User login
Transdermal contraception: update on clinical management
- The transdermal contraceptive system is a combination hormonal patch that contains 6 mg norelgestromin and 0.75 mg ethinyl estradiol (EE2) in a stable adhesive, and delivers a daily dose of 150 μg norelgestromin and 20 μg EE2to the bloodstream.
- Three large contraceptive trials, in which 3,319 women used the contraceptive patch worldwide, reported low pregnancy rates, with an overall Pearl index of 0.88.
- The primary goals of management are increasing correct and consistent use and effectively counseling and educating women about adverse effects, thereby enhancing continuation rates.
- In studies involving more than 70,000 patches, only 4.7% were replaced because of partial or complete detachment. In 1 health-club study, with physical exertion and variable temperature and humidity, only 1 of 87 patches became completely detached.
- Treatment-limiting events were reported in 1% to 2.4% of women in clinical trials and included nausea and/or vomiting, application-site reaction, breast symptoms, headache, and emotional lability.
The delivery of hormones via a transdermal patch is not a new development. Estrogen and estrogen-progestin transdermal systems have been used for a number of years to treat the symptoms of menopause.1-3 However, until now, no patch-delivery system was able to administer sufficient hormones to prevent pregnancy.
Transdermal delivery systems are warranted for drugs that are highly potent and lipophilic.4 Published studies demonstrate some patient preference for transdermal systems over oral dosing, particularly drug-in-adhesive transdermal systems (as opposed to first-generation reservoir systems).5,6 In 1 study, drug-in-adhesive systems were rated as having better cosmetic appearance, better skin adhesion, greater comfort, and less skin irritation.6 These traits also apply to the contraceptive patch. In clinical studies, the transdermal contraceptive system was shown to have statistically better patient compliance than oral contraceptives (OCs); in 1 trial, compliance was better across all age groups (FIGURE 1).7 This may be due in part to the weekly, rather than daily, dosing regimen.
FIGURE 1 Compliance by age group
The basic product
The transdermal contraceptive system is a combination hormonal patch that contains 6 mg norelgestromin and 0.75 mg ethinyl estradiol (EE2) in a stable adhesive, and delivers a daily dose of 150 μg norelgestromin and 20 μg EE2 to the bloodstream.8 The progestin component, norelgestromin, is the primary active metabolite of norgestimate.
The transdermal contraceptive patch is a thin, matrix-type system consisting of 3 layers. The backing, composed of a flexible beige film, provides structural support. It also protects the middle layer from the environment. The middle layer contains the adhesive and active hormones. The third layer is the release liner, which protects the adhesive layer during storage and is removed just prior to application. This matrix system allows for lower delivery doses and bypasses the first-pass hepatic metabolism of oral administration.
Efficacy
The efficacy of the contraceptive patch is well-established. Three large contraceptive trials, in which 3,319 women used the contraceptive patch worldwide, reported low pregnancy rates, with an overall Pearl index of 0.88 (TABLE 1).9 In 1 randomized clinical trial, 811 women who wore the patch (5,240 cycles) had an overall pregnancy rate of 1.2 per 100 woman-years, compared with 605 women who took an OC (4,167 cycles), with an overall pregnancy rate of 2.18 per 100 woman-years.10 This difference is not statistically significant, but it does indicate that the patch performs at least as well as the OC.10,11 This is important when counseling patients, since women are not used to relying on a transdermal system to prevent pregnancy and may be skeptical that a patch can be effective.
For maximum efficacy, the transdermal system should be initiated as directed. That is, the first patch should be applied on the first day of menses. Another start day, such as Sunday (i.e., the first Sunday after menses begins), can be chosen instead. However, the woman then must use a backup method of contraception for the first 7 days of patch use.
TABLE 1
Contraceptive patch efficacy: pregnancies per 100 woman-years
| STUDY | CYCLES (N) | PREGNANCIES (N) | PEARL INDICES | |||
|---|---|---|---|---|---|---|
| OVERALL PEARL* | METHOD PEARL** | |||||
| Smallwood et al16 | 10,994 | 6 | 0.71 | 0.59 | ||
| Hedon et al11 | 5,921 | 4 | 0.88 | 0.66 | ||
| Audet et al10 | 5,240 | 5 | 1.24 | 0.99 | ||
| TOTAL | 22,155 | 15 | 0.88 | 0.70 | ||
| * User failure plus method failure | ||||||
| ** Failure when taken as directed | ||||||
| Source: Zieman M, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system. Fertil Steril. 2002;76:S13. | ||||||
Patient management
The primary goals of management are increasing correct and consistent use and effectively counseling and educating women about adverse effects, thereby enhancing continuation rates.
Although the theoretical pregnancy rate for oral hormonal contraception is extremely low (0.1% for combination pills), the typical annual pregnancy rate is much higher (8%).12 This difference between perfect and typical use derives mainly from patient errors, such as skipping or delaying pills. In addition, the starting and stopping of hormonal contraception may lead to times during which a woman believes she is protected but is actually at risk for pregnancy. In clinical studies, the difference between the transdermal system’s overall failure and method failure probabilities of pregnancy was only 0.2%.9 In other words, when failures occurred, they were seldom due to patient error. This would suggest that typical-use pregnancy rates for the patch are lower than for combination OCs. Typical-use pregnancy rates are usually based on population surveys and studies; these data are not yet available for the patch.
One aspect of the patch that may lead to better compliance and lower levels of user error is the fact that it needs to be changed only once a week. The patch also has some measure of forgiveness built in: each one contains enough active hormones within the reference range for 2 additional days of contraception. This is advantageous when a woman forgets to change her patch on the scheduled day.
Dosage and administration
Initiation.The transdermal contraceptive system uses the familiar 28-day, 4-week cycle, with a new patch applied every 7 days for 3 weeks, followed by a patch-free week. Withdrawal bleeding occurs during the patch-free interval. At the end of that week, a new patch is applied , starting a new 4-week cycle.
For the initial cycle of use, a woman may place her first patch on the first day of her menstrual period or any day thereafter. (Typically the following Sunday is chosen.) A backup method (e.g., diaphragm or condom) should be used for the first 7 days if the patient initiates use any day after the first day of her period.8
Several situations require extra consideration when initiating transdermal contraceptives:
- When switching from an OC, treatment should begin on the first day of withdrawal bleeding. If no withdrawal bleeding occurs within 5 days of the last active tablet, the woman should consult a health-care professional to rule out pregnancy prior to starting the patch.8
- Nonbreastfeeding women should wait 4 weeks after childbirth before initiating the patch, and should use backup contraception for 7 days.8
- The patch may be initiated immediately (the same day) after a first-trimester abortion or miscarriage. Backup contraception is not necessary if the patch is started within 5 days.8
Application. The patient should be instructed to open the foil pouch carefully, avoiding contact with the adhesive surface after the clear protective liner is peeled away. The patch should be applied to clean, dry, healthy skin on the buttocks, abdomen, upper outer arm, or upper torso, as these were the sites included in studies of the contraceptive. The patch should not be applied to skin that is irritated or cut; nor should it be placed on the breast. No make-up, creams, lotions, powders, or other topical products should be applied to the area where the transdermal system is or will soon be placed. The woman should press down firmly on the patch for at least 10 seconds and check that all edges adhere properly. She also should inspect the patch daily for loss or partial detachment.8 Women may be interested in knowing in advance that the patch can develop a ring of color around it with wear, similar to the ring around an adhesive bandage.
