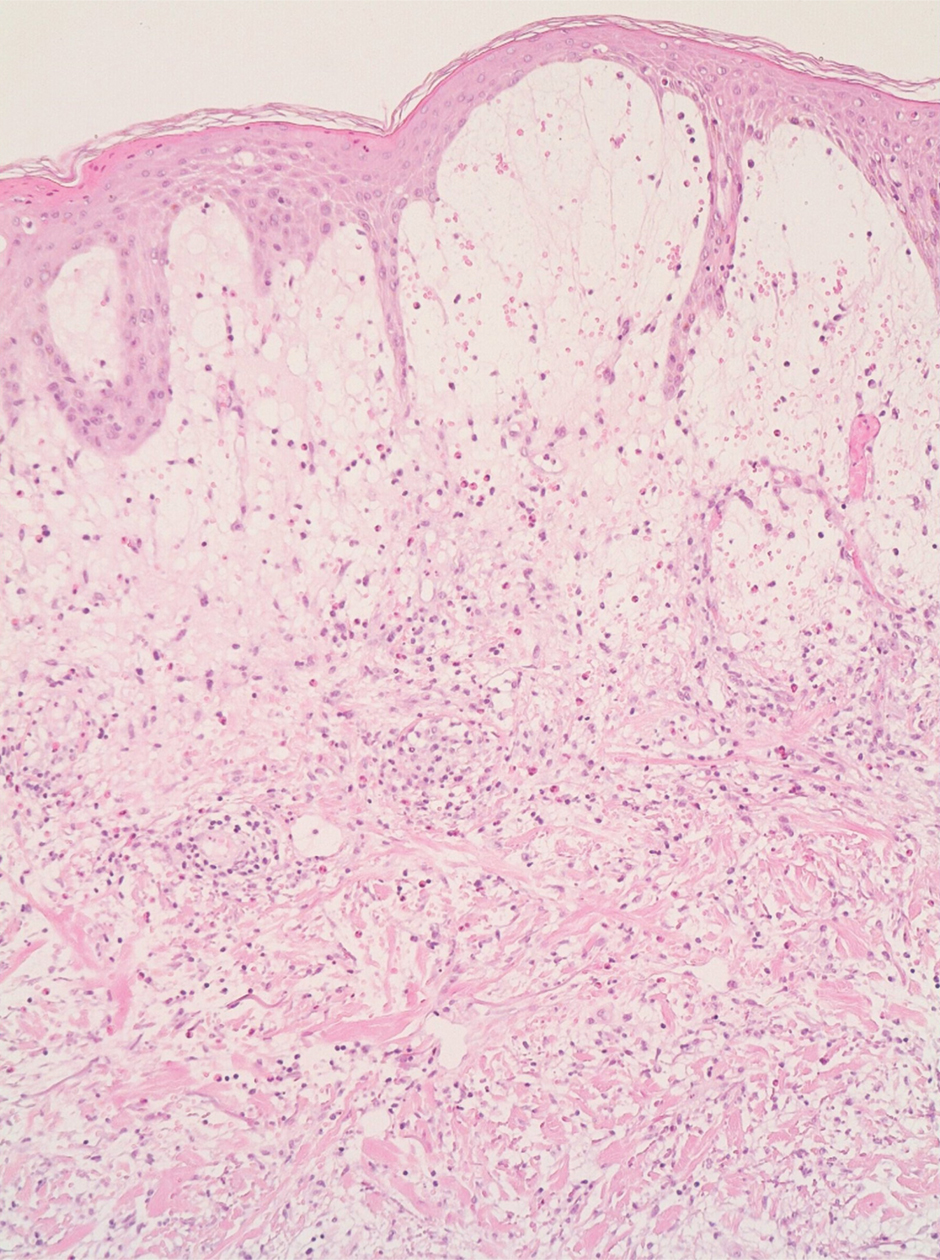
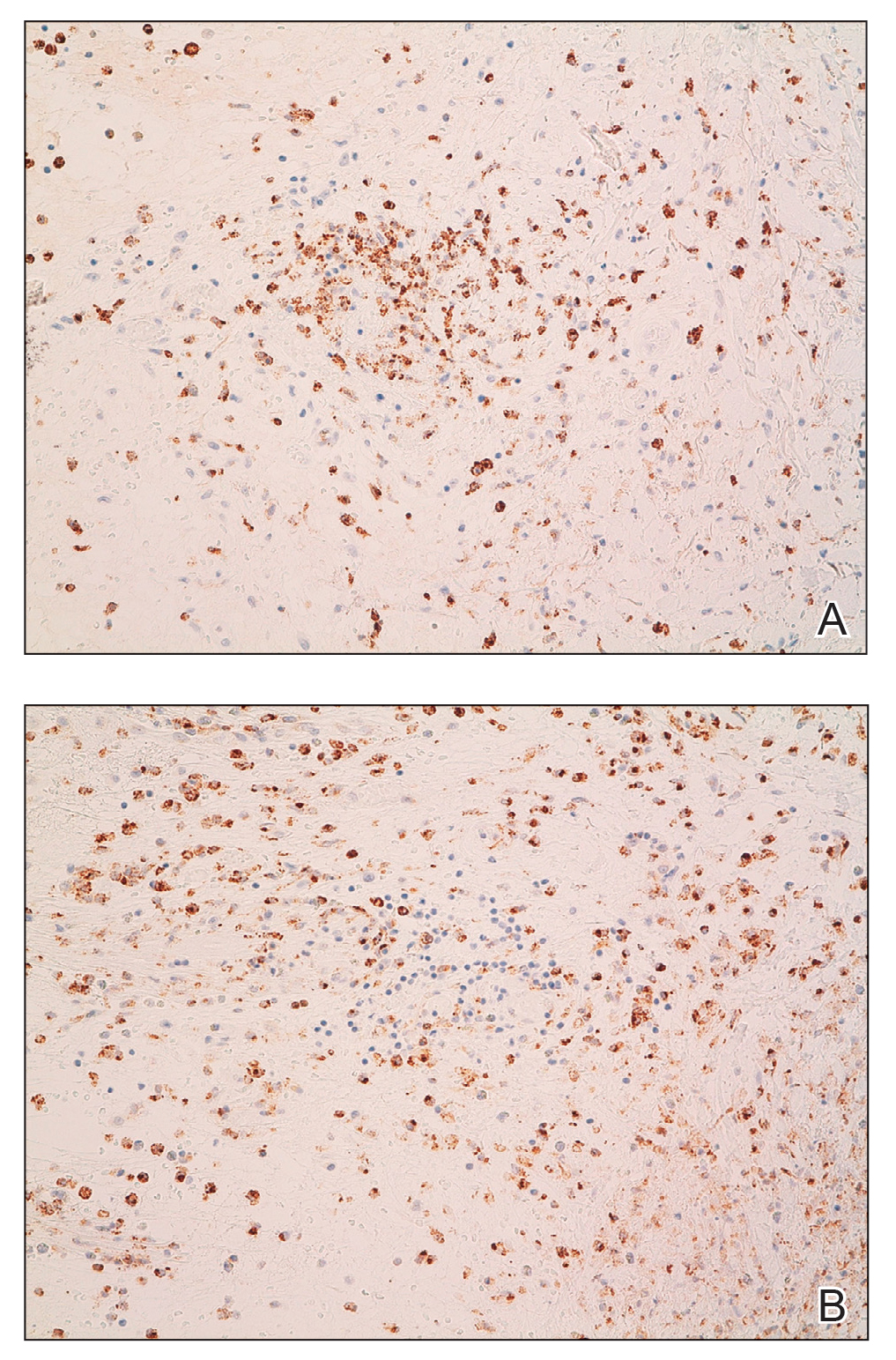
A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.
Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).
The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.

Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).

The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.

Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).

