User login
Continuous Glucose Monitoring vs Fingerstick Monitoring for Hemoglobin A1c Control in Veterans
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
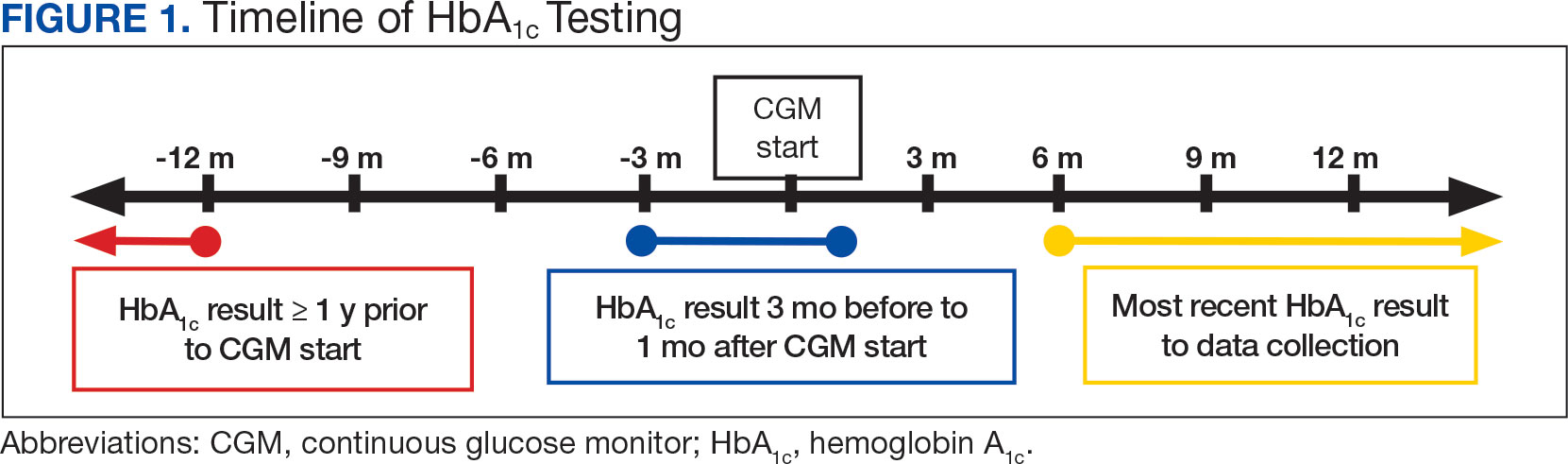