Detachment. The patch delivers an insufficient dose of hormones if it becomes partially or completely detached and remains so. Fortunately, this is not a common problem. In studies involving more than 70,000 patches, only 4.7% were replaced because of partial or complete detachment. In one health-club study where women experienced physical exertion and variable temperature and humidity, only 1 of 87 (1.1%) patches became completely detached.13 Studies also demonstrated that patients learn to use the contraceptive patch more effectively over time, a trend noted in other investigations of transdermal systems.8,14
If a patch partially or fully detaches, the patient can attempt to reapply it. Reapplication should not be attempted if the patch is no longer sticky, becomes stuck to itself or another surface, or has material adhered to it. No other adhesives or wraps should be used to hold the patch in place.8
A replacement program is available in the event a woman needs an extra patch, which can be obtained through the local pharmacy. When the clinician writes the initial prescription for the patch, he or she also writes an additional prescription that can be filled as needed for a single replacement patch. An extra patch would be necessary under the following conditions:
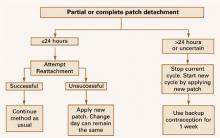
- If the patch has been detached for less than 24 hours, the patient can try to reapply it to the same area. If it does not adhere, it should be replaced with a new patch immediately. No backup contraception is needed, and the patch-change day can remain the same. Alternatively, the day the replacement is placed can become the new patch-change day (FIGURE 2).
- For contraceptive patches detached for more than 24 hours or for an undetermined length of time, a woman may not be protected from pregnancy. She should start a new cycle immediately (new day 1 and new patch-change day) by applying a new patch and using backup contraception for the first week (FIGURE 2). If she had unprotected intercourse, she may be a candidate for emergency contraception.
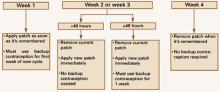
Missed patch-change days. Occasionally, a woman may forget to change the patch on the designated day. As with OCs, the risk of ovulation increases each day beyond the allowed contraceptive-free period (7 days), although, as mentioned earlier, the transdermal system delivers enough hormones to protect a woman for up to 48 additional hours.15 If a woman misses a scheduled change in the middle of her cycle, and less than 48 hours have passed, she should apply a new patch; the patch-change day can remain the same. If more than 48 hours have passed, she may not be protected from pregnancy. She should stop the current cycle and start another 4-week cycle immediately by applying a new patch. (She now has a new patch-change day.) Backup contraception should be used for 1 week (FIGURE 3).8 If she has had unprotected intercourse, she may be a candidate for emergency contraception.
If the third patch is not removed on time, the woman should remove it as soon as she remembers. The next cycle should be started as usual, and no backup contraception is needed. If a woman wants to adjust her patch-change day, she should remove the third contraceptive patch as scheduled and apply a new one on the desired day during the hormone-free week, taking care not to exceed 7 consecutive hormone-free days.8
Visibility. Although the beige patch was designed to minimize visibility, it may be more apparent on women with contrasting skin tones. Until a broad range of colors is available, visibility can be minimized by wearing the patch underneath clothing. (Visibility can sometimes be reassuring to male partners who want to ensure that a contraceptive is being used.)
The designated placement areas (buttocks, abdomen, upper outer arm, or upper torso, excluding the breast), are likely to be covered with clothing and less likely to be noticed, and all sites are therapeutically equivalent. The pharmacokinetics in other application areas is unknown.
Special populations. In 3 large contraceptive trials, the rate of pregnancy did not differ significantly by age or race, or for women with a body weight below 198 lb. However, in women weighing 198 lb or more (approximately 3% of the study population), there was a greater incidence of pregnancy.9 There were only 83 women in this group, yet there were 5 pregnancies. Health-care professionals who consider the contraceptive patch for patients at or above 198 lb should discuss the individual’s needs so that the most appropriate contraceptive, in terms of efficacy and tolerability, can be selected.8
FIGURE 2 Partial or complete patch detachment
FIGURE 3 Patient forgot to apply/change patch (in any patch cycle)
Adverse events
Adverse reactions to the use of hormonal contraception are relatively common, and similar among the different delivery routes. Less serious adverse effects such as breast symptoms, headache, application-site reaction, nausea, and menstrual cramps were reported by women using the contraceptive patch in clinical trials. Treatment-limiting events were reported in 1% to 2.4% of women in clinical trials and included nausea and/or vomiting, application-site reaction, breast symptoms, headache, and emotional lability. The most common adverse effects, as well as those likely to limit treatment in 1 comparative trial, are listed in TABLE 2.10 It is important to counsel patients that these events (with the exception of application-site reaction) are common in users of combination hormonal contraceptives, but tend to resolve by the third cycle of use.
The use of combination hormonal contraceptives also is associated with an increased risk of some serious adverse events. These include venous thromboembolic events, hypertension, gallbladder disease, and hepatic adenomas or benign liver tumors. Since these events are rare, we have limited data specific to the patch. However, the types and incidences of these events appear to be similar to those that occur with combination OCs.
Breast symptoms. These are commonly reported in women using combination hormonal contraceptives. In clinical trials, women who used the patch also reported breast discomfort.10,11 In fact, there was a statistically significant increase in this symptom with the patch in the first 2 cycles of use, compared with OC users, with 1% of participants discontinuing use because of it. However, studies showed that this discomfort was transient and usually resolved by cycle 3. Appropriate counseling about this adverse effect should encourage women to continue use of the method with confidence that the symptoms will likely subside.
Spotting and breakthrough bleeding. Bleeding irregularities are commonly experienced by women who use combination hormonal contraceptives. Breakthrough bleeding and/or spotting (bleeding that occurs on the days that the contraceptive patch is worn) were sometimes encountered by women in clinical trials of the patch.8 Appropriate counseling is recommended so that women are aware of the potential for these irregularities and will continue use of the patch. If breakthrough bleeding persists longer than a few cycles, a cause other than the patch should be considered. In clinical trials, the incidence of breakthrough bleeding was similar in patch and OC users. However, spotting was more common in the first 2 months of patch use.10
In the event of no withdrawal bleeding (bleeding that should occur during the patch-free week), treatment should be resumed on the next scheduled patch-change day. If the patch was initiated and used correctly, the absence of withdrawal bleeding is probably not an indication of pregnancy. Nevertheless, the possibility of pregnancy should be considered, especially if the patch was used incorrectly or with an absence of withdrawal bleeding in 2 consecutive cycles. The contraceptive patch should be discontinued if pregnancy is confirmed. In clinical trials, the incidence of nonpregnancy-related amenorrhea (defined as no withdrawal bleeding for 2 consecutive menstrual periods) was rare (≤0.1%).10
Application-site reactions. These are unique to transdermal delivery systems. If patch use results in uncomfortable irritation, the patch may be removed and a new patch applied to a different location. On the next patch-change day, the application site should be rotated to minimize the risk of skin irritation. As stated earlier, the contraceptive patch should be applied to clean, dry, healthy skin. It should not be placed on skin that has been irritated, cut, or treated with lotion.
TABLE 2
Most common adverse events: patch versus pill
| EVENT | PATCH (N=812) | OC (N=605) | |||
|---|---|---|---|---|---|
| OVERALL (%) | TREATMENT-LIMITING (%) | OVERALL (%) | TREATMENT-LIMITING (%) | ||
| Breast discomfort | 19 | 1.0 | 6 | 0.2 | |
| Headache | 22 | 1.5 | 22 | 0.3 | |
| Application-site reaction | 20 | 2.6 | NA | NA | |
| Nausea | 20 | 1.8 | 18 | 0.8 | |
| Abdominal pain | 8 | 0.2 | 8 | 0.3 | |
| Dysmenorrhea | 13 | 1.5 | 10 | 0.2 | |
| Source: Audet MC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. JAMA. 2001;285:2347-2354. | |||||
Other considerations
Breastfeeding. Nursing mothers should consult a clinician before initiating the transdermal contraceptive system. Minimal levels of hormones are passed on in breast milk, and estrogen may slightly decrease the amount of breast milk. A barrier method or progestin-only contraceptive is preferred, since breastfeeding provides only partial protection from pregnancy. This partial protection decreases significantly when a woman breastfeeds for longer periods of time or supplements with formula or food.
