User login
The Evolution of Insulin Therapy in Diabetes Mellitus
The Evolution of Insulin Therapy in Diabetes Mellitus
Discovery of Insulin
The discovery of insulin in 1921 by Banting and Best ushered in a new age of treatment—and hope—for patients with diabetes mellitus (DM). First administered to 14-year-old Leonard Thompson on January 11, 1922, insulin transformed the lives of patients with type 1 DM (T1DM). No longer were starvation diets the primary mode of treatment.1,2 Life saving in patients with T1DM, insulin has since become an important treatment option in patients with type 2 DM (T2DM) as well.
But as is often the case with medical breakthroughs, the discovery of the hormone that first reversed diabetic coma in dogs was only the beginning. Recognizing the crudeness of the pancreatic extract that he called isletin (after the islets of Langerhans, the insulin-producing tissue of the pancreas), Banting turned to chemist James Collip, also at the University of Toronto, who developed a process to remove the toxins and impurities from the pancreatic extract. Banting also recognized the limitation of using dogs as the source of isletin (the name of which was changed to insulin by the university) so he quickly turned to cattle as a more plentiful source. Not surprisingly, the demand for insulin skyrocketed within months of its first testing in humans by Banting and Best, so, in July 1922, licenses for the manufacture of insulin were given to several pharmaceutical companies.1,2
Evolution of Insulin
While the clinical effects of insulin in patients with T1DM were dramatic, such as waking people from diabetic coma, enabling them to consume a normal diet, and improving long-term prognosis, problems were encountered.2 One was the challenge of balancing normoglycemia without causing hypoglycemia. The early insulin preparations acted relatively quickly and had a peak effect, but they did not provide a continuous, low level of basal insulin in the same manner as did pancreatic β cells. The time-action profile was, therefore, far from physiologically similar to endogenous insulin. The second problem was allergic reactions since the source of the insulin was nonhuman.2 Resolving these issues was the focus of intensive research over many decades.
To better balance normoglycemia without causing hypoglycemia, intermediate- and long-acting insulins were subsequently developed as basal insulins to prolong the duration of effect. Discovered in 1936, neutral protamine Hagedorn (NPH) insulin was released in 1950 as an intermediate-acting basal insulin.3 Although NPH insulin remains widely used today, recent guidelines have recommended against its use since the availability of insulin analogs (detemir and glargine), which provide a relatively flat profile for 24 hours and “yield better reproducibility and consistency, both between patients and within patients, with a corresponding reduction in the risk of hypoglycemia.”4 Other basal insulins such as Lente and Ultralente were introduced in the 1950s and used extensively for many years,3 but they had important limitations, such as wide variability in absorption and duration of effect, which led to inconsistent blood glucose control.
Along with efforts to prolong the duration of action of insulin, much scientific work was undertaken to reduce the risk for the allergic reactions first encountered with canine insulin, and then with bovine and porcine insulins.3 While the purity of these formulations improved over time with advances in chromatography, allergic reactions remained a limitation for some patients. The use of animal-derived insulins eventually gave way to synthetic human insulins, first approved by the US Food and Drug Administration in 1982.5 Consisting of the same amino acid sequence as insulin secreted by the human pancreas, synthetic human insulins are less likely to cause allergic reactions and have a faster onset and shorter duration of action compared with animal-derived insulins. The short-acting regular human insulin has now been largely replaced by rapid-acting insulin analogs (aspart, glulisine, and lispro) because the analogs are more physiologically similar to endogenous insulin and provide improved safety and tolerability.4 While allergic reactions do occur with insulin analogs, the prevalence is low.6-17
Insulin Analogs
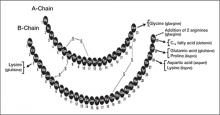
Some of the early insulin formulations included zinc for the binding of insulin to protamine to alter the pharmacokinetic properties of the drug. With the availability of recombinant DNA technology, it became possible to modify the insulin structure so as to yield analogs of human regular insulin with pharmacokinetic and pharmacodynamic properties that more closely mimic the effects of endogenous insulin secreted by the pancreas (FIGURE 1). Two groups of insulin analogs were developed: (1) those with an onset of action more rapid than that of regular human insulin (ie, the rapid-acting insulin analogs); and (2) those with a duration of action longer than that of NPH human insulin (ie, the long-acting basal insulin analogs) (TABLE 1).18-23 Premix insulin formulations are also available that combine a rapid-acting insulin analog with its intermediate-acting protamine suspension.
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32
Diabetes: a journal of the American Diabetes Association by American Diabetes Association; Stanford University. Copyright 2004. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Insulin detemir was reported to have significantly less intraindividual pharmacodynamic variability compared with insulin glargine and NPH insulin. The variability (as assessed by the coefficient of variation) of the glucose infusion rate area under the curve for the first 12 hours was 27% for detemir, 46% for glargine, and 59% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). Over the first 24 hours, the coefficients of variation were 27% for detemir, 48% for glargine, and 68% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). With respect to pharmacokinetics, the coefficients of variation of the maximum plasma insulin concentration were 18% for detemir, 34% for glargine, and 24% for NPH insulin.
Despite these pharmacodynamic and pharmacokinetic differences favoring the basal insulin analogs compared with NPH insulin, evidence-based systematic reviews have concluded that overall glucose control is similar among the 3 basal insulins.28,33 These findings should be interpreted cautiously since the basal insulins were generally administered once daily in the studies included in the systematic reviews, although a few studies used a twice-daily regimen for insulin detemir or NPH insulin.28 Furthermore, some of the studies included in the systematic reviews used a treat-to-target design, in which equal glucose-lowering efficacy was maintained among treatments, thereby allowing comparisons of other insulin properties. An important difference between the basal insulin analogs and NPH insulin identified in the systematic reviews concerns hypoglycemia, particularly nocturnal hypoglycemia. Detemir and glargine were associated with significant reductions in nocturnal hypoglycemia compared with NPH insulin (both, relative risk [RR]=.54; P < .001). The risk for overall hypoglycemia was also reported to be lower with insulin detemir and insulin glargine compared with NPH insulin (RR=.68 and RR=.89, respectively; P < .001 and P=.002). The risk for severe hypoglycemia was similar for insulin glargine or insulin detemir compared with that of NPH insulin.
A recent meta-analysis comparing insulin glargine (once daily) to insulin detemir (once or twice daily) examined data from 4 trials lasting 24 to 52 weeks and involving 2250 people.34 The meta-analysis found no differences between the 2 basal insulin analogs with respect to glycemic control, as measured by the percentage of patients who achieved A1C ≤7.0% with or without hypoglycemia. In addition, no significant differences in overall, severe, and nocturnal hypoglycemia were identified. Insulin detemir was associated with less weight gain and insulin glargine with a lower number of injection-site reactions.
Evolution of Insulin Delivery
In addition to progressive improvements in purity and the time-action profile of insulin, there have been major advances in the devices used to deliver insulin that provide clinicians greater flexibility to meet patients’ needs and to resolve patients’ concerns. Advances in delivery systems include pens with shorter, smaller gauge, highly polished needles; pens with a “dial-a-dose” gauge that is easier to read; easy portability; and insulin-prefilled pens. These advances improve ease of use and dosage accuracy, likely reduce injection pain, facilitate discrete use in public places, and increase patient acceptance and adherence.35-42 Of note, however, insulin pens must never be used in more than one individual, even if a needle has been changed, as is sometimes done in institutions. A clinical reminder from the US Centers for Disease Control and Prevention in January 2012 cautioned against pen reuse and sharing, citing an incident in which more than 2000 individuals were potentially exposed to the transmission of bloodborne pathogens because of inappropriate reuse and sharing of insulin pens.43 Another advance in insulin delivery is insulin-pump therapy, which has become even more promising with the advent of continuous glucose-monitoring devices and the availability of rapid-acting insulin analogs.
Role of Insulin in Diabetes
Recently, insulin has been recognized as a key treatment option for patients with T2DM, and is no longer considered last-line therapy.4,44 When used appropriately, insulin is the most effective glucose-lowering therapy available, with essentially no limit to the magnitude of glucose lowering. Insulin, particularly the insulin analogs, provides many treatment benefits, although some limitations remain.
Benefits of Insulin
Basal-bolus therapy using the combination of a rapid-acting insulin analog and a basal insulin analog may closely mimic the release of insulin from the pancreatic β cells. The use of an insulin pump, which uses only a rapid- or short-acting insulin (rapid-acting analog preferred) may also provide insulin in a pattern that most closely mimics endogenous insulin secretion. The administration of insulin via an insulin pump may be a good treatment option in patients with T1DM or those with T2DM who require intensive basal-bolus therapy.
The reduction of microvascular complications, such as nephropathy, neuropathy, and retinopathy, by achieving intensive glycemic control with the use of insulin, has been well established in patients with T1DM or T2DM.45-48 Nonetheless, the landscape of glycemic control changed with the completion of the Action to Control Cardiovascular Risks in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT).49,50,51 Based on the findings from those trials, caution is advised against the indiscriminate setting of very low glycemic targets. Findings from subanalyses of data from those trials suggest that while most patients are likely to achieve a microvascular benefit from intensive control, others may potentially be harmed by cardiovascular events. Those likely to benefit are those with short-duration DM, a long life expectancy, and no significant cardiovascular disease. Those who may be harmed and in whom an A1C goal <7.0% may not be appropriate are those with a history of severe hypoglycemia, a limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-standing DM in whom the more stringent A1C goal may be difficult to attain.52
Misconceptions and Limitations Regarding Insulin
Insulin therapy is considered by some clinicians and patients to be the most complicated and time-consuming of the glucose-lowering therapies. Concerns about self-injection, the need for dosage adjustment, and cost, as well as the stigma of insulin as last-line therapy, are common. Additionally, in some studies with follow-up to 24 months, patients’ adherence to insulin therapy has been reported to be 54% to 81% in patients with T2DM.53-55 When used properly, insulin is the most efficacious glucose-lowering therapy and, therefore, may help motivate patients to adhere to insulin therapy. Hypoglycemia and weight gain are also common concerns of patients and clinicians, although insulin analogs are an improvement compared with older insulins. The risk for hypoglycemia requires that patients be educated regarding the signs and symptoms and actions to be taken should a hypoglycemic episode occur. Self-monitoring of blood glucose is required and is of crucial importance in patients using multiple insulin injections or insulin-pump therapy.56 Devices for continuous glucose monitoring may also be used to reduce the incidence of hypoglycemia. Because weight gain associated with insulin therapy may be a demotivating factor in patients, lifestyle management and patient education are essential. Education should include consequences of poor glycemic control and disease progression, and the expected benefits with regard to quality of life. Using a collaborative approach to individualize therapy and to match the type of insulin and insulin dosing with a patient’s lifestyle habits, such as food intake and daily activities, fosters patient self-management and may help to minimize the risks and maximize the benefits of insulin therapy.
