User login
Luteal phase deficiency: What we now know
- Luteal phase deficiency (LPD), defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids.
- Wide variation in the reported incidence of LPD—3.7% to 20% in infertile women—reflects lack of agreement about its diagnostic criteria.
- Histologic dating of an endometrial sample is the gold standard for evaluation of the corpus luteum.
- Two main treatment strategies have been suggested: improving follicular dynamics using follicle-maturing drugs such as clomiphene, and use of supplemental progesterone during the luteal phase and first trimester of pregnancy.
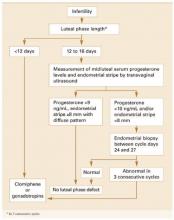
Despite scanty and controversial supporting evidence, evaluation of patients with infertility or recurrent pregnancy loss for possible luteal phase deficiency (LPD) is firmly established in clinical practice. In this article, we examine the data and offer our perspective on the role of LPD in assessing and managing couples with reproductive disorders (FIGURE 1).
FIGURE 1 Diagnosis and treatment of luteal phase deficiency
Many areas of controversy
Although observational and retrospective studies have reported a higher incidence of LPD in women with infertility and recurrent pregnancy losses than in fertile controls,1-4 no prospective study has confirmed these findings. Furthermore, studies have failed to confirm the superiority of any particular therapy.
Once considered an important cause of infertility, LPD has become the subject of debate, with some experts questioning its very existence. Unclear terminology describing this disorder is part of the problem, making it difficult to definitively diagnose the deficiency or determine its incidence. Further, while reasonable consensus exists that endometrial biopsy is the most reliable diagnostic tool, concerns remain about its timing, repetition, and interpretation.
A defect of corpus luteum progesterone output?
LPD is defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, based on the woman’s next menses. It was first described by Jones in 1949.5 One year later, Noyes et al6 published criteria on endometrial dating that became the gold standard for LPD diagnosis.
Pathophysiology. LPD may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids (TABLE). Most experts believe LPD is a defect of corpus luteum progesterone output—both in amount and duration—resulting in inadequate stimulation of the endometrium for implantation of the blastocyst (FIGURE 2).5 Thus, the endometrial histologic pattern is an important bioassay of the corpus luteum steroidogenic function.
Normal embryonic implantation depends on a properly functioning luteal phase, which, in turn, requires optimal secretion of follicle-stimulating hormone (FSH) and adequate follicular development during the follicular phase. Other requirements are a satisfactory luteinizing hormone (LH) surge during ovulation and continuous tonic LH pulses during the luteal phase of the cycle.
LH secretion from the pituitary occurs in a pulsatile fashion,7 which is essential for corpus luteum function.8 During the follicular phase the pulse frequency is high, occurring at a rate of approximately 1 pulse per 90 minutes. However, during the luteal phase and under the influence of progesterone, the pulse frequency is significantly diminished, occurring approximately every 3 to 6 hours, depending on the age of the corpus luteum.7,9 The corpus luteum is unresponsive to LH pulses during the early luteal phase; sensitivity develops about 4 to 6 days after ovulation.7,9
The luteal phase thus involves creation of an optimal hormonal environment as well as adequate endometrial transformation. Alteration of any of the factors that contribute to a normally functioning corpus luteum may thus deleteriously affect the endometrium and embryonic implantation.
Epidemiology. The reported prevalence of LPD ranges from 3.7% to 20% among patients with infertility.10,11 When an out-of-phase endometrium is the diagnostic criterion, prevalence estimates range from 3.5% to 31%.12,13 A short luteal phase is thought to occur in 5% of ovulatory cycles.14Some reports suggest that LPD accounts for 25% to 40% of recurrent pregnancy losses.15
The wide variation in reported incidence of LPD reflects the lack of agreement about its definition and diagnostic criteria. Further, some studies evaluating prevalence have not concurrently tested controls—an important omission since endometrial histology suggestive of LPD occurs in up to 50% of single menstrual cycles and 25% of sequential cycles.16 Thus, the true incidence of the defect may never be known.
TABLE
Etiology of luteal phase deficiency
| FOLLICULAR PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic ovarian defects |
|
| Intrinsic endometrial defects |
|
| LUTEAL PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects |
|
| Intrinsic secretory endometrial defect |
|
| LUTEAL RESCUE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects in early pregnancy |
|
| Intrinsic endometrial defects |
| FSH = follicle-stimulating hormone, hCG = human chorionic gonadotropin, |
| LH = luteinizing hormone |
Seeking a reliable diagnostic tool
Although significant progress has been made in recent years, LPD diagnosis is neither straightforward nor completely accurate. Approaches include measuring the luteal phase duration, taking basal body temperature (BBT), and assessing single or multiple serum progesterone levels, as well as using sonographic imaging and endometrial biopsy.
Luteal phase duration. An abnormally short luteal phase—defined as less than 10 days17,18—occurs in approximately 5% of ovulatory cycles.19 Research has shown such cycles to have low peak serum progesterone levels, suggestive of poor corpus luteum function.18 The relationship between an abnormally short luteal phase and infertility is unclear, however. Smith and colleagues,20 for example, evaluated women with known fertility and women with unexplained infertility and found the prevalence of a short luteal phase to be the same in both groups.
Our own practice is to measure luteal phase parameters in infertile patients. When the luteal phase is shorter than 12 days, we usually treat it.
Basal body temperature. A rise in BBT occurs when progesterone production increases at midcycle. A rise of approximately 2.5 ng/mL of progesterone will result in a temperature elevation of nearly 1°F. Interpretation of the BBT is based on this thermogenic shift. Unfortunately, although BBT may be a sensitive indicator of ovulation, it is a poor indicator of the quality of the luteal phase. Neither the rate nor the magnitude of rise of the postovulatory temperature curve correlates with endometrial histology. Overall correlation varies between 25%21 and 81%.22 Further, an abnormal BBT may occur in 12% of women with normal endometrial histologic dating.23 Because of this lack of specificity and sensitivity, BBT is not an adequate diagnostic tool.
Measuring progesterone and its metabolites. Because progesterone is the principal product of the corpus luteum, its measurement is clinically indicated to evaluate luteal phase abnormalities. For this reason, serum, urine, and salivary progesterone determinations are utilized.
Histologic dating of an endometrial biopsy is considered the gold standard for corpus luteum evaluation.
Although serum progesterone is widely used in the diagnosis of LPD, there is no agreement in the cutoff level for abnormal assays, the number of assays required for diagnosis, or the timing of the test. A number of studies have demonstrated lower progesterone levels in women with “out-of-phase” endometrial biopsies,24,25 but others have noted normal progesterone levels in the presence of abnormal biopsies.26-28
These contradictory findings may be explained by the episodic release of progesterone in response to the slow pulsing of LH during the luteal phase of the cycle. Consequently, there are wide and frequent fluctuations and diurnal variations in progesterone secretion. This makes the use of a single serum progesterone determination—or even a series of single serum measurements—unreliable.
- No standard for ‘normal’ progesterone levels.
- A range of sampling intervals proposed. To reduce the false-positive rate of a single measurement, Wuttke et al15 suggested 2 or 3 blood samples within 3 hours, since low progesterone levels are often observed prior to the occurrence of an LH pulse. Thus, the probability is high that within 3 consecutive hours an LH episode will have stimulated luteal progesterone secretion into the normal range. This approach has not been evaluated clinically. Moreover, it is likely to be time-consuming and inconvenient for the patient.
Biopsy: The ‘gold standard’
Histologic dating of an endometrial biopsy is the gold standard for corpus luteum evaluation because it assesses both quantitative progesterone secretion and the morphologic transformation of the endometrium in preparation for embryo implantation.
The histologic features characteristic of specific days of the menstrual cycle—first described by Noyes and colleagues in 19506—have remained the cornerstone of endometrial dating. The endometrium is considered out of phase when the histologic and chronological dating differ by 3 or more days, provided this difference is present in 2 or more successive cycles. Using these criteria, Noyes and Haman35 showed endometrial biopsy to be accurate, with an interobserver agreement rate of 82% within the 2-day range. Hence, the 3-day out-of-phase criterion.
Variations in results. Recently, the accuracy and reproducibility of endometrial histology in the diagnosis of LPD have been questioned because of considerable intraobserver and interobserver variation in sample readings, as well as variation between cycles of the same patient and timing of the biopsy. Evaluation also can depend on which section of the endometrium is sampled.36 Gibson and colleagues36 showed that 65% of the observed variability in endometrium dating was due to inconsistencies between evaluators, 27% was due to lack of concordance by the same evaluator, and 8% was due to regional differences in the uterus.
In view of these variations, the theoretical probability of changing clinical management is 15% to 28% after the same evaluator reviews a slide37 and 22% to 39% after another observer evaluates the same slide.38
Refresher training fails to improve accuracy. To increase the accuracy and interobserver reproducibility of endometrial dating, Duggan and colleagues39 offered refresher training in histologic criteria after initial endometrial dating. However, they found no improvement in accuracy or inter-observer reproducibility after this training.
Timing of the endometrial biopsy also is important.40 Traditionally, endometrial biopsies have been performed a few days before the presumed onset of menstruation to reflect the maximal influence of progesterone on the endometrium. This practice recently has been questioned, with some researchers favoring biopsies performed in the midluteal phase.40,41
The method of determining the date of ovulation also varies considerably. Traditionally, next-menstrual-period dating is used, whereby the day of menses after the biopsy is labeled day 28 and presumed to have occurred 14 days after ovulation. The use of urine LH to determine the preovulatory LH surge and—15 days later—menstruation, has been suggested as a more precise method.42
Significant intercycle variation may occur within the same individual. Li and colleagues43 reported that within-subject, between-cycle variation of more than 2 days occurred in about 41% of patients.
