User login
Liraglutide improved weight management in overweight and obese adults
When taken daily at a dose of 3 mg in combination with diet and exercise, liraglutide increased weight loss in overweight and obese nondiabetic adults, report Dr. Xavier Pi-Sunyer and coauthors from the Division of Endocrinology and Obesity Research Center at Columbia University in New York.
In a 56-week, double-blind trial of 3,731 overweight adults, patients who received liraglutide lost a mean of 8.4 ± 7.3 kg, compared with 2.8 ± 6.5 kg in the placebo group (a difference of –5.6 kg; 95% CI, –6.0 to –5.1; P < .001).
Additionally, 63.2% of participants in the liraglutide group lost at least 5% of their body weight, compared with 27.1% in the placebo group (P < .001). Furthermore, 33.1% of patients in the liraglutide group and 10.6% of patients in the placebo group lost more than 10% of their body weight (P
“Given previous disappointments with various weight-loss strategies, these are welcome findings,” said Dr. Elias S. Siraj and Dr. Kevin Jon Williams, of the Temple University, Philadelphia, in an accompanying editorial. Though the authors cautioned that “liraglutide is no cure,” the findings suggest that modest weight loss may now be easier to achieve, they concluded.
Read the full article here: http://www.nejm.org/doi/full/10.1056/NEJMoa1411892 and the editorial here: http://www.nejm.org/doi/full/10.1056/NEJMe1506236
When taken daily at a dose of 3 mg in combination with diet and exercise, liraglutide increased weight loss in overweight and obese nondiabetic adults, report Dr. Xavier Pi-Sunyer and coauthors from the Division of Endocrinology and Obesity Research Center at Columbia University in New York.
In a 56-week, double-blind trial of 3,731 overweight adults, patients who received liraglutide lost a mean of 8.4 ± 7.3 kg, compared with 2.8 ± 6.5 kg in the placebo group (a difference of –5.6 kg; 95% CI, –6.0 to –5.1; P < .001).
Additionally, 63.2% of participants in the liraglutide group lost at least 5% of their body weight, compared with 27.1% in the placebo group (P < .001). Furthermore, 33.1% of patients in the liraglutide group and 10.6% of patients in the placebo group lost more than 10% of their body weight (P
“Given previous disappointments with various weight-loss strategies, these are welcome findings,” said Dr. Elias S. Siraj and Dr. Kevin Jon Williams, of the Temple University, Philadelphia, in an accompanying editorial. Though the authors cautioned that “liraglutide is no cure,” the findings suggest that modest weight loss may now be easier to achieve, they concluded.
Read the full article here: http://www.nejm.org/doi/full/10.1056/NEJMoa1411892 and the editorial here: http://www.nejm.org/doi/full/10.1056/NEJMe1506236
When taken daily at a dose of 3 mg in combination with diet and exercise, liraglutide increased weight loss in overweight and obese nondiabetic adults, report Dr. Xavier Pi-Sunyer and coauthors from the Division of Endocrinology and Obesity Research Center at Columbia University in New York.
In a 56-week, double-blind trial of 3,731 overweight adults, patients who received liraglutide lost a mean of 8.4 ± 7.3 kg, compared with 2.8 ± 6.5 kg in the placebo group (a difference of –5.6 kg; 95% CI, –6.0 to –5.1; P < .001).
Additionally, 63.2% of participants in the liraglutide group lost at least 5% of their body weight, compared with 27.1% in the placebo group (P < .001). Furthermore, 33.1% of patients in the liraglutide group and 10.6% of patients in the placebo group lost more than 10% of their body weight (P
“Given previous disappointments with various weight-loss strategies, these are welcome findings,” said Dr. Elias S. Siraj and Dr. Kevin Jon Williams, of the Temple University, Philadelphia, in an accompanying editorial. Though the authors cautioned that “liraglutide is no cure,” the findings suggest that modest weight loss may now be easier to achieve, they concluded.
Read the full article here: http://www.nejm.org/doi/full/10.1056/NEJMoa1411892 and the editorial here: http://www.nejm.org/doi/full/10.1056/NEJMe1506236
Idarucizumab reverses dabigatran’s anticoagulant effects
TORONTO – Idarucizumab is a promising agent that quickly and safely reverses the anticoagulant effects of dabigatran whether the goal is to control serious bleeding or to permit urgent surgery, according to interim results of a multicenter trial.