Conclusions
Since its discovery nearly a century ago, insulin has evolved to greater purity, with pharmacokinetic and pharmacodynamic profiles that more closely resemble insulin secretion by the pancreas. The insulin analogs are now recommended for treatment of patients with T1DM or T2DM because they are better tolerated and more physiologically similar to endogenous insulin compared with older formulations, including human insulins. Insulin analogs delivered and monitored with current pens and devices provide clinicians with improved ability to better manage patients with DM.
1. Echelbarger N, Lanham H, Close K. Diabetes Close Up: DCU Book Review#1. Michael Bliss: The Discovery of Insulin. http://www.closeconcerns.com/dcu/DCU%20BR%20V-1.pdf. Published August 2003. Accessed January 24, 2012.
2. Anderson T, Breecher MM. Science Heroes. Frederick Banting, MD. http://scienceheroes.com/index.php?option=com_content&view=article&id=80&Itemid=115. Published 2012. Accessed January 24, 2012.
3. The history of insulin. Basel, Switzerland; S. Karger AG. http://content.karger.com/ProdukteDB/Katalogteile/isbn3_8055/_83/_53/Insulin_02.pdf. Published 2012. Accessed March 26, 2012.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract 2009;15(7):768-770.] Endocr Pract. 2009;15(6):540-559.
5. US Food and Drug Administration. Humulin R. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HUMULIN%20R. Published 2012. Accessed March 26, 2012.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. American Diabetes Association. Diabetes Forecast. Insulin. http://forecast.diabetes.org/webfm_send/6. Published 2008. Accessed February 6, 2012.
19. NovoLog [package insert]. Princeton, NJ: Novo Nordisk, Inc.; 2011.
20. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
21. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
22. Lantus [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2007.
23. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc.; 2012.
24. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914.
25. Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396-402.
26. Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20(2):152-155.
27. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.-
28. Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1-248.
29. Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8(5):568-573.
30. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
31. Rave K, Nosek L, Heinemann L, Frick A, Becker R. Time-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteers. Diabetes Metab. 2003;29(4 pt 1):430-431.
32. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
33. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.-
34. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383.-
35. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209.
36. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28(1):3-13.
37. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8(10):1259-1269.
38. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8(10):1395-1406.
39. Hofman P, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and ease-of-use test among children and adolescents with diabetes. J Diabetes Sci Technol. 2011;5(6):1480-1487.
40. Siegmund T. Analysis of patient satisfaction with a prefilled insulin injection device in patients with type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1235-1237.
41. Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210-1211.
42. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304.
43. US Centers for Disease Control and Prevention. CDC Clinical Reminder. Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/PDF/Clinical-Reminder-insulin-pen.pdf. Published 2012. Accessed February 7, 2012.
44. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
45. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
46. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
47. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
48. Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294-2303.
49. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
50. Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572.
51. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes [published corrections appear in N Engl J Med. 2009;361(10):1024-1025 N Engl J Med. 2009;361(10):1028-
52. Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association, American College of Cardiology Foundation, American Heart Foundation. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation. 2009;119(25):e605]. Circulation. 2009;119(2):351-357.
53. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
54. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(suppl 5A):27S-34S.
55. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31-41.
56. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
The Evolution of Insulin Therapy in Diabetes Mellitus
Discovery of Insulin
The discovery of insulin in 1921 by Banting and Best ushered in a new age of treatment—and hope—for patients with diabetes mellitus (DM). First administered to 14-year-old Leonard Thompson on January 11, 1922, insulin transformed the lives of patients with type 1 DM (T1DM). No longer were starvation diets the primary mode of treatment.1,2 Life saving in patients with T1DM, insulin has since become an important treatment option in patients with type 2 DM (T2DM) as well.
But as is often the case with medical breakthroughs, the discovery of the hormone that first reversed diabetic coma in dogs was only the beginning. Recognizing the crudeness of the pancreatic extract that he called isletin (after the islets of Langerhans, the insulin-producing tissue of the pancreas), Banting turned to chemist James Collip, also at the University of Toronto, who developed a process to remove the toxins and impurities from the pancreatic extract. Banting also recognized the limitation of using dogs as the source of isletin (the name of which was changed to insulin by the university) so he quickly turned to cattle as a more plentiful source. Not surprisingly, the demand for insulin skyrocketed within months of its first testing in humans by Banting and Best, so, in July 1922, licenses for the manufacture of insulin were given to several pharmaceutical companies.1,2
Evolution of Insulin
While the clinical effects of insulin in patients with T1DM were dramatic, such as waking people from diabetic coma, enabling them to consume a normal diet, and improving long-term prognosis, problems were encountered.2 One was the challenge of balancing normoglycemia without causing hypoglycemia. The early insulin preparations acted relatively quickly and had a peak effect, but they did not provide a continuous, low level of basal insulin in the same manner as did pancreatic β cells. The time-action profile was, therefore, far from physiologically similar to endogenous insulin. The second problem was allergic reactions since the source of the insulin was nonhuman.2 Resolving these issues was the focus of intensive research over many decades.
To better balance normoglycemia without causing hypoglycemia, intermediate- and long-acting insulins were subsequently developed as basal insulins to prolong the duration of effect. Discovered in 1936, neutral protamine Hagedorn (NPH) insulin was released in 1950 as an intermediate-acting basal insulin.3 Although NPH insulin remains widely used today, recent guidelines have recommended against its use since the availability of insulin analogs (detemir and glargine), which provide a relatively flat profile for 24 hours and “yield better reproducibility and consistency, both between patients and within patients, with a corresponding reduction in the risk of hypoglycemia.”4 Other basal insulins such as Lente and Ultralente were introduced in the 1950s and used extensively for many years,3 but they had important limitations, such as wide variability in absorption and duration of effect, which led to inconsistent blood glucose control.
Along with efforts to prolong the duration of action of insulin, much scientific work was undertaken to reduce the risk for the allergic reactions first encountered with canine insulin, and then with bovine and porcine insulins.3 While the purity of these formulations improved over time with advances in chromatography, allergic reactions remained a limitation for some patients. The use of animal-derived insulins eventually gave way to synthetic human insulins, first approved by the US Food and Drug Administration in 1982.5 Consisting of the same amino acid sequence as insulin secreted by the human pancreas, synthetic human insulins are less likely to cause allergic reactions and have a faster onset and shorter duration of action compared with animal-derived insulins. The short-acting regular human insulin has now been largely replaced by rapid-acting insulin analogs (aspart, glulisine, and lispro) because the analogs are more physiologically similar to endogenous insulin and provide improved safety and tolerability.4 While allergic reactions do occur with insulin analogs, the prevalence is low.6-17
Insulin Analogs
Some of the early insulin formulations included zinc for the binding of insulin to protamine to alter the pharmacokinetic properties of the drug. With the availability of recombinant DNA technology, it became possible to modify the insulin structure so as to yield analogs of human regular insulin with pharmacokinetic and pharmacodynamic properties that more closely mimic the effects of endogenous insulin secreted by the pancreas (FIGURE 1). Two groups of insulin analogs were developed: (1) those with an onset of action more rapid than that of regular human insulin (ie, the rapid-acting insulin analogs); and (2) those with a duration of action longer than that of NPH human insulin (ie, the long-acting basal insulin analogs) (TABLE 1).18-23 Premix insulin formulations are also available that combine a rapid-acting insulin analog with its intermediate-acting protamine suspension.
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32
Diabetes: a journal of the American Diabetes Association by American Diabetes Association; Stanford University. Copyright 2004. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Insulin detemir was reported to have significantly less intraindividual pharmacodynamic variability compared with insulin glargine and NPH insulin. The variability (as assessed by the coefficient of variation) of the glucose infusion rate area under the curve for the first 12 hours was 27% for detemir, 46% for glargine, and 59% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). Over the first 24 hours, the coefficients of variation were 27% for detemir, 48% for glargine, and 68% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). With respect to pharmacokinetics, the coefficients of variation of the maximum plasma insulin concentration were 18% for detemir, 34% for glargine, and 24% for NPH insulin.
Despite these pharmacodynamic and pharmacokinetic differences favoring the basal insulin analogs compared with NPH insulin, evidence-based systematic reviews have concluded that overall glucose control is similar among the 3 basal insulins.28,33 These findings should be interpreted cautiously since the basal insulins were generally administered once daily in the studies included in the systematic reviews, although a few studies used a twice-daily regimen for insulin detemir or NPH insulin.28 Furthermore, some of the studies included in the systematic reviews used a treat-to-target design, in which equal glucose-lowering efficacy was maintained among treatments, thereby allowing comparisons of other insulin properties. An important difference between the basal insulin analogs and NPH insulin identified in the systematic reviews concerns hypoglycemia, particularly nocturnal hypoglycemia. Detemir and glargine were associated with significant reductions in nocturnal hypoglycemia compared with NPH insulin (both, relative risk [RR]=.54; P < .001). The risk for overall hypoglycemia was also reported to be lower with insulin detemir and insulin glargine compared with NPH insulin (RR=.68 and RR=.89, respectively; P < .001 and P=.002). The risk for severe hypoglycemia was similar for insulin glargine or insulin detemir compared with that of NPH insulin.
A recent meta-analysis comparing insulin glargine (once daily) to insulin detemir (once or twice daily) examined data from 4 trials lasting 24 to 52 weeks and involving 2250 people.34 The meta-analysis found no differences between the 2 basal insulin analogs with respect to glycemic control, as measured by the percentage of patients who achieved A1C ≤7.0% with or without hypoglycemia. In addition, no significant differences in overall, severe, and nocturnal hypoglycemia were identified. Insulin detemir was associated with less weight gain and insulin glargine with a lower number of injection-site reactions.
