User login
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
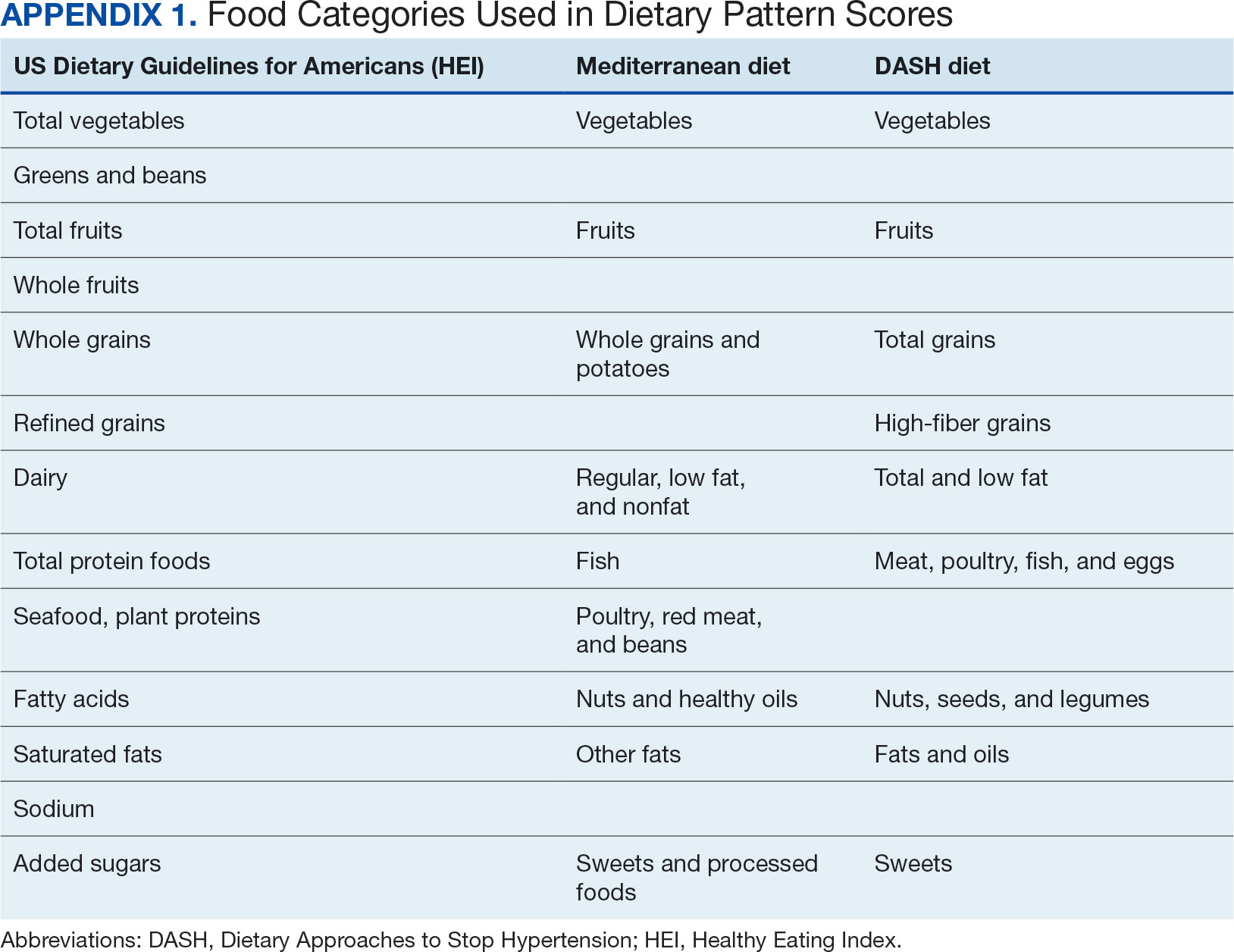
