User login
Gynecologic care of the cancer patient
- Selective serotonin reuptake inhibitors (SSRIs), clonidine hydrochloride, and megestrol alleviate hot flushes in women who opt not to take hormone replacement therapy (HRT) due to concerns about subsequent disease.
- Local estrogen therapy in the form of an estradiol-releasing vaginal tablet or ring effectively improves vaginal atrophy and has a higher patient acceptability than vaginal cream.
- Because a majority of women who have survived breast cancer have not been screened for BRCA1 and BRCA2 mutations, Ob/Gyns should screen survivors to identify those patients at risk for subsequent disease.
- Approximately half of the women who survive breast or a gynecologic cancer report severe, long-lasting sexual problems.
As more is discovered about cancer, caring for the cancer patient has become even more complicated. Women who have survived cancer, even nongynecologic diseases, increasingly are being followed by general obstetrician/gynecologists or primary care physicians. To help Ob/Gyns provide better care, this review will summarize cancer patients’ special needs with regard to menopause and premature menopause, genetic screening techniques, and well-woman care.
Only about 15% of postmenopausal women use HRT.
In evaluating the needs of the cancer patient, an understanding of the type of cancer and the surgical and medical management the woman has undergone is key. This knowledge aids the management of sexual health, fertility, and menopause. Additionally, depending on the type of cancer she has had, the patient may be exposed to health risks not previously identified. In these cases, an understanding of the familial basis of some cancers and hereditary syndromes is essential, as it can help identify those who may be at risk for subsequent malignancies or conditions.
Finally, as with all well-woman care, preventive measures such as bone densitometry, mammography, colonoscopy, exercise, self-breast examination, and smoking cessation must be addressed and discussed.
Menopause
Many young women with a cancer diagnosis face premature menopause. In addition to the psychological issues stemming from premature ovarian failure such as those related to fertility, these women experience many of the typical menopausal symptoms and other health risks well before their healthy counterparts.
In the decades since hormone replacement therapy (HRT) was introduced, there has been increasing controversy and confusion among patients and physicians about its benefits and risks. In particular, because of the possible role of estrogen in the pathogenesis of breast cancer, its use for post-menopausal therapy has been challenged.1-3
Currently, only about 15% of post-menopausal women use HRT, in part due to the concerns about breast cancer. Other reasons include breast engorgement and tenderness and a resumption of vaginal bleeding.
Most health-care providers believe HRT is contraindicated for breast cancer survivors. However, withholding HRT from all postmenopausal women would be a disservice to many, as it would expose them to health risks that would outweigh the risk for breast cancer. One of the clinician’s roles is to identify patients who would benefit from HRT and to avoid exposing others to unreasonable risk. Fortunately, the available alternatives to HRT are rapidly increasing in response to the demand this controversy has generated.
BRCA 1 and BRCA 2 are responsible for most inherited ovarian carcinomas.
Hormone replacement therapy initially was intended for short-term use in the management of vasomotor symptoms. The health benefits that were subsequently identified supported a longer duration of use.4,5 These include improving hot flushes, protecting bone density, and combating urogenital atrophy, but more recently, the potential benefit that hormones may have on cardiac health has come under scrutiny.6-8
Vasomotor symptoms. Menopausal women often complain of debilitating vasomotor flushes. For women who take tamoxifen, e.g., those with a history of breast cancer, vasomotor symptoms may be potentiated.9,10 Traditional HRT regimens, either oral or transdermal, have been effective in treating vasomotor symptoms in 85% to 90% of women.11 Even so, increasing numbers of women are turning to an ever-growing array of nonestrogen agents in an effort to alleviate symptoms and minimize their perceived risk of breast cancer.
Agents that can be used to alleviate hot flushes include selective serotonin reuptake inhibitors (SSRIs) such as venlafaxine hydrochloride in small doses, e.g., 25 to 75 mg daily.12,13 Clonidine hydrochloride, an antihypertensive, also is effective, but its side effects limit its use.14 If the 0.1-mg patch is used, it should be changed weekly, and if the drug is administered orally, the dosage should not exceed 0.1 mg twice daily. Megestrol, a progestin, also has been shown to be effective.15
Recently, phytoestrogens have been sought as substitutes for traditional HRT. While there is some evidence they may improve vasomotor flushes and protect against bone cancer and cardiovascular disease, results have been inconsistent and definitive studies are lacking.16-21
It is beyond the scope of this article to delineate or evaluate the many herbal and nutritional supplements available for the management of vasomotor symptoms. According to the literature, their efficacy is questionable. Nonetheless, the industries that promote these products are among the fastest-growing businesses, largely due to the public’s fears about breast cancer.
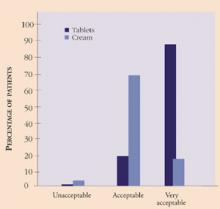
Genitourinary symptoms. Atrophic changes of the vagina are common in the menopausal years and are associated with generalized pruritus and dyspareunia to variable degrees in each individual. This, in addition to the cancer diagnosis and treatment, can lead to symptoms of depression, low self-esteem, and relationship issues surrounding sexuality. It is essential to address symptoms of vaginal atrophy and discuss treatment options with the cancer patient. Local estrogen therapy in the form of a 17-beta estradiol-releasing ring or tablet is an effective method22,23 and has been used successfully in breast cancer survivors at our institution. Since the absorption of estrogen is minimal, it is thought to be safe.24 Further, the risk of endometrial cancer associated with the use of unopposed estrogen is lower with the vaginal ring or tablet than with estrogen cream, as evidenced by endometrial biopsies (TABLE 1).22 In addition, it appears patient satisfaction and acceptability, i.e., ease and comfort of administration, is greater with the tablet than with the cream (Figure 1).
FIGURE 1 Patient satisfaction with vaginal tablets and cream
Source: Rioux JE, Devlin C, Gelfrand M, et al. 17-beta estradiol vaginal tablet versus conjugated equine estrogen cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161.TABLE 1
Safety of vaginal tablets versus cream for the treatment of atrophic vaginitis
| VAGINAL TABLETS (N=80) | VAGINAL CREAM (N=79) | |||
|---|---|---|---|---|
| BASELINE | WEEK 34 | BASELINE | WEEK 24 | |
| Patients with biopsies/stained biopsies | 60 | 49 | 59 | 49 |
| Atrophic endometrium | 34 | 34 | 35 | 15 |
| Weakly proliferative endometrium | 1 | 0 | 3 | 4 |
| Proliferative endometrium | 0 | 1 | 1 | 7 |
| Endometrial hyperplasia | 0 | 0 | 0 | 2 |
| Biopsies with insufficient tissue | 25 | 14 | 20 | 21 |
| Source: Rioux JE, Devlin C, Gelfrand M, et al. 17-beta estradiol vaginal tablet versus conjugated equine estrogen cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161. | ||||
Screening for genetic mutations
Genetic counseling should be offered to all patients who appear to carry an increased cancer risk based on their personal or family histories. Unfortunately, many women with a history of breast or ovarian cancer have never been offered such counseling. Therefore, it is important for Ob/Gyns to screen cancer survivors to identify those patients at risk for subsequent disease.
Although most cancers arise from somatic mutations acquired after birth, approximately 10% of both breast and ovarian cancers are thought to result from inherited gene mutations that are passed down through the maternal or paternal lines. The theory that some breast and ovarian cancers have a genetic basis has been entertained for decades, but only recently have the gene mutations BRCA1 and BRCA2 been identified.25,26 Approximately 85% of hereditary breast carcinomas result from alterations in the BRCA1 and BRCA2 genes.27 These genes also are responsible for most inherited ovarian carcinomas.
Studies also have shown that in families with a history of ovarian cancer or early-onset breast cancer, the risk of developing breast cancer by age 70 is more than 80% for women with BRCA1 or BRCA2 mutations.26,28-30 The risk of developing breast cancer before the age of 50 is 33% to 50% in women with a gene mutation in BRCA1 or BRCA2, versus only 2% in a woman without a gene mutation.30,31
The risk of ovarian cancer conferred by mutations in BRCA1 appears to be greater than that for BRCA2. The BRCA1 mutation is associated with a risk of ovarian cancer between 28% and 44% by age 70, as compared to 1.8% in the general population.28,30,32 The BRCA2-associated risk of ovarian cancer is estimated at 27%, with most cases occurring after the age of 50. TABLE 2 summarizes the risk of cancer conferred by inherited mutations in BRCA1 and BRCA2.
Three forms of hereditary epithelial ovarian cancer exist.26,28,33,34 The most common form appears as a component of the hereditary breast/ovarian cancer syndrome. In these families, multiple cases of breast and ovarian cancer are found, and mutations in the BRCA1 gene are thought to account for the vast majority of them.
The second form of hereditary ovarian cancer occurs as site-specific cancer and is inherited as an autosomal-dominant trait. Mutations in BRCA1 also account for a large proportion of these cases. The third form of hereditary ovarian cancer occurs as a component of the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome. It is characterized by an autosomal-dominant transmission of nonpolyposis colon cancer along with adenocarcinomas of the ovary, uterus, breast, kidney, stomach, pancreas, small bowel, and biliary tree.35
The hallmark of hereditary ovarian cancers is their occurrence at a younger age, the mean being in the mid-40s, with 17% occurring by age 40.36 Therefore, if prophylactic surgery is considered, it generally should be performed prior to the end of the fourth decade of life, after the patient has completed child-bearing. However, since some cases of hereditary ovarian cancer occur in later life, it is thought that even a woman in her 60s or 70s may benefit from prophylactic surgery. Patients undergoing the surgery should be counseled that while their risk is reduced significantly, it is not eliminated. Primary peritoneal carcinomatosis remains a possibility; this risk is estimated at 2% to 11% for women who have undergone prophylactic surgery.37,38
Oral contraceptives (OCs) have been evaluated for their possible role in the prevention of ovarian cancer in the general and high-risk populations. It is estimated that OC use may reduce the risk of hereditary ovarian cancer by as much as 60%.39 However, the benefits of this therapy need to be weighed against the possible increased risk of breast cancer in patients with BRCA1 and BRCA2 mutations.40,41
Due to the low sensitivity and specificity of available screening tests and the relatively low prevalence of ovarian cancer in the general population, routine screening is not recommended, except in women who are at increased risk. Such high-risk women include those who have a personal or family history of ovarian or breast cancer, known mutations in the BRCA1 and BRCA2 genes, or who are of Ashkenazi Jewish descent with personal or family histories of breast or ovarian cancer or both.
No single screening modality has been proven effective. Studies evaluating the role of semiannual pelvic examinations in conjunction with transvaginal sonography (TVS) and CA125 evaluations are under way.42 These modalities currently are the best options in the high-risk population.43-45
For the patient identified as having HNPCC syndrome, screening should include annual endometrial biopsies, breast cancer screening, ovarian cancer screening (as above), colonoscopy, and urine cytology. Prophylactic hysterectomy and oophorectomy may be considered to reduce the gynecologic cancer risks.46
TABLE 3 summarizes surveillance options for women at high risk for familial cancers. Since screening options are limited in sensitivity and specificity, the guidelines are based on expert opinion rather than randomized studies.47
TABLE 2
Cancer risk conferred by inherited mutations in BRCA1 and BRCA2
| CANCER | RISKS |
|---|---|
| Breast | Up to 87% by age 70; 33% to 50% by age 50 |
| BRCA1 or BRCA2 | Risk of contralateral breast cancer, 64% by age 70; 25% within 5 years |
| Ovarian | |
| BRCA1 | 28% to 44% by age 70 to 80 |
| BRCA2 | 27% by age 70 10-fold increased risk following breast cancer |
| Colon | |
| BRCA1 | 3.3-fold increased risk |
| Source: Frank TS. Hereditary risk of breast and ovarian carcinoma: the role of the oncologist. Oncologist. 1998;3:403-412 | |
TABLE 3
Screening options for patients at high risk for familial cancers
| INTERVENTION | RECOMMENDATION | QUALITY OF EVIDENCE* | COMMENTS |
|---|---|---|---|
| BREAST CANCER | |||
| Self-examination | Teach BSE | Level III evidence Expert opinion only | Benefits not proven |
| Clinician breast examination | Annually or semiannually | Level III evidence Expert opinion only | Benefits not proven |
| Mammography | Annually, beginning at age 25 to 35 | Level III evidence Expert opinion only | Risks and benefits not established for women under age 50 |
| OVARIAN CANCER | |||
| Semiannual pelvic examination, TVS, and CA125 | Semiannually, beginning at age 25 to 35 | Level III evidence Expert opinion only | Benefit of TVS, CA125, and pelvic exam levels not proven. Consider prophylactic surgery; significant reduction in risk. Lower estimated ovarian cancer risk in carriers of BRCA2 mutations than in those with BRCA1 mutations. |
| COLON CANCER | |||
| Colonoscopy every 5 to 10 years, hemoccult annually | All patients beginning at age 50. Sigmoidoscopy every 3 to 5 years may be effective in a low-risk population. | Evidence from average-risk populations includes a Level I randomization trial (fecal blood test) and a Level II-2 case- control study (sigmoidoscogy) | If the patient has HNPCC, consider prophylactic total abdominal hysterectomy with bilateral salpingo-oophorectomy |
| Adapted from: Burke et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277(11):915-919. | |||
| *Denotes the highest-quality trial performed | |||
| Level I = highest-quality trials | |||
| Level II = intermediate-quality (nonrandomized) trials and observational studies (Level II-2 indicates a case-control study) | |||
| Level III = lowest-quality (expert opinion and case reports) | |||
Sexual health
Sexual dysfunction is one of the more common, enduring consequences of cancer treatment. Approximately half of the women who survive breast or a gynecologic cancer report severe, long-lasting sexual problems.48 Studies have shown that sexuality issues are prevalent among cancer survivors in general. Anderson reports sexual-functioning morbidity occurs in up to 90% of women with the most prevalent types of cancer.49 Other authors estimate that post-treatment sexual dysfunction ranges from 30% to 100%.50 Disease-free breast cancer survivors admit that sexual problems persist as bothersome and disheartening exceptions to their generally high level of functioning. For other cancers such as Hodgkin’s disease, at least 25% of patients are left with a sexual dysfunction.51
For patients who have vaginal stenosis or shortening due to surgery or irradiation for gynecologic or colorectal cancers, the use of dilators as early as possible is essential to maximize vaginal length and treatment success. In general, initial counseling about the use of these devices is provided by the surgical or radiation oncologist.
Ideally, however, patients with cancer—especially disease involving the vaginal, perineal, and anal areas—who require extensive surgery or irradiation will have had a sexual-health consultation before treatment. At our institution, the patient initially is evaluated by a psychologist, who is a licensed sex therapist, and a gynecologist so that both physical and psychological components can be assessed and a course of treatment planned.
Overall, the ideal approach to sexual dysfunction in the cancer patient is:
- Validating the patient’s need for a sexual relationship;
- Assisting in identifying those factors contributing to the difficulty; and
- Conducting a pretreatment evaluation with the patient and her partner, if possible.
The annual exam
Cervical cancer screening. The most common type of cancer in women is cervical cancer, with approximately 450,000 new cases reported worldwide each year. Screening with Pap smears and advanced colposcopic techniques has dramatically decreased mortality from this disease in developed countries. Further, testing for human Papillomavirus (HPV), in addition to regular Pap smear screening, has improved the sensitivity of cervical cancer screening.52,53
While physicians agree that all women should begin Pap smear evaluations annually beginning at age 18 or at the onset of sexual activity, some argue there may be a role for HPV testing at the initial screening, as well, to reduce the false-negative rate and help identify at-risk individuals earlier. Patients who already have cancer and may be immunosuppressed as a result of their treatment may be at higher risk for HPV, thus warranting routine HPV screening. (In such women, genital warts may be diffuse, requiring surgical treatment.)
Colonoscopy. Colon cancer is the third most common cancer in women. Risk factors include advanced age, living in an industrialized country, a diet high in fat and cholesterol, genetic premalignant polyposis syndromes, a family history of colon cancer or inflammatory bowel disease, and a history of intestinal adenomatous polyps.47
Colonoscopy is recommended for all patients beginning at age 50, with a frequency of every 10 years for those at average risk. Women with any of the risk factors noted above, a personal history of breast or ovarian cancer, or both, should undergo more frequent screening.
Digital rectal examination, as well as a hemoccult, should be part of the annual examination.
Osteoporosis. Osteoporosis is a leading cause of nursing-home admissions and a leading cause of morbidity in post-menopausal women. The cancer survivor needs to be evaluated thoroughly for osteoporosis risk factors, particularly since many of these women will have entered menopause prematurely. Further, these patients may have additional risks associated with treatment with steroids, chemotherapeutic agents, or radiation.
Preventive measures, including bone densitometry and the measurement of bone turnover markers, should be performed at menopause or earlier in cancer survivors. Currently, the National Osteoporosis Foundation recommends HRT as the firstline agent for the treatment and prevention of osteoporosis in the general population. In cancer patients, however, particularly those with estrogen-sensitive cancers, HRT may not be a viable option. Alternatives include antiresorptive agents such as raloxifene hydrochloride, a selective estrogen receptor modulator (SERM). The Multiple Outcomes of Raloxifene Evaluation trial (MORE) found that raloxifene reduced vertebral fracture incidence by approximately 50%, had no adverse gynecologic effects, and may decrease the risk of breast cancer.54
Although it was approved for the treatment of osteoporosis, alendronate, a bisphosphonate with antiresorptive effects, can be used in lower doses as a preventive agent as well.55,56 When alendronate was given, the relative risk for fracture was reduced by 50% in a group of women considered at high risk based on known low bone mass and a history of at least 1 vertebral fracture.54 Incidence of hip fractures also was significantly reduced. However, at the typical dose of 5 to 10 mg daily, alendronate has not been well tolerated, primarily due to gastrointestinal effects. More recently, a 35-mg and 70-mg weekly dose have been used successfully and are better tolerated.
Bone turnover markers, such as urinary N-telopeptide (NTX) levels, can be used to determine if treatment should be instituted and whether it has been effective. NTX is an indicator of what a patient’s bone density will be if she remains untreated or continues her current regimen. A significantly elevated NTX, i.e., 38 BCE (bone collagen excretion), indicates the presence of a metabolic disease, and the clinician should consider initiating antiresorptive therapy.
Regardless of bone mass or bone turnover rates, calcium and vitamin D supplementation should be encouraged. Generally, post-menopausal women should be advised to take 1,000 to 1,500 mg of calcium per day as well as 400 to 800 IU of vitamin D.
Cardiovascular disease. The leading cause of death in postmenopausal women is cardiovascular disease (CVD). Over the remainder of her lifetime, a 50-year-old woman has a 46% probability of developing CVD and a 31% chance of dying from it. The National Cholesterol Education Program (NCEP) Expert Panel [Adult Treatment Panel III (ATP III)] has proposed an algorithm for CVD risk-factor stratification with the goal of treating high-risk women more aggressively.57 When high-density lipoprotein (HDL) cholesterol levels are low, women face a greater risk of CVD than men do at similar levels. Diabetes and hypertriglyceridemia also impart a greater risk of CVD in women.
Cancer survivors often face an even greater risk of CVD than the general population because of the cardiotoxic effects of some cancer therapies. Thus, evaluating cancer survivors for cardiovascular risk factors is essential to their management.
Many agents, including HRT, have been evaluated for the management of hyperlipidemia. The NCEP recommends that statin drugs, rather than HRT, be the first-line treatment for this condition because they have been found to be more effective in lowering low-density lipoprotein (LDL) cholesterol. In fact, recent studies have questioned the safety of estrogen in the secondary prevention of CVD in women with established cardiac disease.7
Endometrial cancer. To date, there is no proven method of screening for endometrial cancer in tamoxifen-treated patients. In these women, the expected annual risk is 2 per 1,000.58 Although this risk is low, endometrial cancer remains a major concern for patients taking the drug.
Endometrial sampling should be reserved for patients who are experiencing abnormal bleeding. In a recent prospective trial measuring the incidence of abnormal endometrial pathology in 111 tamoxifen-treated patients, Barakat et al concluded that the utility of endometrial biopsy is limited in this population.59 In another trial, Gibson and colleagues noted that all cases of endometrial carcinoma detected by dilatation and curettage (D&C) were found in tamoxifen-treated patients who presented with abnormal bleeding.60
Ultrasound also has been evaluated as a screening technique in this population. An endometrial thickness of 8 mm or more is considered abnormal, although this guideline is under evaluation. Hann et al reported that although most tamoxifen-treated women are asymptomatic, endometrial polyps were found in 33% of cases with a lining thicker than 8 mm. In addition, they found a correlation between endometrial thickness and duration of tamoxifen use.61
Another study recently found that transvaginal sonography has a high false-positive rate, even when the cut-off for endometrial thickness is 10 mm instead of 8 mm.62 In this study, 1 asymptomatic endometrial cancer was found. The authors concluded that this low yield does not justify the increased iatrogenic morbidity of transvaginal ultrasound screening in the tamoxifen-treated patient.
Conclusion
This review of the cancer patient’s needs underscores the principle that all patients, regardless of medical history, need thorough evaluations to identify risk factors for other disease entities. As such, all women should undergo an annual examination so that preventive measures such as cardiovascular health assessment, bone densitometry, mammography, colonoscopy, and screening for familial health risks also can be undertaken.
However, a thorough medical evaluation often is not performed in the cancer survivor because her cancer is the focus of most of her physician visits. Thus, preventive measures such as those outlined above are overlooked, putting her at increased risk for fracture, cardiovascular disease, and other dysplasia or malignancies.
Gynecologic care of the cancer patient requires an understanding of the numerous issues that cancer survivors face—many of which are no different than those faced by their healthy counterparts. While a multidisciplinary approach may be required, such health care ideally should be coordinated by a primary-care physician. This responsibility often falls to the gynecologist or internist.
In addition, an understanding of the chemotherapeutic regimens, the effects of radiation therapy, and psychological factors can help the clinician identify or anticipate the problems unique to the cancer patient. The physician’s ability to validate a patient’s concerns, dispel myths, and provide information about treatment options can ease her anxiety and maximize physical and psychological well-being.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Gapstur S, Morrow M, Sellers T. Hormone replacement therapy and risk of breast cancer with a favorable histology. JAMA. 1999;281:2091-2097.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
3. Colditz GA, Hankinson SE, Hunter DJ. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589-1593.
4. Grodstein F, Stampfer M, Colditz D, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769-1775.
5. The Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density: results of the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276(17):1389-1396.
6. Nachtigall LE, Nachtigall MJ. Hormone replacement therapy. Curr Opin Obstet Gynecol. 1992;4(6):907-913.
7. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
8. Mosca L. The role of hormone replacement therapy in the prevention of postmenopausal heart disease. Arch Intern Med. 2000;160:2263-2272.
9. Powles TJ, Jones AL, Ashley SE, et al. The Royal Marsden Hospital pilot tamoxifen chemoprevention trial. Breast Cancer Res Treat. 1994;31(1):73-82.
10. Fisher B, Dignam J, Bryant J, et al. Five years versus more than five years of tamoxifen therapy for breast cancer patients with negative nodes and estrogen-receptor positive tumors. J Natl Cancer Inst. 1996;88(21):1529-1542.
11. Castiel M. Management of menopausal symptoms in the cancer patient. Oncology. 1999;13(10):1363-1372.
12. Loprinzi CL, Kugler JW, Sloan J, et al. Venlafaxine alleviates hot flashes: an NCCTG trial. Progr Proc Am Soc Clin Oncol. 2000;19:2a [abstract].-
13. Loprinzi CL, Quella SK, Sloan JA, et al. Preliminary data from a randomized evaluation of fluoxetine (Prozac) for treating hot flashes in breast cancer survivors. Breast Cancer Res Treat. 1999;57:34 [abstract].-
14. Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in post menopausal patients with breast cancer experiencing tamoxifen induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132(10):788-793.
15. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;11:347-352.
16. Nachtigall L, Fenichel R, La Greg L, et al. The effects of isoflavone-derived red clover on vasomotor symptoms, endometrial thickness, and reproductive hormone concentrations in menopausal women. Proceedings of the 81st annual meeting of the Endocrine Society, June 12-15, 1999, San Diego, Calif.
17. Scambia G, Mango D, Signorile PG. Clinical effects of a standardized soy extract in postmenopausal women: pilot study. Menopause. 2000;7:105-111.
18. Quella S, Loprinzi C, Barton D, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment Group Trial. J Clin Oncol. 2000;18:1068-1074.
19. Adlercreutz CH, Goldin BR, Gorbach SL, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125(3 Suppl):757s-770s.
20. McMichael-Phillips D, Harding C, Morton M, et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431s-1436s.
21. Potter SM, Baum JA, Teng H, et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68:1375s-1379s.
22. Rioux JE, Devlin C, Gelfand M, et al. 17-[beta]-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161.
23. Smith P, Heimer G, Lindskog M, et al. Oestradiol-releasing vaginal ring for treatment of postmenopausal urogenital atrophy. Maturitas. 1993;16:145-154.
24. Bachmann G. Estradiol-releasing vaginal ring delivery system for urogenital atrophy. Experience over the past decade. J Reprod Med. 1998;43:991-998.
25. Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66.-
26. Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789.-
27. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62(3):676-689.
28. Ford D, Easton DF, Bishop DT, et al. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692.-
29. Easton D, Steele L, Fields P, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Human Genet. 1997;61:120-128.
30. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265-271.
31. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401-1408.
32. Whittemore AS, Gong G, Imtyre J. Prevalence and contribution of BRCA1 mutations in breast cancer: results from three US population-based case-control studies of ovarian cancer. Am J Hum Genet. 1997;60:496.-
33. Easton DT, Bishop DT, Ford D, et al. Genetic linkage analysis in familial breast and ovarian cancers: results from 214 families. Am J Hum Genet. 1993;52(4):678-701.
34. Steichen-Gersdorf E, Gallion HH, Ford D, et al. Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet. 1994;55:870-875.
35. Lynch HT, Lynch JF. Hereditary ovarian carcinoma. Hematol Oncol Clin North Am. 1992;6(4):783-811.
36. Struewing J, et al. Prophylactic oophorectomy in inherited breast/ovarian cancer families. J Natl Cancer Inst Monogr. 1995;17:33-35.
37. Piver MS, Jishi MF, Tsukada Y, et al. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. Cancer. 1993;71:2751-2755.
38. Tobacman JK, Greene MH, Tucker MA, et al. Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2(8302):795-797.
39. Narod SA, Risch H, Moslehi R, Dorum A, et al. Oral contraceptives and the risk of hereditary ovarian cancer. N Engl J Med. 1998;339:424-428.
40. Grabrick DM, Hartmann LC, Cerhan JR, et al. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. JAMA. 2000;284:1791-1798.
41. Ursin G, Henderson BG, Haile RW, et al. Does oral contraceptive use increase the risk of breast cancer in women with BRCA1/BRCA2 mutations more than in other women? Cancer Res. 1997;57:3678-3681.
42. NIH Consensus Development Panel on Ovarian Cancer. Ovarian cancer. Screening, treatment, and follow-up. JAMA. 1995;273(6):491-497.
43. Frank TS. Identifying women with inherited risk for ovarian cancer: who and why? Contemp Ob/Gyn. 1998;27-50.
44. Bourne T, Whitehead M, Campbell S, et al. Ultrasound screening for familial ovarian cancer. Gynecol Oncol. 1993;43:92-97.
45. Berchuck A, Cirisano F, Lancaster JM, et al. Role of BRCA1 mutation screening in the management of familial ovarian cancer. Am J Obstet Gynecol. 1996;175:738-746.
46. Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277(11):915-919.
47. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. JAMA. 1997;227:997-1003.
48. Robinson JW. Sexuality and cancer. Breaking the silence. Aust Fam Phys. 1998;27:45-47.
49. Anderson BL. How cancer affects sexual functioning. Oncology. 1990;4:23-42.
50. Anderson BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol. 1997;65:221-229.
51. Van Tulder MW, Aaronson NK, Bruning PF. The quality of life of long-term survivors of Hodgkin’s disease. Ann Oncol. 1994;5:153-158.
52. Wright TC, Jr, Denny L, Kuhn L, et al. HPV testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81-86.
53. Schiffman M, Herrero R, Hildesheim A, et al. HPV DNA testing in cervical cancer screening results from women in a high-risk province of Costa Rica. JAMA. 2000;283:87-93.
54. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
55. Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
56. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial. JAMA. 280;2077-2082.
57. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486-2497.
58. Fisher B, Constantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527.-
59. Barakat RR, Gilewski TA, Almadrones L, et al. Effect of adjuvant tamoxifen on the endometrium in women with breast cancer: a prospective study using office endometrial biopsy. J Clin Oncol. 2000;18:3459-3463.
60. Gibson LE, Barakat RR, Venkatraman ES, Hoskins WJ. Endometrial pathology at dilatation and curettage in breast cancer patients: comparison of tamoxifen users and nonusers. Cancer J Sci Am. 1996;2:35.-
61. Hann LE, Gess CS, Bach AM, et al. Endometrial thickness in tamoxifentreated patients: correlation with clinical and pathologic findings. Am J Roentgenol. 1997;168(3):657-661.
62. Garber B, Krause A, Muller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer a prospective long term study using transvaginal ultrasound. J Clin Oncol. 2000;18:3464-3470.
- Selective serotonin reuptake inhibitors (SSRIs), clonidine hydrochloride, and megestrol alleviate hot flushes in women who opt not to take hormone replacement therapy (HRT) due to concerns about subsequent disease.
- Local estrogen therapy in the form of an estradiol-releasing vaginal tablet or ring effectively improves vaginal atrophy and has a higher patient acceptability than vaginal cream.
- Because a majority of women who have survived breast cancer have not been screened for BRCA1 and BRCA2 mutations, Ob/Gyns should screen survivors to identify those patients at risk for subsequent disease.
- Approximately half of the women who survive breast or a gynecologic cancer report severe, long-lasting sexual problems.
As more is discovered about cancer, caring for the cancer patient has become even more complicated. Women who have survived cancer, even nongynecologic diseases, increasingly are being followed by general obstetrician/gynecologists or primary care physicians. To help Ob/Gyns provide better care, this review will summarize cancer patients’ special needs with regard to menopause and premature menopause, genetic screening techniques, and well-woman care.
Only about 15% of postmenopausal women use HRT.
In evaluating the needs of the cancer patient, an understanding of the type of cancer and the surgical and medical management the woman has undergone is key. This knowledge aids the management of sexual health, fertility, and menopause. Additionally, depending on the type of cancer she has had, the patient may be exposed to health risks not previously identified. In these cases, an understanding of the familial basis of some cancers and hereditary syndromes is essential, as it can help identify those who may be at risk for subsequent malignancies or conditions.
Finally, as with all well-woman care, preventive measures such as bone densitometry, mammography, colonoscopy, exercise, self-breast examination, and smoking cessation must be addressed and discussed.
Menopause
Many young women with a cancer diagnosis face premature menopause. In addition to the psychological issues stemming from premature ovarian failure such as those related to fertility, these women experience many of the typical menopausal symptoms and other health risks well before their healthy counterparts.
In the decades since hormone replacement therapy (HRT) was introduced, there has been increasing controversy and confusion among patients and physicians about its benefits and risks. In particular, because of the possible role of estrogen in the pathogenesis of breast cancer, its use for post-menopausal therapy has been challenged.1-3
Currently, only about 15% of post-menopausal women use HRT, in part due to the concerns about breast cancer. Other reasons include breast engorgement and tenderness and a resumption of vaginal bleeding.
Most health-care providers believe HRT is contraindicated for breast cancer survivors. However, withholding HRT from all postmenopausal women would be a disservice to many, as it would expose them to health risks that would outweigh the risk for breast cancer. One of the clinician’s roles is to identify patients who would benefit from HRT and to avoid exposing others to unreasonable risk. Fortunately, the available alternatives to HRT are rapidly increasing in response to the demand this controversy has generated.
BRCA 1 and BRCA 2 are responsible for most inherited ovarian carcinomas.
Hormone replacement therapy initially was intended for short-term use in the management of vasomotor symptoms. The health benefits that were subsequently identified supported a longer duration of use.4,5 These include improving hot flushes, protecting bone density, and combating urogenital atrophy, but more recently, the potential benefit that hormones may have on cardiac health has come under scrutiny.6-8
Vasomotor symptoms. Menopausal women often complain of debilitating vasomotor flushes. For women who take tamoxifen, e.g., those with a history of breast cancer, vasomotor symptoms may be potentiated.9,10 Traditional HRT regimens, either oral or transdermal, have been effective in treating vasomotor symptoms in 85% to 90% of women.11 Even so, increasing numbers of women are turning to an ever-growing array of nonestrogen agents in an effort to alleviate symptoms and minimize their perceived risk of breast cancer.