Continuous use. To date, no studies have explored continuous use of the patch, i.e., eliminating the patch-free interval. Plans to evaluate use in this setting are in development.
The continuous use of combination OCs is very popular. Since the patch is a monophasic combination method that achieves a steady state of hormones in the bloodstream, it would seem well-suited for continuous use (although this use is not described in the product’s labeling). Pharmacokinetic data have shown that hormone levels in week 2 are similar to week 1. In other words, there is no significant “accumulation” of hormones in the bloodstream.15 However, additional data are needed.
Storage and disposal. Contraceptive patches should be stored at room temperature, not in the refrigerator or freezer. Patches should be removed from their protective pouches only when it is time to apply them to the skin. In addition, women should be counseled that used patches still contain some active hormones. Each used patch should be folded in half so that it adheres to itself before discarding it in a place inaccessible to children and pets.
Drug interactions. The interactions reported for OCs are assumed to pertain to the contraceptive patch, as well. Of clinical importance are drugs that may have an impact on the metabolism of steroidal contraceptives. These include certain antibiotics, antifungals, and anticonvulsants.
A drug-interaction study was conducted to determine what effect, if any, tetracycline had on the pharmacokinetics of the transdermal system. Oral administration of tetracycline hydrochloride (500 mg 4 times daily for 3 days prior to and 7 days during wearing of the patch) did not affect the estrogen or prog-estin components of the patch.15
Conclusion
The contraceptive patch is a new method of hormonal contraception that delivers low and continuous doses of EE2 and norelgestromin. Clinical studies have proven it to be highly effective, with an adverse-effect profile comparable to OCs. In addition, clinical studies reported that compliance was statistically better in patch users than in women taking OCs.
Patient-management issues such as adverse effects and the “how-tos” of application can be easily addressed. With proper use, most mild adverse effects (e.g., breast tenderness, bleeding, and nausea) usually resolve after 3 cycles. Applying the patch in a different area each week will help avoid possible application-site reactions.
Proper counseling about these issues will help women better understand the symptoms they experience, and should encourage continued, correct, and consistent use.
Dr. Zieman serves as a consultant for Ortho-McNeil and Berlex. She is on the speaker’s bureau for Pharmacia, Ortho-McNeil, Berlex, and Wyeth. Dr. Zieman also receives research support from Berlex and Wyeth.
1. Padwick ML, Endacott J, Whitehead MI. Efficacy, acceptability, and metabolic effects of transdermal estradiol in the management of postmenopausal women. Am J Obstet Gynecol. 1985;153:1085-1091.
2. The Transdermal Hormone Replacement Therapy Investigators Group. A randomized study to compare effectiveness, tolerability, and acceptability of two different transdermal estradiol replacement therapies. Int J Fertil. 1993;38:5-11.
3. Whitehead MI, Fraser D, Schenkel L, et al. Transdermal administration of oestrogen/progestagen hormone replacement therapy. Lancet. 1990;335:310-312.
4. Ramachandran C, Fleisher D. Transdermal delivery of drugs for the treatment of bone diseases. Adv Drug Delivery Rev. 2000;42:197-223.
5. O’Neill SO, Kirkegard Y. An Australian experience of transdermal oestradiol patches in a subtropical climate. Aust N Z Obstet Gynaecol. 1993;33:327-329.
6. Lake Y, Pinnock S. Improved patient acceptability with a transdermal drug-in-adhesive oestradiol patch. Aust N Z Obstet Gynaecol. 2000;40:313-315.
7. Archer D, Bigrigg A, Smallwood G, Shangold G, Creasy G, Fisher A. Assessment of compliance with a weekly contraceptive patch (OrthoEvra/Evra) among North American women. Fertil Steril. 2002;77:S27.-
8. Ortho Evra Package Insert Raritan, NJ: Ortho-McNeil Pharmaceutical; 2001.
9. Zieman M, Guillebaud J, Weisberg E, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;76:S13.-
10. Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. JAMA. 2001;285:2347-2354.
11. Hedon B, Helmerhorst FM, Cronje HS, et al. Comparison of efficacy, cycle control, compliance, and safety in users of a contraceptive patch vs an oral contraceptive. Int J Gynecol Obstet. 2000;70(suppl 1):78.-
12. Hatcher RA, Nelson AL, Zieman M, et al. A Pocket Guide to Managing Contraception. Dawsonville, Ga: Bridging the Gap Foundation; 2002.
13. Zacur H, Hedon B, Mansour D, et al. Integrated summary of Ortho Evra/Evra contraceptive patch adhesion in varied climates and conditions. Fertil Steril. 2002;77:S32.-
14. Gomez-Panzani E, Williams MB, Kuznicki JT, et al. Application and maintenance habits do make a difference in adhesion of Alora transdermal systems. Maturitas. 2000;35:57-64.
15. Abrams LS, Skee D, Natarajan J, Wong FA. Pharmacokinetic overview of Ortho Evra/Evra. Fertil Steril. 2002;77(2 Suppl 2):S3-S12.
16. Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. for the Ortho Evra/Evra 002 Study Group. Efficacy and safety of a transdermal contraceptive system. Obstet Gynecol. 2001;98:799-805.
- The transdermal contraceptive system is a combination hormonal patch that contains 6 mg norelgestromin and 0.75 mg ethinyl estradiol (EE2) in a stable adhesive, and delivers a daily dose of 150 μg norelgestromin and 20 μg EE2to the bloodstream.
- Three large contraceptive trials, in which 3,319 women used the contraceptive patch worldwide, reported low pregnancy rates, with an overall Pearl index of 0.88.
- The primary goals of management are increasing correct and consistent use and effectively counseling and educating women about adverse effects, thereby enhancing continuation rates.
- In studies involving more than 70,000 patches, only 4.7% were replaced because of partial or complete detachment. In 1 health-club study, with physical exertion and variable temperature and humidity, only 1 of 87 patches became completely detached.
- Treatment-limiting events were reported in 1% to 2.4% of women in clinical trials and included nausea and/or vomiting, application-site reaction, breast symptoms, headache, and emotional lability.
The delivery of hormones via a transdermal patch is not a new development. Estrogen and estrogen-progestin transdermal systems have been used for a number of years to treat the symptoms of menopause.1-3 However, until now, no patch-delivery system was able to administer sufficient hormones to prevent pregnancy.
Transdermal delivery systems are warranted for drugs that are highly potent and lipophilic.4 Published studies demonstrate some patient preference for transdermal systems over oral dosing, particularly drug-in-adhesive transdermal systems (as opposed to first-generation reservoir systems).5,6 In 1 study, drug-in-adhesive systems were rated as having better cosmetic appearance, better skin adhesion, greater comfort, and less skin irritation.6 These traits also apply to the contraceptive patch. In clinical studies, the transdermal contraceptive system was shown to have statistically better patient compliance than oral contraceptives (OCs); in 1 trial, compliance was better across all age groups (FIGURE 1).7 This may be due in part to the weekly, rather than daily, dosing regimen.
FIGURE 1 Compliance by age group
The basic product
The transdermal contraceptive system is a combination hormonal patch that contains 6 mg norelgestromin and 0.75 mg ethinyl estradiol (EE2) in a stable adhesive, and delivers a daily dose of 150 μg norelgestromin and 20 μg EE2 to the bloodstream.8 The progestin component, norelgestromin, is the primary active metabolite of norgestimate.
