User login
Case Studies in Toxicology: Sippin’ on Some “Sizzurp”
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
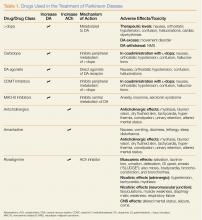
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
Case Studies in Toxicology: A Patchwork of Problems in Parkinson Patients
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
Neonatal Seizure: Sepsis or Toxic Syndrome?
A mother presents to the ED with her 4-day-old daughter after noting abnormal jerking movements of the neonate's upper extremities. She states the baby has had watery stools for the past day, but has been tolerating bottle formula feeds without vomiting and having appropriate urinary output. The patient was born full-term via normal spontaneous vaginal delivery, with Apgar scores of 8 at 1 minute and 9 at 5 minutes. The postdelivery course was uncomplicated, and both mother and baby were discharged home 2 days after delivery.
Initial vital signs are: heart rate, 135 beats/min; respiratory rate (RR), 48 breaths/min; and temperature, 98.7°F; blood glucose was normal. On physical examination, the baby is awake and well-appearing, with a nonbulging anterior fontanelle, soft, supple neck, and flexed and symmetrically mobile extremities. Moro, suck, rooting, and grasp reflexes are all intact. No abnormal movements are noted. The remainder of the examination is unremarkable.
Do the jerking movements indicate a focal seizure? What could cause these movements in a neonate?
As the length of the postpartum hospital stay has decreased over the past 20 years, EDs have experienced an increase in neonatal visits for conditions that traditionally manifested in newborn nurseries. While most presentations are for benign reasons (eg, issues related to feeding, irritability), patients with concerning conditions, including central nervous system (CNS) abnormalities, may also initially present to the ED. Causes of such clinical findings may be structural (eg, cerebral malformations, subdural hematomas, herpes encephalitis) and/or metabolic (eg, hypoglycemia, hypocalcemia, inborn errors). Many early-onset neonatal seizures are benign and resolve by several months of age, but it is essential to identify those that are consequential and treatable.
Case Continuation
In the evaluation of the neonatal patient with suspected seizure, it is important to take a detailed maternal and labor history, and to consider a broad differential in the face of nonspecific findings. In this case, the patient's mother disclosed a personal history of chronic pain, for which she took buprenorphine 2 mg orally in the morning and 4 mg orally at bedtime (total daily dose of 6mg/day) throughout her pregnancy.
How does drug withdrawal present in the neonate?
Neonatal abstinence syndrome (NAS) is the clinical syndrome of withdrawal in a newborn exposed in utero to drugs capable of inducing dependence. Agents associated with NAS include opioids, benzodiazepines, ethanol, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, and nicotine.1,2
Over the past decade, there has been a 330% rise in the diagnosis of opioid-related NAS alone.3 In response to this increase, the US Food and Drug Administration recently added a black-box warning to all extended-release/long-acting opioid preparations detailing this risk.4
Presenting symptoms of NAS are protean, differ from patient to patient, and are a function of drug type, duration, and amount of drug exposure. NAS may mimic other severe life-threatening conditions such as those previously noted, and the inability to obtain an adequate symptom-based medical history from a neonate further complicates the diagnosis. Before making a diagnosis of NAS, other conditions should be carefully considered in the differential.
Neonatal opioid withdrawal manifests primarily with CNS and gastrointestinal (GI) effects since there are high concentrations of opioid receptors in these areas. Although clinical findings are generally similar among opioid agents, the onset and duration following abstinence varies—largely based on individual drug half-life; this helps to differentiate between opioid agents. For example, while babies exposed to heroin in utero present with signs of NAS within 24 hours of birth, those exposed to buprenorphine or methadone tend to present 2 to 6 days after delivery.1 Between 55% to 94% of neonates with in-utero opioid exposure develop NAS.5
Selective Serotonin Reuptake Inhibitors
SSRIs have also been associated with a neonatal syndrome, and largely involve similar signs and symptoms as NAS. Although the specific etiology is not clear, it has been suggested that this syndrome is the result of serotonin toxicity rather than withdrawal; as such, it is often referred to as "serotonin discontinuation syndrome." Clinical findings occur from several hours to several days after birth and usually resolve within 1 to 2 weeks.6
Cocaine Exposure
In-utero cocaine exposure is also associated with neurobehavioral abnormalities in neonates although a withdrawal syndrome is less clearly defined. Findings, however, are consistent with NAS and include increased irritability, tremors, and high-pitched cry—most frequently occurring between 24 and 48 hours postdelivery.6
Neonatal Alcohol Withdrawal Syndrome
Neonatal alcohol withdrawal syndrome, particularly in fetuses exposed to alcohol during the last trimester, is distinct from fetal alcohol syndrome (FAS). The latter is associated with typical dysmorphic features, growth deficiencies, and CNS findings reflective of permanent neurologic sequelae. Neonatal alcohol withdrawal presents with CNS findings similar to those listed for other in-utero exposures—eg, increased irritability, tremors, nystagmus hyperactive reflexes.7
Screening for NAS: The Finnegan Scale
The Finnegan Neonatal Abstinence Scoring System is one of the most commonly employed and validated tools used to screen for NAS. It comprises a 31-item scale, listing the clinical signs and symptoms of NAS, which are scored by severity and organized by system to include neurologic, metabolic, vasomotor, respiratory, and GI disturbances (Figure). Point allocation is based on mild, moderate, or severe symptoms as follows:
- Mild findings (eg, sweating, fever <101°F mottling, nasal stuffiness) each score 1 point.
- Moderate findings (eg, high-pitched cry, hyperactive moro reflex, increased muscle tone, fever >101°F, increased RR >60 with retractions, poor feeding, loose stools) each score 2 points.
- Severe findings (eg, myoclonic jerks, generalized convulsions, projectile vomiting, watery stools) each score 3 points.
While each of the above are independently nonspecific, the constellation of findings, together with the appropriate history, provide for a clinical diagnosis. The Finnegan Scale is therefore designed not only to aid in diagnosis, but also to quantify the severity of NAS and guide management.
Screening for NAS begins at birth in neonates with known in-utero exposure (ie, when risk of NAS is high) or at the time of initial presentation in other circumstances. Scoring is performed every 4 hours; the first two or three scores will determine the need for pharmacotherapy (see below).
| Pharmacotherapy is indicated in the following Finnegan scoring scenarios: |
|
|
|
How is NAS treated?