Evolution of Insulin Delivery
In addition to progressive improvements in purity and the time-action profile of insulin, there have been major advances in the devices used to deliver insulin that provide clinicians greater flexibility to meet patients’ needs and to resolve patients’ concerns. Advances in delivery systems include pens with shorter, smaller gauge, highly polished needles; pens with a “dial-a-dose” gauge that is easier to read; easy portability; and insulin-prefilled pens. These advances improve ease of use and dosage accuracy, likely reduce injection pain, facilitate discrete use in public places, and increase patient acceptance and adherence.35-42 Of note, however, insulin pens must never be used in more than one individual, even if a needle has been changed, as is sometimes done in institutions. A clinical reminder from the US Centers for Disease Control and Prevention in January 2012 cautioned against pen reuse and sharing, citing an incident in which more than 2000 individuals were potentially exposed to the transmission of bloodborne pathogens because of inappropriate reuse and sharing of insulin pens.43 Another advance in insulin delivery is insulin-pump therapy, which has become even more promising with the advent of continuous glucose-monitoring devices and the availability of rapid-acting insulin analogs.
Role of Insulin in Diabetes
Recently, insulin has been recognized as a key treatment option for patients with T2DM, and is no longer considered last-line therapy.4,44 When used appropriately, insulin is the most effective glucose-lowering therapy available, with essentially no limit to the magnitude of glucose lowering. Insulin, particularly the insulin analogs, provides many treatment benefits, although some limitations remain.
Benefits of Insulin
Basal-bolus therapy using the combination of a rapid-acting insulin analog and a basal insulin analog may closely mimic the release of insulin from the pancreatic β cells. The use of an insulin pump, which uses only a rapid- or short-acting insulin (rapid-acting analog preferred) may also provide insulin in a pattern that most closely mimics endogenous insulin secretion. The administration of insulin via an insulin pump may be a good treatment option in patients with T1DM or those with T2DM who require intensive basal-bolus therapy.
The reduction of microvascular complications, such as nephropathy, neuropathy, and retinopathy, by achieving intensive glycemic control with the use of insulin, has been well established in patients with T1DM or T2DM.45-48 Nonetheless, the landscape of glycemic control changed with the completion of the Action to Control Cardiovascular Risks in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT).49,50,51 Based on the findings from those trials, caution is advised against the indiscriminate setting of very low glycemic targets. Findings from subanalyses of data from those trials suggest that while most patients are likely to achieve a microvascular benefit from intensive control, others may potentially be harmed by cardiovascular events. Those likely to benefit are those with short-duration DM, a long life expectancy, and no significant cardiovascular disease. Those who may be harmed and in whom an A1C goal <7.0% may not be appropriate are those with a history of severe hypoglycemia, a limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-standing DM in whom the more stringent A1C goal may be difficult to attain.52
Misconceptions and Limitations Regarding Insulin
Insulin therapy is considered by some clinicians and patients to be the most complicated and time-consuming of the glucose-lowering therapies. Concerns about self-injection, the need for dosage adjustment, and cost, as well as the stigma of insulin as last-line therapy, are common. Additionally, in some studies with follow-up to 24 months, patients’ adherence to insulin therapy has been reported to be 54% to 81% in patients with T2DM.53-55 When used properly, insulin is the most efficacious glucose-lowering therapy and, therefore, may help motivate patients to adhere to insulin therapy. Hypoglycemia and weight gain are also common concerns of patients and clinicians, although insulin analogs are an improvement compared with older insulins. The risk for hypoglycemia requires that patients be educated regarding the signs and symptoms and actions to be taken should a hypoglycemic episode occur. Self-monitoring of blood glucose is required and is of crucial importance in patients using multiple insulin injections or insulin-pump therapy.56 Devices for continuous glucose monitoring may also be used to reduce the incidence of hypoglycemia. Because weight gain associated with insulin therapy may be a demotivating factor in patients, lifestyle management and patient education are essential. Education should include consequences of poor glycemic control and disease progression, and the expected benefits with regard to quality of life. Using a collaborative approach to individualize therapy and to match the type of insulin and insulin dosing with a patient’s lifestyle habits, such as food intake and daily activities, fosters patient self-management and may help to minimize the risks and maximize the benefits of insulin therapy.
Conclusions
Since its discovery nearly a century ago, insulin has evolved to greater purity, with pharmacokinetic and pharmacodynamic profiles that more closely resemble insulin secretion by the pancreas. The insulin analogs are now recommended for treatment of patients with T1DM or T2DM because they are better tolerated and more physiologically similar to endogenous insulin compared with older formulations, including human insulins. Insulin analogs delivered and monitored with current pens and devices provide clinicians with improved ability to better manage patients with DM.
The Evolution of Insulin Therapy in Diabetes Mellitus
Discovery of Insulin
The discovery of insulin in 1921 by Banting and Best ushered in a new age of treatment—and hope—for patients with diabetes mellitus (DM). First administered to 14-year-old Leonard Thompson on January 11, 1922, insulin transformed the lives of patients with type 1 DM (T1DM). No longer were starvation diets the primary mode of treatment.1,2 Life saving in patients with T1DM, insulin has since become an important treatment option in patients with type 2 DM (T2DM) as well.
But as is often the case with medical breakthroughs, the discovery of the hormone that first reversed diabetic coma in dogs was only the beginning. Recognizing the crudeness of the pancreatic extract that he called isletin (after the islets of Langerhans, the insulin-producing tissue of the pancreas), Banting turned to chemist James Collip, also at the University of Toronto, who developed a process to remove the toxins and impurities from the pancreatic extract. Banting also recognized the limitation of using dogs as the source of isletin (the name of which was changed to insulin by the university) so he quickly turned to cattle as a more plentiful source. Not surprisingly, the demand for insulin skyrocketed within months of its first testing in humans by Banting and Best, so, in July 1922, licenses for the manufacture of insulin were given to several pharmaceutical companies.1,2
Evolution of Insulin
While the clinical effects of insulin in patients with T1DM were dramatic, such as waking people from diabetic coma, enabling them to consume a normal diet, and improving long-term prognosis, problems were encountered.2 One was the challenge of balancing normoglycemia without causing hypoglycemia. The early insulin preparations acted relatively quickly and had a peak effect, but they did not provide a continuous, low level of basal insulin in the same manner as did pancreatic β cells. The time-action profile was, therefore, far from physiologically similar to endogenous insulin. The second problem was allergic reactions since the source of the insulin was nonhuman.2 Resolving these issues was the focus of intensive research over many decades.
To better balance normoglycemia without causing hypoglycemia, intermediate- and long-acting insulins were subsequently developed as basal insulins to prolong the duration of effect. Discovered in 1936, neutral protamine Hagedorn (NPH) insulin was released in 1950 as an intermediate-acting basal insulin.3 Although NPH insulin remains widely used today, recent guidelines have recommended against its use since the availability of insulin analogs (detemir and glargine), which provide a relatively flat profile for 24 hours and “yield better reproducibility and consistency, both between patients and within patients, with a corresponding reduction in the risk of hypoglycemia.”4 Other basal insulins such as Lente and Ultralente were introduced in the 1950s and used extensively for many years,3 but they had important limitations, such as wide variability in absorption and duration of effect, which led to inconsistent blood glucose control.
Along with efforts to prolong the duration of action of insulin, much scientific work was undertaken to reduce the risk for the allergic reactions first encountered with canine insulin, and then with bovine and porcine insulins.3 While the purity of these formulations improved over time with advances in chromatography, allergic reactions remained a limitation for some patients. The use of animal-derived insulins eventually gave way to synthetic human insulins, first approved by the US Food and Drug Administration in 1982.5 Consisting of the same amino acid sequence as insulin secreted by the human pancreas, synthetic human insulins are less likely to cause allergic reactions and have a faster onset and shorter duration of action compared with animal-derived insulins. The short-acting regular human insulin has now been largely replaced by rapid-acting insulin analogs (aspart, glulisine, and lispro) because the analogs are more physiologically similar to endogenous insulin and provide improved safety and tolerability.4 While allergic reactions do occur with insulin analogs, the prevalence is low.6-17
Insulin Analogs
Some of the early insulin formulations included zinc for the binding of insulin to protamine to alter the pharmacokinetic properties of the drug. With the availability of recombinant DNA technology, it became possible to modify the insulin structure so as to yield analogs of human regular insulin with pharmacokinetic and pharmacodynamic properties that more closely mimic the effects of endogenous insulin secreted by the pancreas (FIGURE 1). Two groups of insulin analogs were developed: (1) those with an onset of action more rapid than that of regular human insulin (ie, the rapid-acting insulin analogs); and (2) those with a duration of action longer than that of NPH human insulin (ie, the long-acting basal insulin analogs) (TABLE 1).18-23 Premix insulin formulations are also available that combine a rapid-acting insulin analog with its intermediate-acting protamine suspension.
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32
Diabetes: a journal of the American Diabetes Association by American Diabetes Association; Stanford University. Copyright 2004. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Insulin detemir was reported to have significantly less intraindividual pharmacodynamic variability compared with insulin glargine and NPH insulin. The variability (as assessed by the coefficient of variation) of the glucose infusion rate area under the curve for the first 12 hours was 27% for detemir, 46% for glargine, and 59% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). Over the first 24 hours, the coefficients of variation were 27% for detemir, 48% for glargine, and 68% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). With respect to pharmacokinetics, the coefficients of variation of the maximum plasma insulin concentration were 18% for detemir, 34% for glargine, and 24% for NPH insulin.
Despite these pharmacodynamic and pharmacokinetic differences favoring the basal insulin analogs compared with NPH insulin, evidence-based systematic reviews have concluded that overall glucose control is similar among the 3 basal insulins.28,33 These findings should be interpreted cautiously since the basal insulins were generally administered once daily in the studies included in the systematic reviews, although a few studies used a twice-daily regimen for insulin detemir or NPH insulin.28 Furthermore, some of the studies included in the systematic reviews used a treat-to-target design, in which equal glucose-lowering efficacy was maintained among treatments, thereby allowing comparisons of other insulin properties. An important difference between the basal insulin analogs and NPH insulin identified in the systematic reviews concerns hypoglycemia, particularly nocturnal hypoglycemia. Detemir and glargine were associated with significant reductions in nocturnal hypoglycemia compared with NPH insulin (both, relative risk [RR]=.54; P < .001). The risk for overall hypoglycemia was also reported to be lower with insulin detemir and insulin glargine compared with NPH insulin (RR=.68 and RR=.89, respectively; P < .001 and P=.002). The risk for severe hypoglycemia was similar for insulin glargine or insulin detemir compared with that of NPH insulin.