The transdermal contraceptive patch is a thin, matrix-type system consisting of 3 layers. The backing, composed of a flexible beige film, provides structural support. It also protects the middle layer from the environment. The middle layer contains the adhesive and active hormones. The third layer is the release liner, which protects the adhesive layer during storage and is removed just prior to application. This matrix system allows for lower delivery doses and bypasses the first-pass hepatic metabolism of oral administration.
Efficacy
The efficacy of the contraceptive patch is well-established. Three large contraceptive trials, in which 3,319 women used the contraceptive patch worldwide, reported low pregnancy rates, with an overall Pearl index of 0.88 (TABLE 1).9 In 1 randomized clinical trial, 811 women who wore the patch (5,240 cycles) had an overall pregnancy rate of 1.2 per 100 woman-years, compared with 605 women who took an OC (4,167 cycles), with an overall pregnancy rate of 2.18 per 100 woman-years.10 This difference is not statistically significant, but it does indicate that the patch performs at least as well as the OC.10,11 This is important when counseling patients, since women are not used to relying on a transdermal system to prevent pregnancy and may be skeptical that a patch can be effective.
For maximum efficacy, the transdermal system should be initiated as directed. That is, the first patch should be applied on the first day of menses. Another start day, such as Sunday (i.e., the first Sunday after menses begins), can be chosen instead. However, the woman then must use a backup method of contraception for the first 7 days of patch use.
TABLE 1
Contraceptive patch efficacy: pregnancies per 100 woman-years
| STUDY | CYCLES (N) | PREGNANCIES (N) | PEARL INDICES | |||
|---|---|---|---|---|---|---|
| OVERALL PEARL* | METHOD PEARL** | |||||
| Smallwood et al16 | 10,994 | 6 | 0.71 | 0.59 | ||
| Hedon et al11 | 5,921 | 4 | 0.88 | 0.66 | ||
| Audet et al10 | 5,240 | 5 | 1.24 | 0.99 | ||
| TOTAL | 22,155 | 15 | 0.88 | 0.70 | ||
| * User failure plus method failure | ||||||
| ** Failure when taken as directed | ||||||
| Source: Zieman M, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system. Fertil Steril. 2002;76:S13. | ||||||
Patient management
The primary goals of management are increasing correct and consistent use and effectively counseling and educating women about adverse effects, thereby enhancing continuation rates.
Although the theoretical pregnancy rate for oral hormonal contraception is extremely low (0.1% for combination pills), the typical annual pregnancy rate is much higher (8%).12 This difference between perfect and typical use derives mainly from patient errors, such as skipping or delaying pills. In addition, the starting and stopping of hormonal contraception may lead to times during which a woman believes she is protected but is actually at risk for pregnancy. In clinical studies, the difference between the transdermal system’s overall failure and method failure probabilities of pregnancy was only 0.2%.9 In other words, when failures occurred, they were seldom due to patient error. This would suggest that typical-use pregnancy rates for the patch are lower than for combination OCs. Typical-use pregnancy rates are usually based on population surveys and studies; these data are not yet available for the patch.
One aspect of the patch that may lead to better compliance and lower levels of user error is the fact that it needs to be changed only once a week. The patch also has some measure of forgiveness built in: each one contains enough active hormones within the reference range for 2 additional days of contraception. This is advantageous when a woman forgets to change her patch on the scheduled day.
Dosage and administration
Initiation.The transdermal contraceptive system uses the familiar 28-day, 4-week cycle, with a new patch applied every 7 days for 3 weeks, followed by a patch-free week. Withdrawal bleeding occurs during the patch-free interval. At the end of that week, a new patch is applied , starting a new 4-week cycle.
For the initial cycle of use, a woman may place her first patch on the first day of her menstrual period or any day thereafter. (Typically the following Sunday is chosen.) A backup method (e.g., diaphragm or condom) should be used for the first 7 days if the patient initiates use any day after the first day of her period.8
Several situations require extra consideration when initiating transdermal contraceptives:
- When switching from an OC, treatment should begin on the first day of withdrawal bleeding. If no withdrawal bleeding occurs within 5 days of the last active tablet, the woman should consult a health-care professional to rule out pregnancy prior to starting the patch.8
- Nonbreastfeeding women should wait 4 weeks after childbirth before initiating the patch, and should use backup contraception for 7 days.8
- The patch may be initiated immediately (the same day) after a first-trimester abortion or miscarriage. Backup contraception is not necessary if the patch is started within 5 days.8
Application. The patient should be instructed to open the foil pouch carefully, avoiding contact with the adhesive surface after the clear protective liner is peeled away. The patch should be applied to clean, dry, healthy skin on the buttocks, abdomen, upper outer arm, or upper torso, as these were the sites included in studies of the contraceptive. The patch should not be applied to skin that is irritated or cut; nor should it be placed on the breast. No make-up, creams, lotions, powders, or other topical products should be applied to the area where the transdermal system is or will soon be placed. The woman should press down firmly on the patch for at least 10 seconds and check that all edges adhere properly. She also should inspect the patch daily for loss or partial detachment.8 Women may be interested in knowing in advance that the patch can develop a ring of color around it with wear, similar to the ring around an adhesive bandage.
Detachment. The patch delivers an insufficient dose of hormones if it becomes partially or completely detached and remains so. Fortunately, this is not a common problem. In studies involving more than 70,000 patches, only 4.7% were replaced because of partial or complete detachment. In one health-club study where women experienced physical exertion and variable temperature and humidity, only 1 of 87 (1.1%) patches became completely detached.13 Studies also demonstrated that patients learn to use the contraceptive patch more effectively over time, a trend noted in other investigations of transdermal systems.8,14
If a patch partially or fully detaches, the patient can attempt to reapply it. Reapplication should not be attempted if the patch is no longer sticky, becomes stuck to itself or another surface, or has material adhered to it. No other adhesives or wraps should be used to hold the patch in place.8
A replacement program is available in the event a woman needs an extra patch, which can be obtained through the local pharmacy. When the clinician writes the initial prescription for the patch, he or she also writes an additional prescription that can be filled as needed for a single replacement patch. An extra patch would be necessary under the following conditions:
- If the patch has been detached for less than 24 hours, the patient can try to reapply it to the same area. If it does not adhere, it should be replaced with a new patch immediately. No backup contraception is needed, and the patch-change day can remain the same. Alternatively, the day the replacement is placed can become the new patch-change day (FIGURE 2).
- For contraceptive patches detached for more than 24 hours or for an undetermined length of time, a woman may not be protected from pregnancy. She should start a new cycle immediately (new day 1 and new patch-change day) by applying a new patch and using backup contraception for the first week (FIGURE 2). If she had unprotected intercourse, she may be a candidate for emergency contraception.
Missed patch-change days. Occasionally, a woman may forget to change the patch on the designated day. As with OCs, the risk of ovulation increases each day beyond the allowed contraceptive-free period (7 days), although, as mentioned earlier, the transdermal system delivers enough hormones to protect a woman for up to 48 additional hours.15 If a woman misses a scheduled change in the middle of her cycle, and less than 48 hours have passed, she should apply a new patch; the patch-change day can remain the same. If more than 48 hours have passed, she may not be protected from pregnancy. She should stop the current cycle and start another 4-week cycle immediately by applying a new patch. (She now has a new patch-change day.) Backup contraception should be used for 1 week (FIGURE 3).8 If she has had unprotected intercourse, she may be a candidate for emergency contraception.
If the third patch is not removed on time, the woman should remove it as soon as she remembers. The next cycle should be started as usual, and no backup contraception is needed. If a woman wants to adjust her patch-change day, she should remove the third contraceptive patch as scheduled and apply a new one on the desired day during the hormone-free week, taking care not to exceed 7 consecutive hormone-free days.8
Visibility. Although the beige patch was designed to minimize visibility, it may be more apparent on women with contrasting skin tones. Until a broad range of colors is available, visibility can be minimized by wearing the patch underneath clothing. (Visibility can sometimes be reassuring to male partners who want to ensure that a contraceptive is being used.)