The two main goals of management in the treatment of opioid-related NAS are to relieve the signs and symptoms of withdrawal and to prevent complications (eg, fever, weight loss, seizures). Therapy should begin with nonpharmacologic measures that minimize excess external stimuli, such as swaddling, gentle handling, and minimizing noise and light. To prevent weight loss, small hypercaloric feeds may be helpful. If pharmacologic treatment is indicated, oral opioid replacement with morphine is considered by many to be the drug of choice. Oral morphine dosing may be guided by NAS severity based on the Finnegan score; alternatively, initial dosing at 0.1 mg/kg orally every 4 hours has also been recommended.1
Other agents, such methadone 0.1 mg/kg orally every 12 hours and buprenorphine 15.9 mcg/kg divided in three doses orally, may also be used. In patients whose symptoms persist despite opioid treatment, use of adjuncts such as phenobarbital and clonidine may be indicated.
Case Conclusion
The patient was admitted to the neonatal intensive care unit where she appropriately underwent a sepsis workup. Laboratory evaluation, including blood and urine cultures, was obtained. A brain ultrasound was unremarkable, and since lumbar puncture was unsuccessful, the patient was started empirically on meningitis doses of the cefotaxime, vancomycin, and acyclovir.
An initial Finnegan score was calculated. With the exception of soft stools, there were no other persistent symptoms, and patient did not achieve a score indicating a need for pharmacologic management. After 48 hours, she remained afebrile and soft stools resolved. All laboratory values, including cultures, were unremarkable. The patient was discharged on hospital day 3, with a scheduled well-baby follow-up appointment.
| Take Home Points |
|
- Cramton RE, Gruchala NE. Babies breaking bad: neonatal and iatrogenic withdrawal syndromes. Curr Opin Pediatr. 2013;25(4): 532-542.
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574-580. http://dx.doi.org/10.1111/j.1360-0443.2010.03170.x Accessed October 24, 2013.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-40.
- New safety measures announced for extended-release and long-acting opioids. US Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed October 24, 2013.
- Burgos AE, Burke BL Jr. Neonatal abstinence syndrome. NeoReviews. 2009;10(5):e222-e228. http://dx.doi.org/10.1542/neo.10-5-e222. Accessed October 24, 2013.
- Hudak ML, Tan RC. Committee on Drugs. Committee on Fetus and Newborn. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560.
- Coles CD, Smith IE, Fernhoff PM, Falek A. Neonatal ethanol withdrawal: Characteristics in clinically normal nondysmorphic neonates. J Pediatr. 1984;105(3):445-451.
A mother presents to the ED with her 4-day-old daughter after noting abnormal jerking movements of the neonate's upper extremities. She states the baby has had watery stools for the past day, but has been tolerating bottle formula feeds without vomiting and having appropriate urinary output. The patient was born full-term via normal spontaneous vaginal delivery, with Apgar scores of 8 at 1 minute and 9 at 5 minutes. The postdelivery course was uncomplicated, and both mother and baby were discharged home 2 days after delivery.
Initial vital signs are: heart rate, 135 beats/min; respiratory rate (RR), 48 breaths/min; and temperature, 98.7°F; blood glucose was normal. On physical examination, the baby is awake and well-appearing, with a nonbulging anterior fontanelle, soft, supple neck, and flexed and symmetrically mobile extremities. Moro, suck, rooting, and grasp reflexes are all intact. No abnormal movements are noted. The remainder of the examination is unremarkable.
Do the jerking movements indicate a focal seizure? What could cause these movements in a neonate?
As the length of the postpartum hospital stay has decreased over the past 20 years, EDs have experienced an increase in neonatal visits for conditions that traditionally manifested in newborn nurseries. While most presentations are for benign reasons (eg, issues related to feeding, irritability), patients with concerning conditions, including central nervous system (CNS) abnormalities, may also initially present to the ED. Causes of such clinical findings may be structural (eg, cerebral malformations, subdural hematomas, herpes encephalitis) and/or metabolic (eg, hypoglycemia, hypocalcemia, inborn errors). Many early-onset neonatal seizures are benign and resolve by several months of age, but it is essential to identify those that are consequential and treatable.
Case Continuation
In the evaluation of the neonatal patient with suspected seizure, it is important to take a detailed maternal and labor history, and to consider a broad differential in the face of nonspecific findings. In this case, the patient's mother disclosed a personal history of chronic pain, for which she took buprenorphine 2 mg orally in the morning and 4 mg orally at bedtime (total daily dose of 6mg/day) throughout her pregnancy.
How does drug withdrawal present in the neonate?
Neonatal abstinence syndrome (NAS) is the clinical syndrome of withdrawal in a newborn exposed in utero to drugs capable of inducing dependence. Agents associated with NAS include opioids, benzodiazepines, ethanol, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, and nicotine.1,2
Over the past decade, there has been a 330% rise in the diagnosis of opioid-related NAS alone.3 In response to this increase, the US Food and Drug Administration recently added a black-box warning to all extended-release/long-acting opioid preparations detailing this risk.4
Presenting symptoms of NAS are protean, differ from patient to patient, and are a function of drug type, duration, and amount of drug exposure. NAS may mimic other severe life-threatening conditions such as those previously noted, and the inability to obtain an adequate symptom-based medical history from a neonate further complicates the diagnosis. Before making a diagnosis of NAS, other conditions should be carefully considered in the differential.
Neonatal opioid withdrawal manifests primarily with CNS and gastrointestinal (GI) effects since there are high concentrations of opioid receptors in these areas. Although clinical findings are generally similar among opioid agents, the onset and duration following abstinence varies—largely based on individual drug half-life; this helps to differentiate between opioid agents. For example, while babies exposed to heroin in utero present with signs of NAS within 24 hours of birth, those exposed to buprenorphine or methadone tend to present 2 to 6 days after delivery.1 Between 55% to 94% of neonates with in-utero opioid exposure develop NAS.5
Selective Serotonin Reuptake Inhibitors
SSRIs have also been associated with a neonatal syndrome, and largely involve similar signs and symptoms as NAS. Although the specific etiology is not clear, it has been suggested that this syndrome is the result of serotonin toxicity rather than withdrawal; as such, it is often referred to as "serotonin discontinuation syndrome." Clinical findings occur from several hours to several days after birth and usually resolve within 1 to 2 weeks.6
Cocaine Exposure
In-utero cocaine exposure is also associated with neurobehavioral abnormalities in neonates although a withdrawal syndrome is less clearly defined. Findings, however, are consistent with NAS and include increased irritability, tremors, and high-pitched cry—most frequently occurring between 24 and 48 hours postdelivery.6
Neonatal Alcohol Withdrawal Syndrome
Neonatal alcohol withdrawal syndrome, particularly in fetuses exposed to alcohol during the last trimester, is distinct from fetal alcohol syndrome (FAS). The latter is associated with typical dysmorphic features, growth deficiencies, and CNS findings reflective of permanent neurologic sequelae. Neonatal alcohol withdrawal presents with CNS findings similar to those listed for other in-utero exposures—eg, increased irritability, tremors, nystagmus hyperactive reflexes.7
Screening for NAS: The Finnegan Scale
The Finnegan Neonatal Abstinence Scoring System is one of the most commonly employed and validated tools used to screen for NAS. It comprises a 31-item scale, listing the clinical signs and symptoms of NAS, which are scored by severity and organized by system to include neurologic, metabolic, vasomotor, respiratory, and GI disturbances (Figure). Point allocation is based on mild, moderate, or severe symptoms as follows:
- Mild findings (eg, sweating, fever <101°F mottling, nasal stuffiness) each score 1 point.