Idarucizumab is a monoclonal antibody that binds to dabigatran to reverse its activity. The data, presented by Dr. V. Charles Pollack Jr. at the International Society on Thrombosis and Haemostasis congress, involved the first 90 patients of an ongoing trial with a planned enrollment of 300. The data from this trial, called REVERSE-AD, were published online simultaneously with the June 22 presentation at the congress (N. Engl. J. Med 2015 [doi:10.1056/NEJMoa1502000]).
“Non–vitamin K antagonist oral anticoagulants (NOACs) are generally safer than warfarin, and provide similar or improved efficacy in the prevention of stroke in patients with nonvalvular atrial fibrillation and in the prevention and treatment of venous thromboembolism,” Dr. Pollack said in an interview. “Nonetheless, serious bleeding events may occur with NOAC use, and patients taking one of these agents occasionally require urgent surgery or other intervention for which normal hemostasis is required,” added Dr. Pollack, chair of the department of emergency medicine at Pennsylvania Hospital in Philadelphia.
In RE-VERSE AD (a study of the reversal effects of idarucizumab on active dabigatran), the first 90 patients were divided into two distinct groups. Group A, with 51 patients, included those on dabigatran with serious bleeding. Group B, with 39 patients, required reversal of dabigatran for urgent or emergent procedures. In both, idarucizumab provided a median maximum reversal of 100% (95% confidence interval, 100-100) of the anticoagulation effect within 4 hours.
Clotting assays were normalized almost immediately in almost 90% of patients, and the effect was durable, with 80% having measured dabigatran levels reflecting no significant anticoagulation 24 hours later, Dr. Pollack said.
“Clinical outcomes were quite good in this multimorbid patient population, with restoration of hemostasis as reported by local investigators achieved in less than 12 hours when assessable, and with 92% of surgical patients being reported as having normal hemostasis at the time of the procedure,” he said.
Idarucizumab was generally well tolerated in the patient population. “There were no serious adverse events related to the reversal agent ... and only one patient experienced a thrombotic complication within 72 hours, and that patient had not been restarted on any antithrombotic medications,” Dr. Pollack said.
“The study is ongoing,” he added, “but these interim results show rather convincingly that idarucizumab completely and safely reverses the anticoagulant effects of dabigatran within minutes.”
In addition, Dr. Pollack said the availability of a specific reversal agent for dabigatran would enhance its safety margin, and thus alleviate the fears of providers who may hesitate to use a NOAC because of the lack of an “antidote.”
“In fact, most such cases can already be successfully and safely managed with general support and ‘tincture of time’ (the half-life of dabigatran is much shorter than that of warfarin), but having a specific ‘go-to’ option could streamline the care of the most significantly compromised patients,” he said.
Dr. Pollack emphasized, however, that idarucizumab is a specific reversal agent for dabigatran, not an antidote. “To me, the latter would imply that idarucizumab immediately stops bleeding associated with active use of dabigatran,” he said.
Providers should realize that while idarucizumab seems capable of removing dabigatran-induced coagulopathy from the list of concerns when managing a patient with serious bleeding or before a “sharp” procedure, bleeding is a multifaceted issue that also may be due to traumatized blood vessels, other causes of coagulopathy such as liver disease, or concurrent use of antiplatelet medications, he said.
“The patient with a serious or life-threatening bleed on dabigatran will likely need additional care to investigate and manage such concerns,” Dr. Pollack said. “But at least idarucizumab can specifically, safely, and rapidly address the primary consideration.
“The safety of anticoagulation therapy with dabigatran is further enhanced with idarucizumab, a specific reversal agent that won’t need to be used often, but the availability of which would be reassuring to prescribers,” he concluded.
Boehringer Ingelheim sponsored RE-VERSE AD. Idarucizumab was given a fast-track status by the Food and Drug Administration, and BI submitted a new drug application in March 2015, according to the company.
Dr. Pollack reported receiving personal fees from BI, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
TORONTO – Idarucizumab is a promising agent that quickly and safely reverses the anticoagulant effects of dabigatran whether the goal is to control serious bleeding or to permit urgent surgery, according to interim results of a multicenter trial.