A recent meta-analysis comparing insulin glargine (once daily) to insulin detemir (once or twice daily) examined data from 4 trials lasting 24 to 52 weeks and involving 2250 people.34 The meta-analysis found no differences between the 2 basal insulin analogs with respect to glycemic control, as measured by the percentage of patients who achieved A1C ≤7.0% with or without hypoglycemia. In addition, no significant differences in overall, severe, and nocturnal hypoglycemia were identified. Insulin detemir was associated with less weight gain and insulin glargine with a lower number of injection-site reactions.
Evolution of Insulin Delivery
In addition to progressive improvements in purity and the time-action profile of insulin, there have been major advances in the devices used to deliver insulin that provide clinicians greater flexibility to meet patients’ needs and to resolve patients’ concerns. Advances in delivery systems include pens with shorter, smaller gauge, highly polished needles; pens with a “dial-a-dose” gauge that is easier to read; easy portability; and insulin-prefilled pens. These advances improve ease of use and dosage accuracy, likely reduce injection pain, facilitate discrete use in public places, and increase patient acceptance and adherence.35-42 Of note, however, insulin pens must never be used in more than one individual, even if a needle has been changed, as is sometimes done in institutions. A clinical reminder from the US Centers for Disease Control and Prevention in January 2012 cautioned against pen reuse and sharing, citing an incident in which more than 2000 individuals were potentially exposed to the transmission of bloodborne pathogens because of inappropriate reuse and sharing of insulin pens.43 Another advance in insulin delivery is insulin-pump therapy, which has become even more promising with the advent of continuous glucose-monitoring devices and the availability of rapid-acting insulin analogs.
Role of Insulin in Diabetes
Recently, insulin has been recognized as a key treatment option for patients with T2DM, and is no longer considered last-line therapy.4,44 When used appropriately, insulin is the most effective glucose-lowering therapy available, with essentially no limit to the magnitude of glucose lowering. Insulin, particularly the insulin analogs, provides many treatment benefits, although some limitations remain.
Benefits of Insulin
Basal-bolus therapy using the combination of a rapid-acting insulin analog and a basal insulin analog may closely mimic the release of insulin from the pancreatic β cells. The use of an insulin pump, which uses only a rapid- or short-acting insulin (rapid-acting analog preferred) may also provide insulin in a pattern that most closely mimics endogenous insulin secretion. The administration of insulin via an insulin pump may be a good treatment option in patients with T1DM or those with T2DM who require intensive basal-bolus therapy.
The reduction of microvascular complications, such as nephropathy, neuropathy, and retinopathy, by achieving intensive glycemic control with the use of insulin, has been well established in patients with T1DM or T2DM.45-48 Nonetheless, the landscape of glycemic control changed with the completion of the Action to Control Cardiovascular Risks in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT).49,50,51 Based on the findings from those trials, caution is advised against the indiscriminate setting of very low glycemic targets. Findings from subanalyses of data from those trials suggest that while most patients are likely to achieve a microvascular benefit from intensive control, others may potentially be harmed by cardiovascular events. Those likely to benefit are those with short-duration DM, a long life expectancy, and no significant cardiovascular disease. Those who may be harmed and in whom an A1C goal <7.0% may not be appropriate are those with a history of severe hypoglycemia, a limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-standing DM in whom the more stringent A1C goal may be difficult to attain.52
Misconceptions and Limitations Regarding Insulin
Insulin therapy is considered by some clinicians and patients to be the most complicated and time-consuming of the glucose-lowering therapies. Concerns about self-injection, the need for dosage adjustment, and cost, as well as the stigma of insulin as last-line therapy, are common. Additionally, in some studies with follow-up to 24 months, patients’ adherence to insulin therapy has been reported to be 54% to 81% in patients with T2DM.53-55 When used properly, insulin is the most efficacious glucose-lowering therapy and, therefore, may help motivate patients to adhere to insulin therapy. Hypoglycemia and weight gain are also common concerns of patients and clinicians, although insulin analogs are an improvement compared with older insulins. The risk for hypoglycemia requires that patients be educated regarding the signs and symptoms and actions to be taken should a hypoglycemic episode occur. Self-monitoring of blood glucose is required and is of crucial importance in patients using multiple insulin injections or insulin-pump therapy.56 Devices for continuous glucose monitoring may also be used to reduce the incidence of hypoglycemia. Because weight gain associated with insulin therapy may be a demotivating factor in patients, lifestyle management and patient education are essential. Education should include consequences of poor glycemic control and disease progression, and the expected benefits with regard to quality of life. Using a collaborative approach to individualize therapy and to match the type of insulin and insulin dosing with a patient’s lifestyle habits, such as food intake and daily activities, fosters patient self-management and may help to minimize the risks and maximize the benefits of insulin therapy.
Conclusions
Since its discovery nearly a century ago, insulin has evolved to greater purity, with pharmacokinetic and pharmacodynamic profiles that more closely resemble insulin secretion by the pancreas. The insulin analogs are now recommended for treatment of patients with T1DM or T2DM because they are better tolerated and more physiologically similar to endogenous insulin compared with older formulations, including human insulins. Insulin analogs delivered and monitored with current pens and devices provide clinicians with improved ability to better manage patients with DM.
1. Echelbarger N, Lanham H, Close K. Diabetes Close Up: DCU Book Review#1. Michael Bliss: The Discovery of Insulin. http://www.closeconcerns.com/dcu/DCU%20BR%20V-1.pdf. Published August 2003. Accessed January 24, 2012.
2. Anderson T, Breecher MM. Science Heroes. Frederick Banting, MD. http://scienceheroes.com/index.php?option=com_content&view=article&id=80&Itemid=115. Published 2012. Accessed January 24, 2012.
3. The history of insulin. Basel, Switzerland; S. Karger AG. http://content.karger.com/ProdukteDB/Katalogteile/isbn3_8055/_83/_53/Insulin_02.pdf. Published 2012. Accessed March 26, 2012.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract 2009;15(7):768-770.] Endocr Pract. 2009;15(6):540-559.
5. US Food and Drug Administration. Humulin R. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HUMULIN%20R. Published 2012. Accessed March 26, 2012.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. American Diabetes Association. Diabetes Forecast. Insulin. http://forecast.diabetes.org/webfm_send/6. Published 2008. Accessed February 6, 2012.
19. NovoLog [package insert]. Princeton, NJ: Novo Nordisk, Inc.; 2011.
20. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
21. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
22. Lantus [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2007.
23. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc.; 2012.
24. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914.
25. Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396-402.
26. Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20(2):152-155.
27. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.-
28. Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1-248.
29. Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8(5):568-573.
30. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
31. Rave K, Nosek L, Heinemann L, Frick A, Becker R. Time-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteers. Diabetes Metab. 2003;29(4 pt 1):430-431.
32. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
33. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.-
34. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383.-
35. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209.
36. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28(1):3-13.
37. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8(10):1259-1269.
38. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8(10):1395-1406.
39. Hofman P, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and ease-of-use test among children and adolescents with diabetes. J Diabetes Sci Technol. 2011;5(6):1480-1487.
40. Siegmund T. Analysis of patient satisfaction with a prefilled insulin injection device in patients with type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1235-1237.
41. Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210-1211.
42. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304.
43. US Centers for Disease Control and Prevention. CDC Clinical Reminder. Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/PDF/Clinical-Reminder-insulin-pen.pdf. Published 2012. Accessed February 7, 2012.
44. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
45. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
46. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
47. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
48. Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294-2303.
49. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
50. Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572.
51. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes [published corrections appear in N Engl J Med. 2009;361(10):1024-1025 N Engl J Med. 2009;361(10):1028-
52. Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association, American College of Cardiology Foundation, American Heart Foundation. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation. 2009;119(25):e605]. Circulation. 2009;119(2):351-357.
53. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
54. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(suppl 5A):27S-34S.
55. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31-41.
56. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
1. Echelbarger N, Lanham H, Close K. Diabetes Close Up: DCU Book Review#1. Michael Bliss: The Discovery of Insulin. http://www.closeconcerns.com/dcu/DCU%20BR%20V-1.pdf. Published August 2003. Accessed January 24, 2012.
2. Anderson T, Breecher MM. Science Heroes. Frederick Banting, MD. http://scienceheroes.com/index.php?option=com_content&view=article&id=80&Itemid=115. Published 2012. Accessed January 24, 2012.
3. The history of insulin. Basel, Switzerland; S. Karger AG. http://content.karger.com/ProdukteDB/Katalogteile/isbn3_8055/_83/_53/Insulin_02.pdf. Published 2012. Accessed March 26, 2012.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract 2009;15(7):768-770.] Endocr Pract. 2009;15(6):540-559.
5. US Food and Drug Administration. Humulin R. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HUMULIN%20R. Published 2012. Accessed March 26, 2012.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. American Diabetes Association. Diabetes Forecast. Insulin. http://forecast.diabetes.org/webfm_send/6. Published 2008. Accessed February 6, 2012.
19. NovoLog [package insert]. Princeton, NJ: Novo Nordisk, Inc.; 2011.
20. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
21. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
22. Lantus [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2007.
23. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc.; 2012.
24. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914.
25. Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396-402.
26. Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20(2):152-155.
27. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.-
28. Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1-248.
29. Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8(5):568-573.
30. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
31. Rave K, Nosek L, Heinemann L, Frick A, Becker R. Time-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteers. Diabetes Metab. 2003;29(4 pt 1):430-431.
32. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
33. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.-
34. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383.-
35. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209.
36. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28(1):3-13.
37. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8(10):1259-1269.
38. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8(10):1395-1406.
39. Hofman P, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and ease-of-use test among children and adolescents with diabetes. J Diabetes Sci Technol. 2011;5(6):1480-1487.
40. Siegmund T. Analysis of patient satisfaction with a prefilled insulin injection device in patients with type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1235-1237.
41. Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210-1211.
42. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304.
43. US Centers for Disease Control and Prevention. CDC Clinical Reminder. Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/PDF/Clinical-Reminder-insulin-pen.pdf. Published 2012. Accessed February 7, 2012.
44. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
45. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
46. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
47. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
48. Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294-2303.
49. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
50. Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572.
51. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes [published corrections appear in N Engl J Med. 2009;361(10):1024-1025 N Engl J Med. 2009;361(10):1028-
52. Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association, American College of Cardiology Foundation, American Heart Foundation. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation. 2009;119(25):e605]. Circulation. 2009;119(2):351-357.
53. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
54. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(suppl 5A):27S-34S.
55. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31-41.
56. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
The Evolution of Insulin Therapy in Diabetes Mellitus
The Evolution of Insulin Therapy in Diabetes Mellitus
Discovery of Insulin
The discovery of insulin in 1921 by Banting and Best ushered in a new age of treatment—and hope—for patients with diabetes mellitus (DM). First administered to 14-year-old Leonard Thompson on January 11, 1922, insulin transformed the lives of patients with type 1 DM (T1DM). No longer were starvation diets the primary mode of treatment.1,2 Life saving in patients with T1DM, insulin has since become an important treatment option in patients with type 2 DM (T2DM) as well.
But as is often the case with medical breakthroughs, the discovery of the hormone that first reversed diabetic coma in dogs was only the beginning. Recognizing the crudeness of the pancreatic extract that he called isletin (after the islets of Langerhans, the insulin-producing tissue of the pancreas), Banting turned to chemist James Collip, also at the University of Toronto, who developed a process to remove the toxins and impurities from the pancreatic extract. Banting also recognized the limitation of using dogs as the source of isletin (the name of which was changed to insulin by the university) so he quickly turned to cattle as a more plentiful source. Not surprisingly, the demand for insulin skyrocketed within months of its first testing in humans by Banting and Best, so, in July 1922, licenses for the manufacture of insulin were given to several pharmaceutical companies.1,2
Evolution of Insulin
While the clinical effects of insulin in patients with T1DM were dramatic, such as waking people from diabetic coma, enabling them to consume a normal diet, and improving long-term prognosis, problems were encountered.2 One was the challenge of balancing normoglycemia without causing hypoglycemia. The early insulin preparations acted relatively quickly and had a peak effect, but they did not provide a continuous, low level of basal insulin in the same manner as did pancreatic β cells. The time-action profile was, therefore, far from physiologically similar to endogenous insulin. The second problem was allergic reactions since the source of the insulin was nonhuman.2 Resolving these issues was the focus of intensive research over many decades.
To better balance normoglycemia without causing hypoglycemia, intermediate- and long-acting insulins were subsequently developed as basal insulins to prolong the duration of effect. Discovered in 1936, neutral protamine Hagedorn (NPH) insulin was released in 1950 as an intermediate-acting basal insulin.3 Although NPH insulin remains widely used today, recent guidelines have recommended against its use since the availability of insulin analogs (detemir and glargine), which provide a relatively flat profile for 24 hours and “yield better reproducibility and consistency, both between patients and within patients, with a corresponding reduction in the risk of hypoglycemia.”4 Other basal insulins such as Lente and Ultralente were introduced in the 1950s and used extensively for many years,3 but they had important limitations, such as wide variability in absorption and duration of effect, which led to inconsistent blood glucose control.
Along with efforts to prolong the duration of action of insulin, much scientific work was undertaken to reduce the risk for the allergic reactions first encountered with canine insulin, and then with bovine and porcine insulins.3 While the purity of these formulations improved over time with advances in chromatography, allergic reactions remained a limitation for some patients. The use of animal-derived insulins eventually gave way to synthetic human insulins, first approved by the US Food and Drug Administration in 1982.5 Consisting of the same amino acid sequence as insulin secreted by the human pancreas, synthetic human insulins are less likely to cause allergic reactions and have a faster onset and shorter duration of action compared with animal-derived insulins. The short-acting regular human insulin has now been largely replaced by rapid-acting insulin analogs (aspart, glulisine, and lispro) because the analogs are more physiologically similar to endogenous insulin and provide improved safety and tolerability.4 While allergic reactions do occur with insulin analogs, the prevalence is low.6-17
Insulin Analogs
Some of the early insulin formulations included zinc for the binding of insulin to protamine to alter the pharmacokinetic properties of the drug. With the availability of recombinant DNA technology, it became possible to modify the insulin structure so as to yield analogs of human regular insulin with pharmacokinetic and pharmacodynamic properties that more closely mimic the effects of endogenous insulin secreted by the pancreas (FIGURE 1). Two groups of insulin analogs were developed: (1) those with an onset of action more rapid than that of regular human insulin (ie, the rapid-acting insulin analogs); and (2) those with a duration of action longer than that of NPH human insulin (ie, the long-acting basal insulin analogs) (TABLE 1).18-23 Premix insulin formulations are also available that combine a rapid-acting insulin analog with its intermediate-acting protamine suspension.
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32
Diabetes: a journal of the American Diabetes Association by American Diabetes Association; Stanford University. Copyright 2004. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Insulin detemir was reported to have significantly less intraindividual pharmacodynamic variability compared with insulin glargine and NPH insulin. The variability (as assessed by the coefficient of variation) of the glucose infusion rate area under the curve for the first 12 hours was 27% for detemir, 46% for glargine, and 59% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). Over the first 24 hours, the coefficients of variation were 27% for detemir, 48% for glargine, and 68% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). With respect to pharmacokinetics, the coefficients of variation of the maximum plasma insulin concentration were 18% for detemir, 34% for glargine, and 24% for NPH insulin.
Despite these pharmacodynamic and pharmacokinetic differences favoring the basal insulin analogs compared with NPH insulin, evidence-based systematic reviews have concluded that overall glucose control is similar among the 3 basal insulins.28,33 These findings should be interpreted cautiously since the basal insulins were generally administered once daily in the studies included in the systematic reviews, although a few studies used a twice-daily regimen for insulin detemir or NPH insulin.28 Furthermore, some of the studies included in the systematic reviews used a treat-to-target design, in which equal glucose-lowering efficacy was maintained among treatments, thereby allowing comparisons of other insulin properties. An important difference between the basal insulin analogs and NPH insulin identified in the systematic reviews concerns hypoglycemia, particularly nocturnal hypoglycemia. Detemir and glargine were associated with significant reductions in nocturnal hypoglycemia compared with NPH insulin (both, relative risk [RR]=.54; P < .001). The risk for overall hypoglycemia was also reported to be lower with insulin detemir and insulin glargine compared with NPH insulin (RR=.68 and RR=.89, respectively; P < .001 and P=.002). The risk for severe hypoglycemia was similar for insulin glargine or insulin detemir compared with that of NPH insulin.
A recent meta-analysis comparing insulin glargine (once daily) to insulin detemir (once or twice daily) examined data from 4 trials lasting 24 to 52 weeks and involving 2250 people.34 The meta-analysis found no differences between the 2 basal insulin analogs with respect to glycemic control, as measured by the percentage of patients who achieved A1C ≤7.0% with or without hypoglycemia. In addition, no significant differences in overall, severe, and nocturnal hypoglycemia were identified. Insulin detemir was associated with less weight gain and insulin glargine with a lower number of injection-site reactions.
Evolution of Insulin Delivery
In addition to progressive improvements in purity and the time-action profile of insulin, there have been major advances in the devices used to deliver insulin that provide clinicians greater flexibility to meet patients’ needs and to resolve patients’ concerns. Advances in delivery systems include pens with shorter, smaller gauge, highly polished needles; pens with a “dial-a-dose” gauge that is easier to read; easy portability; and insulin-prefilled pens. These advances improve ease of use and dosage accuracy, likely reduce injection pain, facilitate discrete use in public places, and increase patient acceptance and adherence.35-42 Of note, however, insulin pens must never be used in more than one individual, even if a needle has been changed, as is sometimes done in institutions. A clinical reminder from the US Centers for Disease Control and Prevention in January 2012 cautioned against pen reuse and sharing, citing an incident in which more than 2000 individuals were potentially exposed to the transmission of bloodborne pathogens because of inappropriate reuse and sharing of insulin pens.43 Another advance in insulin delivery is insulin-pump therapy, which has become even more promising with the advent of continuous glucose-monitoring devices and the availability of rapid-acting insulin analogs.
Role of Insulin in Diabetes
Recently, insulin has been recognized as a key treatment option for patients with T2DM, and is no longer considered last-line therapy.4,44 When used appropriately, insulin is the most effective glucose-lowering therapy available, with essentially no limit to the magnitude of glucose lowering. Insulin, particularly the insulin analogs, provides many treatment benefits, although some limitations remain.
Benefits of Insulin
Basal-bolus therapy using the combination of a rapid-acting insulin analog and a basal insulin analog may closely mimic the release of insulin from the pancreatic β cells. The use of an insulin pump, which uses only a rapid- or short-acting insulin (rapid-acting analog preferred) may also provide insulin in a pattern that most closely mimics endogenous insulin secretion. The administration of insulin via an insulin pump may be a good treatment option in patients with T1DM or those with T2DM who require intensive basal-bolus therapy.
The reduction of microvascular complications, such as nephropathy, neuropathy, and retinopathy, by achieving intensive glycemic control with the use of insulin, has been well established in patients with T1DM or T2DM.45-48 Nonetheless, the landscape of glycemic control changed with the completion of the Action to Control Cardiovascular Risks in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT).49,50,51 Based on the findings from those trials, caution is advised against the indiscriminate setting of very low glycemic targets. Findings from subanalyses of data from those trials suggest that while most patients are likely to achieve a microvascular benefit from intensive control, others may potentially be harmed by cardiovascular events. Those likely to benefit are those with short-duration DM, a long life expectancy, and no significant cardiovascular disease. Those who may be harmed and in whom an A1C goal <7.0% may not be appropriate are those with a history of severe hypoglycemia, a limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-standing DM in whom the more stringent A1C goal may be difficult to attain.52
Misconceptions and Limitations Regarding Insulin
Insulin therapy is considered by some clinicians and patients to be the most complicated and time-consuming of the glucose-lowering therapies. Concerns about self-injection, the need for dosage adjustment, and cost, as well as the stigma of insulin as last-line therapy, are common. Additionally, in some studies with follow-up to 24 months, patients’ adherence to insulin therapy has been reported to be 54% to 81% in patients with T2DM.53-55 When used properly, insulin is the most efficacious glucose-lowering therapy and, therefore, may help motivate patients to adhere to insulin therapy. Hypoglycemia and weight gain are also common concerns of patients and clinicians, although insulin analogs are an improvement compared with older insulins. The risk for hypoglycemia requires that patients be educated regarding the signs and symptoms and actions to be taken should a hypoglycemic episode occur. Self-monitoring of blood glucose is required and is of crucial importance in patients using multiple insulin injections or insulin-pump therapy.56 Devices for continuous glucose monitoring may also be used to reduce the incidence of hypoglycemia. Because weight gain associated with insulin therapy may be a demotivating factor in patients, lifestyle management and patient education are essential. Education should include consequences of poor glycemic control and disease progression, and the expected benefits with regard to quality of life. Using a collaborative approach to individualize therapy and to match the type of insulin and insulin dosing with a patient’s lifestyle habits, such as food intake and daily activities, fosters patient self-management and may help to minimize the risks and maximize the benefits of insulin therapy.