Uncertain link to infertility. The link between an abnormal biopsy and infertility is questionable. Previous studies have documented abnormal endometrial biopsy results in the range of 31% to 35% in fertile women13,44 —rates almost comparable to those of women with infertility. Earlier investigators also demonstrated no significant differences in pregnancy rates between infertile couples with normal biopsy results, compared with those with abnormal findings.45
Before we consider it clinically significant, we require any LPD to be present in repeated cycles.
Clinical recommendations. Before we consider it clinically significant, we require any LPD to be present in repeated cycles, since approximately 20% to 80% of women with an abnormal biopsy have an “in-phase” endometrium on a repeat biopsy.13,46
The dating of endometrial biopsies should only be done by experienced histopathologists. We perform endometrial biopsies after cycle day 24, and confirm any out-of-phase abnormality in 2 consecutive biopsies. Further, before we diagnose the endometrium as “out of phase,” histologic dating must lag by at least 3 days.
In our practice, all endometrial biopsies are read by the senior author, who has had extensive experience with Noyes’ criteria for endometrial dating.
Is there a proven treatment?
Not surprisingly—given the disagreement about its incidence and diagnosis—LPD treatment also is controversial. Several case series, observational trials, and retrospective studies have explored whether treatment improves the chances of conception, but few randomized trials have taken up the issue.
Two main strategies have been suggested:
- Improving follicular dynamics using drugs such as clomiphene or gonadotropins, which not only produce a follicle but increase progesterone secretion during the luteal phase.
- Administering supplemental progesterone during the luteal phase and first trimester of pregnancy.
Clomiphene and other follicle-maturing drugs. Clomiphene increases FSH secretion and induces development of multiple or larger follicles. As a result, LH receptivity is enhanced. In addition, higher estrogen concentrations in the follicular phase increase steroid receptor content in the endometrium.
Although some studies support the use of clomiphene to treat LPD, in certain cases the drug may actually induce luteal phase defects in 30% to 50% of cycles.47 By its anti-estrogenic activity, clomiphene may suppress progesterone receptor levels, rendering the endometrium out of phase and less responsive to progesterone.
Supplemental progesterone use is supported by research from Lassey and colleagues,48 who demonstrated an association between LPD and abnormal expression of alpha-v-beta-3-integrin, the biomarker of uterine receptivity, which is likely responsible for the window of implantation. The endometrium of women with delayed maturation—that is, LPD—fails to express the alpha-v-beta-3-integrin when biopsied during the window of implantation (days 20 and 21). When given supplemental progesterone, the vast majority of these patients return to normal histologic status and alpha-v-beta-3-integrin expression.48,49
Progesterone versus clomiphene. Clinical trials have not established whether progesterone treatment is more effective than treatment with clomiphene or gonadotropin or no treatment. Problems with design and methodology in trials that do exist make it impossible to draw any firm conclusions from reports published thus far.
The literature includes numerous descriptive studies, most of which involved assessment of pregnancy rates before and after treatment.50 However, pregnancies that occur after therapy do not necessarily imply treatment benefit, but may reflect natural intercycle or biologic variation or spontaneous conception independent of treatment.
Randomized trials. Most of the studies published to date are case series and observational studies, which are subject to methodologic bias. To the best of our knowledge, only a few randomized studies of treatment efficacy have been published.51-53 All included only a few patients and lacked adequate power to detect any differences.50
Pregnancies that occur after therapy do not necessarily imply treatment benefit, but may reflect natural intercycle variation.
For example, Balasch and colleagues52 evaluated treatment with vaginal progesterone suppositories, dehydrogesterone, and no treatment. Although there was a higher pregnancy rate in the treatment groups, it was not statistically significant. However, the power of this trial to detect any differences in pregnancy rates was very low.
Investigating recurrent pregnancy loss. We also lack properly designed studies assessing the role of luteal phase support in women with recurrent miscarriages due to LPD. Two meta-analyses reached different conclusions on the role of progesterone supplementation in patients with recurrent miscarriages.34,54 Studies included in these analyses used various inclusion criteria. Moreover, diagnosis was not based on currently accepted criteria, and the treatment of choice was either 17-OH progesterone or medroxyprogesterone. The trials also included patients at more than 8 weeks’ gestation.
One multicenter randomized study evaluating luteal support with human chorionic gonadotropin (hCG) or placebo found no significant difference in pregnancy rates.55
Targeting the underlying cause. Steroidogenic cells consist of 2 main types: large and small luteal cells. Large luteal cells derive from follicular granulosa cells and secrete autocrine-acting and paracrine-acting peptides and eicosanoids but are not LH-receptive.56 They ensure basal progesterone and estradiol production from the corpus luteum. Small luteal cells derive from follicular thecal cells and acquire LH receptivity; thus, they respond to regularly occurring LH pulses, leading to increased progesterone and estradiol production.15
Only expert histopathologists or reproductive endocrinologists with special training should evaluate samples.
In a series of excellent experiments, Wuttke and colleagues15 studied corpus luteum function by measuring serial LH, progesterone, and estradiol levels. They suggested that, if the underlying cause of LPD can be determined, therapy can be appropriately directed, depending on whether the deficiency is hypothalamic LPD or a defect of small luteal cells or large luteal cells.
In hypothalamic LPD, LH pulses are absent and the hypothalamic gonadotropin-releasing hormone pulse generator appears to be oversuppressed, even though serum progesterone levels are lower than normal. However, during the follicular phase, the pulse generator functions normally.15
When there is a defect of small luteal cells, the corpus luteum responds poorly to normal LH pulses during the luteal phase, resulting in subnormal basal progesterone. Wuttke et al postulated that a defect of small luteal cells occurs because the cells do not differentiate well or fail to acquire LH receptivity.15
A defect of large luteal cells—which ensure the release of basal LH and unstimulated progesterone—occurred in 21% of cases studied. In these cases there was normal corpus luteum response to normal LH pulses, but progesterone secretion decreased to very low levels between LH pulses.15 The follicular-phase LH pulsatility, follicular development, and serum estradiol were all normal.15
Theoretically, stimulation of luteal progesterone secretion by hCG administration should be an effective therapy for hypothalamic LPD as well as a defect of large luteal cells. If the defect involves the small luteal cells, however, hCG therapy will be ineffective, since the corpus luteum is unresponsive to gonadotropins.15 In such patients, progesterone may be the treatment of choice. This may explain, at least in part, the differences in outcomes in the literature, since the underlying causative category has not been identified.
A well-designed, multicenter trial is essential to address remaining questions about LPD, not to mention a clear and uniform definition.
Clinical management
For those who believe, as we do, that LPD is a real and potential cause of reproductive disorders, we recommend that:
- its diagnosis be standardized,
- only expert histopathologists or reproductive endocrinologists with special training and skills in endometrial dating evaluate histologic samples, and
- once a “proper” diagnosis is made, treatment be multifold and as minimally invasive as possible.
Our practice generally is to give patients gonadotropins or clomiphene to improve folliculogenesis and corpus luteum function, and to induce multiple ovulations, which increase serum progesterone levels as well as the chance of pregnancy.
In patients at high risk for multiple gestation, progesterone supplementation as oral tablets or vaginal cream is relatively safe, inexpensive, and well tolerated. There is no need to monitor effects of progesterone treatment, since this natural substance has no known side effects or teratogenicity.
Dr. Engman reports no financial relationship with any companies whose products are mentioned in this article. Dr. Luciano reports that he serves on the speakers bureau and as a consultant for Eli Lilly, and receives research grants from Chitogenics, ML Laboratories, Proctor & Gamble, and Tap Pharmaceuticals.
1. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24-29.
2. Rosemberg SM, Luciano AA, Riddick DH. The luteal phase defect: the relative frequency of, and encouraging response to treatment with vaginal progesterone. Fertil Steril. 1980;34:17-20.
3. Davidson BJ, Thrasher TV, Seraj IM. An analysis of endometrial biopsies performed for infertility. Fertil Steril. 1987;48:770-774.
4. Li TC, Dockery P, Cook ID. Endometrial development in the luteal phase of women with various types of infertility: comparison with women of normal fertility. Hum Reprod. 1991;6:325-330.
5. Jones GS. Some newer aspects of management of infertility. JAMA. 1949;141:1123-1129.
6. Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3-25.
7. Jones GS, Pourmand K. An evaluation of etiologic factors and therapy in 555 private patients with primary infertility. Fertil Steril. 1962;13:398-410.
8. Nash LD. The treatment of luteal phase dysfunction with clomiphene citrate and human chorionic gonadotropin. Infertility. 1982;5:87-104.
9. McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: a critical review. Fertil Steril. 1988;50:1-15.
10. Balasch J, Vanrell JA. Luteal phase deficiency: an inadequate endometrial response to normal hormonal stimulation. Intern J Fertil. 1986;31:368-371.
11. Olive DL. The prevalence and epidemiology of luteal phase deficiency in normal and infertile women. Clin Obstet Gynecol. 1991;34:157-166.
12. Andrews WC. Luteal phase defects. Fertil Steril. 1979;32:501.-
13. Davis OK, Berkeley AS, Naus GJ, Cholst IN, Freeman KS. The incidence of luteal phase defect in normal, fertile women, determined by endometrial biopsies. Fertil Steril. 1989;51:582-586.
14. Filicori M, Butler JP, Crowley WF, Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638-1647.
15. Wuttke W, Pitzel L, Seidlova-Wuttke D, Hinney B. LH pulses and the corpus luteum: the luteal phase deficiency (LPD). Vitam Horm. 2001;63:131-158.
16. Hinney B, Henze C, Wuttke W. Regulation of luteal function by luteinizing hormone and prolactin at different times of the luteal phase. Eur J Endocrinol. 1995;133:701-717.