- Moderate findings (eg, high-pitched cry, hyperactive moro reflex, increased muscle tone, fever >101°F, increased RR >60 with retractions, poor feeding, loose stools) each score 2 points.
- Severe findings (eg, myoclonic jerks, generalized convulsions, projectile vomiting, watery stools) each score 3 points.
While each of the above are independently nonspecific, the constellation of findings, together with the appropriate history, provide for a clinical diagnosis. The Finnegan Scale is therefore designed not only to aid in diagnosis, but also to quantify the severity of NAS and guide management.
Screening for NAS begins at birth in neonates with known in-utero exposure (ie, when risk of NAS is high) or at the time of initial presentation in other circumstances. Scoring is performed every 4 hours; the first two or three scores will determine the need for pharmacotherapy (see below).
| Pharmacotherapy is indicated in the following Finnegan scoring scenarios: |
|
|
|
How is NAS treated?
The two main goals of management in the treatment of opioid-related NAS are to relieve the signs and symptoms of withdrawal and to prevent complications (eg, fever, weight loss, seizures). Therapy should begin with nonpharmacologic measures that minimize excess external stimuli, such as swaddling, gentle handling, and minimizing noise and light. To prevent weight loss, small hypercaloric feeds may be helpful. If pharmacologic treatment is indicated, oral opioid replacement with morphine is considered by many to be the drug of choice. Oral morphine dosing may be guided by NAS severity based on the Finnegan score; alternatively, initial dosing at 0.1 mg/kg orally every 4 hours has also been recommended.1
Other agents, such methadone 0.1 mg/kg orally every 12 hours and buprenorphine 15.9 mcg/kg divided in three doses orally, may also be used. In patients whose symptoms persist despite opioid treatment, use of adjuncts such as phenobarbital and clonidine may be indicated.
Case Conclusion
The patient was admitted to the neonatal intensive care unit where she appropriately underwent a sepsis workup. Laboratory evaluation, including blood and urine cultures, was obtained. A brain ultrasound was unremarkable, and since lumbar puncture was unsuccessful, the patient was started empirically on meningitis doses of the cefotaxime, vancomycin, and acyclovir.
An initial Finnegan score was calculated. With the exception of soft stools, there were no other persistent symptoms, and patient did not achieve a score indicating a need for pharmacologic management. After 48 hours, she remained afebrile and soft stools resolved. All laboratory values, including cultures, were unremarkable. The patient was discharged on hospital day 3, with a scheduled well-baby follow-up appointment.
| Take Home Points |
|
A mother presents to the ED with her 4-day-old daughter after noting abnormal jerking movements of the neonate's upper extremities. She states the baby has had watery stools for the past day, but has been tolerating bottle formula feeds without vomiting and having appropriate urinary output. The patient was born full-term via normal spontaneous vaginal delivery, with Apgar scores of 8 at 1 minute and 9 at 5 minutes. The postdelivery course was uncomplicated, and both mother and baby were discharged home 2 days after delivery.
Initial vital signs are: heart rate, 135 beats/min; respiratory rate (RR), 48 breaths/min; and temperature, 98.7°F; blood glucose was normal. On physical examination, the baby is awake and well-appearing, with a nonbulging anterior fontanelle, soft, supple neck, and flexed and symmetrically mobile extremities. Moro, suck, rooting, and grasp reflexes are all intact. No abnormal movements are noted. The remainder of the examination is unremarkable.
Do the jerking movements indicate a focal seizure? What could cause these movements in a neonate?
As the length of the postpartum hospital stay has decreased over the past 20 years, EDs have experienced an increase in neonatal visits for conditions that traditionally manifested in newborn nurseries. While most presentations are for benign reasons (eg, issues related to feeding, irritability), patients with concerning conditions, including central nervous system (CNS) abnormalities, may also initially present to the ED. Causes of such clinical findings may be structural (eg, cerebral malformations, subdural hematomas, herpes encephalitis) and/or metabolic (eg, hypoglycemia, hypocalcemia, inborn errors). Many early-onset neonatal seizures are benign and resolve by several months of age, but it is essential to identify those that are consequential and treatable.
Case Continuation
In the evaluation of the neonatal patient with suspected seizure, it is important to take a detailed maternal and labor history, and to consider a broad differential in the face of nonspecific findings. In this case, the patient's mother disclosed a personal history of chronic pain, for which she took buprenorphine 2 mg orally in the morning and 4 mg orally at bedtime (total daily dose of 6mg/day) throughout her pregnancy.
How does drug withdrawal present in the neonate?
Neonatal abstinence syndrome (NAS) is the clinical syndrome of withdrawal in a newborn exposed in utero to drugs capable of inducing dependence. Agents associated with NAS include opioids, benzodiazepines, ethanol, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, and nicotine.1,2
Over the past decade, there has been a 330% rise in the diagnosis of opioid-related NAS alone.3 In response to this increase, the US Food and Drug Administration recently added a black-box warning to all extended-release/long-acting opioid preparations detailing this risk.4
Presenting symptoms of NAS are protean, differ from patient to patient, and are a function of drug type, duration, and amount of drug exposure. NAS may mimic other severe life-threatening conditions such as those previously noted, and the inability to obtain an adequate symptom-based medical history from a neonate further complicates the diagnosis. Before making a diagnosis of NAS, other conditions should be carefully considered in the differential.
