User login
Case Studies in Toxicology: DILI Dally
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
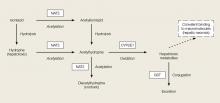
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.