The designated placement areas (buttocks, abdomen, upper outer arm, or upper torso, excluding the breast), are likely to be covered with clothing and less likely to be noticed, and all sites are therapeutically equivalent. The pharmacokinetics in other application areas is unknown.
Special populations. In 3 large contraceptive trials, the rate of pregnancy did not differ significantly by age or race, or for women with a body weight below 198 lb. However, in women weighing 198 lb or more (approximately 3% of the study population), there was a greater incidence of pregnancy.9 There were only 83 women in this group, yet there were 5 pregnancies. Health-care professionals who consider the contraceptive patch for patients at or above 198 lb should discuss the individual’s needs so that the most appropriate contraceptive, in terms of efficacy and tolerability, can be selected.8
FIGURE 2 Partial or complete patch detachment
FIGURE 3 Patient forgot to apply/change patch (in any patch cycle)
Adverse events
Adverse reactions to the use of hormonal contraception are relatively common, and similar among the different delivery routes. Less serious adverse effects such as breast symptoms, headache, application-site reaction, nausea, and menstrual cramps were reported by women using the contraceptive patch in clinical trials. Treatment-limiting events were reported in 1% to 2.4% of women in clinical trials and included nausea and/or vomiting, application-site reaction, breast symptoms, headache, and emotional lability. The most common adverse effects, as well as those likely to limit treatment in 1 comparative trial, are listed in TABLE 2.10 It is important to counsel patients that these events (with the exception of application-site reaction) are common in users of combination hormonal contraceptives, but tend to resolve by the third cycle of use.
The use of combination hormonal contraceptives also is associated with an increased risk of some serious adverse events. These include venous thromboembolic events, hypertension, gallbladder disease, and hepatic adenomas or benign liver tumors. Since these events are rare, we have limited data specific to the patch. However, the types and incidences of these events appear to be similar to those that occur with combination OCs.
Breast symptoms. These are commonly reported in women using combination hormonal contraceptives. In clinical trials, women who used the patch also reported breast discomfort.10,11 In fact, there was a statistically significant increase in this symptom with the patch in the first 2 cycles of use, compared with OC users, with 1% of participants discontinuing use because of it. However, studies showed that this discomfort was transient and usually resolved by cycle 3. Appropriate counseling about this adverse effect should encourage women to continue use of the method with confidence that the symptoms will likely subside.
Spotting and breakthrough bleeding. Bleeding irregularities are commonly experienced by women who use combination hormonal contraceptives. Breakthrough bleeding and/or spotting (bleeding that occurs on the days that the contraceptive patch is worn) were sometimes encountered by women in clinical trials of the patch.8 Appropriate counseling is recommended so that women are aware of the potential for these irregularities and will continue use of the patch. If breakthrough bleeding persists longer than a few cycles, a cause other than the patch should be considered. In clinical trials, the incidence of breakthrough bleeding was similar in patch and OC users. However, spotting was more common in the first 2 months of patch use.10
In the event of no withdrawal bleeding (bleeding that should occur during the patch-free week), treatment should be resumed on the next scheduled patch-change day. If the patch was initiated and used correctly, the absence of withdrawal bleeding is probably not an indication of pregnancy. Nevertheless, the possibility of pregnancy should be considered, especially if the patch was used incorrectly or with an absence of withdrawal bleeding in 2 consecutive cycles. The contraceptive patch should be discontinued if pregnancy is confirmed. In clinical trials, the incidence of nonpregnancy-related amenorrhea (defined as no withdrawal bleeding for 2 consecutive menstrual periods) was rare (≤0.1%).10
Application-site reactions. These are unique to transdermal delivery systems. If patch use results in uncomfortable irritation, the patch may be removed and a new patch applied to a different location. On the next patch-change day, the application site should be rotated to minimize the risk of skin irritation. As stated earlier, the contraceptive patch should be applied to clean, dry, healthy skin. It should not be placed on skin that has been irritated, cut, or treated with lotion.
TABLE 2
Most common adverse events: patch versus pill
| EVENT | PATCH (N=812) | OC (N=605) | |||
|---|---|---|---|---|---|
| OVERALL (%) | TREATMENT-LIMITING (%) | OVERALL (%) | TREATMENT-LIMITING (%) | ||
| Breast discomfort | 19 | 1.0 | 6 | 0.2 | |
| Headache | 22 | 1.5 | 22 | 0.3 | |
| Application-site reaction | 20 | 2.6 | NA | NA | |
| Nausea | 20 | 1.8 | 18 | 0.8 | |
| Abdominal pain | 8 | 0.2 | 8 | 0.3 | |
| Dysmenorrhea | 13 | 1.5 | 10 | 0.2 | |
| Source: Audet MC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. JAMA. 2001;285:2347-2354. | |||||
Other considerations
Breastfeeding. Nursing mothers should consult a clinician before initiating the transdermal contraceptive system. Minimal levels of hormones are passed on in breast milk, and estrogen may slightly decrease the amount of breast milk. A barrier method or progestin-only contraceptive is preferred, since breastfeeding provides only partial protection from pregnancy. This partial protection decreases significantly when a woman breastfeeds for longer periods of time or supplements with formula or food.
Continuous use. To date, no studies have explored continuous use of the patch, i.e., eliminating the patch-free interval. Plans to evaluate use in this setting are in development.
The continuous use of combination OCs is very popular. Since the patch is a monophasic combination method that achieves a steady state of hormones in the bloodstream, it would seem well-suited for continuous use (although this use is not described in the product’s labeling). Pharmacokinetic data have shown that hormone levels in week 2 are similar to week 1. In other words, there is no significant “accumulation” of hormones in the bloodstream.15 However, additional data are needed.
Storage and disposal. Contraceptive patches should be stored at room temperature, not in the refrigerator or freezer. Patches should be removed from their protective pouches only when it is time to apply them to the skin. In addition, women should be counseled that used patches still contain some active hormones. Each used patch should be folded in half so that it adheres to itself before discarding it in a place inaccessible to children and pets.
Drug interactions. The interactions reported for OCs are assumed to pertain to the contraceptive patch, as well. Of clinical importance are drugs that may have an impact on the metabolism of steroidal contraceptives. These include certain antibiotics, antifungals, and anticonvulsants.
A drug-interaction study was conducted to determine what effect, if any, tetracycline had on the pharmacokinetics of the transdermal system. Oral administration of tetracycline hydrochloride (500 mg 4 times daily for 3 days prior to and 7 days during wearing of the patch) did not affect the estrogen or prog-estin components of the patch.15
Conclusion
The contraceptive patch is a new method of hormonal contraception that delivers low and continuous doses of EE2 and norelgestromin. Clinical studies have proven it to be highly effective, with an adverse-effect profile comparable to OCs. In addition, clinical studies reported that compliance was statistically better in patch users than in women taking OCs.
Patient-management issues such as adverse effects and the “how-tos” of application can be easily addressed. With proper use, most mild adverse effects (e.g., breast tenderness, bleeding, and nausea) usually resolve after 3 cycles. Applying the patch in a different area each week will help avoid possible application-site reactions.
Proper counseling about these issues will help women better understand the symptoms they experience, and should encourage continued, correct, and consistent use.
Dr. Zieman serves as a consultant for Ortho-McNeil and Berlex. She is on the speaker’s bureau for Pharmacia, Ortho-McNeil, Berlex, and Wyeth. Dr. Zieman also receives research support from Berlex and Wyeth.
- The transdermal contraceptive system is a combination hormonal patch that contains 6 mg norelgestromin and 0.75 mg ethinyl estradiol (EE2) in a stable adhesive, and delivers a daily dose of 150 μg norelgestromin and 20 μg EE2to the bloodstream.