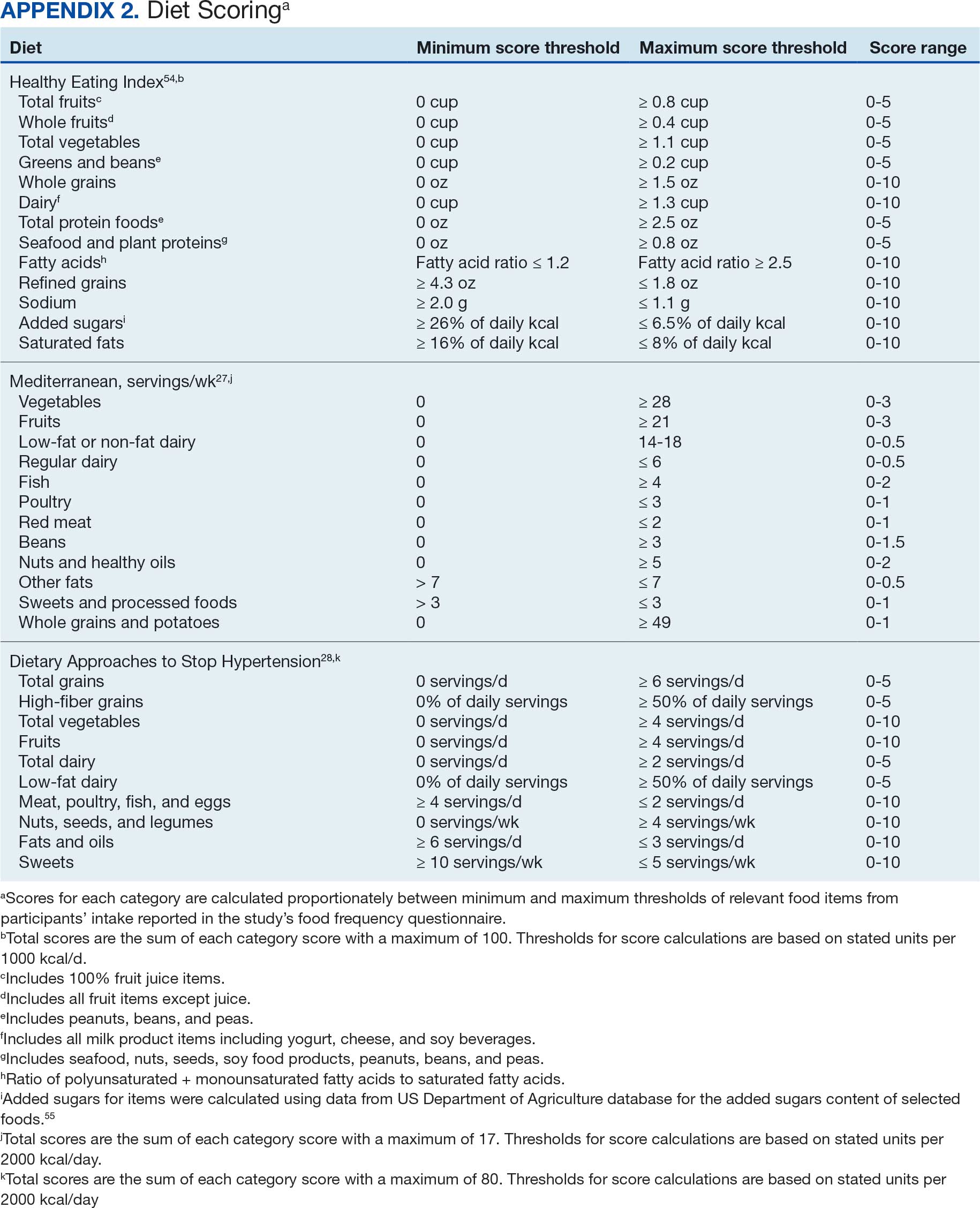
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.
Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
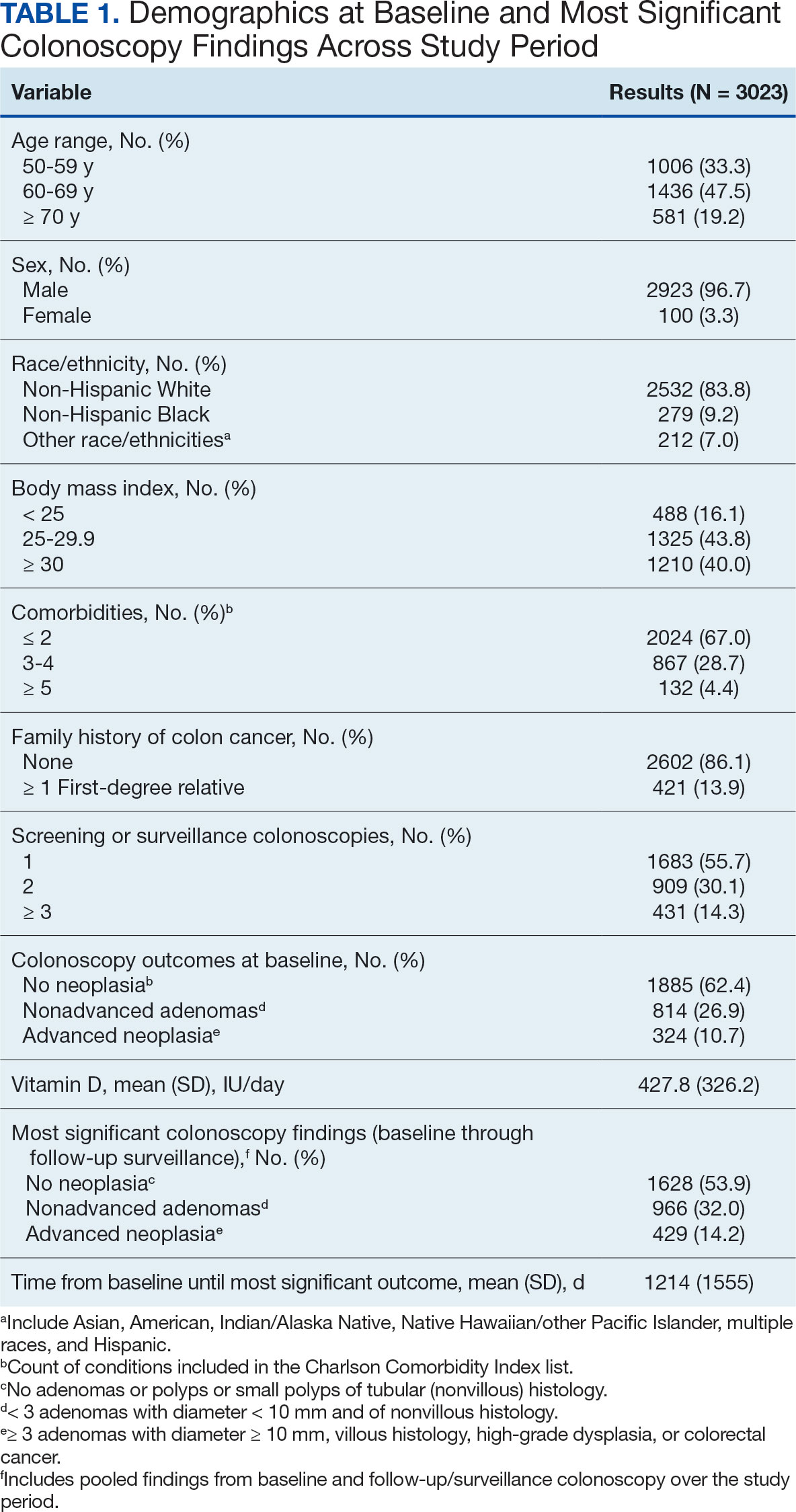
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.