17. Sherman BM, Korenman SG. Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. J Clin Endocrinol Metab. 1974;38:89.-
18. Strott CA, Cargille CM, Ross GT, Lipsett MD. The short luteal phase. J Clin Endocrinol Metab. 1970;30:246.-
19. Lenton EA, Landgren BM, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol. 1984;91:685-689.
20. Smith SK, Lenton EA, Landgren BM, Cooke ID. The short luteal phase and infertility. Br J Obstet Gynaecol. 1984;91:1120-1122.
21. Downs KA, Gibson M. Basal body temperature graph and the luteal phase defect. Fertil Steril. 1983;40:466-468.
22. Soules MR, Wiebe RH, Aksel S, Hammond CB. The diagnosis and therapy of luteal phase deficiency. Fertil Steril. 1977;28:1033.-
23. Johansson EAB, Larsson-Cohn U, Gemzell C. Monophasic basal body temperature in ovulatory menstrual cycles. Am J Obstet Gynecol. 1972;113:933.-
24. Cummings DC, Honore LH, Scott JC, Williams KP. The late luteal phase in infertile women: comparison of simultaneous endometrial biopsy and progesterone levels. Fertil Steril. 1985;43:715-719.
25. Daya S, Ward S. Diagnostic test properties of serum progesterone in the evaluation of luteal phase defects. Fertil Steril. 1988;49:168-170.
26. Batista MC, Carteledge TP, Merino MJ, et al. Midluteal phase endometrial biopsy does not accurately predict luteal function. Fertil Steril. 1993;59:294-300.
27. Rosenfeld DL, Garcia CR. A comparison of endometrial histology with simultaneous plasma progesterone determinations in infertile women. Fertil Steril. 1976;27:1256-1261.
28. Shangold M, Berkeley A, Gray J. Both midluteal serum progesterone levels and late luteal endometrial histology should be assessed in all infertile women. Fertil Steril. 1983;40:627-630.
29. Hensleigh PA, Fainstat T. Corpus luteum dysfunction: serum progesterone levels in diagnosis and assessment of therapy for recurrent and threatened abortion. Fertil Steril. 1979;32:396-400.
30. Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94:89-98.
31. Hull MGR, Savage PE, Bromham DR, et al. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle (“ovulation”) derived from treated and untreated conception cycles. Fertil Steril. 1982;37:355-360.
32. Abraham GE, Maroulis GB, Marshall JR. Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet Gynecol. 1974;44:522-525.
33. Wu CH, Minassian SS. The integrated luteal progesterone: an assessment of luteal function. Fertil Steril. 1987;48:937-940.
34. Daya S. Efficacy of progesterone support for pregnancy in women with recurrent miscarriage. A meta-analysis of controlled trials. Br J Obstet Gynaecol. 1989;96:275-280.
35. Noyes RW, Haman JO. Accuracy of endometrial dating. Fertil Steril. 1953;4:504.-
36. Gibson M, Badger GJ, Byrn F, Lee KR, Korson R, Trainer TD. Error in histologic dating of secretory endometrium: variance component analysis. Fertil Steril. 1991;56:242-247.
37. Scott RT, Snyder RR, Bagnall JW, Reed KD, Adair CF, Hensley SD. Evaluation of the impact of intraobserver variability on endometrial dating and the diagnosis of luteal phase defects. Fertil Steril. 1993;60:652-657.
38. Scott RT, Snyder RR, Strickland DM, et al. The effect of interobserver variation in dating endometrial histology on the diagnosis of luteal phase defects. Fertil Steril. 1988;50:888-892.
39. Duggan MA, Brashert P, Ostor A, et al. The accuracy and interobserver reproducibility of endometrial dating. Pathology. 2001;33:292-297.
40. Castelbaum AJ, Wheeler J, Coutifaris CB, Mastroianni L, Jr, Lessey BA. Timing of the endometrial biopsy may be critical for the accurate diagnosis of luteal phase deficiency. Fertil Steril. 1994;61:443-447.
41. Johannisson E, Landgren BM, Rohr HP, Diczfalusy E. Endometrial morphology and peripheral hormone levels in women with regular menstrual cycles. Fertil Steril. 1987;48:401-408.
42. Li TC, Rogers AW, Lenton EA, et al. A comparison between two methods of chronological dating of human endometrial biopsies during the luteal phase and their correlation with histologic dating. Fertil Steril. 1987;48:928-932.
43. Li TC, Dockery P, Rogers AW, Cooke ID. How precise is histologic dating of endometrium using the standard dating criteria? Fertil Steril. 1989;51:759-763.
44. Shoupe D, Mishell DR, Jr, Lacarra M, et al. Correlation of endometrial maturation with four methods of estimating day of ovulation. Obstet Gynecol. 1989;73:88-92.
45. Driessen F, Holwerda PJ, vd Putte SC, Kremer J. The significance of dating on endometrial biopsy for the prognosis of the infertile couple. Int J Fertil. 1980;25:112-116.
46. Wentz AC. Endometrial biopsy in the evaluation of infertility. Fertil Steril. 1980;33:121-124.
47. Keenan JW, Herbert CM, Bush JR, Wentz AC. Diagnosis and management of out-of-phase endometrial biopsies among patients receiving clomiphene citrate for ovulation induction. Fertil Steril. 1989;51:964.-
48. Lassey BA, Castlebaum AJ, Sawin SJ, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63:535-542.
49. Lassey BA, Damjanovich L, Coutifaris C, et al. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188-195.
50. Karamardian LM, Grimes DA. Luteal phase deficiency: effect of treatment on pregnancy rates. Am J Obstet Gynecol. 1992;167:1391-1398.
51. Echt CR, Romberger FT, Goodman JA. Clomiphene citrate in the treatment of luteal phase defects. Fertil Steril. 1969;20:564-571.
52. Balasch J, Vanrell JA, Marquez M, Burzaco I, Gonzalez-Merlo J. Dehydrogesterone versus vaginal progesterone in the treatment of the endometrial luteal phase deficiency. Fertil Steril. 1982;37:751-754.
53. Huang KE. The primary treatment of luteal phase inadequacy: progesterone versus clomiphene citrate. Am J Obstet Gynecol. 1986;155:824-828.
54. Goldstein P, Berrier J, Rosen S, Sacks HS, Chalmers TC. A meta-analysis of randomized control trials of progestational agents in pregnancy. Br J Obstet Gynaecol. 1989;96:265-274.
55. Harrison RF. Human chorionic gonadotrophin (hCG) in the management of recurrent abortion; results of a multicentre placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 1992;47:175-179.
56. Ohara A, Mori T, Taii S, Ban C, Narimoto K. Functional differentiation in steroidogenesis of two types of luteal cells isolated from mature human corpora lutea of menstrual cycle. J Clin Endocrinol Metab. 1987;65:1192-1200.
- Luteal phase deficiency (LPD), defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids.
- Wide variation in the reported incidence of LPD—3.7% to 20% in infertile women—reflects lack of agreement about its diagnostic criteria.
- Histologic dating of an endometrial sample is the gold standard for evaluation of the corpus luteum.
- Two main treatment strategies have been suggested: improving follicular dynamics using follicle-maturing drugs such as clomiphene, and use of supplemental progesterone during the luteal phase and first trimester of pregnancy.
Despite scanty and controversial supporting evidence, evaluation of patients with infertility or recurrent pregnancy loss for possible luteal phase deficiency (LPD) is firmly established in clinical practice. In this article, we examine the data and offer our perspective on the role of LPD in assessing and managing couples with reproductive disorders (FIGURE 1).
FIGURE 1 Diagnosis and treatment of luteal phase deficiency
Many areas of controversy
Although observational and retrospective studies have reported a higher incidence of LPD in women with infertility and recurrent pregnancy losses than in fertile controls,1-4 no prospective study has confirmed these findings. Furthermore, studies have failed to confirm the superiority of any particular therapy.
Once considered an important cause of infertility, LPD has become the subject of debate, with some experts questioning its very existence. Unclear terminology describing this disorder is part of the problem, making it difficult to definitively diagnose the deficiency or determine its incidence. Further, while reasonable consensus exists that endometrial biopsy is the most reliable diagnostic tool, concerns remain about its timing, repetition, and interpretation.
A defect of corpus luteum progesterone output?
LPD is defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, based on the woman’s next menses. It was first described by Jones in 1949.5 One year later, Noyes et al6 published criteria on endometrial dating that became the gold standard for LPD diagnosis.
Pathophysiology. LPD may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids (TABLE). Most experts believe LPD is a defect of corpus luteum progesterone output—both in amount and duration—resulting in inadequate stimulation of the endometrium for implantation of the blastocyst (FIGURE 2).5 Thus, the endometrial histologic pattern is an important bioassay of the corpus luteum steroidogenic function.
Normal embryonic implantation depends on a properly functioning luteal phase, which, in turn, requires optimal secretion of follicle-stimulating hormone (FSH) and adequate follicular development during the follicular phase. Other requirements are a satisfactory luteinizing hormone (LH) surge during ovulation and continuous tonic LH pulses during the luteal phase of the cycle.
LH secretion from the pituitary occurs in a pulsatile fashion,7 which is essential for corpus luteum function.8 During the follicular phase the pulse frequency is high, occurring at a rate of approximately 1 pulse per 90 minutes. However, during the luteal phase and under the influence of progesterone, the pulse frequency is significantly diminished, occurring approximately every 3 to 6 hours, depending on the age of the corpus luteum.7,9 The corpus luteum is unresponsive to LH pulses during the early luteal phase; sensitivity develops about 4 to 6 days after ovulation.7,9
The luteal phase thus involves creation of an optimal hormonal environment as well as adequate endometrial transformation. Alteration of any of the factors that contribute to a normally functioning corpus luteum may thus deleteriously affect the endometrium and embryonic implantation.