Conclusions
Since its discovery nearly a century ago, insulin has evolved to greater purity, with pharmacokinetic and pharmacodynamic profiles that more closely resemble insulin secretion by the pancreas. The insulin analogs are now recommended for treatment of patients with T1DM or T2DM because they are better tolerated and more physiologically similar to endogenous insulin compared with older formulations, including human insulins. Insulin analogs delivered and monitored with current pens and devices provide clinicians with improved ability to better manage patients with DM.
1. Echelbarger N, Lanham H, Close K. Diabetes Close Up: DCU Book Review#1. Michael Bliss: The Discovery of Insulin. http://www.closeconcerns.com/dcu/DCU%20BR%20V-1.pdf. Published August 2003. Accessed January 24, 2012.
2. Anderson T, Breecher MM. Science Heroes. Frederick Banting, MD. http://scienceheroes.com/index.php?option=com_content&view=article&id=80&Itemid=115. Published 2012. Accessed January 24, 2012.
3. The history of insulin. Basel, Switzerland; S. Karger AG. http://content.karger.com/ProdukteDB/Katalogteile/isbn3_8055/_83/_53/Insulin_02.pdf. Published 2012. Accessed March 26, 2012.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract 2009;15(7):768-770.] Endocr Pract. 2009;15(6):540-559.
5. US Food and Drug Administration. Humulin R. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HUMULIN%20R. Published 2012. Accessed March 26, 2012.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. American Diabetes Association. Diabetes Forecast. Insulin. http://forecast.diabetes.org/webfm_send/6. Published 2008. Accessed February 6, 2012.
19. NovoLog [package insert]. Princeton, NJ: Novo Nordisk, Inc.; 2011.
20. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
21. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
22. Lantus [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2007.
23. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc.; 2012.
24. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914.
25. Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396-402.
26. Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20(2):152-155.
27. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.-
28. Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1-248.
29. Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8(5):568-573.
30. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
31. Rave K, Nosek L, Heinemann L, Frick A, Becker R. Time-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteers. Diabetes Metab. 2003;29(4 pt 1):430-431.
32. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
33. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.-
34. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383.-
35. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209.
36. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28(1):3-13.
37. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8(10):1259-1269.
38. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8(10):1395-1406.
39. Hofman P, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and ease-of-use test among children and adolescents with diabetes. J Diabetes Sci Technol. 2011;5(6):1480-1487.
40. Siegmund T. Analysis of patient satisfaction with a prefilled insulin injection device in patients with type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1235-1237.
41. Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210-1211.
42. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304.
43. US Centers for Disease Control and Prevention. CDC Clinical Reminder. Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/PDF/Clinical-Reminder-insulin-pen.pdf. Published 2012. Accessed February 7, 2012.
44. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
45. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
46. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
47. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
48. Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294-2303.
49. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
50. Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572.
51. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes [published corrections appear in N Engl J Med. 2009;361(10):1024-1025 N Engl J Med. 2009;361(10):1028-
52. Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association, American College of Cardiology Foundation, American Heart Foundation. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation. 2009;119(25):e605]. Circulation. 2009;119(2):351-357.
53. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
54. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(suppl 5A):27S-34S.
55. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31-41.
56. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
The Evolution of Insulin Therapy in Diabetes Mellitus
Discovery of Insulin
The discovery of insulin in 1921 by Banting and Best ushered in a new age of treatment—and hope—for patients with diabetes mellitus (DM). First administered to 14-year-old Leonard Thompson on January 11, 1922, insulin transformed the lives of patients with type 1 DM (T1DM). No longer were starvation diets the primary mode of treatment.1,2 Life saving in patients with T1DM, insulin has since become an important treatment option in patients with type 2 DM (T2DM) as well.
But as is often the case with medical breakthroughs, the discovery of the hormone that first reversed diabetic coma in dogs was only the beginning. Recognizing the crudeness of the pancreatic extract that he called isletin (after the islets of Langerhans, the insulin-producing tissue of the pancreas), Banting turned to chemist James Collip, also at the University of Toronto, who developed a process to remove the toxins and impurities from the pancreatic extract. Banting also recognized the limitation of using dogs as the source of isletin (the name of which was changed to insulin by the university) so he quickly turned to cattle as a more plentiful source. Not surprisingly, the demand for insulin skyrocketed within months of its first testing in humans by Banting and Best, so, in July 1922, licenses for the manufacture of insulin were given to several pharmaceutical companies.1,2
Evolution of Insulin
While the clinical effects of insulin in patients with T1DM were dramatic, such as waking people from diabetic coma, enabling them to consume a normal diet, and improving long-term prognosis, problems were encountered.2 One was the challenge of balancing normoglycemia without causing hypoglycemia. The early insulin preparations acted relatively quickly and had a peak effect, but they did not provide a continuous, low level of basal insulin in the same manner as did pancreatic β cells. The time-action profile was, therefore, far from physiologically similar to endogenous insulin. The second problem was allergic reactions since the source of the insulin was nonhuman.2 Resolving these issues was the focus of intensive research over many decades.
To better balance normoglycemia without causing hypoglycemia, intermediate- and long-acting insulins were subsequently developed as basal insulins to prolong the duration of effect. Discovered in 1936, neutral protamine Hagedorn (NPH) insulin was released in 1950 as an intermediate-acting basal insulin.3 Although NPH insulin remains widely used today, recent guidelines have recommended against its use since the availability of insulin analogs (detemir and glargine), which provide a relatively flat profile for 24 hours and “yield better reproducibility and consistency, both between patients and within patients, with a corresponding reduction in the risk of hypoglycemia.”4 Other basal insulins such as Lente and Ultralente were introduced in the 1950s and used extensively for many years,3 but they had important limitations, such as wide variability in absorption and duration of effect, which led to inconsistent blood glucose control.
Along with efforts to prolong the duration of action of insulin, much scientific work was undertaken to reduce the risk for the allergic reactions first encountered with canine insulin, and then with bovine and porcine insulins.3 While the purity of these formulations improved over time with advances in chromatography, allergic reactions remained a limitation for some patients. The use of animal-derived insulins eventually gave way to synthetic human insulins, first approved by the US Food and Drug Administration in 1982.5 Consisting of the same amino acid sequence as insulin secreted by the human pancreas, synthetic human insulins are less likely to cause allergic reactions and have a faster onset and shorter duration of action compared with animal-derived insulins. The short-acting regular human insulin has now been largely replaced by rapid-acting insulin analogs (aspart, glulisine, and lispro) because the analogs are more physiologically similar to endogenous insulin and provide improved safety and tolerability.4 While allergic reactions do occur with insulin analogs, the prevalence is low.6-17
Insulin Analogs
Some of the early insulin formulations included zinc for the binding of insulin to protamine to alter the pharmacokinetic properties of the drug. With the availability of recombinant DNA technology, it became possible to modify the insulin structure so as to yield analogs of human regular insulin with pharmacokinetic and pharmacodynamic properties that more closely mimic the effects of endogenous insulin secreted by the pancreas (FIGURE 1). Two groups of insulin analogs were developed: (1) those with an onset of action more rapid than that of regular human insulin (ie, the rapid-acting insulin analogs); and (2) those with a duration of action longer than that of NPH human insulin (ie, the long-acting basal insulin analogs) (TABLE 1).18-23 Premix insulin formulations are also available that combine a rapid-acting insulin analog with its intermediate-acting protamine suspension.
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32
Diabetes: a journal of the American Diabetes Association by American Diabetes Association; Stanford University. Copyright 2004. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Insulin detemir was reported to have significantly less intraindividual pharmacodynamic variability compared with insulin glargine and NPH insulin. The variability (as assessed by the coefficient of variation) of the glucose infusion rate area under the curve for the first 12 hours was 27% for detemir, 46% for glargine, and 59% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). Over the first 24 hours, the coefficients of variation were 27% for detemir, 48% for glargine, and 68% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). With respect to pharmacokinetics, the coefficients of variation of the maximum plasma insulin concentration were 18% for detemir, 34% for glargine, and 24% for NPH insulin.
Despite these pharmacodynamic and pharmacokinetic differences favoring the basal insulin analogs compared with NPH insulin, evidence-based systematic reviews have concluded that overall glucose control is similar among the 3 basal insulins.28,33 These findings should be interpreted cautiously since the basal insulins were generally administered once daily in the studies included in the systematic reviews, although a few studies used a twice-daily regimen for insulin detemir or NPH insulin.28 Furthermore, some of the studies included in the systematic reviews used a treat-to-target design, in which equal glucose-lowering efficacy was maintained among treatments, thereby allowing comparisons of other insulin properties. An important difference between the basal insulin analogs and NPH insulin identified in the systematic reviews concerns hypoglycemia, particularly nocturnal hypoglycemia. Detemir and glargine were associated with significant reductions in nocturnal hypoglycemia compared with NPH insulin (both, relative risk [RR]=.54; P < .001). The risk for overall hypoglycemia was also reported to be lower with insulin detemir and insulin glargine compared with NPH insulin (RR=.68 and RR=.89, respectively; P < .001 and P=.002). The risk for severe hypoglycemia was similar for insulin glargine or insulin detemir compared with that of NPH insulin.
A recent meta-analysis comparing insulin glargine (once daily) to insulin detemir (once or twice daily) examined data from 4 trials lasting 24 to 52 weeks and involving 2250 people.34 The meta-analysis found no differences between the 2 basal insulin analogs with respect to glycemic control, as measured by the percentage of patients who achieved A1C ≤7.0% with or without hypoglycemia. In addition, no significant differences in overall, severe, and nocturnal hypoglycemia were identified. Insulin detemir was associated with less weight gain and insulin glargine with a lower number of injection-site reactions.