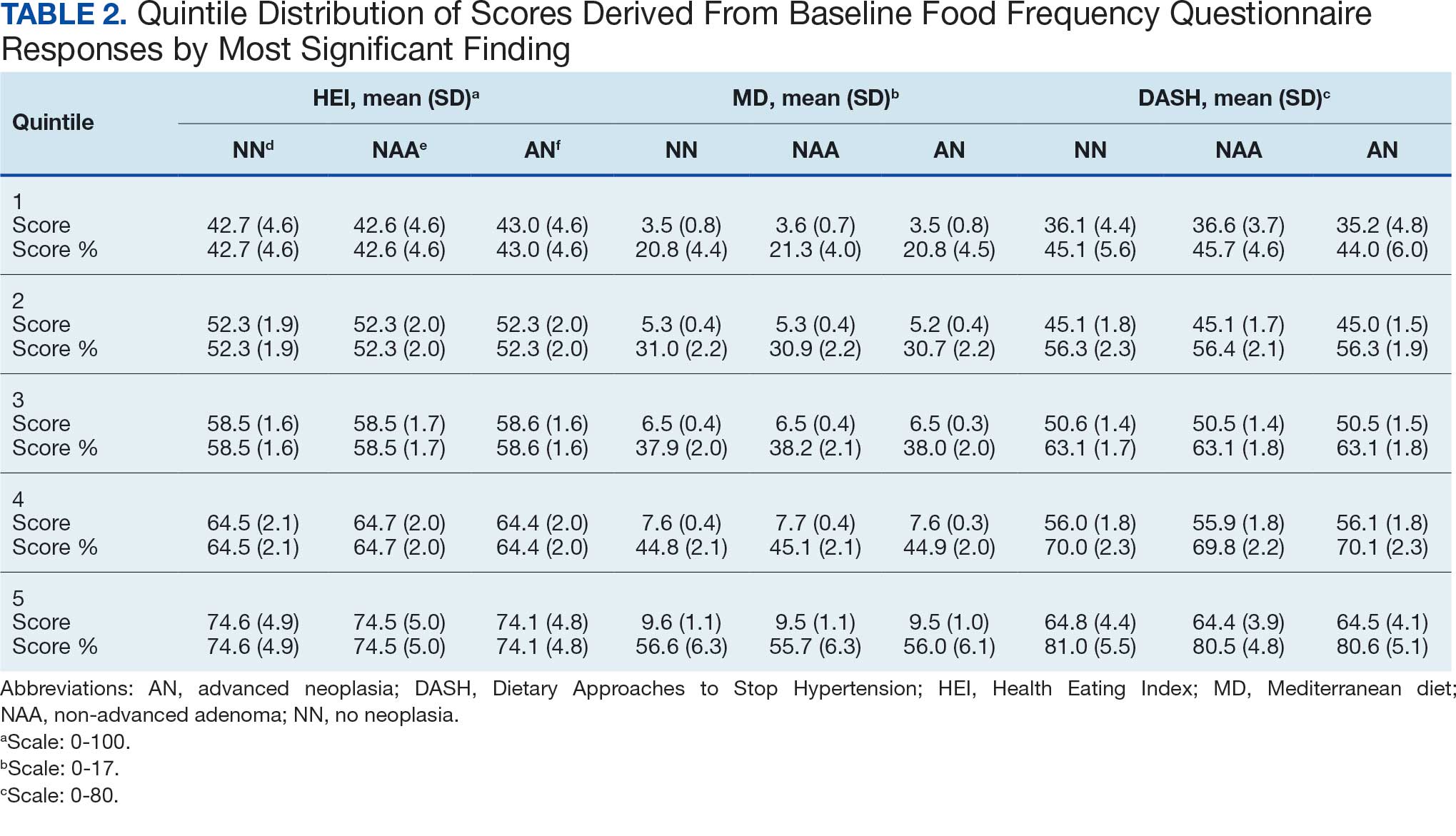
Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).
Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).
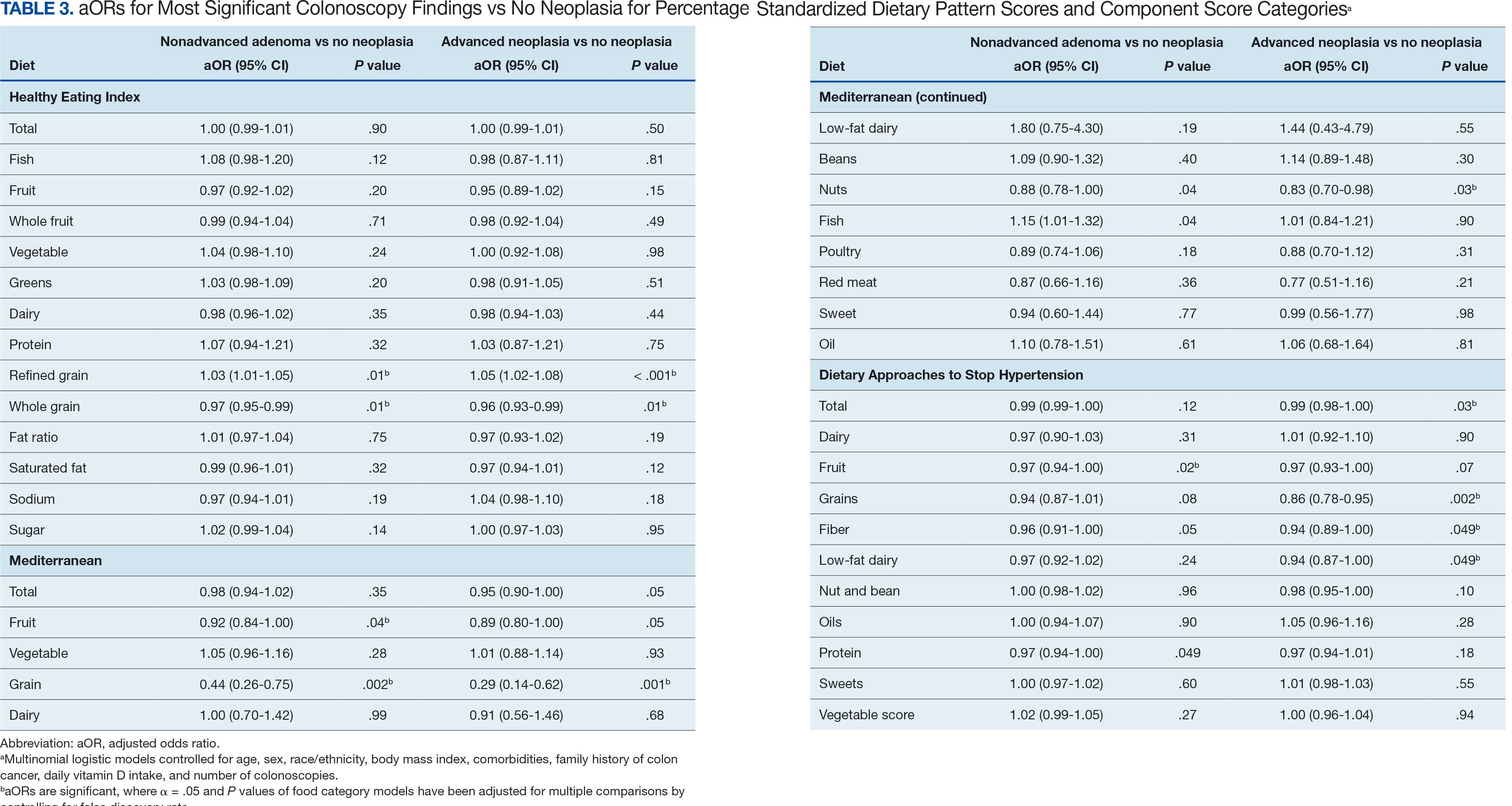
We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.


Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.

Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).

Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).

We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.


Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.

Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).

Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).

We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