Neonatal opioid withdrawal manifests primarily with CNS and gastrointestinal (GI) effects since there are high concentrations of opioid receptors in these areas. Although clinical findings are generally similar among opioid agents, the onset and duration following abstinence varies—largely based on individual drug half-life; this helps to differentiate between opioid agents. For example, while babies exposed to heroin in utero present with signs of NAS within 24 hours of birth, those exposed to buprenorphine or methadone tend to present 2 to 6 days after delivery.1 Between 55% to 94% of neonates with in-utero opioid exposure develop NAS.5
Selective Serotonin Reuptake Inhibitors
SSRIs have also been associated with a neonatal syndrome, and largely involve similar signs and symptoms as NAS. Although the specific etiology is not clear, it has been suggested that this syndrome is the result of serotonin toxicity rather than withdrawal; as such, it is often referred to as "serotonin discontinuation syndrome." Clinical findings occur from several hours to several days after birth and usually resolve within 1 to 2 weeks.6
Cocaine Exposure
In-utero cocaine exposure is also associated with neurobehavioral abnormalities in neonates although a withdrawal syndrome is less clearly defined. Findings, however, are consistent with NAS and include increased irritability, tremors, and high-pitched cry—most frequently occurring between 24 and 48 hours postdelivery.6
Neonatal Alcohol Withdrawal Syndrome
Neonatal alcohol withdrawal syndrome, particularly in fetuses exposed to alcohol during the last trimester, is distinct from fetal alcohol syndrome (FAS). The latter is associated with typical dysmorphic features, growth deficiencies, and CNS findings reflective of permanent neurologic sequelae. Neonatal alcohol withdrawal presents with CNS findings similar to those listed for other in-utero exposures—eg, increased irritability, tremors, nystagmus hyperactive reflexes.7
Screening for NAS: The Finnegan Scale
The Finnegan Neonatal Abstinence Scoring System is one of the most commonly employed and validated tools used to screen for NAS. It comprises a 31-item scale, listing the clinical signs and symptoms of NAS, which are scored by severity and organized by system to include neurologic, metabolic, vasomotor, respiratory, and GI disturbances (Figure). Point allocation is based on mild, moderate, or severe symptoms as follows:
- Mild findings (eg, sweating, fever <101°F mottling, nasal stuffiness) each score 1 point.
- Moderate findings (eg, high-pitched cry, hyperactive moro reflex, increased muscle tone, fever >101°F, increased RR >60 with retractions, poor feeding, loose stools) each score 2 points.
- Severe findings (eg, myoclonic jerks, generalized convulsions, projectile vomiting, watery stools) each score 3 points.
While each of the above are independently nonspecific, the constellation of findings, together with the appropriate history, provide for a clinical diagnosis. The Finnegan Scale is therefore designed not only to aid in diagnosis, but also to quantify the severity of NAS and guide management.
Screening for NAS begins at birth in neonates with known in-utero exposure (ie, when risk of NAS is high) or at the time of initial presentation in other circumstances. Scoring is performed every 4 hours; the first two or three scores will determine the need for pharmacotherapy (see below).
| Pharmacotherapy is indicated in the following Finnegan scoring scenarios: |
|
|
|
How is NAS treated?
The two main goals of management in the treatment of opioid-related NAS are to relieve the signs and symptoms of withdrawal and to prevent complications (eg, fever, weight loss, seizures). Therapy should begin with nonpharmacologic measures that minimize excess external stimuli, such as swaddling, gentle handling, and minimizing noise and light. To prevent weight loss, small hypercaloric feeds may be helpful. If pharmacologic treatment is indicated, oral opioid replacement with morphine is considered by many to be the drug of choice. Oral morphine dosing may be guided by NAS severity based on the Finnegan score; alternatively, initial dosing at 0.1 mg/kg orally every 4 hours has also been recommended.1
Other agents, such methadone 0.1 mg/kg orally every 12 hours and buprenorphine 15.9 mcg/kg divided in three doses orally, may also be used. In patients whose symptoms persist despite opioid treatment, use of adjuncts such as phenobarbital and clonidine may be indicated.
Case Conclusion
The patient was admitted to the neonatal intensive care unit where she appropriately underwent a sepsis workup. Laboratory evaluation, including blood and urine cultures, was obtained. A brain ultrasound was unremarkable, and since lumbar puncture was unsuccessful, the patient was started empirically on meningitis doses of the cefotaxime, vancomycin, and acyclovir.
An initial Finnegan score was calculated. With the exception of soft stools, there were no other persistent symptoms, and patient did not achieve a score indicating a need for pharmacologic management. After 48 hours, she remained afebrile and soft stools resolved. All laboratory values, including cultures, were unremarkable. The patient was discharged on hospital day 3, with a scheduled well-baby follow-up appointment.
| Take Home Points |
|
- Cramton RE, Gruchala NE. Babies breaking bad: neonatal and iatrogenic withdrawal syndromes. Curr Opin Pediatr. 2013;25(4): 532-542.
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574-580. http://dx.doi.org/10.1111/j.1360-0443.2010.03170.x Accessed October 24, 2013.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-40.
- New safety measures announced for extended-release and long-acting opioids. US Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed October 24, 2013.
- Burgos AE, Burke BL Jr. Neonatal abstinence syndrome. NeoReviews. 2009;10(5):e222-e228. http://dx.doi.org/10.1542/neo.10-5-e222. Accessed October 24, 2013.
- Hudak ML, Tan RC. Committee on Drugs. Committee on Fetus and Newborn. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560.
- Coles CD, Smith IE, Fernhoff PM, Falek A. Neonatal ethanol withdrawal: Characteristics in clinically normal nondysmorphic neonates. J Pediatr. 1984;105(3):445-451.
- Cramton RE, Gruchala NE. Babies breaking bad: neonatal and iatrogenic withdrawal syndromes. Curr Opin Pediatr. 2013;25(4): 532-542.
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574-580. http://dx.doi.org/10.1111/j.1360-0443.2010.03170.x Accessed October 24, 2013.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-40.
- New safety measures announced for extended-release and long-acting opioids. US Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed October 24, 2013.
- Burgos AE, Burke BL Jr. Neonatal abstinence syndrome. NeoReviews. 2009;10(5):e222-e228. http://dx.doi.org/10.1542/neo.10-5-e222. Accessed October 24, 2013.
- Hudak ML, Tan RC. Committee on Drugs. Committee on Fetus and Newborn. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560.
- Coles CD, Smith IE, Fernhoff PM, Falek A. Neonatal ethanol withdrawal: Characteristics in clinically normal nondysmorphic neonates. J Pediatr. 1984;105(3):445-451.