Epidemiology. The reported prevalence of LPD ranges from 3.7% to 20% among patients with infertility.10,11 When an out-of-phase endometrium is the diagnostic criterion, prevalence estimates range from 3.5% to 31%.12,13 A short luteal phase is thought to occur in 5% of ovulatory cycles.14Some reports suggest that LPD accounts for 25% to 40% of recurrent pregnancy losses.15
The wide variation in reported incidence of LPD reflects the lack of agreement about its definition and diagnostic criteria. Further, some studies evaluating prevalence have not concurrently tested controls—an important omission since endometrial histology suggestive of LPD occurs in up to 50% of single menstrual cycles and 25% of sequential cycles.16 Thus, the true incidence of the defect may never be known.
TABLE
Etiology of luteal phase deficiency
| FOLLICULAR PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic ovarian defects |
|
| Intrinsic endometrial defects |
|
| LUTEAL PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects |
|
| Intrinsic secretory endometrial defect |
|
| LUTEAL RESCUE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects in early pregnancy |
|
| Intrinsic endometrial defects |
| FSH = follicle-stimulating hormone, hCG = human chorionic gonadotropin, |
| LH = luteinizing hormone |
Seeking a reliable diagnostic tool
Although significant progress has been made in recent years, LPD diagnosis is neither straightforward nor completely accurate. Approaches include measuring the luteal phase duration, taking basal body temperature (BBT), and assessing single or multiple serum progesterone levels, as well as using sonographic imaging and endometrial biopsy.
Luteal phase duration. An abnormally short luteal phase—defined as less than 10 days17,18—occurs in approximately 5% of ovulatory cycles.19 Research has shown such cycles to have low peak serum progesterone levels, suggestive of poor corpus luteum function.18 The relationship between an abnormally short luteal phase and infertility is unclear, however. Smith and colleagues,20 for example, evaluated women with known fertility and women with unexplained infertility and found the prevalence of a short luteal phase to be the same in both groups.
Our own practice is to measure luteal phase parameters in infertile patients. When the luteal phase is shorter than 12 days, we usually treat it.
Basal body temperature. A rise in BBT occurs when progesterone production increases at midcycle. A rise of approximately 2.5 ng/mL of progesterone will result in a temperature elevation of nearly 1°F. Interpretation of the BBT is based on this thermogenic shift. Unfortunately, although BBT may be a sensitive indicator of ovulation, it is a poor indicator of the quality of the luteal phase. Neither the rate nor the magnitude of rise of the postovulatory temperature curve correlates with endometrial histology. Overall correlation varies between 25%21 and 81%.22 Further, an abnormal BBT may occur in 12% of women with normal endometrial histologic dating.23 Because of this lack of specificity and sensitivity, BBT is not an adequate diagnostic tool.
Measuring progesterone and its metabolites. Because progesterone is the principal product of the corpus luteum, its measurement is clinically indicated to evaluate luteal phase abnormalities. For this reason, serum, urine, and salivary progesterone determinations are utilized.
Histologic dating of an endometrial biopsy is considered the gold standard for corpus luteum evaluation.
Although serum progesterone is widely used in the diagnosis of LPD, there is no agreement in the cutoff level for abnormal assays, the number of assays required for diagnosis, or the timing of the test. A number of studies have demonstrated lower progesterone levels in women with “out-of-phase” endometrial biopsies,24,25 but others have noted normal progesterone levels in the presence of abnormal biopsies.26-28
These contradictory findings may be explained by the episodic release of progesterone in response to the slow pulsing of LH during the luteal phase of the cycle. Consequently, there are wide and frequent fluctuations and diurnal variations in progesterone secretion. This makes the use of a single serum progesterone determination—or even a series of single serum measurements—unreliable.
- No standard for ‘normal’ progesterone levels.
- A range of sampling intervals proposed. To reduce the false-positive rate of a single measurement, Wuttke et al15 suggested 2 or 3 blood samples within 3 hours, since low progesterone levels are often observed prior to the occurrence of an LH pulse. Thus, the probability is high that within 3 consecutive hours an LH episode will have stimulated luteal progesterone secretion into the normal range. This approach has not been evaluated clinically. Moreover, it is likely to be time-consuming and inconvenient for the patient.
Biopsy: The ‘gold standard’
Histologic dating of an endometrial biopsy is the gold standard for corpus luteum evaluation because it assesses both quantitative progesterone secretion and the morphologic transformation of the endometrium in preparation for embryo implantation.
The histologic features characteristic of specific days of the menstrual cycle—first described by Noyes and colleagues in 19506—have remained the cornerstone of endometrial dating. The endometrium is considered out of phase when the histologic and chronological dating differ by 3 or more days, provided this difference is present in 2 or more successive cycles. Using these criteria, Noyes and Haman35 showed endometrial biopsy to be accurate, with an interobserver agreement rate of 82% within the 2-day range. Hence, the 3-day out-of-phase criterion.
Variations in results. Recently, the accuracy and reproducibility of endometrial histology in the diagnosis of LPD have been questioned because of considerable intraobserver and interobserver variation in sample readings, as well as variation between cycles of the same patient and timing of the biopsy. Evaluation also can depend on which section of the endometrium is sampled.36 Gibson and colleagues36 showed that 65% of the observed variability in endometrium dating was due to inconsistencies between evaluators, 27% was due to lack of concordance by the same evaluator, and 8% was due to regional differences in the uterus.
In view of these variations, the theoretical probability of changing clinical management is 15% to 28% after the same evaluator reviews a slide37 and 22% to 39% after another observer evaluates the same slide.38
Refresher training fails to improve accuracy. To increase the accuracy and interobserver reproducibility of endometrial dating, Duggan and colleagues39 offered refresher training in histologic criteria after initial endometrial dating. However, they found no improvement in accuracy or inter-observer reproducibility after this training.
Timing of the endometrial biopsy also is important.40 Traditionally, endometrial biopsies have been performed a few days before the presumed onset of menstruation to reflect the maximal influence of progesterone on the endometrium. This practice recently has been questioned, with some researchers favoring biopsies performed in the midluteal phase.40,41
The method of determining the date of ovulation also varies considerably. Traditionally, next-menstrual-period dating is used, whereby the day of menses after the biopsy is labeled day 28 and presumed to have occurred 14 days after ovulation. The use of urine LH to determine the preovulatory LH surge and—15 days later—menstruation, has been suggested as a more precise method.42
Significant intercycle variation may occur within the same individual. Li and colleagues43 reported that within-subject, between-cycle variation of more than 2 days occurred in about 41% of patients.
Uncertain link to infertility. The link between an abnormal biopsy and infertility is questionable. Previous studies have documented abnormal endometrial biopsy results in the range of 31% to 35% in fertile women13,44 —rates almost comparable to those of women with infertility. Earlier investigators also demonstrated no significant differences in pregnancy rates between infertile couples with normal biopsy results, compared with those with abnormal findings.45
Before we consider it clinically significant, we require any LPD to be present in repeated cycles.
Clinical recommendations. Before we consider it clinically significant, we require any LPD to be present in repeated cycles, since approximately 20% to 80% of women with an abnormal biopsy have an “in-phase” endometrium on a repeat biopsy.13,46
The dating of endometrial biopsies should only be done by experienced histopathologists. We perform endometrial biopsies after cycle day 24, and confirm any out-of-phase abnormality in 2 consecutive biopsies. Further, before we diagnose the endometrium as “out of phase,” histologic dating must lag by at least 3 days.
In our practice, all endometrial biopsies are read by the senior author, who has had extensive experience with Noyes’ criteria for endometrial dating.
Is there a proven treatment?
Not surprisingly—given the disagreement about its incidence and diagnosis—LPD treatment also is controversial. Several case series, observational trials, and retrospective studies have explored whether treatment improves the chances of conception, but few randomized trials have taken up the issue.
Two main strategies have been suggested:
- Improving follicular dynamics using drugs such as clomiphene or gonadotropins, which not only produce a follicle but increase progesterone secretion during the luteal phase.
- Administering supplemental progesterone during the luteal phase and first trimester of pregnancy.
Clomiphene and other follicle-maturing drugs. Clomiphene increases FSH secretion and induces development of multiple or larger follicles. As a result, LH receptivity is enhanced. In addition, higher estrogen concentrations in the follicular phase increase steroid receptor content in the endometrium.
Although some studies support the use of clomiphene to treat LPD, in certain cases the drug may actually induce luteal phase defects in 30% to 50% of cycles.47 By its anti-estrogenic activity, clomiphene may suppress progesterone receptor levels, rendering the endometrium out of phase and less responsive to progesterone.
Supplemental progesterone use is supported by research from Lassey and colleagues,48 who demonstrated an association between LPD and abnormal expression of alpha-v-beta-3-integrin, the biomarker of uterine receptivity, which is likely responsible for the window of implantation. The endometrium of women with delayed maturation—that is, LPD—fails to express the alpha-v-beta-3-integrin when biopsied during the window of implantation (days 20 and 21). When given supplemental progesterone, the vast majority of these patients return to normal histologic status and alpha-v-beta-3-integrin expression.48,49
Progesterone versus clomiphene. Clinical trials have not established whether progesterone treatment is more effective than treatment with clomiphene or gonadotropin or no treatment. Problems with design and methodology in trials that do exist make it impossible to draw any firm conclusions from reports published thus far.
The literature includes numerous descriptive studies, most of which involved assessment of pregnancy rates before and after treatment.50 However, pregnancies that occur after therapy do not necessarily imply treatment benefit, but may reflect natural intercycle or biologic variation or spontaneous conception independent of treatment.
Randomized trials. Most of the studies published to date are case series and observational studies, which are subject to methodologic bias. To the best of our knowledge, only a few randomized studies of treatment efficacy have been published.51-53 All included only a few patients and lacked adequate power to detect any differences.50
Pregnancies that occur after therapy do not necessarily imply treatment benefit, but may reflect natural intercycle variation.