Idarucizumab is a monoclonal antibody that binds to dabigatran to reverse its activity. The data, presented by Dr. V. Charles Pollack Jr. at the International Society on Thrombosis and Haemostasis congress, involved the first 90 patients of an ongoing trial with a planned enrollment of 300. The data from this trial, called REVERSE-AD, were published online simultaneously with the June 22 presentation at the congress (N. Engl. J. Med 2015 [doi:10.1056/NEJMoa1502000]).
“Non–vitamin K antagonist oral anticoagulants (NOACs) are generally safer than warfarin, and provide similar or improved efficacy in the prevention of stroke in patients with nonvalvular atrial fibrillation and in the prevention and treatment of venous thromboembolism,” Dr. Pollack said in an interview. “Nonetheless, serious bleeding events may occur with NOAC use, and patients taking one of these agents occasionally require urgent surgery or other intervention for which normal hemostasis is required,” added Dr. Pollack, chair of the department of emergency medicine at Pennsylvania Hospital in Philadelphia.
In RE-VERSE AD (a study of the reversal effects of idarucizumab on active dabigatran), the first 90 patients were divided into two distinct groups. Group A, with 51 patients, included those on dabigatran with serious bleeding. Group B, with 39 patients, required reversal of dabigatran for urgent or emergent procedures. In both, idarucizumab provided a median maximum reversal of 100% (95% confidence interval, 100-100) of the anticoagulation effect within 4 hours.
Clotting assays were normalized almost immediately in almost 90% of patients, and the effect was durable, with 80% having measured dabigatran levels reflecting no significant anticoagulation 24 hours later, Dr. Pollack said.
“Clinical outcomes were quite good in this multimorbid patient population, with restoration of hemostasis as reported by local investigators achieved in less than 12 hours when assessable, and with 92% of surgical patients being reported as having normal hemostasis at the time of the procedure,” he said.
Idarucizumab was generally well tolerated in the patient population. “There were no serious adverse events related to the reversal agent ... and only one patient experienced a thrombotic complication within 72 hours, and that patient had not been restarted on any antithrombotic medications,” Dr. Pollack said.
“The study is ongoing,” he added, “but these interim results show rather convincingly that idarucizumab completely and safely reverses the anticoagulant effects of dabigatran within minutes.”
In addition, Dr. Pollack said the availability of a specific reversal agent for dabigatran would enhance its safety margin, and thus alleviate the fears of providers who may hesitate to use a NOAC because of the lack of an “antidote.”
“In fact, most such cases can already be successfully and safely managed with general support and ‘tincture of time’ (the half-life of dabigatran is much shorter than that of warfarin), but having a specific ‘go-to’ option could streamline the care of the most significantly compromised patients,” he said.
Dr. Pollack emphasized, however, that idarucizumab is a specific reversal agent for dabigatran, not an antidote. “To me, the latter would imply that idarucizumab immediately stops bleeding associated with active use of dabigatran,” he said.
Providers should realize that while idarucizumab seems capable of removing dabigatran-induced coagulopathy from the list of concerns when managing a patient with serious bleeding or before a “sharp” procedure, bleeding is a multifaceted issue that also may be due to traumatized blood vessels, other causes of coagulopathy such as liver disease, or concurrent use of antiplatelet medications, he said.
“The patient with a serious or life-threatening bleed on dabigatran will likely need additional care to investigate and manage such concerns,” Dr. Pollack said. “But at least idarucizumab can specifically, safely, and rapidly address the primary consideration.
“The safety of anticoagulation therapy with dabigatran is further enhanced with idarucizumab, a specific reversal agent that won’t need to be used often, but the availability of which would be reassuring to prescribers,” he concluded.
Boehringer Ingelheim sponsored RE-VERSE AD. Idarucizumab was given a fast-track status by the Food and Drug Administration, and BI submitted a new drug application in March 2015, according to the company.
Dr. Pollack reported receiving personal fees from BI, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
TORONTO – Idarucizumab is a promising agent that quickly and safely reverses the anticoagulant effects of dabigatran whether the goal is to control serious bleeding or to permit urgent surgery, according to interim results of a multicenter trial.