Case Studies in ToxicologyNeonatal Seizure: Sepsis or Toxic Syndrome?
Neonatal Seizure: Sepsis or Toxic Syndrome?
A mother presents to the ED with her 4-day-old daughter after noting abnormal jerking movements of the neonate's upper extremities. She states the baby has had watery stools for the past day, but has been tolerating bottle formula feeds without vomiting and having appropriate urinary output. The patient was born full-term via normal spontaneous vaginal delivery, with Apgar scores of 8 at 1 minute and 9 at 5 minutes. The postdelivery course was uncomplicated, and both mother and baby were discharged home 2 days after delivery.
Initial vital signs are: heart rate, 135 beats/min; respiratory rate (RR), 48 breaths/min; and temperature, 98.7°F; blood glucose was normal. On physical examination, the baby is awake and well-appearing, with a nonbulging anterior fontanelle, soft, supple neck, and flexed and symmetrically mobile extremities. Moro, suck, rooting, and grasp reflexes are all intact. No abnormal movements are noted. The remainder of the examination is unremarkable.
Do the jerking movements indicate a focal seizure? What could cause these movements in a neonate?
As the length of the postpartum hospital stay has decreased over the past 20 years, EDs have experienced an increase in neonatal visits for conditions that traditionally manifested in newborn nurseries. While most presentations are for benign reasons (eg, issues related to feeding, irritability), patients with concerning conditions, including central nervous system (CNS) abnormalities, may also initially present to the ED. Causes of such clinical findings may be structural (eg, cerebral malformations, subdural hematomas, herpes encephalitis) and/or metabolic (eg, hypoglycemia, hypocalcemia, inborn errors). Many early-onset neonatal seizures are benign and resolve by several months of age, but it is essential to identify those that are consequential and treatable.
Case Continuation
In the evaluation of the neonatal patient with suspected seizure, it is important to take a detailed maternal and labor history, and to consider a broad differential in the face of nonspecific findings. In this case, the patient's mother disclosed a personal history of chronic pain, for which she took buprenorphine 2 mg orally in the morning and 4 mg orally at bedtime (total daily dose of 6mg/day) throughout her pregnancy.
How does drug withdrawal present in the neonate?
Neonatal abstinence syndrome (NAS) is the clinical syndrome of withdrawal in a newborn exposed in utero to drugs capable of inducing dependence. Agents associated with NAS include opioids, benzodiazepines, ethanol, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, and nicotine.1,2
Over the past decade, there has been a 330% rise in the diagnosis of opioid-related NAS alone.3 In response to this increase, the US Food and Drug Administration recently added a black-box warning to all extended-release/long-acting opioid preparations detailing this risk.4
Presenting symptoms of NAS are protean, differ from patient to patient, and are a function of drug type, duration, and amount of drug exposure. NAS may mimic other severe life-threatening conditions such as those previously noted, and the inability to obtain an adequate symptom-based medical history from a neonate further complicates the diagnosis. Before making a diagnosis of NAS, other conditions should be carefully considered in the differential.
Take Home Points |
|
Neonatal opioid withdrawal manifests primarily with CNS and gastrointestinal (GI) effects since there are high concentrations of opioid receptors in these areas. Although clinical findings are generally similar among opioid agents, the onset and duration following abstinence varies—largely based on individual drug half-life; this helps to differentiate between opioid agents. For example, while babies exposed to heroin in utero present with signs of NAS within 24 hours of birth, those exposed to buprenorphine or methadone tend to present 2 to 6 days after delivery.1 Between 55% to 94% of neonates with in-utero opioid exposure develop NAS.5
Select Serotonin Reuptake Inhibitors
SSRIs have also been associated with a neonatal syndrome, and largely involve similar signs and symptoms as NAS. Although the specific etiology is not clear, it has been suggested that this syndrome is the result of serotonin toxicity rather than withdrawal; as such, it is often referred to as "serotonin discontinuation syndrome." Clinical findings occur from several hours to several days after birth and usually resolve within 1 to 2 weeks.6
Cocaine Exposure
In-utero cocaine exposure is also associated with neurobehavioral abnormalities in neonates although a withdrawal syndrome is less clearly defined. Findings, however, are consistent with NAS and include increased irritability, tremors, and high-pitched cry—most frequently occurring between 24 and 48 hours postdelivery.6
Neonatal Alcohol Withdrawal Syndrome
Neonatal alcohol withdrawal syndrome, particularly in fetuses exposed to alcohol during the last trimester, is distinct from fetal alcohol syndrome (FAS). The latter is associated with typical dysmorphic features, growth deficiencies, and CNS findings reflective of permanent neurologic sequelae. Neonatal alcohol withdrawal presents with CNS findings similar to those listed for other in-utero exposures—eg, increased irritability, tremors, nystagmus hyperactive reflexes.7
Screening for NAS: The Finnegan Scale
The Finnegan Neonatal Abstinence Scoring System is one of the most commonly employed and validated tools used to screen for NAS. It comprises a 31-item scale, listing the clinical signs and symptoms of NAS, which are scored by severity and organized by system to include neurologic, metabolic, vasomotor, respiratory, and GI disturbances (Figure). Point allocation is based on mild, moderate, or severe symptoms as follows:
- Mild findings (eg, sweating, fever <101°F mottling, nasal stuffiness) each score 1 point.
- Moderate findings (eg, high-pitched cry, hyperactive moro reflex, increased muscle tone, fever >101°F, increased RR >60 with retractions, poor feeding, loose stools) each score 2 points.
- Severe findings (eg, myoclonic jerks, generalized convulsions, projectile vomiting, watery stools) each score 3 points.
While each of the above are independently nonspecific, the constellation of findings, together with the appropriate history, provide for a clinical diagnosis. The Finnegan Scale is therefore designed not only to aid in diagnosis, but also to quantify the severity of NAS and guide management.
Screening for NAS begins at birth in neonates with known in-utero exposure (ie, when risk of NAS is high) or at the time of initial presentation in other circumstances. Scoring is performed every 4 hours; the first two or three scores will determine the need for pharmacotherapy (see Table).
| Table |
Pharmacotherapy is indicated in the following Finnegan scoring scenarios: |
|
|
|
How is NAS treated?
The two main goals of management in the treatment of opioid-related NAS are to relieve the signs and symptoms of withdrawal and to prevent complications (eg, fever, weight loss, seizures). Therapy should begin with nonpharmacologic measures that minimize excess external stimuli, such as swaddling, gentle handling, and minimizing noise and light. To prevent weight loss, small hypercaloric feeds may be helpful. If pharmacologic treatment is indicated, oral opioid replacement with morphine is considered by many to be the drug of choice. Oral morphine dosing may be guided by NAS severity based on the Finnegan score; alternatively, initial dosing at 0.1 mg/kg orally every 4 hours has also been recommended.1
Other agents, such methadone 0.1 mg/kg orally every 12 hours and buprenorphine 15.9 mcg/kg divided in three doses orally, may also be used. In patients whose symptoms persist despite opioid treatment, use of adjuncts such as phenobarbital and clonidine may be indicated.
Case Conclusion
The patient was admitted to the neonatal intensive care unit where she appropriately underwent a sepsis workup. Laboratory evaluation, including blood and urine cultures, was obtained. A brain ultrasound was unremarkable, and since lumbar puncture was unsuccessful, the patient was started empirically on meningitis doses of the cefotaxime, vancomycin, and acyclovir.
An initial Finnegan score was calculated. With the exception of soft stools, there were no other persistent symptoms, and patient did not achieve a score indicating a need for pharmacologic management. After 48 hours, she remained afebrile and soft stools resolved. All laboratory values, including cultures, were unremarkable. The patient was discharged on hospital day 3, with a scheduled well-baby follow-up appointment.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of "Case Studies in Toxicology," is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Cramton RE, Gruchala NE. Babies breaking bad: neonatal and iatrogenic withdrawal syndromes. Curr Opin Pediatr. 2013;25(4): 532-542.
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574-580. http://dx.doi.org/10.1111/j.1360-0443.2010.03170.x Accessed October 24, 2013.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-40.
- New safety measures announced for extended-release and long-acting opioids. US Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed October 24, 2013.
- Burgos AE, Burke BL Jr. Neonatal abstinence syndrome. NeoReviews. 2009;10(5):e222-e228. http://dx.doi.org/10.1542/neo.10-5-e222. Accessed October 24, 2013.
- Hudak ML, Tan RC. Committee on Drugs. Committee on Fetus and Newborn. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560.
- Coles CD, Smith IE, Fernhoff PM, Falek A. Neonatal ethanol withdrawal: Characteristics in clinically normal nondysmorphic neonates. J Pediatr. 1984;105(3):445-451.
A mother presents to the ED with her 4-day-old daughter after noting abnormal jerking movements of the neonate's upper extremities. She states the baby has had watery stools for the past day, but has been tolerating bottle formula feeds without vomiting and having appropriate urinary output. The patient was born full-term via normal spontaneous vaginal delivery, with Apgar scores of 8 at 1 minute and 9 at 5 minutes. The postdelivery course was uncomplicated, and both mother and baby were discharged home 2 days after delivery.
Initial vital signs are: heart rate, 135 beats/min; respiratory rate (RR), 48 breaths/min; and temperature, 98.7°F; blood glucose was normal. On physical examination, the baby is awake and well-appearing, with a nonbulging anterior fontanelle, soft, supple neck, and flexed and symmetrically mobile extremities. Moro, suck, rooting, and grasp reflexes are all intact. No abnormal movements are noted. The remainder of the examination is unremarkable.
Do the jerking movements indicate a focal seizure? What could cause these movements in a neonate?
As the length of the postpartum hospital stay has decreased over the past 20 years, EDs have experienced an increase in neonatal visits for conditions that traditionally manifested in newborn nurseries. While most presentations are for benign reasons (eg, issues related to feeding, irritability), patients with concerning conditions, including central nervous system (CNS) abnormalities, may also initially present to the ED. Causes of such clinical findings may be structural (eg, cerebral malformations, subdural hematomas, herpes encephalitis) and/or metabolic (eg, hypoglycemia, hypocalcemia, inborn errors). Many early-onset neonatal seizures are benign and resolve by several months of age, but it is essential to identify those that are consequential and treatable.
Case Continuation
In the evaluation of the neonatal patient with suspected seizure, it is important to take a detailed maternal and labor history, and to consider a broad differential in the face of nonspecific findings. In this case, the patient's mother disclosed a personal history of chronic pain, for which she took buprenorphine 2 mg orally in the morning and 4 mg orally at bedtime (total daily dose of 6mg/day) throughout her pregnancy.
How does drug withdrawal present in the neonate?
Neonatal abstinence syndrome (NAS) is the clinical syndrome of withdrawal in a newborn exposed in utero to drugs capable of inducing dependence. Agents associated with NAS include opioids, benzodiazepines, ethanol, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, and nicotine.1,2
Over the past decade, there has been a 330% rise in the diagnosis of opioid-related NAS alone.3 In response to this increase, the US Food and Drug Administration recently added a black-box warning to all extended-release/long-acting opioid preparations detailing this risk.4
Presenting symptoms of NAS are protean, differ from patient to patient, and are a function of drug type, duration, and amount of drug exposure. NAS may mimic other severe life-threatening conditions such as those previously noted, and the inability to obtain an adequate symptom-based medical history from a neonate further complicates the diagnosis. Before making a diagnosis of NAS, other conditions should be carefully considered in the differential.
Take Home Points |
|
Neonatal opioid withdrawal manifests primarily with CNS and gastrointestinal (GI) effects since there are high concentrations of opioid receptors in these areas. Although clinical findings are generally similar among opioid agents, the onset and duration following abstinence varies—largely based on individual drug half-life; this helps to differentiate between opioid agents. For example, while babies exposed to heroin in utero present with signs of NAS within 24 hours of birth, those exposed to buprenorphine or methadone tend to present 2 to 6 days after delivery.1 Between 55% to 94% of neonates with in-utero opioid exposure develop NAS.5
Select Serotonin Reuptake Inhibitors
SSRIs have also been associated with a neonatal syndrome, and largely involve similar signs and symptoms as NAS. Although the specific etiology is not clear, it has been suggested that this syndrome is the result of serotonin toxicity rather than withdrawal; as such, it is often referred to as "serotonin discontinuation syndrome." Clinical findings occur from several hours to several days after birth and usually resolve within 1 to 2 weeks.6
Cocaine Exposure
In-utero cocaine exposure is also associated with neurobehavioral abnormalities in neonates although a withdrawal syndrome is less clearly defined. Findings, however, are consistent with NAS and include increased irritability, tremors, and high-pitched cry—most frequently occurring between 24 and 48 hours postdelivery.6
Neonatal Alcohol Withdrawal Syndrome
Neonatal alcohol withdrawal syndrome, particularly in fetuses exposed to alcohol during the last trimester, is distinct from fetal alcohol syndrome (FAS). The latter is associated with typical dysmorphic features, growth deficiencies, and CNS findings reflective of permanent neurologic sequelae. Neonatal alcohol withdrawal presents with CNS findings similar to those listed for other in-utero exposures—eg, increased irritability, tremors, nystagmus hyperactive reflexes.7
Screening for NAS: The Finnegan Scale
The Finnegan Neonatal Abstinence Scoring System is one of the most commonly employed and validated tools used to screen for NAS. It comprises a 31-item scale, listing the clinical signs and symptoms of NAS, which are scored by severity and organized by system to include neurologic, metabolic, vasomotor, respiratory, and GI disturbances (Figure). Point allocation is based on mild, moderate, or severe symptoms as follows:
- Mild findings (eg, sweating, fever <101°F mottling, nasal stuffiness) each score 1 point.