For example, Balasch and colleagues52 evaluated treatment with vaginal progesterone suppositories, dehydrogesterone, and no treatment. Although there was a higher pregnancy rate in the treatment groups, it was not statistically significant. However, the power of this trial to detect any differences in pregnancy rates was very low.
Investigating recurrent pregnancy loss. We also lack properly designed studies assessing the role of luteal phase support in women with recurrent miscarriages due to LPD. Two meta-analyses reached different conclusions on the role of progesterone supplementation in patients with recurrent miscarriages.34,54 Studies included in these analyses used various inclusion criteria. Moreover, diagnosis was not based on currently accepted criteria, and the treatment of choice was either 17-OH progesterone or medroxyprogesterone. The trials also included patients at more than 8 weeks’ gestation.
One multicenter randomized study evaluating luteal support with human chorionic gonadotropin (hCG) or placebo found no significant difference in pregnancy rates.55
Targeting the underlying cause. Steroidogenic cells consist of 2 main types: large and small luteal cells. Large luteal cells derive from follicular granulosa cells and secrete autocrine-acting and paracrine-acting peptides and eicosanoids but are not LH-receptive.56 They ensure basal progesterone and estradiol production from the corpus luteum. Small luteal cells derive from follicular thecal cells and acquire LH receptivity; thus, they respond to regularly occurring LH pulses, leading to increased progesterone and estradiol production.15
Only expert histopathologists or reproductive endocrinologists with special training should evaluate samples.
In a series of excellent experiments, Wuttke and colleagues15 studied corpus luteum function by measuring serial LH, progesterone, and estradiol levels. They suggested that, if the underlying cause of LPD can be determined, therapy can be appropriately directed, depending on whether the deficiency is hypothalamic LPD or a defect of small luteal cells or large luteal cells.
In hypothalamic LPD, LH pulses are absent and the hypothalamic gonadotropin-releasing hormone pulse generator appears to be oversuppressed, even though serum progesterone levels are lower than normal. However, during the follicular phase, the pulse generator functions normally.15
When there is a defect of small luteal cells, the corpus luteum responds poorly to normal LH pulses during the luteal phase, resulting in subnormal basal progesterone. Wuttke et al postulated that a defect of small luteal cells occurs because the cells do not differentiate well or fail to acquire LH receptivity.15
A defect of large luteal cells—which ensure the release of basal LH and unstimulated progesterone—occurred in 21% of cases studied. In these cases there was normal corpus luteum response to normal LH pulses, but progesterone secretion decreased to very low levels between LH pulses.15 The follicular-phase LH pulsatility, follicular development, and serum estradiol were all normal.15
Theoretically, stimulation of luteal progesterone secretion by hCG administration should be an effective therapy for hypothalamic LPD as well as a defect of large luteal cells. If the defect involves the small luteal cells, however, hCG therapy will be ineffective, since the corpus luteum is unresponsive to gonadotropins.15 In such patients, progesterone may be the treatment of choice. This may explain, at least in part, the differences in outcomes in the literature, since the underlying causative category has not been identified.
A well-designed, multicenter trial is essential to address remaining questions about LPD, not to mention a clear and uniform definition.
Clinical management
For those who believe, as we do, that LPD is a real and potential cause of reproductive disorders, we recommend that:
- its diagnosis be standardized,
- only expert histopathologists or reproductive endocrinologists with special training and skills in endometrial dating evaluate histologic samples, and
- once a “proper” diagnosis is made, treatment be multifold and as minimally invasive as possible.
Our practice generally is to give patients gonadotropins or clomiphene to improve folliculogenesis and corpus luteum function, and to induce multiple ovulations, which increase serum progesterone levels as well as the chance of pregnancy.
In patients at high risk for multiple gestation, progesterone supplementation as oral tablets or vaginal cream is relatively safe, inexpensive, and well tolerated. There is no need to monitor effects of progesterone treatment, since this natural substance has no known side effects or teratogenicity.
Dr. Engman reports no financial relationship with any companies whose products are mentioned in this article. Dr. Luciano reports that he serves on the speakers bureau and as a consultant for Eli Lilly, and receives research grants from Chitogenics, ML Laboratories, Proctor & Gamble, and Tap Pharmaceuticals.
- Luteal phase deficiency (LPD), defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids.
- Wide variation in the reported incidence of LPD—3.7% to 20% in infertile women—reflects lack of agreement about its diagnostic criteria.
- Histologic dating of an endometrial sample is the gold standard for evaluation of the corpus luteum.
- Two main treatment strategies have been suggested: improving follicular dynamics using follicle-maturing drugs such as clomiphene, and use of supplemental progesterone during the luteal phase and first trimester of pregnancy.
Despite scanty and controversial supporting evidence, evaluation of patients with infertility or recurrent pregnancy loss for possible luteal phase deficiency (LPD) is firmly established in clinical practice. In this article, we examine the data and offer our perspective on the role of LPD in assessing and managing couples with reproductive disorders (FIGURE 1).
FIGURE 1 Diagnosis and treatment of luteal phase deficiency
Many areas of controversy
Although observational and retrospective studies have reported a higher incidence of LPD in women with infertility and recurrent pregnancy losses than in fertile controls,1-4 no prospective study has confirmed these findings. Furthermore, studies have failed to confirm the superiority of any particular therapy.
Once considered an important cause of infertility, LPD has become the subject of debate, with some experts questioning its very existence. Unclear terminology describing this disorder is part of the problem, making it difficult to definitively diagnose the deficiency or determine its incidence. Further, while reasonable consensus exists that endometrial biopsy is the most reliable diagnostic tool, concerns remain about its timing, repetition, and interpretation.
A defect of corpus luteum progesterone output?
LPD is defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, based on the woman’s next menses. It was first described by Jones in 1949.5 One year later, Noyes et al6 published criteria on endometrial dating that became the gold standard for LPD diagnosis.
Pathophysiology. LPD may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids (TABLE). Most experts believe LPD is a defect of corpus luteum progesterone output—both in amount and duration—resulting in inadequate stimulation of the endometrium for implantation of the blastocyst (FIGURE 2).5 Thus, the endometrial histologic pattern is an important bioassay of the corpus luteum steroidogenic function.
Normal embryonic implantation depends on a properly functioning luteal phase, which, in turn, requires optimal secretion of follicle-stimulating hormone (FSH) and adequate follicular development during the follicular phase. Other requirements are a satisfactory luteinizing hormone (LH) surge during ovulation and continuous tonic LH pulses during the luteal phase of the cycle.
LH secretion from the pituitary occurs in a pulsatile fashion,7 which is essential for corpus luteum function.8 During the follicular phase the pulse frequency is high, occurring at a rate of approximately 1 pulse per 90 minutes. However, during the luteal phase and under the influence of progesterone, the pulse frequency is significantly diminished, occurring approximately every 3 to 6 hours, depending on the age of the corpus luteum.7,9 The corpus luteum is unresponsive to LH pulses during the early luteal phase; sensitivity develops about 4 to 6 days after ovulation.7,9
The luteal phase thus involves creation of an optimal hormonal environment as well as adequate endometrial transformation. Alteration of any of the factors that contribute to a normally functioning corpus luteum may thus deleteriously affect the endometrium and embryonic implantation.
Epidemiology. The reported prevalence of LPD ranges from 3.7% to 20% among patients with infertility.10,11 When an out-of-phase endometrium is the diagnostic criterion, prevalence estimates range from 3.5% to 31%.12,13 A short luteal phase is thought to occur in 5% of ovulatory cycles.14Some reports suggest that LPD accounts for 25% to 40% of recurrent pregnancy losses.15
The wide variation in reported incidence of LPD reflects the lack of agreement about its definition and diagnostic criteria. Further, some studies evaluating prevalence have not concurrently tested controls—an important omission since endometrial histology suggestive of LPD occurs in up to 50% of single menstrual cycles and 25% of sequential cycles.16 Thus, the true incidence of the defect may never be known.
TABLE
Etiology of luteal phase deficiency
| FOLLICULAR PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic ovarian defects |
|
| Intrinsic endometrial defects |
|
| LUTEAL PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects |
|
| Intrinsic secretory endometrial defect |
|
| LUTEAL RESCUE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects in early pregnancy |
|
| Intrinsic endometrial defects |
| FSH = follicle-stimulating hormone, hCG = human chorionic gonadotropin, |
| LH = luteinizing hormone |
Seeking a reliable diagnostic tool
Although significant progress has been made in recent years, LPD diagnosis is neither straightforward nor completely accurate. Approaches include measuring the luteal phase duration, taking basal body temperature (BBT), and assessing single or multiple serum progesterone levels, as well as using sonographic imaging and endometrial biopsy.
Luteal phase duration. An abnormally short luteal phase—defined as less than 10 days17,18—occurs in approximately 5% of ovulatory cycles.19 Research has shown such cycles to have low peak serum progesterone levels, suggestive of poor corpus luteum function.18 The relationship between an abnormally short luteal phase and infertility is unclear, however. Smith and colleagues,20 for example, evaluated women with known fertility and women with unexplained infertility and found the prevalence of a short luteal phase to be the same in both groups.
Our own practice is to measure luteal phase parameters in infertile patients. When the luteal phase is shorter than 12 days, we usually treat it.
Basal body temperature. A rise in BBT occurs when progesterone production increases at midcycle. A rise of approximately 2.5 ng/mL of progesterone will result in a temperature elevation of nearly 1°F. Interpretation of the BBT is based on this thermogenic shift. Unfortunately, although BBT may be a sensitive indicator of ovulation, it is a poor indicator of the quality of the luteal phase. Neither the rate nor the magnitude of rise of the postovulatory temperature curve correlates with endometrial histology. Overall correlation varies between 25%21 and 81%.22 Further, an abnormal BBT may occur in 12% of women with normal endometrial histologic dating.23 Because of this lack of specificity and sensitivity, BBT is not an adequate diagnostic tool.