Idarucizumab is a monoclonal antibody that binds to dabigatran to reverse its activity. The data, presented by Dr. V. Charles Pollack Jr. at the International Society on Thrombosis and Haemostasis congress, involved the first 90 patients of an ongoing trial with a planned enrollment of 300. The data from this trial, called REVERSE-AD, were published online simultaneously with the June 22 presentation at the congress (N. Engl. J. Med 2015 [doi:10.1056/NEJMoa1502000]).
“Non–vitamin K antagonist oral anticoagulants (NOACs) are generally safer than warfarin, and provide similar or improved efficacy in the prevention of stroke in patients with nonvalvular atrial fibrillation and in the prevention and treatment of venous thromboembolism,” Dr. Pollack said in an interview. “Nonetheless, serious bleeding events may occur with NOAC use, and patients taking one of these agents occasionally require urgent surgery or other intervention for which normal hemostasis is required,” added Dr. Pollack, chair of the department of emergency medicine at Pennsylvania Hospital in Philadelphia.
In RE-VERSE AD (a study of the reversal effects of idarucizumab on active dabigatran), the first 90 patients were divided into two distinct groups. Group A, with 51 patients, included those on dabigatran with serious bleeding. Group B, with 39 patients, required reversal of dabigatran for urgent or emergent procedures. In both, idarucizumab provided a median maximum reversal of 100% (95% confidence interval, 100-100) of the anticoagulation effect within 4 hours.
Clotting assays were normalized almost immediately in almost 90% of patients, and the effect was durable, with 80% having measured dabigatran levels reflecting no significant anticoagulation 24 hours later, Dr. Pollack said.
“Clinical outcomes were quite good in this multimorbid patient population, with restoration of hemostasis as reported by local investigators achieved in less than 12 hours when assessable, and with 92% of surgical patients being reported as having normal hemostasis at the time of the procedure,” he said.
Idarucizumab was generally well tolerated in the patient population. “There were no serious adverse events related to the reversal agent ... and only one patient experienced a thrombotic complication within 72 hours, and that patient had not been restarted on any antithrombotic medications,” Dr. Pollack said.
“The study is ongoing,” he added, “but these interim results show rather convincingly that idarucizumab completely and safely reverses the anticoagulant effects of dabigatran within minutes.”
In addition, Dr. Pollack said the availability of a specific reversal agent for dabigatran would enhance its safety margin, and thus alleviate the fears of providers who may hesitate to use a NOAC because of the lack of an “antidote.”
“In fact, most such cases can already be successfully and safely managed with general support and ‘tincture of time’ (the half-life of dabigatran is much shorter than that of warfarin), but having a specific ‘go-to’ option could streamline the care of the most significantly compromised patients,” he said.
Dr. Pollack emphasized, however, that idarucizumab is a specific reversal agent for dabigatran, not an antidote. “To me, the latter would imply that idarucizumab immediately stops bleeding associated with active use of dabigatran,” he said.
Providers should realize that while idarucizumab seems capable of removing dabigatran-induced coagulopathy from the list of concerns when managing a patient with serious bleeding or before a “sharp” procedure, bleeding is a multifaceted issue that also may be due to traumatized blood vessels, other causes of coagulopathy such as liver disease, or concurrent use of antiplatelet medications, he said.
“The patient with a serious or life-threatening bleed on dabigatran will likely need additional care to investigate and manage such concerns,” Dr. Pollack said. “But at least idarucizumab can specifically, safely, and rapidly address the primary consideration.
“The safety of anticoagulation therapy with dabigatran is further enhanced with idarucizumab, a specific reversal agent that won’t need to be used often, but the availability of which would be reassuring to prescribers,” he concluded.
Boehringer Ingelheim sponsored RE-VERSE AD. Idarucizumab was given a fast-track status by the Food and Drug Administration, and BI submitted a new drug application in March 2015, according to the company.
Dr. Pollack reported receiving personal fees from BI, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
AT 2015 ISTH CONGRESS
Key clinical point: The investigational monoclonal antibody idarucizumab reversed the anticoagulant effects of dabigatran.
Major finding: Idarucizumab provided a median maximum dabigatran reversal of 100% (95% CI, 100-100) of the anticoagulation effect within 4 hours in an interim analysis.