Evolution of Insulin Delivery
In addition to progressive improvements in purity and the time-action profile of insulin, there have been major advances in the devices used to deliver insulin that provide clinicians greater flexibility to meet patients’ needs and to resolve patients’ concerns. Advances in delivery systems include pens with shorter, smaller gauge, highly polished needles; pens with a “dial-a-dose” gauge that is easier to read; easy portability; and insulin-prefilled pens. These advances improve ease of use and dosage accuracy, likely reduce injection pain, facilitate discrete use in public places, and increase patient acceptance and adherence.35-42 Of note, however, insulin pens must never be used in more than one individual, even if a needle has been changed, as is sometimes done in institutions. A clinical reminder from the US Centers for Disease Control and Prevention in January 2012 cautioned against pen reuse and sharing, citing an incident in which more than 2000 individuals were potentially exposed to the transmission of bloodborne pathogens because of inappropriate reuse and sharing of insulin pens.43 Another advance in insulin delivery is insulin-pump therapy, which has become even more promising with the advent of continuous glucose-monitoring devices and the availability of rapid-acting insulin analogs.
Role of Insulin in Diabetes
Recently, insulin has been recognized as a key treatment option for patients with T2DM, and is no longer considered last-line therapy.4,44 When used appropriately, insulin is the most effective glucose-lowering therapy available, with essentially no limit to the magnitude of glucose lowering. Insulin, particularly the insulin analogs, provides many treatment benefits, although some limitations remain.
Benefits of Insulin
Basal-bolus therapy using the combination of a rapid-acting insulin analog and a basal insulin analog may closely mimic the release of insulin from the pancreatic β cells. The use of an insulin pump, which uses only a rapid- or short-acting insulin (rapid-acting analog preferred) may also provide insulin in a pattern that most closely mimics endogenous insulin secretion. The administration of insulin via an insulin pump may be a good treatment option in patients with T1DM or those with T2DM who require intensive basal-bolus therapy.
The reduction of microvascular complications, such as nephropathy, neuropathy, and retinopathy, by achieving intensive glycemic control with the use of insulin, has been well established in patients with T1DM or T2DM.45-48 Nonetheless, the landscape of glycemic control changed with the completion of the Action to Control Cardiovascular Risks in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT).49,50,51 Based on the findings from those trials, caution is advised against the indiscriminate setting of very low glycemic targets. Findings from subanalyses of data from those trials suggest that while most patients are likely to achieve a microvascular benefit from intensive control, others may potentially be harmed by cardiovascular events. Those likely to benefit are those with short-duration DM, a long life expectancy, and no significant cardiovascular disease. Those who may be harmed and in whom an A1C goal <7.0% may not be appropriate are those with a history of severe hypoglycemia, a limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-standing DM in whom the more stringent A1C goal may be difficult to attain.52
Misconceptions and Limitations Regarding Insulin
Insulin therapy is considered by some clinicians and patients to be the most complicated and time-consuming of the glucose-lowering therapies. Concerns about self-injection, the need for dosage adjustment, and cost, as well as the stigma of insulin as last-line therapy, are common. Additionally, in some studies with follow-up to 24 months, patients’ adherence to insulin therapy has been reported to be 54% to 81% in patients with T2DM.53-55 When used properly, insulin is the most efficacious glucose-lowering therapy and, therefore, may help motivate patients to adhere to insulin therapy. Hypoglycemia and weight gain are also common concerns of patients and clinicians, although insulin analogs are an improvement compared with older insulins. The risk for hypoglycemia requires that patients be educated regarding the signs and symptoms and actions to be taken should a hypoglycemic episode occur. Self-monitoring of blood glucose is required and is of crucial importance in patients using multiple insulin injections or insulin-pump therapy.56 Devices for continuous glucose monitoring may also be used to reduce the incidence of hypoglycemia. Because weight gain associated with insulin therapy may be a demotivating factor in patients, lifestyle management and patient education are essential. Education should include consequences of poor glycemic control and disease progression, and the expected benefits with regard to quality of life. Using a collaborative approach to individualize therapy and to match the type of insulin and insulin dosing with a patient’s lifestyle habits, such as food intake and daily activities, fosters patient self-management and may help to minimize the risks and maximize the benefits of insulin therapy.
Conclusions
Since its discovery nearly a century ago, insulin has evolved to greater purity, with pharmacokinetic and pharmacodynamic profiles that more closely resemble insulin secretion by the pancreas. The insulin analogs are now recommended for treatment of patients with T1DM or T2DM because they are better tolerated and more physiologically similar to endogenous insulin compared with older formulations, including human insulins. Insulin analogs delivered and monitored with current pens and devices provide clinicians with improved ability to better manage patients with DM.
The Evolution of Insulin Therapy in Diabetes Mellitus
Discovery of Insulin
The discovery of insulin in 1921 by Banting and Best ushered in a new age of treatment—and hope—for patients with diabetes mellitus (DM). First administered to 14-year-old Leonard Thompson on January 11, 1922, insulin transformed the lives of patients with type 1 DM (T1DM). No longer were starvation diets the primary mode of treatment.1,2 Life saving in patients with T1DM, insulin has since become an important treatment option in patients with type 2 DM (T2DM) as well.
But as is often the case with medical breakthroughs, the discovery of the hormone that first reversed diabetic coma in dogs was only the beginning. Recognizing the crudeness of the pancreatic extract that he called isletin (after the islets of Langerhans, the insulin-producing tissue of the pancreas), Banting turned to chemist James Collip, also at the University of Toronto, who developed a process to remove the toxins and impurities from the pancreatic extract. Banting also recognized the limitation of using dogs as the source of isletin (the name of which was changed to insulin by the university) so he quickly turned to cattle as a more plentiful source. Not surprisingly, the demand for insulin skyrocketed within months of its first testing in humans by Banting and Best, so, in July 1922, licenses for the manufacture of insulin were given to several pharmaceutical companies.1,2
Evolution of Insulin
While the clinical effects of insulin in patients with T1DM were dramatic, such as waking people from diabetic coma, enabling them to consume a normal diet, and improving long-term prognosis, problems were encountered.2 One was the challenge of balancing normoglycemia without causing hypoglycemia. The early insulin preparations acted relatively quickly and had a peak effect, but they did not provide a continuous, low level of basal insulin in the same manner as did pancreatic β cells. The time-action profile was, therefore, far from physiologically similar to endogenous insulin. The second problem was allergic reactions since the source of the insulin was nonhuman.2 Resolving these issues was the focus of intensive research over many decades.
To better balance normoglycemia without causing hypoglycemia, intermediate- and long-acting insulins were subsequently developed as basal insulins to prolong the duration of effect. Discovered in 1936, neutral protamine Hagedorn (NPH) insulin was released in 1950 as an intermediate-acting basal insulin.3 Although NPH insulin remains widely used today, recent guidelines have recommended against its use since the availability of insulin analogs (detemir and glargine), which provide a relatively flat profile for 24 hours and “yield better reproducibility and consistency, both between patients and within patients, with a corresponding reduction in the risk of hypoglycemia.”4 Other basal insulins such as Lente and Ultralente were introduced in the 1950s and used extensively for many years,3 but they had important limitations, such as wide variability in absorption and duration of effect, which led to inconsistent blood glucose control.
Along with efforts to prolong the duration of action of insulin, much scientific work was undertaken to reduce the risk for the allergic reactions first encountered with canine insulin, and then with bovine and porcine insulins.3 While the purity of these formulations improved over time with advances in chromatography, allergic reactions remained a limitation for some patients. The use of animal-derived insulins eventually gave way to synthetic human insulins, first approved by the US Food and Drug Administration in 1982.5 Consisting of the same amino acid sequence as insulin secreted by the human pancreas, synthetic human insulins are less likely to cause allergic reactions and have a faster onset and shorter duration of action compared with animal-derived insulins. The short-acting regular human insulin has now been largely replaced by rapid-acting insulin analogs (aspart, glulisine, and lispro) because the analogs are more physiologically similar to endogenous insulin and provide improved safety and tolerability.4 While allergic reactions do occur with insulin analogs, the prevalence is low.6-17
Insulin Analogs
Some of the early insulin formulations included zinc for the binding of insulin to protamine to alter the pharmacokinetic properties of the drug. With the availability of recombinant DNA technology, it became possible to modify the insulin structure so as to yield analogs of human regular insulin with pharmacokinetic and pharmacodynamic properties that more closely mimic the effects of endogenous insulin secreted by the pancreas (FIGURE 1). Two groups of insulin analogs were developed: (1) those with an onset of action more rapid than that of regular human insulin (ie, the rapid-acting insulin analogs); and (2) those with a duration of action longer than that of NPH human insulin (ie, the long-acting basal insulin analogs) (TABLE 1).18-23 Premix insulin formulations are also available that combine a rapid-acting insulin analog with its intermediate-acting protamine suspension.
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32
Diabetes: a journal of the American Diabetes Association by American Diabetes Association; Stanford University. Copyright 2004. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Insulin detemir was reported to have significantly less intraindividual pharmacodynamic variability compared with insulin glargine and NPH insulin. The variability (as assessed by the coefficient of variation) of the glucose infusion rate area under the curve for the first 12 hours was 27% for detemir, 46% for glargine, and 59% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). Over the first 24 hours, the coefficients of variation were 27% for detemir, 48% for glargine, and 68% for NPH insulin (P < .001 vs insulin glargine and NPH insulin). With respect to pharmacokinetics, the coefficients of variation of the maximum plasma insulin concentration were 18% for detemir, 34% for glargine, and 24% for NPH insulin.
Despite these pharmacodynamic and pharmacokinetic differences favoring the basal insulin analogs compared with NPH insulin, evidence-based systematic reviews have concluded that overall glucose control is similar among the 3 basal insulins.28,33 These findings should be interpreted cautiously since the basal insulins were generally administered once daily in the studies included in the systematic reviews, although a few studies used a twice-daily regimen for insulin detemir or NPH insulin.28 Furthermore, some of the studies included in the systematic reviews used a treat-to-target design, in which equal glucose-lowering efficacy was maintained among treatments, thereby allowing comparisons of other insulin properties. An important difference between the basal insulin analogs and NPH insulin identified in the systematic reviews concerns hypoglycemia, particularly nocturnal hypoglycemia. Detemir and glargine were associated with significant reductions in nocturnal hypoglycemia compared with NPH insulin (both, relative risk [RR]=.54; P < .001). The risk for overall hypoglycemia was also reported to be lower with insulin detemir and insulin glargine compared with NPH insulin (RR=.68 and RR=.89, respectively; P < .001 and P=.002). The risk for severe hypoglycemia was similar for insulin glargine or insulin detemir compared with that of NPH insulin.