Measuring progesterone and its metabolites. Because progesterone is the principal product of the corpus luteum, its measurement is clinically indicated to evaluate luteal phase abnormalities. For this reason, serum, urine, and salivary progesterone determinations are utilized.
Histologic dating of an endometrial biopsy is considered the gold standard for corpus luteum evaluation.
Although serum progesterone is widely used in the diagnosis of LPD, there is no agreement in the cutoff level for abnormal assays, the number of assays required for diagnosis, or the timing of the test. A number of studies have demonstrated lower progesterone levels in women with “out-of-phase” endometrial biopsies,24,25 but others have noted normal progesterone levels in the presence of abnormal biopsies.26-28
These contradictory findings may be explained by the episodic release of progesterone in response to the slow pulsing of LH during the luteal phase of the cycle. Consequently, there are wide and frequent fluctuations and diurnal variations in progesterone secretion. This makes the use of a single serum progesterone determination—or even a series of single serum measurements—unreliable.
- No standard for ‘normal’ progesterone levels.
- A range of sampling intervals proposed. To reduce the false-positive rate of a single measurement, Wuttke et al15 suggested 2 or 3 blood samples within 3 hours, since low progesterone levels are often observed prior to the occurrence of an LH pulse. Thus, the probability is high that within 3 consecutive hours an LH episode will have stimulated luteal progesterone secretion into the normal range. This approach has not been evaluated clinically. Moreover, it is likely to be time-consuming and inconvenient for the patient.
Biopsy: The ‘gold standard’
Histologic dating of an endometrial biopsy is the gold standard for corpus luteum evaluation because it assesses both quantitative progesterone secretion and the morphologic transformation of the endometrium in preparation for embryo implantation.
The histologic features characteristic of specific days of the menstrual cycle—first described by Noyes and colleagues in 19506—have remained the cornerstone of endometrial dating. The endometrium is considered out of phase when the histologic and chronological dating differ by 3 or more days, provided this difference is present in 2 or more successive cycles. Using these criteria, Noyes and Haman35 showed endometrial biopsy to be accurate, with an interobserver agreement rate of 82% within the 2-day range. Hence, the 3-day out-of-phase criterion.
Variations in results. Recently, the accuracy and reproducibility of endometrial histology in the diagnosis of LPD have been questioned because of considerable intraobserver and interobserver variation in sample readings, as well as variation between cycles of the same patient and timing of the biopsy. Evaluation also can depend on which section of the endometrium is sampled.36 Gibson and colleagues36 showed that 65% of the observed variability in endometrium dating was due to inconsistencies between evaluators, 27% was due to lack of concordance by the same evaluator, and 8% was due to regional differences in the uterus.
In view of these variations, the theoretical probability of changing clinical management is 15% to 28% after the same evaluator reviews a slide37 and 22% to 39% after another observer evaluates the same slide.38
Refresher training fails to improve accuracy. To increase the accuracy and interobserver reproducibility of endometrial dating, Duggan and colleagues39 offered refresher training in histologic criteria after initial endometrial dating. However, they found no improvement in accuracy or inter-observer reproducibility after this training.
Timing of the endometrial biopsy also is important.40 Traditionally, endometrial biopsies have been performed a few days before the presumed onset of menstruation to reflect the maximal influence of progesterone on the endometrium. This practice recently has been questioned, with some researchers favoring biopsies performed in the midluteal phase.40,41
The method of determining the date of ovulation also varies considerably. Traditionally, next-menstrual-period dating is used, whereby the day of menses after the biopsy is labeled day 28 and presumed to have occurred 14 days after ovulation. The use of urine LH to determine the preovulatory LH surge and—15 days later—menstruation, has been suggested as a more precise method.42
Significant intercycle variation may occur within the same individual. Li and colleagues43 reported that within-subject, between-cycle variation of more than 2 days occurred in about 41% of patients.
Uncertain link to infertility. The link between an abnormal biopsy and infertility is questionable. Previous studies have documented abnormal endometrial biopsy results in the range of 31% to 35% in fertile women13,44 —rates almost comparable to those of women with infertility. Earlier investigators also demonstrated no significant differences in pregnancy rates between infertile couples with normal biopsy results, compared with those with abnormal findings.45
Before we consider it clinically significant, we require any LPD to be present in repeated cycles.
Clinical recommendations. Before we consider it clinically significant, we require any LPD to be present in repeated cycles, since approximately 20% to 80% of women with an abnormal biopsy have an “in-phase” endometrium on a repeat biopsy.13,46
The dating of endometrial biopsies should only be done by experienced histopathologists. We perform endometrial biopsies after cycle day 24, and confirm any out-of-phase abnormality in 2 consecutive biopsies. Further, before we diagnose the endometrium as “out of phase,” histologic dating must lag by at least 3 days.
In our practice, all endometrial biopsies are read by the senior author, who has had extensive experience with Noyes’ criteria for endometrial dating.
Is there a proven treatment?
Not surprisingly—given the disagreement about its incidence and diagnosis—LPD treatment also is controversial. Several case series, observational trials, and retrospective studies have explored whether treatment improves the chances of conception, but few randomized trials have taken up the issue.
Two main strategies have been suggested:
- Improving follicular dynamics using drugs such as clomiphene or gonadotropins, which not only produce a follicle but increase progesterone secretion during the luteal phase.
- Administering supplemental progesterone during the luteal phase and first trimester of pregnancy.
Clomiphene and other follicle-maturing drugs. Clomiphene increases FSH secretion and induces development of multiple or larger follicles. As a result, LH receptivity is enhanced. In addition, higher estrogen concentrations in the follicular phase increase steroid receptor content in the endometrium.
Although some studies support the use of clomiphene to treat LPD, in certain cases the drug may actually induce luteal phase defects in 30% to 50% of cycles.47 By its anti-estrogenic activity, clomiphene may suppress progesterone receptor levels, rendering the endometrium out of phase and less responsive to progesterone.
Supplemental progesterone use is supported by research from Lassey and colleagues,48 who demonstrated an association between LPD and abnormal expression of alpha-v-beta-3-integrin, the biomarker of uterine receptivity, which is likely responsible for the window of implantation. The endometrium of women with delayed maturation—that is, LPD—fails to express the alpha-v-beta-3-integrin when biopsied during the window of implantation (days 20 and 21). When given supplemental progesterone, the vast majority of these patients return to normal histologic status and alpha-v-beta-3-integrin expression.48,49
Progesterone versus clomiphene. Clinical trials have not established whether progesterone treatment is more effective than treatment with clomiphene or gonadotropin or no treatment. Problems with design and methodology in trials that do exist make it impossible to draw any firm conclusions from reports published thus far.
The literature includes numerous descriptive studies, most of which involved assessment of pregnancy rates before and after treatment.50 However, pregnancies that occur after therapy do not necessarily imply treatment benefit, but may reflect natural intercycle or biologic variation or spontaneous conception independent of treatment.
Randomized trials. Most of the studies published to date are case series and observational studies, which are subject to methodologic bias. To the best of our knowledge, only a few randomized studies of treatment efficacy have been published.51-53 All included only a few patients and lacked adequate power to detect any differences.50
Pregnancies that occur after therapy do not necessarily imply treatment benefit, but may reflect natural intercycle variation.
For example, Balasch and colleagues52 evaluated treatment with vaginal progesterone suppositories, dehydrogesterone, and no treatment. Although there was a higher pregnancy rate in the treatment groups, it was not statistically significant. However, the power of this trial to detect any differences in pregnancy rates was very low.
Investigating recurrent pregnancy loss. We also lack properly designed studies assessing the role of luteal phase support in women with recurrent miscarriages due to LPD. Two meta-analyses reached different conclusions on the role of progesterone supplementation in patients with recurrent miscarriages.34,54 Studies included in these analyses used various inclusion criteria. Moreover, diagnosis was not based on currently accepted criteria, and the treatment of choice was either 17-OH progesterone or medroxyprogesterone. The trials also included patients at more than 8 weeks’ gestation.
One multicenter randomized study evaluating luteal support with human chorionic gonadotropin (hCG) or placebo found no significant difference in pregnancy rates.55
Targeting the underlying cause. Steroidogenic cells consist of 2 main types: large and small luteal cells. Large luteal cells derive from follicular granulosa cells and secrete autocrine-acting and paracrine-acting peptides and eicosanoids but are not LH-receptive.56 They ensure basal progesterone and estradiol production from the corpus luteum. Small luteal cells derive from follicular thecal cells and acquire LH receptivity; thus, they respond to regularly occurring LH pulses, leading to increased progesterone and estradiol production.15
Only expert histopathologists or reproductive endocrinologists with special training should evaluate samples.
In a series of excellent experiments, Wuttke and colleagues15 studied corpus luteum function by measuring serial LH, progesterone, and estradiol levels. They suggested that, if the underlying cause of LPD can be determined, therapy can be appropriately directed, depending on whether the deficiency is hypothalamic LPD or a defect of small luteal cells or large luteal cells.
In hypothalamic LPD, LH pulses are absent and the hypothalamic gonadotropin-releasing hormone pulse generator appears to be oversuppressed, even though serum progesterone levels are lower than normal. However, during the follicular phase, the pulse generator functions normally.15
When there is a defect of small luteal cells, the corpus luteum responds poorly to normal LH pulses during the luteal phase, resulting in subnormal basal progesterone. Wuttke et al postulated that a defect of small luteal cells occurs because the cells do not differentiate well or fail to acquire LH receptivity.15
A defect of large luteal cells—which ensure the release of basal LH and unstimulated progesterone—occurred in 21% of cases studied. In these cases there was normal corpus luteum response to normal LH pulses, but progesterone secretion decreased to very low levels between LH pulses.15 The follicular-phase LH pulsatility, follicular development, and serum estradiol were all normal.15
Theoretically, stimulation of luteal progesterone secretion by hCG administration should be an effective therapy for hypothalamic LPD as well as a defect of large luteal cells. If the defect involves the small luteal cells, however, hCG therapy will be ineffective, since the corpus luteum is unresponsive to gonadotropins.15 In such patients, progesterone may be the treatment of choice. This may explain, at least in part, the differences in outcomes in the literature, since the underlying causative category has not been identified.