Data source: RE-VERSE AD, a prospective cohort study in which 90 patients treated with dabigatran who had uncontrolled bleeding or required emergency surgery or procedures were given 5.0 g idarucizumab.
Disclosures: Boehringer Ingelheim sponsored RE-VERSE AD. Dr. Pollack reported receiving personal fees from Boehringer Ingelheim, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
ACIP Recommends MenB Vaccination for At-risk Groups
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
ACIP recommends MenB vaccination for at-risk groups
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
Most teens get health information online but prefer parents as primary source
The majority of teenagers in the United States rely on the Internet for health information, although parents remain their most common source of guidance, according to results published online from the Center on Media and Human Development at the Northwestern University School of Communication.
In a national survey of teens aged 13-18 years, 84% reported having obtained health information online and 21% said they have used health-related mobile apps, reported Ellen Wartella, Ph.D., of Northwestern University and her coauthors. Additionally, 32% of teens reported changing their behavior as a result of digital tools or health information found online.
Parents, however, remained the most common source of health information, with 55% of teens reporting that they receive “a lot” of information from parents, compared with health classes (32%), doctors and nurses (29%), and the Internet (25%).
Dr. Wartella and her colleagues called the findings “heartening,” because “even when it comes to sensitive health topics, teens are just as likely to want to speak with their parents as they are to want to look information up online.”
Additionally, the study “underscores the importance of making sure there is accurate, appropriate, and easily accessible health information available to teens online,” the authors concluded.
Read the full report here.
The majority of teenagers in the United States rely on the Internet for health information, although parents remain their most common source of guidance, according to results published online from the Center on Media and Human Development at the Northwestern University School of Communication.
In a national survey of teens aged 13-18 years, 84% reported having obtained health information online and 21% said they have used health-related mobile apps, reported Ellen Wartella, Ph.D., of Northwestern University and her coauthors. Additionally, 32% of teens reported changing their behavior as a result of digital tools or health information found online.
Parents, however, remained the most common source of health information, with 55% of teens reporting that they receive “a lot” of information from parents, compared with health classes (32%), doctors and nurses (29%), and the Internet (25%).
Dr. Wartella and her colleagues called the findings “heartening,” because “even when it comes to sensitive health topics, teens are just as likely to want to speak with their parents as they are to want to look information up online.”
Additionally, the study “underscores the importance of making sure there is accurate, appropriate, and easily accessible health information available to teens online,” the authors concluded.
Read the full report here.
The majority of teenagers in the United States rely on the Internet for health information, although parents remain their most common source of guidance, according to results published online from the Center on Media and Human Development at the Northwestern University School of Communication.
In a national survey of teens aged 13-18 years, 84% reported having obtained health information online and 21% said they have used health-related mobile apps, reported Ellen Wartella, Ph.D., of Northwestern University and her coauthors. Additionally, 32% of teens reported changing their behavior as a result of digital tools or health information found online.
Parents, however, remained the most common source of health information, with 55% of teens reporting that they receive “a lot” of information from parents, compared with health classes (32%), doctors and nurses (29%), and the Internet (25%).
Dr. Wartella and her colleagues called the findings “heartening,” because “even when it comes to sensitive health topics, teens are just as likely to want to speak with their parents as they are to want to look information up online.”
Additionally, the study “underscores the importance of making sure there is accurate, appropriate, and easily accessible health information available to teens online,” the authors concluded.
Read the full report here.
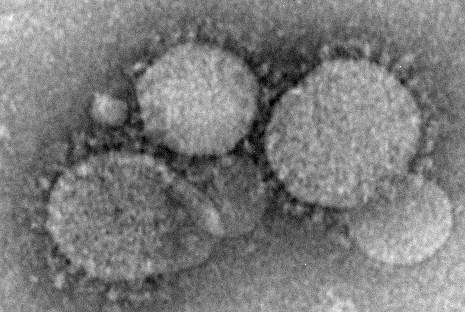
Prevention measures critical in avoiding global spread of MERS
Although currently considered an “endemic, low-level public health threat,” Middle East respiratory syndrome coronavirus (MERS-CoV) has the potential to mutate to have an increased capacity for human transmission, making preventive measures among health care workers crucial, reported Dr. Alimuddin Zumla and his colleagues from University College London, England.