- Moderate findings (eg, high-pitched cry, hyperactive moro reflex, increased muscle tone, fever >101°F, increased RR >60 with retractions, poor feeding, loose stools) each score 2 points.
- Severe findings (eg, myoclonic jerks, generalized convulsions, projectile vomiting, watery stools) each score 3 points.
While each of the above are independently nonspecific, the constellation of findings, together with the appropriate history, provide for a clinical diagnosis. The Finnegan Scale is therefore designed not only to aid in diagnosis, but also to quantify the severity of NAS and guide management.
Screening for NAS begins at birth in neonates with known in-utero exposure (ie, when risk of NAS is high) or at the time of initial presentation in other circumstances. Scoring is performed every 4 hours; the first two or three scores will determine the need for pharmacotherapy (see Table).
| Table |
Pharmacotherapy is indicated in the following Finnegan scoring scenarios: |
|
|
|
How is NAS treated?
The two main goals of management in the treatment of opioid-related NAS are to relieve the signs and symptoms of withdrawal and to prevent complications (eg, fever, weight loss, seizures). Therapy should begin with nonpharmacologic measures that minimize excess external stimuli, such as swaddling, gentle handling, and minimizing noise and light. To prevent weight loss, small hypercaloric feeds may be helpful. If pharmacologic treatment is indicated, oral opioid replacement with morphine is considered by many to be the drug of choice. Oral morphine dosing may be guided by NAS severity based on the Finnegan score; alternatively, initial dosing at 0.1 mg/kg orally every 4 hours has also been recommended.1
Other agents, such methadone 0.1 mg/kg orally every 12 hours and buprenorphine 15.9 mcg/kg divided in three doses orally, may also be used. In patients whose symptoms persist despite opioid treatment, use of adjuncts such as phenobarbital and clonidine may be indicated.
Case Conclusion
The patient was admitted to the neonatal intensive care unit where she appropriately underwent a sepsis workup. Laboratory evaluation, including blood and urine cultures, was obtained. A brain ultrasound was unremarkable, and since lumbar puncture was unsuccessful, the patient was started empirically on meningitis doses of the cefotaxime, vancomycin, and acyclovir.
An initial Finnegan score was calculated. With the exception of soft stools, there were no other persistent symptoms, and patient did not achieve a score indicating a need for pharmacologic management. After 48 hours, she remained afebrile and soft stools resolved. All laboratory values, including cultures, were unremarkable. The patient was discharged on hospital day 3, with a scheduled well-baby follow-up appointment.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of "Case Studies in Toxicology," is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
A mother presents to the ED with her 4-day-old daughter after noting abnormal jerking movements of the neonate's upper extremities. She states the baby has had watery stools for the past day, but has been tolerating bottle formula feeds without vomiting and having appropriate urinary output. The patient was born full-term via normal spontaneous vaginal delivery, with Apgar scores of 8 at 1 minute and 9 at 5 minutes. The postdelivery course was uncomplicated, and both mother and baby were discharged home 2 days after delivery.
Initial vital signs are: heart rate, 135 beats/min; respiratory rate (RR), 48 breaths/min; and temperature, 98.7°F; blood glucose was normal. On physical examination, the baby is awake and well-appearing, with a nonbulging anterior fontanelle, soft, supple neck, and flexed and symmetrically mobile extremities. Moro, suck, rooting, and grasp reflexes are all intact. No abnormal movements are noted. The remainder of the examination is unremarkable.
Do the jerking movements indicate a focal seizure? What could cause these movements in a neonate?
As the length of the postpartum hospital stay has decreased over the past 20 years, EDs have experienced an increase in neonatal visits for conditions that traditionally manifested in newborn nurseries. While most presentations are for benign reasons (eg, issues related to feeding, irritability), patients with concerning conditions, including central nervous system (CNS) abnormalities, may also initially present to the ED. Causes of such clinical findings may be structural (eg, cerebral malformations, subdural hematomas, herpes encephalitis) and/or metabolic (eg, hypoglycemia, hypocalcemia, inborn errors). Many early-onset neonatal seizures are benign and resolve by several months of age, but it is essential to identify those that are consequential and treatable.
Case Continuation
In the evaluation of the neonatal patient with suspected seizure, it is important to take a detailed maternal and labor history, and to consider a broad differential in the face of nonspecific findings. In this case, the patient's mother disclosed a personal history of chronic pain, for which she took buprenorphine 2 mg orally in the morning and 4 mg orally at bedtime (total daily dose of 6mg/day) throughout her pregnancy.
How does drug withdrawal present in the neonate?
Neonatal abstinence syndrome (NAS) is the clinical syndrome of withdrawal in a newborn exposed in utero to drugs capable of inducing dependence. Agents associated with NAS include opioids, benzodiazepines, ethanol, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, and nicotine.1,2
Over the past decade, there has been a 330% rise in the diagnosis of opioid-related NAS alone.3 In response to this increase, the US Food and Drug Administration recently added a black-box warning to all extended-release/long-acting opioid preparations detailing this risk.4
Presenting symptoms of NAS are protean, differ from patient to patient, and are a function of drug type, duration, and amount of drug exposure. NAS may mimic other severe life-threatening conditions such as those previously noted, and the inability to obtain an adequate symptom-based medical history from a neonate further complicates the diagnosis. Before making a diagnosis of NAS, other conditions should be carefully considered in the differential.
