User login
Bimatoprost-Induced Iris Hyperpigmentation: Beauty in the Darkened Eye of the Beholder
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
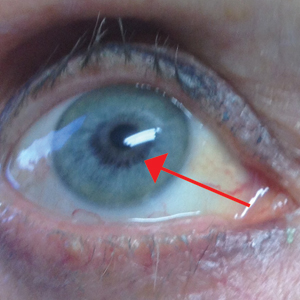
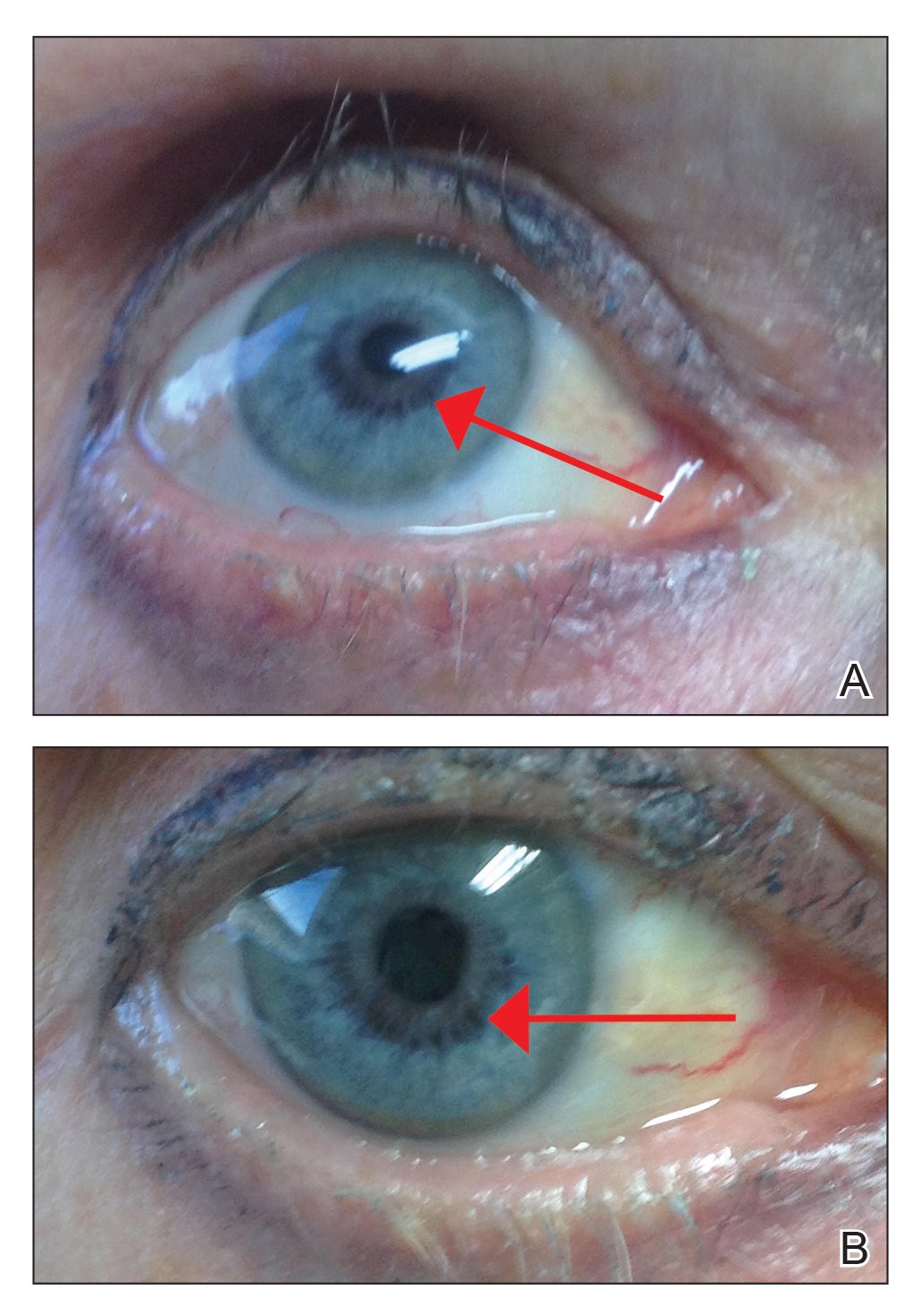
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).
The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).

The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).

The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
Practice Points
- Bimatoprost ophthalmic solution 0.03% was approved by the US Food and Drug Administration in 2008 as an eyelash solution with an eyelid applicator for treatment of eyelash hypotrichosis.
- Iris hyperpigmentation can occur when bimatoprost eye drops are applied to the eyes for treatment of ocular hypertension and glaucoma, but reports associated with bimatoprost eyelash solution are rare.
- It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information to avoid potential adverse events. The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye.