User login
Why celiac disease is so easy to miss
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
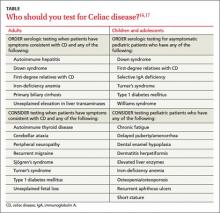
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; pdowling@mednet.ucla.edu
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; pdowling@mednet.ucla.edu
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; pdowling@mednet.ucla.edu
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.