Take Home Points |
|
Neonatal opioid withdrawal manifests primarily with CNS and gastrointestinal (GI) effects since there are high concentrations of opioid receptors in these areas. Although clinical findings are generally similar among opioid agents, the onset and duration following abstinence varies—largely based on individual drug half-life; this helps to differentiate between opioid agents. For example, while babies exposed to heroin in utero present with signs of NAS within 24 hours of birth, those exposed to buprenorphine or methadone tend to present 2 to 6 days after delivery.1 Between 55% to 94% of neonates with in-utero opioid exposure develop NAS.5
Select Serotonin Reuptake Inhibitors
SSRIs have also been associated with a neonatal syndrome, and largely involve similar signs and symptoms as NAS. Although the specific etiology is not clear, it has been suggested that this syndrome is the result of serotonin toxicity rather than withdrawal; as such, it is often referred to as "serotonin discontinuation syndrome." Clinical findings occur from several hours to several days after birth and usually resolve within 1 to 2 weeks.6
Cocaine Exposure
In-utero cocaine exposure is also associated with neurobehavioral abnormalities in neonates although a withdrawal syndrome is less clearly defined. Findings, however, are consistent with NAS and include increased irritability, tremors, and high-pitched cry—most frequently occurring between 24 and 48 hours postdelivery.6
Neonatal Alcohol Withdrawal Syndrome
Neonatal alcohol withdrawal syndrome, particularly in fetuses exposed to alcohol during the last trimester, is distinct from fetal alcohol syndrome (FAS). The latter is associated with typical dysmorphic features, growth deficiencies, and CNS findings reflective of permanent neurologic sequelae. Neonatal alcohol withdrawal presents with CNS findings similar to those listed for other in-utero exposures—eg, increased irritability, tremors, nystagmus hyperactive reflexes.7
Screening for NAS: The Finnegan Scale
The Finnegan Neonatal Abstinence Scoring System is one of the most commonly employed and validated tools used to screen for NAS. It comprises a 31-item scale, listing the clinical signs and symptoms of NAS, which are scored by severity and organized by system to include neurologic, metabolic, vasomotor, respiratory, and GI disturbances (Figure). Point allocation is based on mild, moderate, or severe symptoms as follows:
- Mild findings (eg, sweating, fever <101°F mottling, nasal stuffiness) each score 1 point.
- Moderate findings (eg, high-pitched cry, hyperactive moro reflex, increased muscle tone, fever >101°F, increased RR >60 with retractions, poor feeding, loose stools) each score 2 points.
- Severe findings (eg, myoclonic jerks, generalized convulsions, projectile vomiting, watery stools) each score 3 points.
While each of the above are independently nonspecific, the constellation of findings, together with the appropriate history, provide for a clinical diagnosis. The Finnegan Scale is therefore designed not only to aid in diagnosis, but also to quantify the severity of NAS and guide management.
Screening for NAS begins at birth in neonates with known in-utero exposure (ie, when risk of NAS is high) or at the time of initial presentation in other circumstances. Scoring is performed every 4 hours; the first two or three scores will determine the need for pharmacotherapy (see Table).
| Table |
Pharmacotherapy is indicated in the following Finnegan scoring scenarios: |
|
|
|
How is NAS treated?
The two main goals of management in the treatment of opioid-related NAS are to relieve the signs and symptoms of withdrawal and to prevent complications (eg, fever, weight loss, seizures). Therapy should begin with nonpharmacologic measures that minimize excess external stimuli, such as swaddling, gentle handling, and minimizing noise and light. To prevent weight loss, small hypercaloric feeds may be helpful. If pharmacologic treatment is indicated, oral opioid replacement with morphine is considered by many to be the drug of choice. Oral morphine dosing may be guided by NAS severity based on the Finnegan score; alternatively, initial dosing at 0.1 mg/kg orally every 4 hours has also been recommended.1
Other agents, such methadone 0.1 mg/kg orally every 12 hours and buprenorphine 15.9 mcg/kg divided in three doses orally, may also be used. In patients whose symptoms persist despite opioid treatment, use of adjuncts such as phenobarbital and clonidine may be indicated.
Case Conclusion
The patient was admitted to the neonatal intensive care unit where she appropriately underwent a sepsis workup. Laboratory evaluation, including blood and urine cultures, was obtained. A brain ultrasound was unremarkable, and since lumbar puncture was unsuccessful, the patient was started empirically on meningitis doses of the cefotaxime, vancomycin, and acyclovir.
An initial Finnegan score was calculated. With the exception of soft stools, there were no other persistent symptoms, and patient did not achieve a score indicating a need for pharmacologic management. After 48 hours, she remained afebrile and soft stools resolved. All laboratory values, including cultures, were unremarkable. The patient was discharged on hospital day 3, with a scheduled well-baby follow-up appointment.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of "Case Studies in Toxicology," is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Cramton RE, Gruchala NE. Babies breaking bad: neonatal and iatrogenic withdrawal syndromes. Curr Opin Pediatr. 2013;25(4): 532-542.
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574-580. http://dx.doi.org/10.1111/j.1360-0443.2010.03170.x Accessed October 24, 2013.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-40.
- New safety measures announced for extended-release and long-acting opioids. US Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed October 24, 2013.
- Burgos AE, Burke BL Jr. Neonatal abstinence syndrome. NeoReviews. 2009;10(5):e222-e228. http://dx.doi.org/10.1542/neo.10-5-e222. Accessed October 24, 2013.
- Hudak ML, Tan RC. Committee on Drugs. Committee on Fetus and Newborn. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560.
- Coles CD, Smith IE, Fernhoff PM, Falek A. Neonatal ethanol withdrawal: Characteristics in clinically normal nondysmorphic neonates. J Pediatr. 1984;105(3):445-451.
- Cramton RE, Gruchala NE. Babies breaking bad: neonatal and iatrogenic withdrawal syndromes. Curr Opin Pediatr. 2013;25(4): 532-542.
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574-580. http://dx.doi.org/10.1111/j.1360-0443.2010.03170.x Accessed October 24, 2013.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-40.
- New safety measures announced for extended-release and long-acting opioids. US Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed October 24, 2013.
- Burgos AE, Burke BL Jr. Neonatal abstinence syndrome. NeoReviews. 2009;10(5):e222-e228. http://dx.doi.org/10.1542/neo.10-5-e222. Accessed October 24, 2013.
- Hudak ML, Tan RC. Committee on Drugs. Committee on Fetus and Newborn. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560.
- Coles CD, Smith IE, Fernhoff PM, Falek A. Neonatal ethanol withdrawal: Characteristics in clinically normal nondysmorphic neonates. J Pediatr. 1984;105(3):445-451.
Neonatal Seizure: Sepsis or Toxic Syndrome?
Neonatal Seizure: Sepsis or Toxic Syndrome?