Agents that can be used to alleviate hot flushes include selective serotonin reuptake inhibitors (SSRIs) such as venlafaxine hydrochloride in small doses, e.g., 25 to 75 mg daily.12,13 Clonidine hydrochloride, an antihypertensive, also is effective, but its side effects limit its use.14 If the 0.1-mg patch is used, it should be changed weekly, and if the drug is administered orally, the dosage should not exceed 0.1 mg twice daily. Megestrol, a progestin, also has been shown to be effective.15
Recently, phytoestrogens have been sought as substitutes for traditional HRT. While there is some evidence they may improve vasomotor flushes and protect against bone cancer and cardiovascular disease, results have been inconsistent and definitive studies are lacking.16-21
It is beyond the scope of this article to delineate or evaluate the many herbal and nutritional supplements available for the management of vasomotor symptoms. According to the literature, their efficacy is questionable. Nonetheless, the industries that promote these products are among the fastest-growing businesses, largely due to the public’s fears about breast cancer.
Genitourinary symptoms. Atrophic changes of the vagina are common in the menopausal years and are associated with generalized pruritus and dyspareunia to variable degrees in each individual. This, in addition to the cancer diagnosis and treatment, can lead to symptoms of depression, low self-esteem, and relationship issues surrounding sexuality. It is essential to address symptoms of vaginal atrophy and discuss treatment options with the cancer patient. Local estrogen therapy in the form of a 17-beta estradiol-releasing ring or tablet is an effective method22,23 and has been used successfully in breast cancer survivors at our institution. Since the absorption of estrogen is minimal, it is thought to be safe.24 Further, the risk of endometrial cancer associated with the use of unopposed estrogen is lower with the vaginal ring or tablet than with estrogen cream, as evidenced by endometrial biopsies (TABLE 1).22 In addition, it appears patient satisfaction and acceptability, i.e., ease and comfort of administration, is greater with the tablet than with the cream (Figure 1).
FIGURE 1 Patient satisfaction with vaginal tablets and cream
Source: Rioux JE, Devlin C, Gelfrand M, et al. 17-beta estradiol vaginal tablet versus conjugated equine estrogen cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161.TABLE 1
Safety of vaginal tablets versus cream for the treatment of atrophic vaginitis
| VAGINAL TABLETS (N=80) | VAGINAL CREAM (N=79) | |||
|---|---|---|---|---|
| BASELINE | WEEK 34 | BASELINE | WEEK 24 | |
| Patients with biopsies/stained biopsies | 60 | 49 | 59 | 49 |
| Atrophic endometrium | 34 | 34 | 35 | 15 |
| Weakly proliferative endometrium | 1 | 0 | 3 | 4 |
| Proliferative endometrium | 0 | 1 | 1 | 7 |
| Endometrial hyperplasia | 0 | 0 | 0 | 2 |
| Biopsies with insufficient tissue | 25 | 14 | 20 | 21 |
| Source: Rioux JE, Devlin C, Gelfrand M, et al. 17-beta estradiol vaginal tablet versus conjugated equine estrogen cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161. | ||||
Screening for genetic mutations
Genetic counseling should be offered to all patients who appear to carry an increased cancer risk based on their personal or family histories. Unfortunately, many women with a history of breast or ovarian cancer have never been offered such counseling. Therefore, it is important for Ob/Gyns to screen cancer survivors to identify those patients at risk for subsequent disease.
Although most cancers arise from somatic mutations acquired after birth, approximately 10% of both breast and ovarian cancers are thought to result from inherited gene mutations that are passed down through the maternal or paternal lines. The theory that some breast and ovarian cancers have a genetic basis has been entertained for decades, but only recently have the gene mutations BRCA1 and BRCA2 been identified.25,26 Approximately 85% of hereditary breast carcinomas result from alterations in the BRCA1 and BRCA2 genes.27 These genes also are responsible for most inherited ovarian carcinomas.
Studies also have shown that in families with a history of ovarian cancer or early-onset breast cancer, the risk of developing breast cancer by age 70 is more than 80% for women with BRCA1 or BRCA2 mutations.26,28-30 The risk of developing breast cancer before the age of 50 is 33% to 50% in women with a gene mutation in BRCA1 or BRCA2, versus only 2% in a woman without a gene mutation.30,31
The risk of ovarian cancer conferred by mutations in BRCA1 appears to be greater than that for BRCA2. The BRCA1 mutation is associated with a risk of ovarian cancer between 28% and 44% by age 70, as compared to 1.8% in the general population.28,30,32 The BRCA2-associated risk of ovarian cancer is estimated at 27%, with most cases occurring after the age of 50. TABLE 2 summarizes the risk of cancer conferred by inherited mutations in BRCA1 and BRCA2.
Three forms of hereditary epithelial ovarian cancer exist.26,28,33,34 The most common form appears as a component of the hereditary breast/ovarian cancer syndrome. In these families, multiple cases of breast and ovarian cancer are found, and mutations in the BRCA1 gene are thought to account for the vast majority of them.
The second form of hereditary ovarian cancer occurs as site-specific cancer and is inherited as an autosomal-dominant trait. Mutations in BRCA1 also account for a large proportion of these cases. The third form of hereditary ovarian cancer occurs as a component of the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome. It is characterized by an autosomal-dominant transmission of nonpolyposis colon cancer along with adenocarcinomas of the ovary, uterus, breast, kidney, stomach, pancreas, small bowel, and biliary tree.35
The hallmark of hereditary ovarian cancers is their occurrence at a younger age, the mean being in the mid-40s, with 17% occurring by age 40.36 Therefore, if prophylactic surgery is considered, it generally should be performed prior to the end of the fourth decade of life, after the patient has completed child-bearing. However, since some cases of hereditary ovarian cancer occur in later life, it is thought that even a woman in her 60s or 70s may benefit from prophylactic surgery. Patients undergoing the surgery should be counseled that while their risk is reduced significantly, it is not eliminated. Primary peritoneal carcinomatosis remains a possibility; this risk is estimated at 2% to 11% for women who have undergone prophylactic surgery.37,38
Oral contraceptives (OCs) have been evaluated for their possible role in the prevention of ovarian cancer in the general and high-risk populations. It is estimated that OC use may reduce the risk of hereditary ovarian cancer by as much as 60%.39 However, the benefits of this therapy need to be weighed against the possible increased risk of breast cancer in patients with BRCA1 and BRCA2 mutations.40,41
Due to the low sensitivity and specificity of available screening tests and the relatively low prevalence of ovarian cancer in the general population, routine screening is not recommended, except in women who are at increased risk. Such high-risk women include those who have a personal or family history of ovarian or breast cancer, known mutations in the BRCA1 and BRCA2 genes, or who are of Ashkenazi Jewish descent with personal or family histories of breast or ovarian cancer or both.
No single screening modality has been proven effective. Studies evaluating the role of semiannual pelvic examinations in conjunction with transvaginal sonography (TVS) and CA125 evaluations are under way.42 These modalities currently are the best options in the high-risk population.43-45
For the patient identified as having HNPCC syndrome, screening should include annual endometrial biopsies, breast cancer screening, ovarian cancer screening (as above), colonoscopy, and urine cytology. Prophylactic hysterectomy and oophorectomy may be considered to reduce the gynecologic cancer risks.46
TABLE 3 summarizes surveillance options for women at high risk for familial cancers. Since screening options are limited in sensitivity and specificity, the guidelines are based on expert opinion rather than randomized studies.47
TABLE 2
Cancer risk conferred by inherited mutations in BRCA1 and BRCA2
| CANCER | RISKS |
|---|---|
| Breast | Up to 87% by age 70; 33% to 50% by age 50 |
| BRCA1 or BRCA2 | Risk of contralateral breast cancer, 64% by age 70; 25% within 5 years |
| Ovarian | |
| BRCA1 | 28% to 44% by age 70 to 80 |
| BRCA2 | 27% by age 70 10-fold increased risk following breast cancer |
| Colon | |
| BRCA1 | 3.3-fold increased risk |
| Source: Frank TS. Hereditary risk of breast and ovarian carcinoma: the role of the oncologist. Oncologist. 1998;3:403-412 | |
TABLE 3
Screening options for patients at high risk for familial cancers
| INTERVENTION | RECOMMENDATION | QUALITY OF EVIDENCE* | COMMENTS |
|---|---|---|---|
| BREAST CANCER | |||
| Self-examination | Teach BSE | Level III evidence Expert opinion only | Benefits not proven |
| Clinician breast examination | Annually or semiannually | Level III evidence Expert opinion only | Benefits not proven |
| Mammography | Annually, beginning at age 25 to 35 | Level III evidence Expert opinion only | Risks and benefits not established for women under age 50 |
| OVARIAN CANCER | |||
| Semiannual pelvic examination, TVS, and CA125 | Semiannually, beginning at age 25 to 35 | Level III evidence Expert opinion only | Benefit of TVS, CA125, and pelvic exam levels not proven. Consider prophylactic surgery; significant reduction in risk. Lower estimated ovarian cancer risk in carriers of BRCA2 mutations than in those with BRCA1 mutations. |
| COLON CANCER | |||
| Colonoscopy every 5 to 10 years, hemoccult annually | All patients beginning at age 50. Sigmoidoscopy every 3 to 5 years may be effective in a low-risk population. | Evidence from average-risk populations includes a Level I randomization trial (fecal blood test) and a Level II-2 case- control study (sigmoidoscogy) | If the patient has HNPCC, consider prophylactic total abdominal hysterectomy with bilateral salpingo-oophorectomy |
| Adapted from: Burke et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277(11):915-919. | |||
| *Denotes the highest-quality trial performed | |||
| Level I = highest-quality trials | |||
| Level II = intermediate-quality (nonrandomized) trials and observational studies (Level II-2 indicates a case-control study) | |||
| Level III = lowest-quality (expert opinion and case reports) | |||
Sexual health
Sexual dysfunction is one of the more common, enduring consequences of cancer treatment. Approximately half of the women who survive breast or a gynecologic cancer report severe, long-lasting sexual problems.48 Studies have shown that sexuality issues are prevalent among cancer survivors in general. Anderson reports sexual-functioning morbidity occurs in up to 90% of women with the most prevalent types of cancer.49 Other authors estimate that post-treatment sexual dysfunction ranges from 30% to 100%.50 Disease-free breast cancer survivors admit that sexual problems persist as bothersome and disheartening exceptions to their generally high level of functioning. For other cancers such as Hodgkin’s disease, at least 25% of patients are left with a sexual dysfunction.51
For patients who have vaginal stenosis or shortening due to surgery or irradiation for gynecologic or colorectal cancers, the use of dilators as early as possible is essential to maximize vaginal length and treatment success. In general, initial counseling about the use of these devices is provided by the surgical or radiation oncologist.
Ideally, however, patients with cancer—especially disease involving the vaginal, perineal, and anal areas—who require extensive surgery or irradiation will have had a sexual-health consultation before treatment. At our institution, the patient initially is evaluated by a psychologist, who is a licensed sex therapist, and a gynecologist so that both physical and psychological components can be assessed and a course of treatment planned.