- Three large contraceptive trials, in which 3,319 women used the contraceptive patch worldwide, reported low pregnancy rates, with an overall Pearl index of 0.88.
- The primary goals of management are increasing correct and consistent use and effectively counseling and educating women about adverse effects, thereby enhancing continuation rates.
- In studies involving more than 70,000 patches, only 4.7% were replaced because of partial or complete detachment. In 1 health-club study, with physical exertion and variable temperature and humidity, only 1 of 87 patches became completely detached.
- Treatment-limiting events were reported in 1% to 2.4% of women in clinical trials and included nausea and/or vomiting, application-site reaction, breast symptoms, headache, and emotional lability.
The delivery of hormones via a transdermal patch is not a new development. Estrogen and estrogen-progestin transdermal systems have been used for a number of years to treat the symptoms of menopause.1-3 However, until now, no patch-delivery system was able to administer sufficient hormones to prevent pregnancy.
Transdermal delivery systems are warranted for drugs that are highly potent and lipophilic.4 Published studies demonstrate some patient preference for transdermal systems over oral dosing, particularly drug-in-adhesive transdermal systems (as opposed to first-generation reservoir systems).5,6 In 1 study, drug-in-adhesive systems were rated as having better cosmetic appearance, better skin adhesion, greater comfort, and less skin irritation.6 These traits also apply to the contraceptive patch. In clinical studies, the transdermal contraceptive system was shown to have statistically better patient compliance than oral contraceptives (OCs); in 1 trial, compliance was better across all age groups (FIGURE 1).7 This may be due in part to the weekly, rather than daily, dosing regimen.
FIGURE 1 Compliance by age group
The basic product
The transdermal contraceptive system is a combination hormonal patch that contains 6 mg norelgestromin and 0.75 mg ethinyl estradiol (EE2) in a stable adhesive, and delivers a daily dose of 150 μg norelgestromin and 20 μg EE2 to the bloodstream.8 The progestin component, norelgestromin, is the primary active metabolite of norgestimate.
The transdermal contraceptive patch is a thin, matrix-type system consisting of 3 layers. The backing, composed of a flexible beige film, provides structural support. It also protects the middle layer from the environment. The middle layer contains the adhesive and active hormones. The third layer is the release liner, which protects the adhesive layer during storage and is removed just prior to application. This matrix system allows for lower delivery doses and bypasses the first-pass hepatic metabolism of oral administration.
Efficacy
The efficacy of the contraceptive patch is well-established. Three large contraceptive trials, in which 3,319 women used the contraceptive patch worldwide, reported low pregnancy rates, with an overall Pearl index of 0.88 (TABLE 1).9 In 1 randomized clinical trial, 811 women who wore the patch (5,240 cycles) had an overall pregnancy rate of 1.2 per 100 woman-years, compared with 605 women who took an OC (4,167 cycles), with an overall pregnancy rate of 2.18 per 100 woman-years.10 This difference is not statistically significant, but it does indicate that the patch performs at least as well as the OC.10,11 This is important when counseling patients, since women are not used to relying on a transdermal system to prevent pregnancy and may be skeptical that a patch can be effective.
For maximum efficacy, the transdermal system should be initiated as directed. That is, the first patch should be applied on the first day of menses. Another start day, such as Sunday (i.e., the first Sunday after menses begins), can be chosen instead. However, the woman then must use a backup method of contraception for the first 7 days of patch use.
TABLE 1
Contraceptive patch efficacy: pregnancies per 100 woman-years
| STUDY | CYCLES (N) | PREGNANCIES (N) | PEARL INDICES | |||
|---|---|---|---|---|---|---|
| OVERALL PEARL* | METHOD PEARL** | |||||
| Smallwood et al16 | 10,994 | 6 | 0.71 | 0.59 | ||
| Hedon et al11 | 5,921 | 4 | 0.88 | 0.66 | ||
| Audet et al10 | 5,240 | 5 | 1.24 | 0.99 | ||
| TOTAL | 22,155 | 15 | 0.88 | 0.70 | ||
| * User failure plus method failure | ||||||
| ** Failure when taken as directed | ||||||
| Source: Zieman M, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system. Fertil Steril. 2002;76:S13. | ||||||
Patient management
The primary goals of management are increasing correct and consistent use and effectively counseling and educating women about adverse effects, thereby enhancing continuation rates.
Although the theoretical pregnancy rate for oral hormonal contraception is extremely low (0.1% for combination pills), the typical annual pregnancy rate is much higher (8%).12 This difference between perfect and typical use derives mainly from patient errors, such as skipping or delaying pills. In addition, the starting and stopping of hormonal contraception may lead to times during which a woman believes she is protected but is actually at risk for pregnancy. In clinical studies, the difference between the transdermal system’s overall failure and method failure probabilities of pregnancy was only 0.2%.9 In other words, when failures occurred, they were seldom due to patient error. This would suggest that typical-use pregnancy rates for the patch are lower than for combination OCs. Typical-use pregnancy rates are usually based on population surveys and studies; these data are not yet available for the patch.
One aspect of the patch that may lead to better compliance and lower levels of user error is the fact that it needs to be changed only once a week. The patch also has some measure of forgiveness built in: each one contains enough active hormones within the reference range for 2 additional days of contraception. This is advantageous when a woman forgets to change her patch on the scheduled day.
Dosage and administration
Initiation.The transdermal contraceptive system uses the familiar 28-day, 4-week cycle, with a new patch applied every 7 days for 3 weeks, followed by a patch-free week. Withdrawal bleeding occurs during the patch-free interval. At the end of that week, a new patch is applied , starting a new 4-week cycle.
For the initial cycle of use, a woman may place her first patch on the first day of her menstrual period or any day thereafter. (Typically the following Sunday is chosen.) A backup method (e.g., diaphragm or condom) should be used for the first 7 days if the patient initiates use any day after the first day of her period.8
Several situations require extra consideration when initiating transdermal contraceptives:
- When switching from an OC, treatment should begin on the first day of withdrawal bleeding. If no withdrawal bleeding occurs within 5 days of the last active tablet, the woman should consult a health-care professional to rule out pregnancy prior to starting the patch.8
- Nonbreastfeeding women should wait 4 weeks after childbirth before initiating the patch, and should use backup contraception for 7 days.8
- The patch may be initiated immediately (the same day) after a first-trimester abortion or miscarriage. Backup contraception is not necessary if the patch is started within 5 days.8
Application. The patient should be instructed to open the foil pouch carefully, avoiding contact with the adhesive surface after the clear protective liner is peeled away. The patch should be applied to clean, dry, healthy skin on the buttocks, abdomen, upper outer arm, or upper torso, as these were the sites included in studies of the contraceptive. The patch should not be applied to skin that is irritated or cut; nor should it be placed on the breast. No make-up, creams, lotions, powders, or other topical products should be applied to the area where the transdermal system is or will soon be placed. The woman should press down firmly on the patch for at least 10 seconds and check that all edges adhere properly. She also should inspect the patch daily for loss or partial detachment.8 Women may be interested in knowing in advance that the patch can develop a ring of color around it with wear, similar to the ring around an adhesive bandage.
