User login
Porocarcinoma Development in a Prior Trauma Site
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.
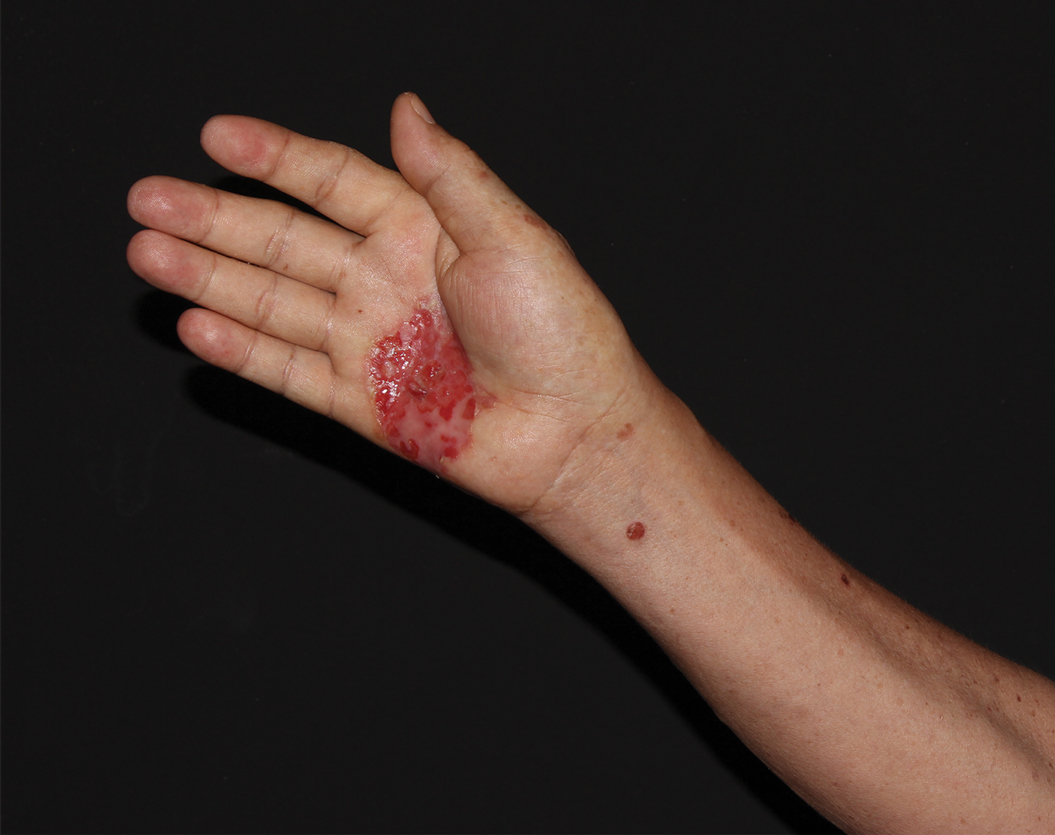
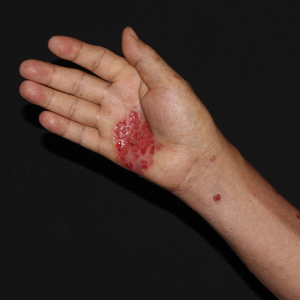
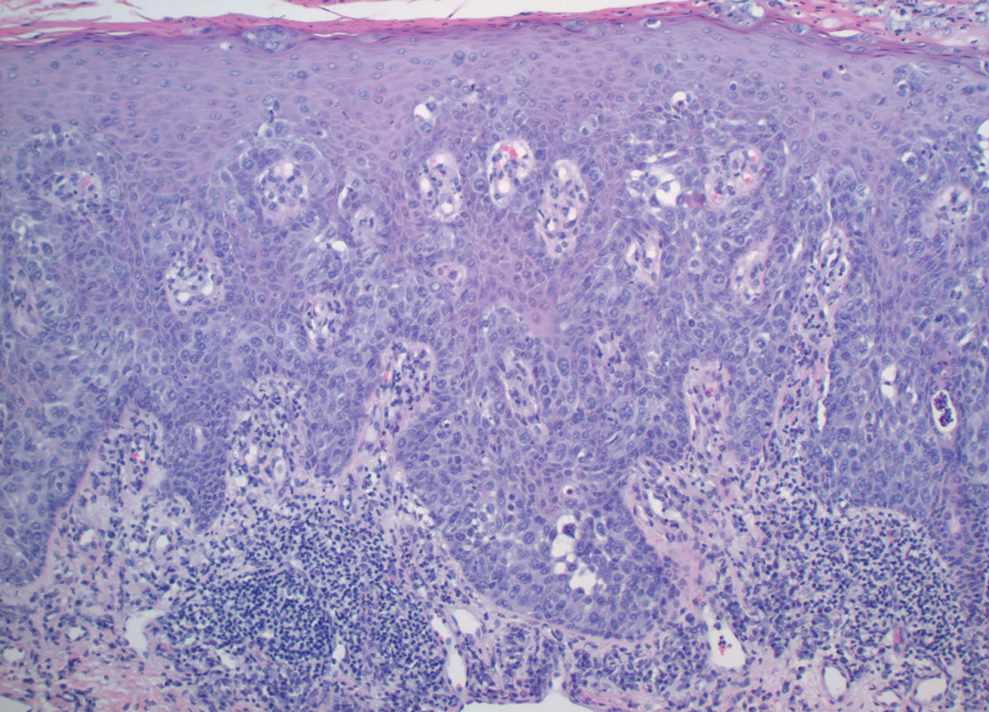
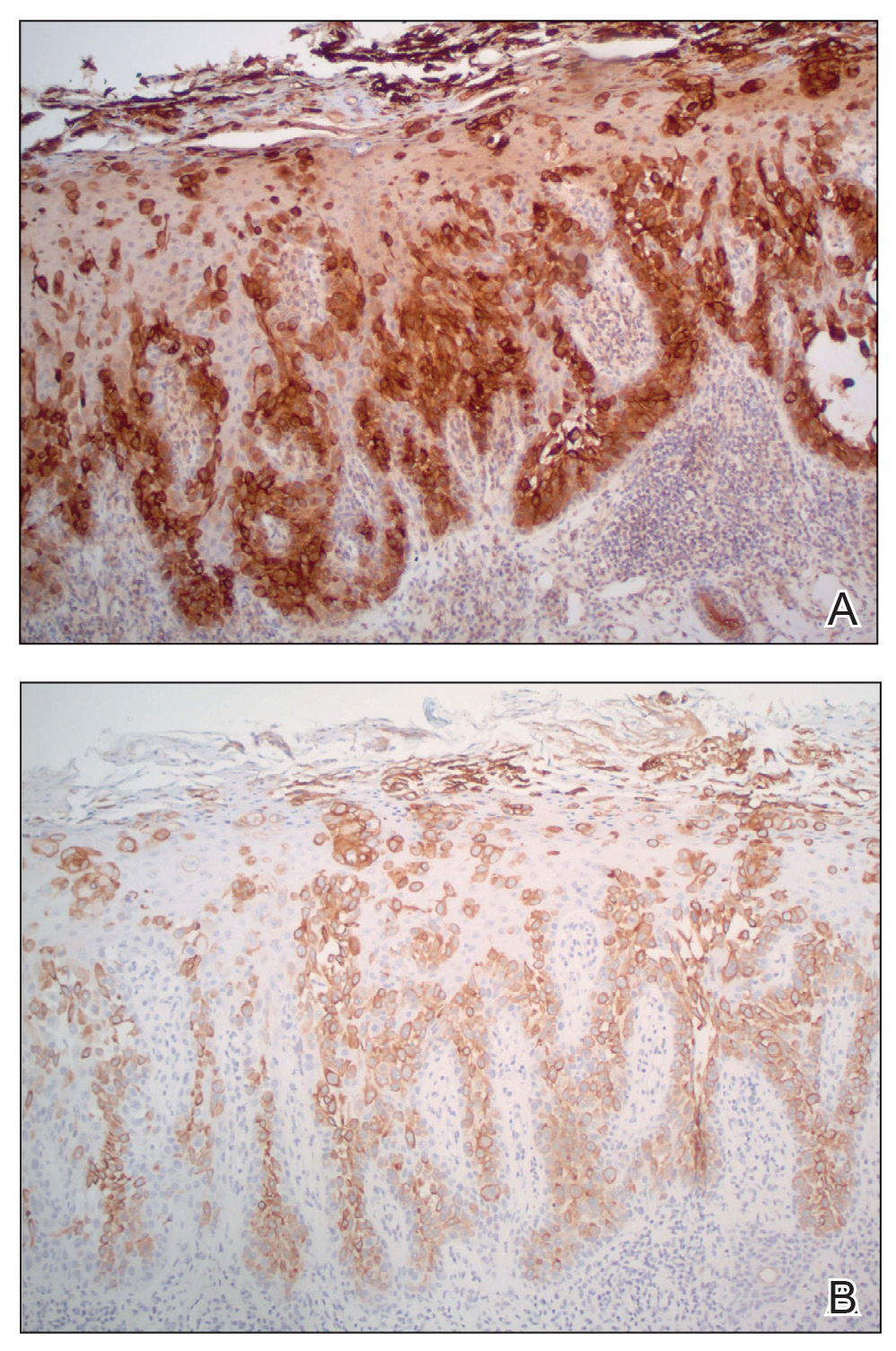
Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.
The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.
Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
Practice Points
- Porocarcinoma is a rare, potentially aggressive, glandular malignancy that should be a clinical consideration in patients presenting with a cutaneous neoplasm.
- Although wide local excision historically has been the treatment of choice for porocarcinoma, Mohs micrographic surgery has demonstrated excellent cure rates.
- Patients with unresectable or metastatic porocarcinomas have a poor prognosis but may respond to combination chemotherapy regimens.