Overall, the ideal approach to sexual dysfunction in the cancer patient is:
- Validating the patient’s need for a sexual relationship;
- Assisting in identifying those factors contributing to the difficulty; and
- Conducting a pretreatment evaluation with the patient and her partner, if possible.
The annual exam
Cervical cancer screening. The most common type of cancer in women is cervical cancer, with approximately 450,000 new cases reported worldwide each year. Screening with Pap smears and advanced colposcopic techniques has dramatically decreased mortality from this disease in developed countries. Further, testing for human Papillomavirus (HPV), in addition to regular Pap smear screening, has improved the sensitivity of cervical cancer screening.52,53
While physicians agree that all women should begin Pap smear evaluations annually beginning at age 18 or at the onset of sexual activity, some argue there may be a role for HPV testing at the initial screening, as well, to reduce the false-negative rate and help identify at-risk individuals earlier. Patients who already have cancer and may be immunosuppressed as a result of their treatment may be at higher risk for HPV, thus warranting routine HPV screening. (In such women, genital warts may be diffuse, requiring surgical treatment.)
Colonoscopy. Colon cancer is the third most common cancer in women. Risk factors include advanced age, living in an industrialized country, a diet high in fat and cholesterol, genetic premalignant polyposis syndromes, a family history of colon cancer or inflammatory bowel disease, and a history of intestinal adenomatous polyps.47
Colonoscopy is recommended for all patients beginning at age 50, with a frequency of every 10 years for those at average risk. Women with any of the risk factors noted above, a personal history of breast or ovarian cancer, or both, should undergo more frequent screening.
Digital rectal examination, as well as a hemoccult, should be part of the annual examination.
Osteoporosis. Osteoporosis is a leading cause of nursing-home admissions and a leading cause of morbidity in post-menopausal women. The cancer survivor needs to be evaluated thoroughly for osteoporosis risk factors, particularly since many of these women will have entered menopause prematurely. Further, these patients may have additional risks associated with treatment with steroids, chemotherapeutic agents, or radiation.
Preventive measures, including bone densitometry and the measurement of bone turnover markers, should be performed at menopause or earlier in cancer survivors. Currently, the National Osteoporosis Foundation recommends HRT as the firstline agent for the treatment and prevention of osteoporosis in the general population. In cancer patients, however, particularly those with estrogen-sensitive cancers, HRT may not be a viable option. Alternatives include antiresorptive agents such as raloxifene hydrochloride, a selective estrogen receptor modulator (SERM). The Multiple Outcomes of Raloxifene Evaluation trial (MORE) found that raloxifene reduced vertebral fracture incidence by approximately 50%, had no adverse gynecologic effects, and may decrease the risk of breast cancer.54
Although it was approved for the treatment of osteoporosis, alendronate, a bisphosphonate with antiresorptive effects, can be used in lower doses as a preventive agent as well.55,56 When alendronate was given, the relative risk for fracture was reduced by 50% in a group of women considered at high risk based on known low bone mass and a history of at least 1 vertebral fracture.54 Incidence of hip fractures also was significantly reduced. However, at the typical dose of 5 to 10 mg daily, alendronate has not been well tolerated, primarily due to gastrointestinal effects. More recently, a 35-mg and 70-mg weekly dose have been used successfully and are better tolerated.
Bone turnover markers, such as urinary N-telopeptide (NTX) levels, can be used to determine if treatment should be instituted and whether it has been effective. NTX is an indicator of what a patient’s bone density will be if she remains untreated or continues her current regimen. A significantly elevated NTX, i.e., 38 BCE (bone collagen excretion), indicates the presence of a metabolic disease, and the clinician should consider initiating antiresorptive therapy.
Regardless of bone mass or bone turnover rates, calcium and vitamin D supplementation should be encouraged. Generally, post-menopausal women should be advised to take 1,000 to 1,500 mg of calcium per day as well as 400 to 800 IU of vitamin D.
Cardiovascular disease. The leading cause of death in postmenopausal women is cardiovascular disease (CVD). Over the remainder of her lifetime, a 50-year-old woman has a 46% probability of developing CVD and a 31% chance of dying from it. The National Cholesterol Education Program (NCEP) Expert Panel [Adult Treatment Panel III (ATP III)] has proposed an algorithm for CVD risk-factor stratification with the goal of treating high-risk women more aggressively.57 When high-density lipoprotein (HDL) cholesterol levels are low, women face a greater risk of CVD than men do at similar levels. Diabetes and hypertriglyceridemia also impart a greater risk of CVD in women.
Cancer survivors often face an even greater risk of CVD than the general population because of the cardiotoxic effects of some cancer therapies. Thus, evaluating cancer survivors for cardiovascular risk factors is essential to their management.
Many agents, including HRT, have been evaluated for the management of hyperlipidemia. The NCEP recommends that statin drugs, rather than HRT, be the first-line treatment for this condition because they have been found to be more effective in lowering low-density lipoprotein (LDL) cholesterol. In fact, recent studies have questioned the safety of estrogen in the secondary prevention of CVD in women with established cardiac disease.7
Endometrial cancer. To date, there is no proven method of screening for endometrial cancer in tamoxifen-treated patients. In these women, the expected annual risk is 2 per 1,000.58 Although this risk is low, endometrial cancer remains a major concern for patients taking the drug.
Endometrial sampling should be reserved for patients who are experiencing abnormal bleeding. In a recent prospective trial measuring the incidence of abnormal endometrial pathology in 111 tamoxifen-treated patients, Barakat et al concluded that the utility of endometrial biopsy is limited in this population.59 In another trial, Gibson and colleagues noted that all cases of endometrial carcinoma detected by dilatation and curettage (D&C) were found in tamoxifen-treated patients who presented with abnormal bleeding.60
Ultrasound also has been evaluated as a screening technique in this population. An endometrial thickness of 8 mm or more is considered abnormal, although this guideline is under evaluation. Hann et al reported that although most tamoxifen-treated women are asymptomatic, endometrial polyps were found in 33% of cases with a lining thicker than 8 mm. In addition, they found a correlation between endometrial thickness and duration of tamoxifen use.61
Another study recently found that transvaginal sonography has a high false-positive rate, even when the cut-off for endometrial thickness is 10 mm instead of 8 mm.62 In this study, 1 asymptomatic endometrial cancer was found. The authors concluded that this low yield does not justify the increased iatrogenic morbidity of transvaginal ultrasound screening in the tamoxifen-treated patient.
Conclusion
This review of the cancer patient’s needs underscores the principle that all patients, regardless of medical history, need thorough evaluations to identify risk factors for other disease entities. As such, all women should undergo an annual examination so that preventive measures such as cardiovascular health assessment, bone densitometry, mammography, colonoscopy, and screening for familial health risks also can be undertaken.
However, a thorough medical evaluation often is not performed in the cancer survivor because her cancer is the focus of most of her physician visits. Thus, preventive measures such as those outlined above are overlooked, putting her at increased risk for fracture, cardiovascular disease, and other dysplasia or malignancies.
Gynecologic care of the cancer patient requires an understanding of the numerous issues that cancer survivors face—many of which are no different than those faced by their healthy counterparts. While a multidisciplinary approach may be required, such health care ideally should be coordinated by a primary-care physician. This responsibility often falls to the gynecologist or internist.
In addition, an understanding of the chemotherapeutic regimens, the effects of radiation therapy, and psychological factors can help the clinician identify or anticipate the problems unique to the cancer patient. The physician’s ability to validate a patient’s concerns, dispel myths, and provide information about treatment options can ease her anxiety and maximize physical and psychological well-being.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Selective serotonin reuptake inhibitors (SSRIs), clonidine hydrochloride, and megestrol alleviate hot flushes in women who opt not to take hormone replacement therapy (HRT) due to concerns about subsequent disease.
- Local estrogen therapy in the form of an estradiol-releasing vaginal tablet or ring effectively improves vaginal atrophy and has a higher patient acceptability than vaginal cream.
- Because a majority of women who have survived breast cancer have not been screened for BRCA1 and BRCA2 mutations, Ob/Gyns should screen survivors to identify those patients at risk for subsequent disease.
- Approximately half of the women who survive breast or a gynecologic cancer report severe, long-lasting sexual problems.
As more is discovered about cancer, caring for the cancer patient has become even more complicated. Women who have survived cancer, even nongynecologic diseases, increasingly are being followed by general obstetrician/gynecologists or primary care physicians. To help Ob/Gyns provide better care, this review will summarize cancer patients’ special needs with regard to menopause and premature menopause, genetic screening techniques, and well-woman care.
Only about 15% of postmenopausal women use HRT.
In evaluating the needs of the cancer patient, an understanding of the type of cancer and the surgical and medical management the woman has undergone is key. This knowledge aids the management of sexual health, fertility, and menopause. Additionally, depending on the type of cancer she has had, the patient may be exposed to health risks not previously identified. In these cases, an understanding of the familial basis of some cancers and hereditary syndromes is essential, as it can help identify those who may be at risk for subsequent malignancies or conditions.
Finally, as with all well-woman care, preventive measures such as bone densitometry, mammography, colonoscopy, exercise, self-breast examination, and smoking cessation must be addressed and discussed.
Menopause
Many young women with a cancer diagnosis face premature menopause. In addition to the psychological issues stemming from premature ovarian failure such as those related to fertility, these women experience many of the typical menopausal symptoms and other health risks well before their healthy counterparts.
In the decades since hormone replacement therapy (HRT) was introduced, there has been increasing controversy and confusion among patients and physicians about its benefits and risks. In particular, because of the possible role of estrogen in the pathogenesis of breast cancer, its use for post-menopausal therapy has been challenged.1-3
Currently, only about 15% of post-menopausal women use HRT, in part due to the concerns about breast cancer. Other reasons include breast engorgement and tenderness and a resumption of vaginal bleeding.
Most health-care providers believe HRT is contraindicated for breast cancer survivors. However, withholding HRT from all postmenopausal women would be a disservice to many, as it would expose them to health risks that would outweigh the risk for breast cancer. One of the clinician’s roles is to identify patients who would benefit from HRT and to avoid exposing others to unreasonable risk. Fortunately, the available alternatives to HRT are rapidly increasing in response to the demand this controversy has generated.
BRCA 1 and BRCA 2 are responsible for most inherited ovarian carcinomas.
Hormone replacement therapy initially was intended for short-term use in the management of vasomotor symptoms. The health benefits that were subsequently identified supported a longer duration of use.4,5 These include improving hot flushes, protecting bone density, and combating urogenital atrophy, but more recently, the potential benefit that hormones may have on cardiac health has come under scrutiny.6-8
Vasomotor symptoms. Menopausal women often complain of debilitating vasomotor flushes. For women who take tamoxifen, e.g., those with a history of breast cancer, vasomotor symptoms may be potentiated.9,10 Traditional HRT regimens, either oral or transdermal, have been effective in treating vasomotor symptoms in 85% to 90% of women.11 Even so, increasing numbers of women are turning to an ever-growing array of nonestrogen agents in an effort to alleviate symptoms and minimize their perceived risk of breast cancer.