A well-designed, multicenter trial is essential to address remaining questions about LPD, not to mention a clear and uniform definition.
Clinical management
For those who believe, as we do, that LPD is a real and potential cause of reproductive disorders, we recommend that:
- its diagnosis be standardized,
- only expert histopathologists or reproductive endocrinologists with special training and skills in endometrial dating evaluate histologic samples, and
- once a “proper” diagnosis is made, treatment be multifold and as minimally invasive as possible.
Our practice generally is to give patients gonadotropins or clomiphene to improve folliculogenesis and corpus luteum function, and to induce multiple ovulations, which increase serum progesterone levels as well as the chance of pregnancy.
In patients at high risk for multiple gestation, progesterone supplementation as oral tablets or vaginal cream is relatively safe, inexpensive, and well tolerated. There is no need to monitor effects of progesterone treatment, since this natural substance has no known side effects or teratogenicity.
Dr. Engman reports no financial relationship with any companies whose products are mentioned in this article. Dr. Luciano reports that he serves on the speakers bureau and as a consultant for Eli Lilly, and receives research grants from Chitogenics, ML Laboratories, Proctor & Gamble, and Tap Pharmaceuticals.
1. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24-29.
2. Rosemberg SM, Luciano AA, Riddick DH. The luteal phase defect: the relative frequency of, and encouraging response to treatment with vaginal progesterone. Fertil Steril. 1980;34:17-20.
3. Davidson BJ, Thrasher TV, Seraj IM. An analysis of endometrial biopsies performed for infertility. Fertil Steril. 1987;48:770-774.
4. Li TC, Dockery P, Cook ID. Endometrial development in the luteal phase of women with various types of infertility: comparison with women of normal fertility. Hum Reprod. 1991;6:325-330.
5. Jones GS. Some newer aspects of management of infertility. JAMA. 1949;141:1123-1129.
6. Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3-25.
7. Jones GS, Pourmand K. An evaluation of etiologic factors and therapy in 555 private patients with primary infertility. Fertil Steril. 1962;13:398-410.
8. Nash LD. The treatment of luteal phase dysfunction with clomiphene citrate and human chorionic gonadotropin. Infertility. 1982;5:87-104.
9. McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: a critical review. Fertil Steril. 1988;50:1-15.
10. Balasch J, Vanrell JA. Luteal phase deficiency: an inadequate endometrial response to normal hormonal stimulation. Intern J Fertil. 1986;31:368-371.
11. Olive DL. The prevalence and epidemiology of luteal phase deficiency in normal and infertile women. Clin Obstet Gynecol. 1991;34:157-166.
12. Andrews WC. Luteal phase defects. Fertil Steril. 1979;32:501.-
13. Davis OK, Berkeley AS, Naus GJ, Cholst IN, Freeman KS. The incidence of luteal phase defect in normal, fertile women, determined by endometrial biopsies. Fertil Steril. 1989;51:582-586.
14. Filicori M, Butler JP, Crowley WF, Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638-1647.
15. Wuttke W, Pitzel L, Seidlova-Wuttke D, Hinney B. LH pulses and the corpus luteum: the luteal phase deficiency (LPD). Vitam Horm. 2001;63:131-158.
16. Hinney B, Henze C, Wuttke W. Regulation of luteal function by luteinizing hormone and prolactin at different times of the luteal phase. Eur J Endocrinol. 1995;133:701-717.
17. Sherman BM, Korenman SG. Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. J Clin Endocrinol Metab. 1974;38:89.-
18. Strott CA, Cargille CM, Ross GT, Lipsett MD. The short luteal phase. J Clin Endocrinol Metab. 1970;30:246.-
19. Lenton EA, Landgren BM, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol. 1984;91:685-689.
20. Smith SK, Lenton EA, Landgren BM, Cooke ID. The short luteal phase and infertility. Br J Obstet Gynaecol. 1984;91:1120-1122.
21. Downs KA, Gibson M. Basal body temperature graph and the luteal phase defect. Fertil Steril. 1983;40:466-468.
22. Soules MR, Wiebe RH, Aksel S, Hammond CB. The diagnosis and therapy of luteal phase deficiency. Fertil Steril. 1977;28:1033.-
23. Johansson EAB, Larsson-Cohn U, Gemzell C. Monophasic basal body temperature in ovulatory menstrual cycles. Am J Obstet Gynecol. 1972;113:933.-
24. Cummings DC, Honore LH, Scott JC, Williams KP. The late luteal phase in infertile women: comparison of simultaneous endometrial biopsy and progesterone levels. Fertil Steril. 1985;43:715-719.
25. Daya S, Ward S. Diagnostic test properties of serum progesterone in the evaluation of luteal phase defects. Fertil Steril. 1988;49:168-170.
26. Batista MC, Carteledge TP, Merino MJ, et al. Midluteal phase endometrial biopsy does not accurately predict luteal function. Fertil Steril. 1993;59:294-300.
27. Rosenfeld DL, Garcia CR. A comparison of endometrial histology with simultaneous plasma progesterone determinations in infertile women. Fertil Steril. 1976;27:1256-1261.
28. Shangold M, Berkeley A, Gray J. Both midluteal serum progesterone levels and late luteal endometrial histology should be assessed in all infertile women. Fertil Steril. 1983;40:627-630.
29. Hensleigh PA, Fainstat T. Corpus luteum dysfunction: serum progesterone levels in diagnosis and assessment of therapy for recurrent and threatened abortion. Fertil Steril. 1979;32:396-400.
30. Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94:89-98.
31. Hull MGR, Savage PE, Bromham DR, et al. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle (“ovulation”) derived from treated and untreated conception cycles. Fertil Steril. 1982;37:355-360.
32. Abraham GE, Maroulis GB, Marshall JR. Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet Gynecol. 1974;44:522-525.
33. Wu CH, Minassian SS. The integrated luteal progesterone: an assessment of luteal function. Fertil Steril. 1987;48:937-940.
34. Daya S. Efficacy of progesterone support for pregnancy in women with recurrent miscarriage. A meta-analysis of controlled trials. Br J Obstet Gynaecol. 1989;96:275-280.
35. Noyes RW, Haman JO. Accuracy of endometrial dating. Fertil Steril. 1953;4:504.-
36. Gibson M, Badger GJ, Byrn F, Lee KR, Korson R, Trainer TD. Error in histologic dating of secretory endometrium: variance component analysis. Fertil Steril. 1991;56:242-247.
37. Scott RT, Snyder RR, Bagnall JW, Reed KD, Adair CF, Hensley SD. Evaluation of the impact of intraobserver variability on endometrial dating and the diagnosis of luteal phase defects. Fertil Steril. 1993;60:652-657.
38. Scott RT, Snyder RR, Strickland DM, et al. The effect of interobserver variation in dating endometrial histology on the diagnosis of luteal phase defects. Fertil Steril. 1988;50:888-892.
39. Duggan MA, Brashert P, Ostor A, et al. The accuracy and interobserver reproducibility of endometrial dating. Pathology. 2001;33:292-297.
40. Castelbaum AJ, Wheeler J, Coutifaris CB, Mastroianni L, Jr, Lessey BA. Timing of the endometrial biopsy may be critical for the accurate diagnosis of luteal phase deficiency. Fertil Steril. 1994;61:443-447.
41. Johannisson E, Landgren BM, Rohr HP, Diczfalusy E. Endometrial morphology and peripheral hormone levels in women with regular menstrual cycles. Fertil Steril. 1987;48:401-408.
42. Li TC, Rogers AW, Lenton EA, et al. A comparison between two methods of chronological dating of human endometrial biopsies during the luteal phase and their correlation with histologic dating. Fertil Steril. 1987;48:928-932.
43. Li TC, Dockery P, Rogers AW, Cooke ID. How precise is histologic dating of endometrium using the standard dating criteria? Fertil Steril. 1989;51:759-763.
44. Shoupe D, Mishell DR, Jr, Lacarra M, et al. Correlation of endometrial maturation with four methods of estimating day of ovulation. Obstet Gynecol. 1989;73:88-92.
45. Driessen F, Holwerda PJ, vd Putte SC, Kremer J. The significance of dating on endometrial biopsy for the prognosis of the infertile couple. Int J Fertil. 1980;25:112-116.
46. Wentz AC. Endometrial biopsy in the evaluation of infertility. Fertil Steril. 1980;33:121-124.
47. Keenan JW, Herbert CM, Bush JR, Wentz AC. Diagnosis and management of out-of-phase endometrial biopsies among patients receiving clomiphene citrate for ovulation induction. Fertil Steril. 1989;51:964.-
48. Lassey BA, Castlebaum AJ, Sawin SJ, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63:535-542.
49. Lassey BA, Damjanovich L, Coutifaris C, et al. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188-195.
50. Karamardian LM, Grimes DA. Luteal phase deficiency: effect of treatment on pregnancy rates. Am J Obstet Gynecol. 1992;167:1391-1398.
51. Echt CR, Romberger FT, Goodman JA. Clomiphene citrate in the treatment of luteal phase defects. Fertil Steril. 1969;20:564-571.
52. Balasch J, Vanrell JA, Marquez M, Burzaco I, Gonzalez-Merlo J. Dehydrogesterone versus vaginal progesterone in the treatment of the endometrial luteal phase deficiency. Fertil Steril. 1982;37:751-754.