As no drug treatment exists for the disease, which can range from mild to severe with acute respiratory distress syndrome and organ failure leading to death, clinicians are strongly urged to put several preventive measures in place to avoid its spread in health care facilities, the authors said. Recommended measures include droplet precautions such as wearing a surgical mask, using eye protection, and taking contact and respiratory precautions, Dr. Zumla and his colleagues said in the report.
MERS cases reported to the World Health Organization as of May 31, 2015, total 1,149, including 431 deaths, according to a Lancet press release.
The disease is believed to be spread through direct or indirect contact with dromedary camels, and most cases have been concentrated in Saudi Arabia, with added cases reported in Europe, the United States, and Asia in individuals who traveled from the Middle East, or their contacts, the authors said. The exact mechanism of transmission remains unknown.
“Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential,” they said.
Read the full article here.
Although currently considered an “endemic, low-level public health threat,” Middle East respiratory syndrome coronavirus (MERS-CoV) has the potential to mutate to have an increased capacity for human transmission, making preventive measures among health care workers crucial, reported Dr. Alimuddin Zumla and his colleagues from University College London, England.
As no drug treatment exists for the disease, which can range from mild to severe with acute respiratory distress syndrome and organ failure leading to death, clinicians are strongly urged to put several preventive measures in place to avoid its spread in health care facilities, the authors said. Recommended measures include droplet precautions such as wearing a surgical mask, using eye protection, and taking contact and respiratory precautions, Dr. Zumla and his colleagues said in the report.
MERS cases reported to the World Health Organization as of May 31, 2015, total 1,149, including 431 deaths, according to a Lancet press release.
The disease is believed to be spread through direct or indirect contact with dromedary camels, and most cases have been concentrated in Saudi Arabia, with added cases reported in Europe, the United States, and Asia in individuals who traveled from the Middle East, or their contacts, the authors said. The exact mechanism of transmission remains unknown.
“Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential,” they said.
Read the full article here.
Although currently considered an “endemic, low-level public health threat,” Middle East respiratory syndrome coronavirus (MERS-CoV) has the potential to mutate to have an increased capacity for human transmission, making preventive measures among health care workers crucial, reported Dr. Alimuddin Zumla and his colleagues from University College London, England.
As no drug treatment exists for the disease, which can range from mild to severe with acute respiratory distress syndrome and organ failure leading to death, clinicians are strongly urged to put several preventive measures in place to avoid its spread in health care facilities, the authors said. Recommended measures include droplet precautions such as wearing a surgical mask, using eye protection, and taking contact and respiratory precautions, Dr. Zumla and his colleagues said in the report.
MERS cases reported to the World Health Organization as of May 31, 2015, total 1,149, including 431 deaths, according to a Lancet press release.
The disease is believed to be spread through direct or indirect contact with dromedary camels, and most cases have been concentrated in Saudi Arabia, with added cases reported in Europe, the United States, and Asia in individuals who traveled from the Middle East, or their contacts, the authors said. The exact mechanism of transmission remains unknown.
“Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential,” they said.
Read the full article here.
Creativity and ambition linked in bipolar patients
Creativity is linked to ambition in patients with bipolar disorder, according to study results published in the Journal of Affective Disorders.
Sheri L. Johnson, Ph.D., and her colleagues in the department of psychology at the University of California, Berkeley, performed two studies: The first assessed accomplishment in 22 patients with bipolar disorder who self-identified as highly creative; the second study examined creative accomplishment and mania risk in 221 undergraduates.
The results from the first study showed that WASSUP scores, a measure of mania risk, were higher in highly creative bipolar patients (27.45) than in normative bipolar patients (14.10) and patients with no mood disorder (9.06). The second study found that mania risk and ambition were both linked to greater creativity, the authors reported.
“Findings across two studies strongly suggest that across the bipolar spectrum, ambition and creativity are linked,” Dr. Johnson and her associates wrote.
Read the full article in the Journal of Affective Disorders.
Creativity is linked to ambition in patients with bipolar disorder, according to study results published in the Journal of Affective Disorders.
Sheri L. Johnson, Ph.D., and her colleagues in the department of psychology at the University of California, Berkeley, performed two studies: The first assessed accomplishment in 22 patients with bipolar disorder who self-identified as highly creative; the second study examined creative accomplishment and mania risk in 221 undergraduates.