Agents that can be used to alleviate hot flushes include selective serotonin reuptake inhibitors (SSRIs) such as venlafaxine hydrochloride in small doses, e.g., 25 to 75 mg daily.12,13 Clonidine hydrochloride, an antihypertensive, also is effective, but its side effects limit its use.14 If the 0.1-mg patch is used, it should be changed weekly, and if the drug is administered orally, the dosage should not exceed 0.1 mg twice daily. Megestrol, a progestin, also has been shown to be effective.15
Recently, phytoestrogens have been sought as substitutes for traditional HRT. While there is some evidence they may improve vasomotor flushes and protect against bone cancer and cardiovascular disease, results have been inconsistent and definitive studies are lacking.16-21
It is beyond the scope of this article to delineate or evaluate the many herbal and nutritional supplements available for the management of vasomotor symptoms. According to the literature, their efficacy is questionable. Nonetheless, the industries that promote these products are among the fastest-growing businesses, largely due to the public’s fears about breast cancer.
Genitourinary symptoms. Atrophic changes of the vagina are common in the menopausal years and are associated with generalized pruritus and dyspareunia to variable degrees in each individual. This, in addition to the cancer diagnosis and treatment, can lead to symptoms of depression, low self-esteem, and relationship issues surrounding sexuality. It is essential to address symptoms of vaginal atrophy and discuss treatment options with the cancer patient. Local estrogen therapy in the form of a 17-beta estradiol-releasing ring or tablet is an effective method22,23 and has been used successfully in breast cancer survivors at our institution. Since the absorption of estrogen is minimal, it is thought to be safe.24 Further, the risk of endometrial cancer associated with the use of unopposed estrogen is lower with the vaginal ring or tablet than with estrogen cream, as evidenced by endometrial biopsies (TABLE 1).22 In addition, it appears patient satisfaction and acceptability, i.e., ease and comfort of administration, is greater with the tablet than with the cream (Figure 1).
FIGURE 1 Patient satisfaction with vaginal tablets and cream
Source: Rioux JE, Devlin C, Gelfrand M, et al. 17-beta estradiol vaginal tablet versus conjugated equine estrogen cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161.TABLE 1
Safety of vaginal tablets versus cream for the treatment of atrophic vaginitis
| VAGINAL TABLETS (N=80) | VAGINAL CREAM (N=79) | |||
|---|---|---|---|---|
| BASELINE | WEEK 34 | BASELINE | WEEK 24 | |
| Patients with biopsies/stained biopsies | 60 | 49 | 59 | 49 |
| Atrophic endometrium | 34 | 34 | 35 | 15 |
| Weakly proliferative endometrium | 1 | 0 | 3 | 4 |
| Proliferative endometrium | 0 | 1 | 1 | 7 |
| Endometrial hyperplasia | 0 | 0 | 0 | 2 |
| Biopsies with insufficient tissue | 25 | 14 | 20 | 21 |
| Source: Rioux JE, Devlin C, Gelfrand M, et al. 17-beta estradiol vaginal tablet versus conjugated equine estrogen cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161. | ||||
Screening for genetic mutations
Genetic counseling should be offered to all patients who appear to carry an increased cancer risk based on their personal or family histories. Unfortunately, many women with a history of breast or ovarian cancer have never been offered such counseling. Therefore, it is important for Ob/Gyns to screen cancer survivors to identify those patients at risk for subsequent disease.
Although most cancers arise from somatic mutations acquired after birth, approximately 10% of both breast and ovarian cancers are thought to result from inherited gene mutations that are passed down through the maternal or paternal lines. The theory that some breast and ovarian cancers have a genetic basis has been entertained for decades, but only recently have the gene mutations BRCA1 and BRCA2 been identified.25,26 Approximately 85% of hereditary breast carcinomas result from alterations in the BRCA1 and BRCA2 genes.27 These genes also are responsible for most inherited ovarian carcinomas.
Studies also have shown that in families with a history of ovarian cancer or early-onset breast cancer, the risk of developing breast cancer by age 70 is more than 80% for women with BRCA1 or BRCA2 mutations.26,28-30 The risk of developing breast cancer before the age of 50 is 33% to 50% in women with a gene mutation in BRCA1 or BRCA2, versus only 2% in a woman without a gene mutation.30,31
The risk of ovarian cancer conferred by mutations in BRCA1 appears to be greater than that for BRCA2. The BRCA1 mutation is associated with a risk of ovarian cancer between 28% and 44% by age 70, as compared to 1.8% in the general population.28,30,32 The BRCA2-associated risk of ovarian cancer is estimated at 27%, with most cases occurring after the age of 50. TABLE 2 summarizes the risk of cancer conferred by inherited mutations in BRCA1 and BRCA2.
Three forms of hereditary epithelial ovarian cancer exist.26,28,33,34 The most common form appears as a component of the hereditary breast/ovarian cancer syndrome. In these families, multiple cases of breast and ovarian cancer are found, and mutations in the BRCA1 gene are thought to account for the vast majority of them.
The second form of hereditary ovarian cancer occurs as site-specific cancer and is inherited as an autosomal-dominant trait. Mutations in BRCA1 also account for a large proportion of these cases. The third form of hereditary ovarian cancer occurs as a component of the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome. It is characterized by an autosomal-dominant transmission of nonpolyposis colon cancer along with adenocarcinomas of the ovary, uterus, breast, kidney, stomach, pancreas, small bowel, and biliary tree.35
The hallmark of hereditary ovarian cancers is their occurrence at a younger age, the mean being in the mid-40s, with 17% occurring by age 40.36 Therefore, if prophylactic surgery is considered, it generally should be performed prior to the end of the fourth decade of life, after the patient has completed child-bearing. However, since some cases of hereditary ovarian cancer occur in later life, it is thought that even a woman in her 60s or 70s may benefit from prophylactic surgery. Patients undergoing the surgery should be counseled that while their risk is reduced significantly, it is not eliminated. Primary peritoneal carcinomatosis remains a possibility; this risk is estimated at 2% to 11% for women who have undergone prophylactic surgery.37,38
Oral contraceptives (OCs) have been evaluated for their possible role in the prevention of ovarian cancer in the general and high-risk populations. It is estimated that OC use may reduce the risk of hereditary ovarian cancer by as much as 60%.39 However, the benefits of this therapy need to be weighed against the possible increased risk of breast cancer in patients with BRCA1 and BRCA2 mutations.40,41
Due to the low sensitivity and specificity of available screening tests and the relatively low prevalence of ovarian cancer in the general population, routine screening is not recommended, except in women who are at increased risk. Such high-risk women include those who have a personal or family history of ovarian or breast cancer, known mutations in the BRCA1 and BRCA2 genes, or who are of Ashkenazi Jewish descent with personal or family histories of breast or ovarian cancer or both.
No single screening modality has been proven effective. Studies evaluating the role of semiannual pelvic examinations in conjunction with transvaginal sonography (TVS) and CA125 evaluations are under way.42 These modalities currently are the best options in the high-risk population.43-45
For the patient identified as having HNPCC syndrome, screening should include annual endometrial biopsies, breast cancer screening, ovarian cancer screening (as above), colonoscopy, and urine cytology. Prophylactic hysterectomy and oophorectomy may be considered to reduce the gynecologic cancer risks.46
TABLE 3 summarizes surveillance options for women at high risk for familial cancers. Since screening options are limited in sensitivity and specificity, the guidelines are based on expert opinion rather than randomized studies.47
TABLE 2
Cancer risk conferred by inherited mutations in BRCA1 and BRCA2
| CANCER | RISKS |
|---|---|
| Breast | Up to 87% by age 70; 33% to 50% by age 50 |
| BRCA1 or BRCA2 | Risk of contralateral breast cancer, 64% by age 70; 25% within 5 years |
| Ovarian | |
| BRCA1 | 28% to 44% by age 70 to 80 |
| BRCA2 | 27% by age 70 10-fold increased risk following breast cancer |
| Colon | |
| BRCA1 | 3.3-fold increased risk |
| Source: Frank TS. Hereditary risk of breast and ovarian carcinoma: the role of the oncologist. Oncologist. 1998;3:403-412 | |
TABLE 3
Screening options for patients at high risk for familial cancers
| INTERVENTION | RECOMMENDATION | QUALITY OF EVIDENCE* | COMMENTS |
|---|---|---|---|
| BREAST CANCER | |||
| Self-examination | Teach BSE | Level III evidence Expert opinion only | Benefits not proven |
| Clinician breast examination | Annually or semiannually | Level III evidence Expert opinion only | Benefits not proven |
| Mammography | Annually, beginning at age 25 to 35 | Level III evidence Expert opinion only | Risks and benefits not established for women under age 50 |
| OVARIAN CANCER | |||
| Semiannual pelvic examination, TVS, and CA125 | Semiannually, beginning at age 25 to 35 | Level III evidence Expert opinion only | Benefit of TVS, CA125, and pelvic exam levels not proven. Consider prophylactic surgery; significant reduction in risk. Lower estimated ovarian cancer risk in carriers of BRCA2 mutations than in those with BRCA1 mutations. |
| COLON CANCER | |||
| Colonoscopy every 5 to 10 years, hemoccult annually | All patients beginning at age 50. Sigmoidoscopy every 3 to 5 years may be effective in a low-risk population. | Evidence from average-risk populations includes a Level I randomization trial (fecal blood test) and a Level II-2 case- control study (sigmoidoscogy) | If the patient has HNPCC, consider prophylactic total abdominal hysterectomy with bilateral salpingo-oophorectomy |
| Adapted from: Burke et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277(11):915-919. | |||
| *Denotes the highest-quality trial performed | |||
| Level I = highest-quality trials | |||
| Level II = intermediate-quality (nonrandomized) trials and observational studies (Level II-2 indicates a case-control study) | |||
| Level III = lowest-quality (expert opinion and case reports) | |||
Sexual health
Sexual dysfunction is one of the more common, enduring consequences of cancer treatment. Approximately half of the women who survive breast or a gynecologic cancer report severe, long-lasting sexual problems.48 Studies have shown that sexuality issues are prevalent among cancer survivors in general. Anderson reports sexual-functioning morbidity occurs in up to 90% of women with the most prevalent types of cancer.49 Other authors estimate that post-treatment sexual dysfunction ranges from 30% to 100%.50 Disease-free breast cancer survivors admit that sexual problems persist as bothersome and disheartening exceptions to their generally high level of functioning. For other cancers such as Hodgkin’s disease, at least 25% of patients are left with a sexual dysfunction.51
For patients who have vaginal stenosis or shortening due to surgery or irradiation for gynecologic or colorectal cancers, the use of dilators as early as possible is essential to maximize vaginal length and treatment success. In general, initial counseling about the use of these devices is provided by the surgical or radiation oncologist.
Ideally, however, patients with cancer—especially disease involving the vaginal, perineal, and anal areas—who require extensive surgery or irradiation will have had a sexual-health consultation before treatment. At our institution, the patient initially is evaluated by a psychologist, who is a licensed sex therapist, and a gynecologist so that both physical and psychological components can be assessed and a course of treatment planned.