The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
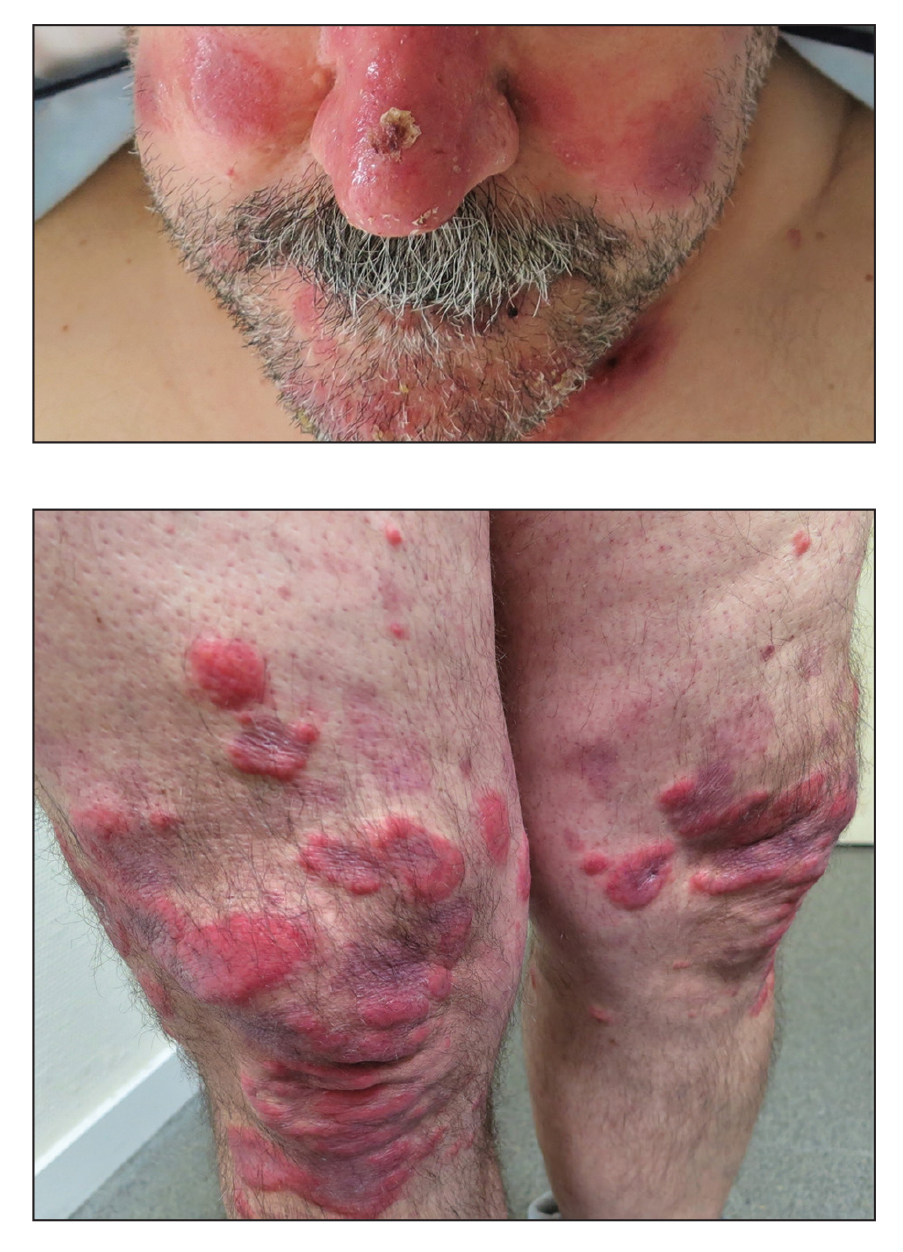
A 53-year-old man presented to the emergency department with a fever and painful skin lesions of 2 days’ duration. He reported a medical history of an upper respiratory infection 4 weeks prior. Physical examination was notable for erythematous-violet edematous papules, necrotic lesions, and pseudovesicles located on the face (top), head, neck, arms, and legs (bottom). Hemorrhagic splinters were evidenced in multiple nail sections. Urgent blood work revealed microcytic anemia (hemoglobin, 12.6 g/dL [reference range, 14.0–17.5 g/dL]) and elevated C-reactive protein (58 mg/L [reference range, 0.0–5.0 mg/L]).