The results from the first study showed that WASSUP scores, a measure of mania risk, were higher in highly creative bipolar patients (27.45) than in normative bipolar patients (14.10) and patients with no mood disorder (9.06). The second study found that mania risk and ambition were both linked to greater creativity, the authors reported.
“Findings across two studies strongly suggest that across the bipolar spectrum, ambition and creativity are linked,” Dr. Johnson and her associates wrote.
Read the full article in the Journal of Affective Disorders.
Creativity is linked to ambition in patients with bipolar disorder, according to study results published in the Journal of Affective Disorders.
Sheri L. Johnson, Ph.D., and her colleagues in the department of psychology at the University of California, Berkeley, performed two studies: The first assessed accomplishment in 22 patients with bipolar disorder who self-identified as highly creative; the second study examined creative accomplishment and mania risk in 221 undergraduates.
The results from the first study showed that WASSUP scores, a measure of mania risk, were higher in highly creative bipolar patients (27.45) than in normative bipolar patients (14.10) and patients with no mood disorder (9.06). The second study found that mania risk and ambition were both linked to greater creativity, the authors reported.
“Findings across two studies strongly suggest that across the bipolar spectrum, ambition and creativity are linked,” Dr. Johnson and her associates wrote.
Read the full article in the Journal of Affective Disorders.
FROM JOURNAL OF AFFECTIVE DISORDERS
CDC investigating accidental anthrax shipment to labs
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
FDA approves Viberzi and Xifaxan for IBS
The Food and Drug Administration has approved Viberzi (eluxadoline) and Xifaxan (rifaximin) to treat irritable bowel syndrome with diarrhea (IBS-D) in adults, the agency announced May 27.
Viberzi is a locally active mixed mu-opioid–receptor agonist and delta-opioid–receptor antagonist that targets nervous system receptors to lessen bowel contractions. The medication is taken twice daily with food, the FDA said in a statement.
Phase III clinical trial results presented at the 2014 Digestive Disease Week showed that 31% of IBS-D patients who took Viberzi had improved abdominal pain and stool consistency at 26 weeks, compared with 19.5% of placebo patients. Xifaxan is an antibiotic originally approved as a therapy for travelers’ diarrhea caused by Escherichia coli, and for recurring overt hepatic encephalopathy. It is taken orally three times per day for 14 days, and can be re-administered up to two times in patients who experience symptom recurrence. Its efficacy was established in three clinical trials, including a phase III trial of 636 patients with recurrence which showed greater improvement of symptoms in patients taking Xifaxan than in those who received placebo, the FDA statement said.
Viberzi should not be taken by patients with a history of bile duct obstruction, pancreatitis, severe liver impairment, or severe constipation, or patients who drink more than three alcoholic beverages per day. Common side effects in patients treated with Viberzi were constipation, nausea, and abdominal pain.
In patients treated with Xifaxan for IBS-D, common side effects included nausea and an increase in the liver enzyme alanine aminotransferase (ALT).
Viberzi is manufactured by Patheon Pharmaceuticals. Xifaxan is marketed by Salix Pharmaceuticals, based in Raleigh, N.C.
For more safety information, please visit the FDA website.
The Food and Drug Administration has approved Viberzi (eluxadoline) and Xifaxan (rifaximin) to treat irritable bowel syndrome with diarrhea (IBS-D) in adults, the agency announced May 27.
Viberzi is a locally active mixed mu-opioid–receptor agonist and delta-opioid–receptor antagonist that targets nervous system receptors to lessen bowel contractions. The medication is taken twice daily with food, the FDA said in a statement.
Phase III clinical trial results presented at the 2014 Digestive Disease Week showed that 31% of IBS-D patients who took Viberzi had improved abdominal pain and stool consistency at 26 weeks, compared with 19.5% of placebo patients. Xifaxan is an antibiotic originally approved as a therapy for travelers’ diarrhea caused by Escherichia coli, and for recurring overt hepatic encephalopathy. It is taken orally three times per day for 14 days, and can be re-administered up to two times in patients who experience symptom recurrence. Its efficacy was established in three clinical trials, including a phase III trial of 636 patients with recurrence which showed greater improvement of symptoms in patients taking Xifaxan than in those who received placebo, the FDA statement said.