Detachment. The patch delivers an insufficient dose of hormones if it becomes partially or completely detached and remains so. Fortunately, this is not a common problem. In studies involving more than 70,000 patches, only 4.7% were replaced because of partial or complete detachment. In one health-club study where women experienced physical exertion and variable temperature and humidity, only 1 of 87 (1.1%) patches became completely detached.13 Studies also demonstrated that patients learn to use the contraceptive patch more effectively over time, a trend noted in other investigations of transdermal systems.8,14
If a patch partially or fully detaches, the patient can attempt to reapply it. Reapplication should not be attempted if the patch is no longer sticky, becomes stuck to itself or another surface, or has material adhered to it. No other adhesives or wraps should be used to hold the patch in place.8
A replacement program is available in the event a woman needs an extra patch, which can be obtained through the local pharmacy. When the clinician writes the initial prescription for the patch, he or she also writes an additional prescription that can be filled as needed for a single replacement patch. An extra patch would be necessary under the following conditions:
- If the patch has been detached for less than 24 hours, the patient can try to reapply it to the same area. If it does not adhere, it should be replaced with a new patch immediately. No backup contraception is needed, and the patch-change day can remain the same. Alternatively, the day the replacement is placed can become the new patch-change day (FIGURE 2).
- For contraceptive patches detached for more than 24 hours or for an undetermined length of time, a woman may not be protected from pregnancy. She should start a new cycle immediately (new day 1 and new patch-change day) by applying a new patch and using backup contraception for the first week (FIGURE 2). If she had unprotected intercourse, she may be a candidate for emergency contraception.
Missed patch-change days. Occasionally, a woman may forget to change the patch on the designated day. As with OCs, the risk of ovulation increases each day beyond the allowed contraceptive-free period (7 days), although, as mentioned earlier, the transdermal system delivers enough hormones to protect a woman for up to 48 additional hours.15 If a woman misses a scheduled change in the middle of her cycle, and less than 48 hours have passed, she should apply a new patch; the patch-change day can remain the same. If more than 48 hours have passed, she may not be protected from pregnancy. She should stop the current cycle and start another 4-week cycle immediately by applying a new patch. (She now has a new patch-change day.) Backup contraception should be used for 1 week (FIGURE 3).8 If she has had unprotected intercourse, she may be a candidate for emergency contraception.
If the third patch is not removed on time, the woman should remove it as soon as she remembers. The next cycle should be started as usual, and no backup contraception is needed. If a woman wants to adjust her patch-change day, she should remove the third contraceptive patch as scheduled and apply a new one on the desired day during the hormone-free week, taking care not to exceed 7 consecutive hormone-free days.8
Visibility. Although the beige patch was designed to minimize visibility, it may be more apparent on women with contrasting skin tones. Until a broad range of colors is available, visibility can be minimized by wearing the patch underneath clothing. (Visibility can sometimes be reassuring to male partners who want to ensure that a contraceptive is being used.)
The designated placement areas (buttocks, abdomen, upper outer arm, or upper torso, excluding the breast), are likely to be covered with clothing and less likely to be noticed, and all sites are therapeutically equivalent. The pharmacokinetics in other application areas is unknown.
Special populations. In 3 large contraceptive trials, the rate of pregnancy did not differ significantly by age or race, or for women with a body weight below 198 lb. However, in women weighing 198 lb or more (approximately 3% of the study population), there was a greater incidence of pregnancy.9 There were only 83 women in this group, yet there were 5 pregnancies. Health-care professionals who consider the contraceptive patch for patients at or above 198 lb should discuss the individual’s needs so that the most appropriate contraceptive, in terms of efficacy and tolerability, can be selected.8
FIGURE 2 Partial or complete patch detachment
FIGURE 3 Patient forgot to apply/change patch (in any patch cycle)
Adverse events
Adverse reactions to the use of hormonal contraception are relatively common, and similar among the different delivery routes. Less serious adverse effects such as breast symptoms, headache, application-site reaction, nausea, and menstrual cramps were reported by women using the contraceptive patch in clinical trials. Treatment-limiting events were reported in 1% to 2.4% of women in clinical trials and included nausea and/or vomiting, application-site reaction, breast symptoms, headache, and emotional lability. The most common adverse effects, as well as those likely to limit treatment in 1 comparative trial, are listed in TABLE 2.10 It is important to counsel patients that these events (with the exception of application-site reaction) are common in users of combination hormonal contraceptives, but tend to resolve by the third cycle of use.
The use of combination hormonal contraceptives also is associated with an increased risk of some serious adverse events. These include venous thromboembolic events, hypertension, gallbladder disease, and hepatic adenomas or benign liver tumors. Since these events are rare, we have limited data specific to the patch. However, the types and incidences of these events appear to be similar to those that occur with combination OCs.
Breast symptoms. These are commonly reported in women using combination hormonal contraceptives. In clinical trials, women who used the patch also reported breast discomfort.10,11 In fact, there was a statistically significant increase in this symptom with the patch in the first 2 cycles of use, compared with OC users, with 1% of participants discontinuing use because of it. However, studies showed that this discomfort was transient and usually resolved by cycle 3. Appropriate counseling about this adverse effect should encourage women to continue use of the method with confidence that the symptoms will likely subside.
Spotting and breakthrough bleeding. Bleeding irregularities are commonly experienced by women who use combination hormonal contraceptives. Breakthrough bleeding and/or spotting (bleeding that occurs on the days that the contraceptive patch is worn) were sometimes encountered by women in clinical trials of the patch.8 Appropriate counseling is recommended so that women are aware of the potential for these irregularities and will continue use of the patch. If breakthrough bleeding persists longer than a few cycles, a cause other than the patch should be considered. In clinical trials, the incidence of breakthrough bleeding was similar in patch and OC users. However, spotting was more common in the first 2 months of patch use.10
In the event of no withdrawal bleeding (bleeding that should occur during the patch-free week), treatment should be resumed on the next scheduled patch-change day. If the patch was initiated and used correctly, the absence of withdrawal bleeding is probably not an indication of pregnancy. Nevertheless, the possibility of pregnancy should be considered, especially if the patch was used incorrectly or with an absence of withdrawal bleeding in 2 consecutive cycles. The contraceptive patch should be discontinued if pregnancy is confirmed. In clinical trials, the incidence of nonpregnancy-related amenorrhea (defined as no withdrawal bleeding for 2 consecutive menstrual periods) was rare (≤0.1%).10
Application-site reactions. These are unique to transdermal delivery systems. If patch use results in uncomfortable irritation, the patch may be removed and a new patch applied to a different location. On the next patch-change day, the application site should be rotated to minimize the risk of skin irritation. As stated earlier, the contraceptive patch should be applied to clean, dry, healthy skin. It should not be placed on skin that has been irritated, cut, or treated with lotion.
TABLE 2
Most common adverse events: patch versus pill
| EVENT | PATCH (N=812) | OC (N=605) | |||
|---|---|---|---|---|---|
| OVERALL (%) | TREATMENT-LIMITING (%) | OVERALL (%) | TREATMENT-LIMITING (%) | ||
| Breast discomfort | 19 | 1.0 | 6 | 0.2 | |
| Headache | 22 | 1.5 | 22 | 0.3 | |
| Application-site reaction | 20 | 2.6 | NA | NA | |
| Nausea | 20 | 1.8 | 18 | 0.8 | |
| Abdominal pain | 8 | 0.2 | 8 | 0.3 | |
| Dysmenorrhea | 13 | 1.5 | 10 | 0.2 | |
| Source: Audet MC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. JAMA. 2001;285:2347-2354. | |||||
Other considerations
Breastfeeding. Nursing mothers should consult a clinician before initiating the transdermal contraceptive system. Minimal levels of hormones are passed on in breast milk, and estrogen may slightly decrease the amount of breast milk. A barrier method or progestin-only contraceptive is preferred, since breastfeeding provides only partial protection from pregnancy. This partial protection decreases significantly when a woman breastfeeds for longer periods of time or supplements with formula or food.
Continuous use. To date, no studies have explored continuous use of the patch, i.e., eliminating the patch-free interval. Plans to evaluate use in this setting are in development.