A recent meta-analysis comparing insulin glargine (once daily) to insulin detemir (once or twice daily) examined data from 4 trials lasting 24 to 52 weeks and involving 2250 people.34 The meta-analysis found no differences between the 2 basal insulin analogs with respect to glycemic control, as measured by the percentage of patients who achieved A1C ≤7.0% with or without hypoglycemia. In addition, no significant differences in overall, severe, and nocturnal hypoglycemia were identified. Insulin detemir was associated with less weight gain and insulin glargine with a lower number of injection-site reactions.
Evolution of Insulin Delivery
In addition to progressive improvements in purity and the time-action profile of insulin, there have been major advances in the devices used to deliver insulin that provide clinicians greater flexibility to meet patients’ needs and to resolve patients’ concerns. Advances in delivery systems include pens with shorter, smaller gauge, highly polished needles; pens with a “dial-a-dose” gauge that is easier to read; easy portability; and insulin-prefilled pens. These advances improve ease of use and dosage accuracy, likely reduce injection pain, facilitate discrete use in public places, and increase patient acceptance and adherence.35-42 Of note, however, insulin pens must never be used in more than one individual, even if a needle has been changed, as is sometimes done in institutions. A clinical reminder from the US Centers for Disease Control and Prevention in January 2012 cautioned against pen reuse and sharing, citing an incident in which more than 2000 individuals were potentially exposed to the transmission of bloodborne pathogens because of inappropriate reuse and sharing of insulin pens.43 Another advance in insulin delivery is insulin-pump therapy, which has become even more promising with the advent of continuous glucose-monitoring devices and the availability of rapid-acting insulin analogs.
Role of Insulin in Diabetes
Recently, insulin has been recognized as a key treatment option for patients with T2DM, and is no longer considered last-line therapy.4,44 When used appropriately, insulin is the most effective glucose-lowering therapy available, with essentially no limit to the magnitude of glucose lowering. Insulin, particularly the insulin analogs, provides many treatment benefits, although some limitations remain.
Benefits of Insulin
Basal-bolus therapy using the combination of a rapid-acting insulin analog and a basal insulin analog may closely mimic the release of insulin from the pancreatic β cells. The use of an insulin pump, which uses only a rapid- or short-acting insulin (rapid-acting analog preferred) may also provide insulin in a pattern that most closely mimics endogenous insulin secretion. The administration of insulin via an insulin pump may be a good treatment option in patients with T1DM or those with T2DM who require intensive basal-bolus therapy.
The reduction of microvascular complications, such as nephropathy, neuropathy, and retinopathy, by achieving intensive glycemic control with the use of insulin, has been well established in patients with T1DM or T2DM.45-48 Nonetheless, the landscape of glycemic control changed with the completion of the Action to Control Cardiovascular Risks in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT).49,50,51 Based on the findings from those trials, caution is advised against the indiscriminate setting of very low glycemic targets. Findings from subanalyses of data from those trials suggest that while most patients are likely to achieve a microvascular benefit from intensive control, others may potentially be harmed by cardiovascular events. Those likely to benefit are those with short-duration DM, a long life expectancy, and no significant cardiovascular disease. Those who may be harmed and in whom an A1C goal <7.0% may not be appropriate are those with a history of severe hypoglycemia, a limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-standing DM in whom the more stringent A1C goal may be difficult to attain.52
Misconceptions and Limitations Regarding Insulin
Insulin therapy is considered by some clinicians and patients to be the most complicated and time-consuming of the glucose-lowering therapies. Concerns about self-injection, the need for dosage adjustment, and cost, as well as the stigma of insulin as last-line therapy, are common. Additionally, in some studies with follow-up to 24 months, patients’ adherence to insulin therapy has been reported to be 54% to 81% in patients with T2DM.53-55 When used properly, insulin is the most efficacious glucose-lowering therapy and, therefore, may help motivate patients to adhere to insulin therapy. Hypoglycemia and weight gain are also common concerns of patients and clinicians, although insulin analogs are an improvement compared with older insulins. The risk for hypoglycemia requires that patients be educated regarding the signs and symptoms and actions to be taken should a hypoglycemic episode occur. Self-monitoring of blood glucose is required and is of crucial importance in patients using multiple insulin injections or insulin-pump therapy.56 Devices for continuous glucose monitoring may also be used to reduce the incidence of hypoglycemia. Because weight gain associated with insulin therapy may be a demotivating factor in patients, lifestyle management and patient education are essential. Education should include consequences of poor glycemic control and disease progression, and the expected benefits with regard to quality of life. Using a collaborative approach to individualize therapy and to match the type of insulin and insulin dosing with a patient’s lifestyle habits, such as food intake and daily activities, fosters patient self-management and may help to minimize the risks and maximize the benefits of insulin therapy.
Conclusions
Since its discovery nearly a century ago, insulin has evolved to greater purity, with pharmacokinetic and pharmacodynamic profiles that more closely resemble insulin secretion by the pancreas. The insulin analogs are now recommended for treatment of patients with T1DM or T2DM because they are better tolerated and more physiologically similar to endogenous insulin compared with older formulations, including human insulins. Insulin analogs delivered and monitored with current pens and devices provide clinicians with improved ability to better manage patients with DM.
1. Echelbarger N, Lanham H, Close K. Diabetes Close Up: DCU Book Review#1. Michael Bliss: The Discovery of Insulin. http://www.closeconcerns.com/dcu/DCU%20BR%20V-1.pdf. Published August 2003. Accessed January 24, 2012.
2. Anderson T, Breecher MM. Science Heroes. Frederick Banting, MD. http://scienceheroes.com/index.php?option=com_content&view=article&id=80&Itemid=115. Published 2012. Accessed January 24, 2012.
3. The history of insulin. Basel, Switzerland; S. Karger AG. http://content.karger.com/ProdukteDB/Katalogteile/isbn3_8055/_83/_53/Insulin_02.pdf. Published 2012. Accessed March 26, 2012.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract 2009;15(7):768-770.] Endocr Pract. 2009;15(6):540-559.
5. US Food and Drug Administration. Humulin R. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HUMULIN%20R. Published 2012. Accessed March 26, 2012.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. American Diabetes Association. Diabetes Forecast. Insulin. http://forecast.diabetes.org/webfm_send/6. Published 2008. Accessed February 6, 2012.
19. NovoLog [package insert]. Princeton, NJ: Novo Nordisk, Inc.; 2011.
20. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
21. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
22. Lantus [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2007.
23. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc.; 2012.
24. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914.
25. Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396-402.
26. Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20(2):152-155.
27. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.-
28. Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1-248.
29. Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8(5):568-573.
30. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
31. Rave K, Nosek L, Heinemann L, Frick A, Becker R. Time-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteers. Diabetes Metab. 2003;29(4 pt 1):430-431.
32. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
33. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.-
34. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383.-
35. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209.
36. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28(1):3-13.
37. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8(10):1259-1269.
38. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8(10):1395-1406.
39. Hofman P, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and ease-of-use test among children and adolescents with diabetes. J Diabetes Sci Technol. 2011;5(6):1480-1487.
40. Siegmund T. Analysis of patient satisfaction with a prefilled insulin injection device in patients with type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1235-1237.
41. Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210-1211.
42. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304.
43. US Centers for Disease Control and Prevention. CDC Clinical Reminder. Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/PDF/Clinical-Reminder-insulin-pen.pdf. Published 2012. Accessed February 7, 2012.
44. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
45. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
46. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
47. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
48. Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294-2303.
49. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
50. Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572.
51. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes [published corrections appear in N Engl J Med. 2009;361(10):1024-1025 N Engl J Med. 2009;361(10):1028-
52. Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association, American College of Cardiology Foundation, American Heart Foundation. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation. 2009;119(25):e605]. Circulation. 2009;119(2):351-357.
53. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
54. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(suppl 5A):27S-34S.
55. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31-41.
56. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
1. Echelbarger N, Lanham H, Close K. Diabetes Close Up: DCU Book Review#1. Michael Bliss: The Discovery of Insulin. http://www.closeconcerns.com/dcu/DCU%20BR%20V-1.pdf. Published August 2003. Accessed January 24, 2012.
2. Anderson T, Breecher MM. Science Heroes. Frederick Banting, MD. http://scienceheroes.com/index.php?option=com_content&view=article&id=80&Itemid=115. Published 2012. Accessed January 24, 2012.
3. The history of insulin. Basel, Switzerland; S. Karger AG. http://content.karger.com/ProdukteDB/Katalogteile/isbn3_8055/_83/_53/Insulin_02.pdf. Published 2012. Accessed March 26, 2012.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract 2009;15(7):768-770.] Endocr Pract. 2009;15(6):540-559.
5. US Food and Drug Administration. Humulin R. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HUMULIN%20R. Published 2012. Accessed March 26, 2012.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. American Diabetes Association. Diabetes Forecast. Insulin. http://forecast.diabetes.org/webfm_send/6. Published 2008. Accessed February 6, 2012.
19. NovoLog [package insert]. Princeton, NJ: Novo Nordisk, Inc.; 2011.
20. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
21. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
22. Lantus [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2007.
23. Levemir [package insert]. Princeton, NJ: Novo Nordisk Inc.; 2012.
24. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914.
25. Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396-402.
26. Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20(2):152-155.
27. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.-
28. Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1-248.
29. Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8(5):568-573.
30. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
31. Rave K, Nosek L, Heinemann L, Frick A, Becker R. Time-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteers. Diabetes Metab. 2003;29(4 pt 1):430-431.
32. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
33. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.-
34. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383.-
35. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209.
36. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28(1):3-13.
37. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8(10):1259-1269.
38. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8(10):1395-1406.
39. Hofman P, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and ease-of-use test among children and adolescents with diabetes. J Diabetes Sci Technol. 2011;5(6):1480-1487.
40. Siegmund T. Analysis of patient satisfaction with a prefilled insulin injection device in patients with type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1235-1237.
41. Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210-1211.
42. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304.
43. US Centers for Disease Control and Prevention. CDC Clinical Reminder. Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/PDF/Clinical-Reminder-insulin-pen.pdf. Published 2012. Accessed February 7, 2012.
44. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
45. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
46. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
47. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
48. Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294-2303.
49. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
50. Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572.
51. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes [published corrections appear in N Engl J Med. 2009;361(10):1024-1025 N Engl J Med. 2009;361(10):1028-
52. Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association, American College of Cardiology Foundation, American Heart Foundation. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation. 2009;119(25):e605]. Circulation. 2009;119(2):351-357.
53. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
54. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(suppl 5A):27S-34S.
55. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31-41.
56. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.