53. Huang KE. The primary treatment of luteal phase inadequacy: progesterone versus clomiphene citrate. Am J Obstet Gynecol. 1986;155:824-828.
54. Goldstein P, Berrier J, Rosen S, Sacks HS, Chalmers TC. A meta-analysis of randomized control trials of progestational agents in pregnancy. Br J Obstet Gynaecol. 1989;96:265-274.
55. Harrison RF. Human chorionic gonadotrophin (hCG) in the management of recurrent abortion; results of a multicentre placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 1992;47:175-179.
56. Ohara A, Mori T, Taii S, Ban C, Narimoto K. Functional differentiation in steroidogenesis of two types of luteal cells isolated from mature human corpora lutea of menstrual cycle. J Clin Endocrinol Metab. 1987;65:1192-1200.
1. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24-29.
2. Rosemberg SM, Luciano AA, Riddick DH. The luteal phase defect: the relative frequency of, and encouraging response to treatment with vaginal progesterone. Fertil Steril. 1980;34:17-20.
3. Davidson BJ, Thrasher TV, Seraj IM. An analysis of endometrial biopsies performed for infertility. Fertil Steril. 1987;48:770-774.
4. Li TC, Dockery P, Cook ID. Endometrial development in the luteal phase of women with various types of infertility: comparison with women of normal fertility. Hum Reprod. 1991;6:325-330.
5. Jones GS. Some newer aspects of management of infertility. JAMA. 1949;141:1123-1129.
6. Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3-25.
7. Jones GS, Pourmand K. An evaluation of etiologic factors and therapy in 555 private patients with primary infertility. Fertil Steril. 1962;13:398-410.
8. Nash LD. The treatment of luteal phase dysfunction with clomiphene citrate and human chorionic gonadotropin. Infertility. 1982;5:87-104.
9. McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: a critical review. Fertil Steril. 1988;50:1-15.
10. Balasch J, Vanrell JA. Luteal phase deficiency: an inadequate endometrial response to normal hormonal stimulation. Intern J Fertil. 1986;31:368-371.
11. Olive DL. The prevalence and epidemiology of luteal phase deficiency in normal and infertile women. Clin Obstet Gynecol. 1991;34:157-166.
12. Andrews WC. Luteal phase defects. Fertil Steril. 1979;32:501.-
13. Davis OK, Berkeley AS, Naus GJ, Cholst IN, Freeman KS. The incidence of luteal phase defect in normal, fertile women, determined by endometrial biopsies. Fertil Steril. 1989;51:582-586.
14. Filicori M, Butler JP, Crowley WF, Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638-1647.
15. Wuttke W, Pitzel L, Seidlova-Wuttke D, Hinney B. LH pulses and the corpus luteum: the luteal phase deficiency (LPD). Vitam Horm. 2001;63:131-158.
16. Hinney B, Henze C, Wuttke W. Regulation of luteal function by luteinizing hormone and prolactin at different times of the luteal phase. Eur J Endocrinol. 1995;133:701-717.
17. Sherman BM, Korenman SG. Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. J Clin Endocrinol Metab. 1974;38:89.-
18. Strott CA, Cargille CM, Ross GT, Lipsett MD. The short luteal phase. J Clin Endocrinol Metab. 1970;30:246.-
19. Lenton EA, Landgren BM, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol. 1984;91:685-689.
20. Smith SK, Lenton EA, Landgren BM, Cooke ID. The short luteal phase and infertility. Br J Obstet Gynaecol. 1984;91:1120-1122.
21. Downs KA, Gibson M. Basal body temperature graph and the luteal phase defect. Fertil Steril. 1983;40:466-468.
22. Soules MR, Wiebe RH, Aksel S, Hammond CB. The diagnosis and therapy of luteal phase deficiency. Fertil Steril. 1977;28:1033.-
23. Johansson EAB, Larsson-Cohn U, Gemzell C. Monophasic basal body temperature in ovulatory menstrual cycles. Am J Obstet Gynecol. 1972;113:933.-
24. Cummings DC, Honore LH, Scott JC, Williams KP. The late luteal phase in infertile women: comparison of simultaneous endometrial biopsy and progesterone levels. Fertil Steril. 1985;43:715-719.
25. Daya S, Ward S. Diagnostic test properties of serum progesterone in the evaluation of luteal phase defects. Fertil Steril. 1988;49:168-170.
26. Batista MC, Carteledge TP, Merino MJ, et al. Midluteal phase endometrial biopsy does not accurately predict luteal function. Fertil Steril. 1993;59:294-300.
27. Rosenfeld DL, Garcia CR. A comparison of endometrial histology with simultaneous plasma progesterone determinations in infertile women. Fertil Steril. 1976;27:1256-1261.
28. Shangold M, Berkeley A, Gray J. Both midluteal serum progesterone levels and late luteal endometrial histology should be assessed in all infertile women. Fertil Steril. 1983;40:627-630.
29. Hensleigh PA, Fainstat T. Corpus luteum dysfunction: serum progesterone levels in diagnosis and assessment of therapy for recurrent and threatened abortion. Fertil Steril. 1979;32:396-400.
30. Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94:89-98.
31. Hull MGR, Savage PE, Bromham DR, et al. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle (“ovulation”) derived from treated and untreated conception cycles. Fertil Steril. 1982;37:355-360.
32. Abraham GE, Maroulis GB, Marshall JR. Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet Gynecol. 1974;44:522-525.
33. Wu CH, Minassian SS. The integrated luteal progesterone: an assessment of luteal function. Fertil Steril. 1987;48:937-940.
34. Daya S. Efficacy of progesterone support for pregnancy in women with recurrent miscarriage. A meta-analysis of controlled trials. Br J Obstet Gynaecol. 1989;96:275-280.
35. Noyes RW, Haman JO. Accuracy of endometrial dating. Fertil Steril. 1953;4:504.-
36. Gibson M, Badger GJ, Byrn F, Lee KR, Korson R, Trainer TD. Error in histologic dating of secretory endometrium: variance component analysis. Fertil Steril. 1991;56:242-247.
37. Scott RT, Snyder RR, Bagnall JW, Reed KD, Adair CF, Hensley SD. Evaluation of the impact of intraobserver variability on endometrial dating and the diagnosis of luteal phase defects. Fertil Steril. 1993;60:652-657.
38. Scott RT, Snyder RR, Strickland DM, et al. The effect of interobserver variation in dating endometrial histology on the diagnosis of luteal phase defects. Fertil Steril. 1988;50:888-892.
39. Duggan MA, Brashert P, Ostor A, et al. The accuracy and interobserver reproducibility of endometrial dating. Pathology. 2001;33:292-297.
40. Castelbaum AJ, Wheeler J, Coutifaris CB, Mastroianni L, Jr, Lessey BA. Timing of the endometrial biopsy may be critical for the accurate diagnosis of luteal phase deficiency. Fertil Steril. 1994;61:443-447.
41. Johannisson E, Landgren BM, Rohr HP, Diczfalusy E. Endometrial morphology and peripheral hormone levels in women with regular menstrual cycles. Fertil Steril. 1987;48:401-408.
42. Li TC, Rogers AW, Lenton EA, et al. A comparison between two methods of chronological dating of human endometrial biopsies during the luteal phase and their correlation with histologic dating. Fertil Steril. 1987;48:928-932.
43. Li TC, Dockery P, Rogers AW, Cooke ID. How precise is histologic dating of endometrium using the standard dating criteria? Fertil Steril. 1989;51:759-763.
44. Shoupe D, Mishell DR, Jr, Lacarra M, et al. Correlation of endometrial maturation with four methods of estimating day of ovulation. Obstet Gynecol. 1989;73:88-92.
45. Driessen F, Holwerda PJ, vd Putte SC, Kremer J. The significance of dating on endometrial biopsy for the prognosis of the infertile couple. Int J Fertil. 1980;25:112-116.
46. Wentz AC. Endometrial biopsy in the evaluation of infertility. Fertil Steril. 1980;33:121-124.
47. Keenan JW, Herbert CM, Bush JR, Wentz AC. Diagnosis and management of out-of-phase endometrial biopsies among patients receiving clomiphene citrate for ovulation induction. Fertil Steril. 1989;51:964.-
48. Lassey BA, Castlebaum AJ, Sawin SJ, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63:535-542.
49. Lassey BA, Damjanovich L, Coutifaris C, et al. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188-195.
50. Karamardian LM, Grimes DA. Luteal phase deficiency: effect of treatment on pregnancy rates. Am J Obstet Gynecol. 1992;167:1391-1398.
51. Echt CR, Romberger FT, Goodman JA. Clomiphene citrate in the treatment of luteal phase defects. Fertil Steril. 1969;20:564-571.
52. Balasch J, Vanrell JA, Marquez M, Burzaco I, Gonzalez-Merlo J. Dehydrogesterone versus vaginal progesterone in the treatment of the endometrial luteal phase deficiency. Fertil Steril. 1982;37:751-754.
53. Huang KE. The primary treatment of luteal phase inadequacy: progesterone versus clomiphene citrate. Am J Obstet Gynecol. 1986;155:824-828.
54. Goldstein P, Berrier J, Rosen S, Sacks HS, Chalmers TC. A meta-analysis of randomized control trials of progestational agents in pregnancy. Br J Obstet Gynaecol. 1989;96:265-274.
55. Harrison RF. Human chorionic gonadotrophin (hCG) in the management of recurrent abortion; results of a multicentre placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 1992;47:175-179.
56. Ohara A, Mori T, Taii S, Ban C, Narimoto K. Functional differentiation in steroidogenesis of two types of luteal cells isolated from mature human corpora lutea of menstrual cycle. J Clin Endocrinol Metab. 1987;65:1192-1200.