Overall, the ideal approach to sexual dysfunction in the cancer patient is:
- Validating the patient’s need for a sexual relationship;
- Assisting in identifying those factors contributing to the difficulty; and
- Conducting a pretreatment evaluation with the patient and her partner, if possible.
The annual exam
Cervical cancer screening. The most common type of cancer in women is cervical cancer, with approximately 450,000 new cases reported worldwide each year. Screening with Pap smears and advanced colposcopic techniques has dramatically decreased mortality from this disease in developed countries. Further, testing for human Papillomavirus (HPV), in addition to regular Pap smear screening, has improved the sensitivity of cervical cancer screening.52,53
While physicians agree that all women should begin Pap smear evaluations annually beginning at age 18 or at the onset of sexual activity, some argue there may be a role for HPV testing at the initial screening, as well, to reduce the false-negative rate and help identify at-risk individuals earlier. Patients who already have cancer and may be immunosuppressed as a result of their treatment may be at higher risk for HPV, thus warranting routine HPV screening. (In such women, genital warts may be diffuse, requiring surgical treatment.)
Colonoscopy. Colon cancer is the third most common cancer in women. Risk factors include advanced age, living in an industrialized country, a diet high in fat and cholesterol, genetic premalignant polyposis syndromes, a family history of colon cancer or inflammatory bowel disease, and a history of intestinal adenomatous polyps.47
Colonoscopy is recommended for all patients beginning at age 50, with a frequency of every 10 years for those at average risk. Women with any of the risk factors noted above, a personal history of breast or ovarian cancer, or both, should undergo more frequent screening.
Digital rectal examination, as well as a hemoccult, should be part of the annual examination.
Osteoporosis. Osteoporosis is a leading cause of nursing-home admissions and a leading cause of morbidity in post-menopausal women. The cancer survivor needs to be evaluated thoroughly for osteoporosis risk factors, particularly since many of these women will have entered menopause prematurely. Further, these patients may have additional risks associated with treatment with steroids, chemotherapeutic agents, or radiation.
Preventive measures, including bone densitometry and the measurement of bone turnover markers, should be performed at menopause or earlier in cancer survivors. Currently, the National Osteoporosis Foundation recommends HRT as the firstline agent for the treatment and prevention of osteoporosis in the general population. In cancer patients, however, particularly those with estrogen-sensitive cancers, HRT may not be a viable option. Alternatives include antiresorptive agents such as raloxifene hydrochloride, a selective estrogen receptor modulator (SERM). The Multiple Outcomes of Raloxifene Evaluation trial (MORE) found that raloxifene reduced vertebral fracture incidence by approximately 50%, had no adverse gynecologic effects, and may decrease the risk of breast cancer.54
Although it was approved for the treatment of osteoporosis, alendronate, a bisphosphonate with antiresorptive effects, can be used in lower doses as a preventive agent as well.55,56 When alendronate was given, the relative risk for fracture was reduced by 50% in a group of women considered at high risk based on known low bone mass and a history of at least 1 vertebral fracture.54 Incidence of hip fractures also was significantly reduced. However, at the typical dose of 5 to 10 mg daily, alendronate has not been well tolerated, primarily due to gastrointestinal effects. More recently, a 35-mg and 70-mg weekly dose have been used successfully and are better tolerated.
Bone turnover markers, such as urinary N-telopeptide (NTX) levels, can be used to determine if treatment should be instituted and whether it has been effective. NTX is an indicator of what a patient’s bone density will be if she remains untreated or continues her current regimen. A significantly elevated NTX, i.e., 38 BCE (bone collagen excretion), indicates the presence of a metabolic disease, and the clinician should consider initiating antiresorptive therapy.
Regardless of bone mass or bone turnover rates, calcium and vitamin D supplementation should be encouraged. Generally, post-menopausal women should be advised to take 1,000 to 1,500 mg of calcium per day as well as 400 to 800 IU of vitamin D.
Cardiovascular disease. The leading cause of death in postmenopausal women is cardiovascular disease (CVD). Over the remainder of her lifetime, a 50-year-old woman has a 46% probability of developing CVD and a 31% chance of dying from it. The National Cholesterol Education Program (NCEP) Expert Panel [Adult Treatment Panel III (ATP III)] has proposed an algorithm for CVD risk-factor stratification with the goal of treating high-risk women more aggressively.57 When high-density lipoprotein (HDL) cholesterol levels are low, women face a greater risk of CVD than men do at similar levels. Diabetes and hypertriglyceridemia also impart a greater risk of CVD in women.
Cancer survivors often face an even greater risk of CVD than the general population because of the cardiotoxic effects of some cancer therapies. Thus, evaluating cancer survivors for cardiovascular risk factors is essential to their management.
Many agents, including HRT, have been evaluated for the management of hyperlipidemia. The NCEP recommends that statin drugs, rather than HRT, be the first-line treatment for this condition because they have been found to be more effective in lowering low-density lipoprotein (LDL) cholesterol. In fact, recent studies have questioned the safety of estrogen in the secondary prevention of CVD in women with established cardiac disease.7
Endometrial cancer. To date, there is no proven method of screening for endometrial cancer in tamoxifen-treated patients. In these women, the expected annual risk is 2 per 1,000.58 Although this risk is low, endometrial cancer remains a major concern for patients taking the drug.
Endometrial sampling should be reserved for patients who are experiencing abnormal bleeding. In a recent prospective trial measuring the incidence of abnormal endometrial pathology in 111 tamoxifen-treated patients, Barakat et al concluded that the utility of endometrial biopsy is limited in this population.59 In another trial, Gibson and colleagues noted that all cases of endometrial carcinoma detected by dilatation and curettage (D&C) were found in tamoxifen-treated patients who presented with abnormal bleeding.60
Ultrasound also has been evaluated as a screening technique in this population. An endometrial thickness of 8 mm or more is considered abnormal, although this guideline is under evaluation. Hann et al reported that although most tamoxifen-treated women are asymptomatic, endometrial polyps were found in 33% of cases with a lining thicker than 8 mm. In addition, they found a correlation between endometrial thickness and duration of tamoxifen use.61
Another study recently found that transvaginal sonography has a high false-positive rate, even when the cut-off for endometrial thickness is 10 mm instead of 8 mm.62 In this study, 1 asymptomatic endometrial cancer was found. The authors concluded that this low yield does not justify the increased iatrogenic morbidity of transvaginal ultrasound screening in the tamoxifen-treated patient.
Conclusion
This review of the cancer patient’s needs underscores the principle that all patients, regardless of medical history, need thorough evaluations to identify risk factors for other disease entities. As such, all women should undergo an annual examination so that preventive measures such as cardiovascular health assessment, bone densitometry, mammography, colonoscopy, and screening for familial health risks also can be undertaken.
However, a thorough medical evaluation often is not performed in the cancer survivor because her cancer is the focus of most of her physician visits. Thus, preventive measures such as those outlined above are overlooked, putting her at increased risk for fracture, cardiovascular disease, and other dysplasia or malignancies.
Gynecologic care of the cancer patient requires an understanding of the numerous issues that cancer survivors face—many of which are no different than those faced by their healthy counterparts. While a multidisciplinary approach may be required, such health care ideally should be coordinated by a primary-care physician. This responsibility often falls to the gynecologist or internist.
In addition, an understanding of the chemotherapeutic regimens, the effects of radiation therapy, and psychological factors can help the clinician identify or anticipate the problems unique to the cancer patient. The physician’s ability to validate a patient’s concerns, dispel myths, and provide information about treatment options can ease her anxiety and maximize physical and psychological well-being.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Gapstur S, Morrow M, Sellers T. Hormone replacement therapy and risk of breast cancer with a favorable histology. JAMA. 1999;281:2091-2097.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
3. Colditz GA, Hankinson SE, Hunter DJ. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589-1593.
4. Grodstein F, Stampfer M, Colditz D, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769-1775.
5. The Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density: results of the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276(17):1389-1396.
6. Nachtigall LE, Nachtigall MJ. Hormone replacement therapy. Curr Opin Obstet Gynecol. 1992;4(6):907-913.
7. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
8. Mosca L. The role of hormone replacement therapy in the prevention of postmenopausal heart disease. Arch Intern Med. 2000;160:2263-2272.
9. Powles TJ, Jones AL, Ashley SE, et al. The Royal Marsden Hospital pilot tamoxifen chemoprevention trial. Breast Cancer Res Treat. 1994;31(1):73-82.
10. Fisher B, Dignam J, Bryant J, et al. Five years versus more than five years of tamoxifen therapy for breast cancer patients with negative nodes and estrogen-receptor positive tumors. J Natl Cancer Inst. 1996;88(21):1529-1542.
11. Castiel M. Management of menopausal symptoms in the cancer patient. Oncology. 1999;13(10):1363-1372.
12. Loprinzi CL, Kugler JW, Sloan J, et al. Venlafaxine alleviates hot flashes: an NCCTG trial. Progr Proc Am Soc Clin Oncol. 2000;19:2a [abstract].-
13. Loprinzi CL, Quella SK, Sloan JA, et al. Preliminary data from a randomized evaluation of fluoxetine (Prozac) for treating hot flashes in breast cancer survivors. Breast Cancer Res Treat. 1999;57:34 [abstract].-
14. Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in post menopausal patients with breast cancer experiencing tamoxifen induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132(10):788-793.
15. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;11:347-352.
16. Nachtigall L, Fenichel R, La Greg L, et al. The effects of isoflavone-derived red clover on vasomotor symptoms, endometrial thickness, and reproductive hormone concentrations in menopausal women. Proceedings of the 81st annual meeting of the Endocrine Society, June 12-15, 1999, San Diego, Calif.
17. Scambia G, Mango D, Signorile PG. Clinical effects of a standardized soy extract in postmenopausal women: pilot study. Menopause. 2000;7:105-111.
18. Quella S, Loprinzi C, Barton D, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment Group Trial. J Clin Oncol. 2000;18:1068-1074.
19. Adlercreutz CH, Goldin BR, Gorbach SL, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125(3 Suppl):757s-770s.
20. McMichael-Phillips D, Harding C, Morton M, et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431s-1436s.
21. Potter SM, Baum JA, Teng H, et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68:1375s-1379s.
22. Rioux JE, Devlin C, Gelfand M, et al. 17-[beta]-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161.
23. Smith P, Heimer G, Lindskog M, et al. Oestradiol-releasing vaginal ring for treatment of postmenopausal urogenital atrophy. Maturitas. 1993;16:145-154.
24. Bachmann G. Estradiol-releasing vaginal ring delivery system for urogenital atrophy. Experience over the past decade. J Reprod Med. 1998;43:991-998.
25. Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66.-
26. Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789.-
27. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62(3):676-689.
28. Ford D, Easton DF, Bishop DT, et al. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692.-
29. Easton D, Steele L, Fields P, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Human Genet. 1997;61:120-128.
30. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265-271.
31. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401-1408.
32. Whittemore AS, Gong G, Imtyre J. Prevalence and contribution of BRCA1 mutations in breast cancer: results from three US population-based case-control studies of ovarian cancer. Am J Hum Genet. 1997;60:496.-
33. Easton DT, Bishop DT, Ford D, et al. Genetic linkage analysis in familial breast and ovarian cancers: results from 214 families. Am J Hum Genet. 1993;52(4):678-701.