The continuous use of combination OCs is very popular. Since the patch is a monophasic combination method that achieves a steady state of hormones in the bloodstream, it would seem well-suited for continuous use (although this use is not described in the product’s labeling). Pharmacokinetic data have shown that hormone levels in week 2 are similar to week 1. In other words, there is no significant “accumulation” of hormones in the bloodstream.15 However, additional data are needed.
Storage and disposal. Contraceptive patches should be stored at room temperature, not in the refrigerator or freezer. Patches should be removed from their protective pouches only when it is time to apply them to the skin. In addition, women should be counseled that used patches still contain some active hormones. Each used patch should be folded in half so that it adheres to itself before discarding it in a place inaccessible to children and pets.
Drug interactions. The interactions reported for OCs are assumed to pertain to the contraceptive patch, as well. Of clinical importance are drugs that may have an impact on the metabolism of steroidal contraceptives. These include certain antibiotics, antifungals, and anticonvulsants.
A drug-interaction study was conducted to determine what effect, if any, tetracycline had on the pharmacokinetics of the transdermal system. Oral administration of tetracycline hydrochloride (500 mg 4 times daily for 3 days prior to and 7 days during wearing of the patch) did not affect the estrogen or prog-estin components of the patch.15
Conclusion
The contraceptive patch is a new method of hormonal contraception that delivers low and continuous doses of EE2 and norelgestromin. Clinical studies have proven it to be highly effective, with an adverse-effect profile comparable to OCs. In addition, clinical studies reported that compliance was statistically better in patch users than in women taking OCs.
Patient-management issues such as adverse effects and the “how-tos” of application can be easily addressed. With proper use, most mild adverse effects (e.g., breast tenderness, bleeding, and nausea) usually resolve after 3 cycles. Applying the patch in a different area each week will help avoid possible application-site reactions.
Proper counseling about these issues will help women better understand the symptoms they experience, and should encourage continued, correct, and consistent use.
Dr. Zieman serves as a consultant for Ortho-McNeil and Berlex. She is on the speaker’s bureau for Pharmacia, Ortho-McNeil, Berlex, and Wyeth. Dr. Zieman also receives research support from Berlex and Wyeth.
1. Padwick ML, Endacott J, Whitehead MI. Efficacy, acceptability, and metabolic effects of transdermal estradiol in the management of postmenopausal women. Am J Obstet Gynecol. 1985;153:1085-1091.
2. The Transdermal Hormone Replacement Therapy Investigators Group. A randomized study to compare effectiveness, tolerability, and acceptability of two different transdermal estradiol replacement therapies. Int J Fertil. 1993;38:5-11.
3. Whitehead MI, Fraser D, Schenkel L, et al. Transdermal administration of oestrogen/progestagen hormone replacement therapy. Lancet. 1990;335:310-312.
4. Ramachandran C, Fleisher D. Transdermal delivery of drugs for the treatment of bone diseases. Adv Drug Delivery Rev. 2000;42:197-223.
5. O’Neill SO, Kirkegard Y. An Australian experience of transdermal oestradiol patches in a subtropical climate. Aust N Z Obstet Gynaecol. 1993;33:327-329.
6. Lake Y, Pinnock S. Improved patient acceptability with a transdermal drug-in-adhesive oestradiol patch. Aust N Z Obstet Gynaecol. 2000;40:313-315.
7. Archer D, Bigrigg A, Smallwood G, Shangold G, Creasy G, Fisher A. Assessment of compliance with a weekly contraceptive patch (OrthoEvra/Evra) among North American women. Fertil Steril. 2002;77:S27.-
8. Ortho Evra Package Insert Raritan, NJ: Ortho-McNeil Pharmaceutical; 2001.
9. Zieman M, Guillebaud J, Weisberg E, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;76:S13.-
10. Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. JAMA. 2001;285:2347-2354.
11. Hedon B, Helmerhorst FM, Cronje HS, et al. Comparison of efficacy, cycle control, compliance, and safety in users of a contraceptive patch vs an oral contraceptive. Int J Gynecol Obstet. 2000;70(suppl 1):78.-
12. Hatcher RA, Nelson AL, Zieman M, et al. A Pocket Guide to Managing Contraception. Dawsonville, Ga: Bridging the Gap Foundation; 2002.
13. Zacur H, Hedon B, Mansour D, et al. Integrated summary of Ortho Evra/Evra contraceptive patch adhesion in varied climates and conditions. Fertil Steril. 2002;77:S32.-
14. Gomez-Panzani E, Williams MB, Kuznicki JT, et al. Application and maintenance habits do make a difference in adhesion of Alora transdermal systems. Maturitas. 2000;35:57-64.
15. Abrams LS, Skee D, Natarajan J, Wong FA. Pharmacokinetic overview of Ortho Evra/Evra. Fertil Steril. 2002;77(2 Suppl 2):S3-S12.
16. Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. for the Ortho Evra/Evra 002 Study Group. Efficacy and safety of a transdermal contraceptive system. Obstet Gynecol. 2001;98:799-805.
1. Padwick ML, Endacott J, Whitehead MI. Efficacy, acceptability, and metabolic effects of transdermal estradiol in the management of postmenopausal women. Am J Obstet Gynecol. 1985;153:1085-1091.
2. The Transdermal Hormone Replacement Therapy Investigators Group. A randomized study to compare effectiveness, tolerability, and acceptability of two different transdermal estradiol replacement therapies. Int J Fertil. 1993;38:5-11.
3. Whitehead MI, Fraser D, Schenkel L, et al. Transdermal administration of oestrogen/progestagen hormone replacement therapy. Lancet. 1990;335:310-312.
4. Ramachandran C, Fleisher D. Transdermal delivery of drugs for the treatment of bone diseases. Adv Drug Delivery Rev. 2000;42:197-223.
5. O’Neill SO, Kirkegard Y. An Australian experience of transdermal oestradiol patches in a subtropical climate. Aust N Z Obstet Gynaecol. 1993;33:327-329.
6. Lake Y, Pinnock S. Improved patient acceptability with a transdermal drug-in-adhesive oestradiol patch. Aust N Z Obstet Gynaecol. 2000;40:313-315.
7. Archer D, Bigrigg A, Smallwood G, Shangold G, Creasy G, Fisher A. Assessment of compliance with a weekly contraceptive patch (OrthoEvra/Evra) among North American women. Fertil Steril. 2002;77:S27.-
8. Ortho Evra Package Insert Raritan, NJ: Ortho-McNeil Pharmaceutical; 2001.
9. Zieman M, Guillebaud J, Weisberg E, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;76:S13.-
10. Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. JAMA. 2001;285:2347-2354.
11. Hedon B, Helmerhorst FM, Cronje HS, et al. Comparison of efficacy, cycle control, compliance, and safety in users of a contraceptive patch vs an oral contraceptive. Int J Gynecol Obstet. 2000;70(suppl 1):78.-
12. Hatcher RA, Nelson AL, Zieman M, et al. A Pocket Guide to Managing Contraception. Dawsonville, Ga: Bridging the Gap Foundation; 2002.
13. Zacur H, Hedon B, Mansour D, et al. Integrated summary of Ortho Evra/Evra contraceptive patch adhesion in varied climates and conditions. Fertil Steril. 2002;77:S32.-
14. Gomez-Panzani E, Williams MB, Kuznicki JT, et al. Application and maintenance habits do make a difference in adhesion of Alora transdermal systems. Maturitas. 2000;35:57-64.
15. Abrams LS, Skee D, Natarajan J, Wong FA. Pharmacokinetic overview of Ortho Evra/Evra. Fertil Steril. 2002;77(2 Suppl 2):S3-S12.
16. Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. for the Ortho Evra/Evra 002 Study Group. Efficacy and safety of a transdermal contraceptive system. Obstet Gynecol. 2001;98:799-805.