Viberzi should not be taken by patients with a history of bile duct obstruction, pancreatitis, severe liver impairment, or severe constipation, or patients who drink more than three alcoholic beverages per day. Common side effects in patients treated with Viberzi were constipation, nausea, and abdominal pain.
In patients treated with Xifaxan for IBS-D, common side effects included nausea and an increase in the liver enzyme alanine aminotransferase (ALT).
Viberzi is manufactured by Patheon Pharmaceuticals. Xifaxan is marketed by Salix Pharmaceuticals, based in Raleigh, N.C.
For more safety information, please visit the FDA website.
The Food and Drug Administration has approved Viberzi (eluxadoline) and Xifaxan (rifaximin) to treat irritable bowel syndrome with diarrhea (IBS-D) in adults, the agency announced May 27.
Viberzi is a locally active mixed mu-opioid–receptor agonist and delta-opioid–receptor antagonist that targets nervous system receptors to lessen bowel contractions. The medication is taken twice daily with food, the FDA said in a statement.
Phase III clinical trial results presented at the 2014 Digestive Disease Week showed that 31% of IBS-D patients who took Viberzi had improved abdominal pain and stool consistency at 26 weeks, compared with 19.5% of placebo patients. Xifaxan is an antibiotic originally approved as a therapy for travelers’ diarrhea caused by Escherichia coli, and for recurring overt hepatic encephalopathy. It is taken orally three times per day for 14 days, and can be re-administered up to two times in patients who experience symptom recurrence. Its efficacy was established in three clinical trials, including a phase III trial of 636 patients with recurrence which showed greater improvement of symptoms in patients taking Xifaxan than in those who received placebo, the FDA statement said.
Viberzi should not be taken by patients with a history of bile duct obstruction, pancreatitis, severe liver impairment, or severe constipation, or patients who drink more than three alcoholic beverages per day. Common side effects in patients treated with Viberzi were constipation, nausea, and abdominal pain.
In patients treated with Xifaxan for IBS-D, common side effects included nausea and an increase in the liver enzyme alanine aminotransferase (ALT).
Viberzi is manufactured by Patheon Pharmaceuticals. Xifaxan is marketed by Salix Pharmaceuticals, based in Raleigh, N.C.
For more safety information, please visit the FDA website.
European Medicines Agency recommends evolocumab for cholesterol control
The European Medicines Agency (EMA) has recommended the use of evolocumab (Repatha) to treat high cholesterol in patients who do not respond to statin therapy, the agency announced in a statement.
Evolocumab is a monoclonal antibody that blocks proprotein convertase subtilisin/kexin type 9 (PCSK9); it is the first drug in this class available for lowering cholesterol.
In clinical trials of more than 5,000 patients, Repatha lowered LDL cholesterol both in patients with hypercholesterolemia and mixed dyslipidemia, and in patients with homozygous familial hypercholesterolemia, the agency said in its announcement.
Read more information on the EMA website.
The European Medicines Agency (EMA) has recommended the use of evolocumab (Repatha) to treat high cholesterol in patients who do not respond to statin therapy, the agency announced in a statement.
Evolocumab is a monoclonal antibody that blocks proprotein convertase subtilisin/kexin type 9 (PCSK9); it is the first drug in this class available for lowering cholesterol.
In clinical trials of more than 5,000 patients, Repatha lowered LDL cholesterol both in patients with hypercholesterolemia and mixed dyslipidemia, and in patients with homozygous familial hypercholesterolemia, the agency said in its announcement.
Read more information on the EMA website.
The European Medicines Agency (EMA) has recommended the use of evolocumab (Repatha) to treat high cholesterol in patients who do not respond to statin therapy, the agency announced in a statement.
Evolocumab is a monoclonal antibody that blocks proprotein convertase subtilisin/kexin type 9 (PCSK9); it is the first drug in this class available for lowering cholesterol.
In clinical trials of more than 5,000 patients, Repatha lowered LDL cholesterol both in patients with hypercholesterolemia and mixed dyslipidemia, and in patients with homozygous familial hypercholesterolemia, the agency said in its announcement.
Read more information on the EMA website.