34. Steichen-Gersdorf E, Gallion HH, Ford D, et al. Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet. 1994;55:870-875.
35. Lynch HT, Lynch JF. Hereditary ovarian carcinoma. Hematol Oncol Clin North Am. 1992;6(4):783-811.
36. Struewing J, et al. Prophylactic oophorectomy in inherited breast/ovarian cancer families. J Natl Cancer Inst Monogr. 1995;17:33-35.
37. Piver MS, Jishi MF, Tsukada Y, et al. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. Cancer. 1993;71:2751-2755.
38. Tobacman JK, Greene MH, Tucker MA, et al. Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2(8302):795-797.
39. Narod SA, Risch H, Moslehi R, Dorum A, et al. Oral contraceptives and the risk of hereditary ovarian cancer. N Engl J Med. 1998;339:424-428.
40. Grabrick DM, Hartmann LC, Cerhan JR, et al. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. JAMA. 2000;284:1791-1798.
41. Ursin G, Henderson BG, Haile RW, et al. Does oral contraceptive use increase the risk of breast cancer in women with BRCA1/BRCA2 mutations more than in other women? Cancer Res. 1997;57:3678-3681.
42. NIH Consensus Development Panel on Ovarian Cancer. Ovarian cancer. Screening, treatment, and follow-up. JAMA. 1995;273(6):491-497.
43. Frank TS. Identifying women with inherited risk for ovarian cancer: who and why? Contemp Ob/Gyn. 1998;27-50.
44. Bourne T, Whitehead M, Campbell S, et al. Ultrasound screening for familial ovarian cancer. Gynecol Oncol. 1993;43:92-97.
45. Berchuck A, Cirisano F, Lancaster JM, et al. Role of BRCA1 mutation screening in the management of familial ovarian cancer. Am J Obstet Gynecol. 1996;175:738-746.
46. Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277(11):915-919.
47. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. JAMA. 1997;227:997-1003.
48. Robinson JW. Sexuality and cancer. Breaking the silence. Aust Fam Phys. 1998;27:45-47.
49. Anderson BL. How cancer affects sexual functioning. Oncology. 1990;4:23-42.
50. Anderson BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol. 1997;65:221-229.
51. Van Tulder MW, Aaronson NK, Bruning PF. The quality of life of long-term survivors of Hodgkin’s disease. Ann Oncol. 1994;5:153-158.
52. Wright TC, Jr, Denny L, Kuhn L, et al. HPV testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81-86.
53. Schiffman M, Herrero R, Hildesheim A, et al. HPV DNA testing in cervical cancer screening results from women in a high-risk province of Costa Rica. JAMA. 2000;283:87-93.
54. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
55. Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
56. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial. JAMA. 280;2077-2082.
57. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486-2497.
58. Fisher B, Constantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527.-
59. Barakat RR, Gilewski TA, Almadrones L, et al. Effect of adjuvant tamoxifen on the endometrium in women with breast cancer: a prospective study using office endometrial biopsy. J Clin Oncol. 2000;18:3459-3463.
60. Gibson LE, Barakat RR, Venkatraman ES, Hoskins WJ. Endometrial pathology at dilatation and curettage in breast cancer patients: comparison of tamoxifen users and nonusers. Cancer J Sci Am. 1996;2:35.-
61. Hann LE, Gess CS, Bach AM, et al. Endometrial thickness in tamoxifentreated patients: correlation with clinical and pathologic findings. Am J Roentgenol. 1997;168(3):657-661.
62. Garber B, Krause A, Muller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer a prospective long term study using transvaginal ultrasound. J Clin Oncol. 2000;18:3464-3470.
1. Gapstur S, Morrow M, Sellers T. Hormone replacement therapy and risk of breast cancer with a favorable histology. JAMA. 1999;281:2091-2097.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
3. Colditz GA, Hankinson SE, Hunter DJ. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589-1593.
4. Grodstein F, Stampfer M, Colditz D, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769-1775.
5. The Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density: results of the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276(17):1389-1396.
6. Nachtigall LE, Nachtigall MJ. Hormone replacement therapy. Curr Opin Obstet Gynecol. 1992;4(6):907-913.
7. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
8. Mosca L. The role of hormone replacement therapy in the prevention of postmenopausal heart disease. Arch Intern Med. 2000;160:2263-2272.
9. Powles TJ, Jones AL, Ashley SE, et al. The Royal Marsden Hospital pilot tamoxifen chemoprevention trial. Breast Cancer Res Treat. 1994;31(1):73-82.
10. Fisher B, Dignam J, Bryant J, et al. Five years versus more than five years of tamoxifen therapy for breast cancer patients with negative nodes and estrogen-receptor positive tumors. J Natl Cancer Inst. 1996;88(21):1529-1542.
11. Castiel M. Management of menopausal symptoms in the cancer patient. Oncology. 1999;13(10):1363-1372.
12. Loprinzi CL, Kugler JW, Sloan J, et al. Venlafaxine alleviates hot flashes: an NCCTG trial. Progr Proc Am Soc Clin Oncol. 2000;19:2a [abstract].-
13. Loprinzi CL, Quella SK, Sloan JA, et al. Preliminary data from a randomized evaluation of fluoxetine (Prozac) for treating hot flashes in breast cancer survivors. Breast Cancer Res Treat. 1999;57:34 [abstract].-
14. Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in post menopausal patients with breast cancer experiencing tamoxifen induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132(10):788-793.
15. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;11:347-352.
16. Nachtigall L, Fenichel R, La Greg L, et al. The effects of isoflavone-derived red clover on vasomotor symptoms, endometrial thickness, and reproductive hormone concentrations in menopausal women. Proceedings of the 81st annual meeting of the Endocrine Society, June 12-15, 1999, San Diego, Calif.
17. Scambia G, Mango D, Signorile PG. Clinical effects of a standardized soy extract in postmenopausal women: pilot study. Menopause. 2000;7:105-111.
18. Quella S, Loprinzi C, Barton D, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment Group Trial. J Clin Oncol. 2000;18:1068-1074.
19. Adlercreutz CH, Goldin BR, Gorbach SL, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125(3 Suppl):757s-770s.
20. McMichael-Phillips D, Harding C, Morton M, et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431s-1436s.
21. Potter SM, Baum JA, Teng H, et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68:1375s-1379s.
22. Rioux JE, Devlin C, Gelfand M, et al. 17-[beta]-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156-161.
23. Smith P, Heimer G, Lindskog M, et al. Oestradiol-releasing vaginal ring for treatment of postmenopausal urogenital atrophy. Maturitas. 1993;16:145-154.
24. Bachmann G. Estradiol-releasing vaginal ring delivery system for urogenital atrophy. Experience over the past decade. J Reprod Med. 1998;43:991-998.
25. Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66.-
26. Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789.-
27. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62(3):676-689.
28. Ford D, Easton DF, Bishop DT, et al. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692.-
29. Easton D, Steele L, Fields P, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Human Genet. 1997;61:120-128.
30. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265-271.
31. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401-1408.
32. Whittemore AS, Gong G, Imtyre J. Prevalence and contribution of BRCA1 mutations in breast cancer: results from three US population-based case-control studies of ovarian cancer. Am J Hum Genet. 1997;60:496.-
33. Easton DT, Bishop DT, Ford D, et al. Genetic linkage analysis in familial breast and ovarian cancers: results from 214 families. Am J Hum Genet. 1993;52(4):678-701.
34. Steichen-Gersdorf E, Gallion HH, Ford D, et al. Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet. 1994;55:870-875.
35. Lynch HT, Lynch JF. Hereditary ovarian carcinoma. Hematol Oncol Clin North Am. 1992;6(4):783-811.
36. Struewing J, et al. Prophylactic oophorectomy in inherited breast/ovarian cancer families. J Natl Cancer Inst Monogr. 1995;17:33-35.
37. Piver MS, Jishi MF, Tsukada Y, et al. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. Cancer. 1993;71:2751-2755.
38. Tobacman JK, Greene MH, Tucker MA, et al. Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2(8302):795-797.
39. Narod SA, Risch H, Moslehi R, Dorum A, et al. Oral contraceptives and the risk of hereditary ovarian cancer. N Engl J Med. 1998;339:424-428.
40. Grabrick DM, Hartmann LC, Cerhan JR, et al. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. JAMA. 2000;284:1791-1798.
41. Ursin G, Henderson BG, Haile RW, et al. Does oral contraceptive use increase the risk of breast cancer in women with BRCA1/BRCA2 mutations more than in other women? Cancer Res. 1997;57:3678-3681.
42. NIH Consensus Development Panel on Ovarian Cancer. Ovarian cancer. Screening, treatment, and follow-up. JAMA. 1995;273(6):491-497.
43. Frank TS. Identifying women with inherited risk for ovarian cancer: who and why? Contemp Ob/Gyn. 1998;27-50.
44. Bourne T, Whitehead M, Campbell S, et al. Ultrasound screening for familial ovarian cancer. Gynecol Oncol. 1993;43:92-97.
45. Berchuck A, Cirisano F, Lancaster JM, et al. Role of BRCA1 mutation screening in the management of familial ovarian cancer. Am J Obstet Gynecol. 1996;175:738-746.
46. Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277(11):915-919.
47. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. JAMA. 1997;227:997-1003.
48. Robinson JW. Sexuality and cancer. Breaking the silence. Aust Fam Phys. 1998;27:45-47.
49. Anderson BL. How cancer affects sexual functioning. Oncology. 1990;4:23-42.
50. Anderson BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol. 1997;65:221-229.
51. Van Tulder MW, Aaronson NK, Bruning PF. The quality of life of long-term survivors of Hodgkin’s disease. Ann Oncol. 1994;5:153-158.
52. Wright TC, Jr, Denny L, Kuhn L, et al. HPV testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81-86.
53. Schiffman M, Herrero R, Hildesheim A, et al. HPV DNA testing in cervical cancer screening results from women in a high-risk province of Costa Rica. JAMA. 2000;283:87-93.
54. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
55. Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
56. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial. JAMA. 280;2077-2082.
57. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486-2497.
58. Fisher B, Constantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527.-
59. Barakat RR, Gilewski TA, Almadrones L, et al. Effect of adjuvant tamoxifen on the endometrium in women with breast cancer: a prospective study using office endometrial biopsy. J Clin Oncol. 2000;18:3459-3463.
60. Gibson LE, Barakat RR, Venkatraman ES, Hoskins WJ. Endometrial pathology at dilatation and curettage in breast cancer patients: comparison of tamoxifen users and nonusers. Cancer J Sci Am. 1996;2:35.-
61. Hann LE, Gess CS, Bach AM, et al. Endometrial thickness in tamoxifentreated patients: correlation with clinical and pathologic findings. Am J Roentgenol. 1997;168(3):657-661.
62. Garber B, Krause A, Muller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer a prospective long term study using transvaginal ultrasound. J Clin Oncol. 2000;18:3464-3470.