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.
This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.
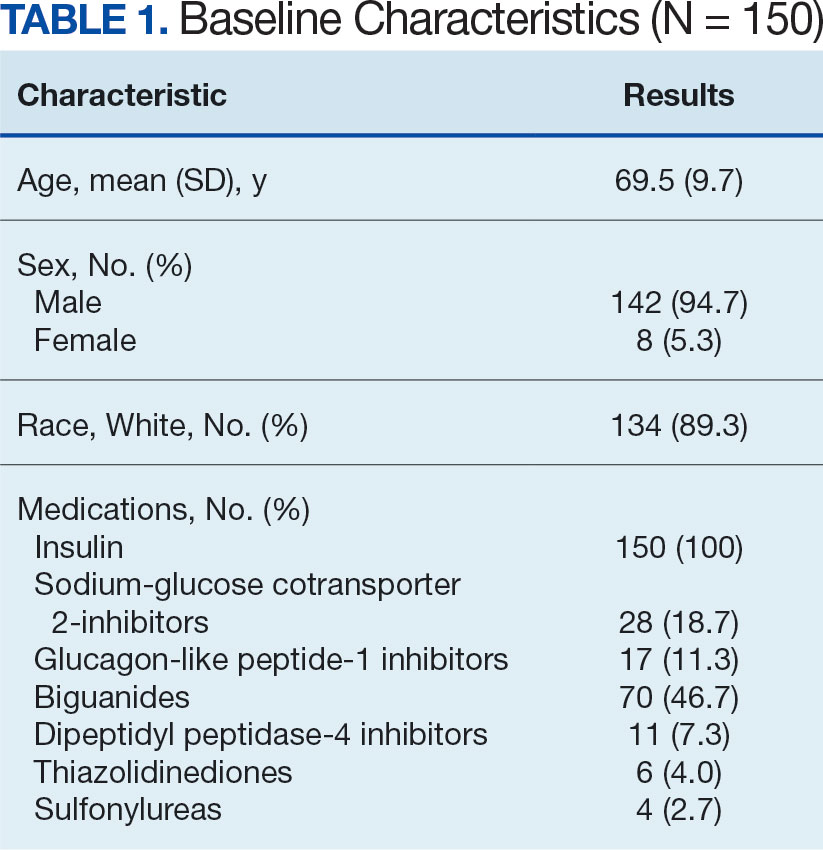
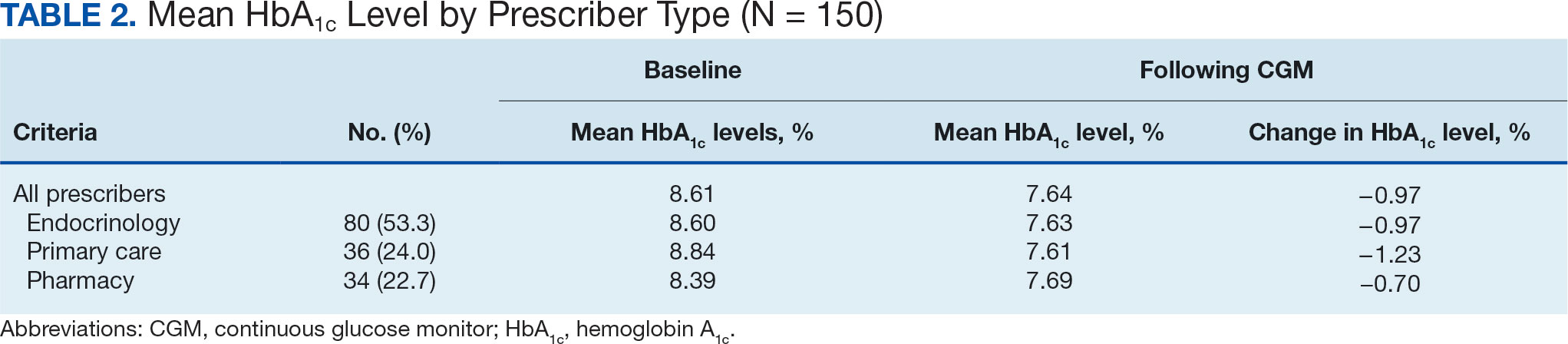
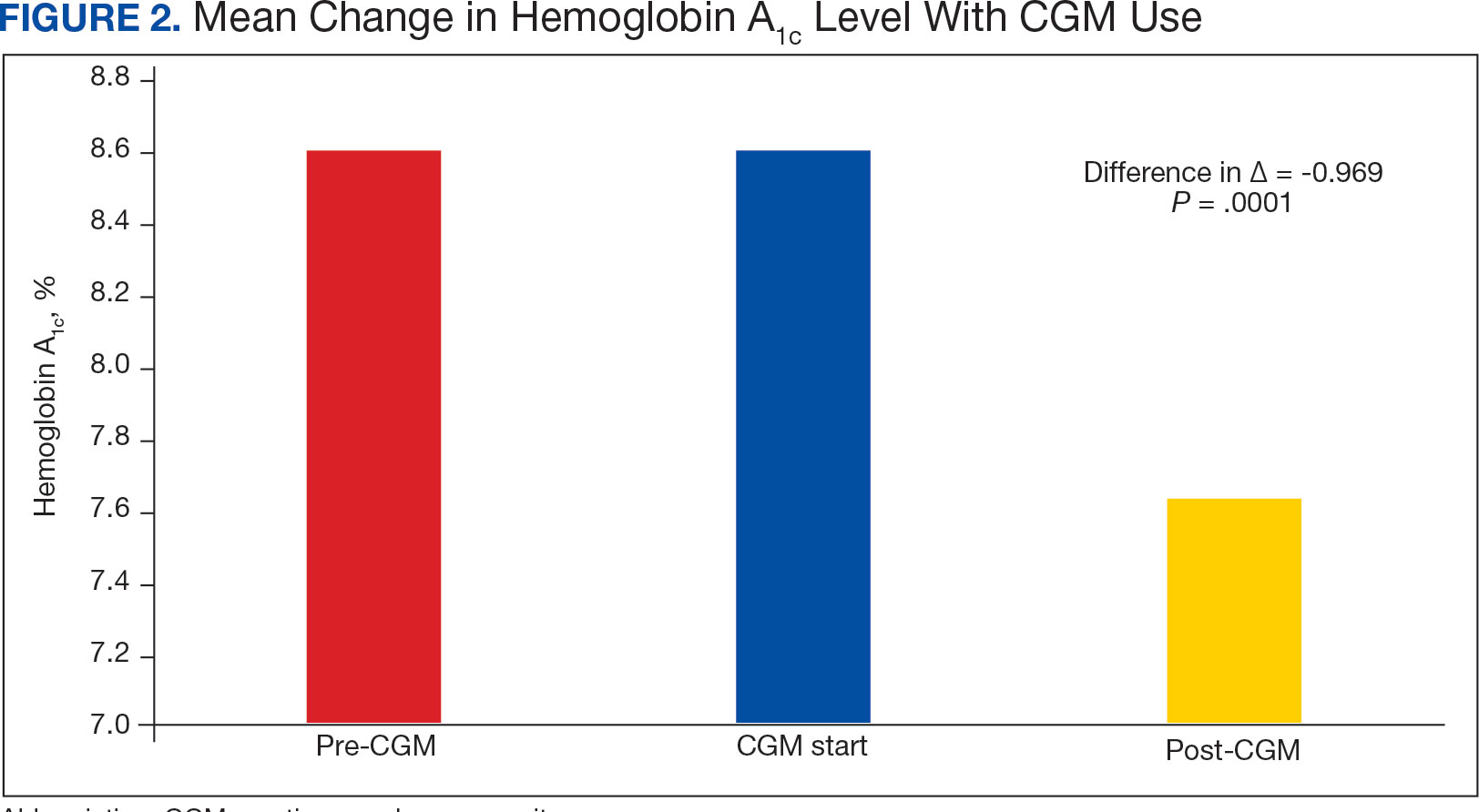
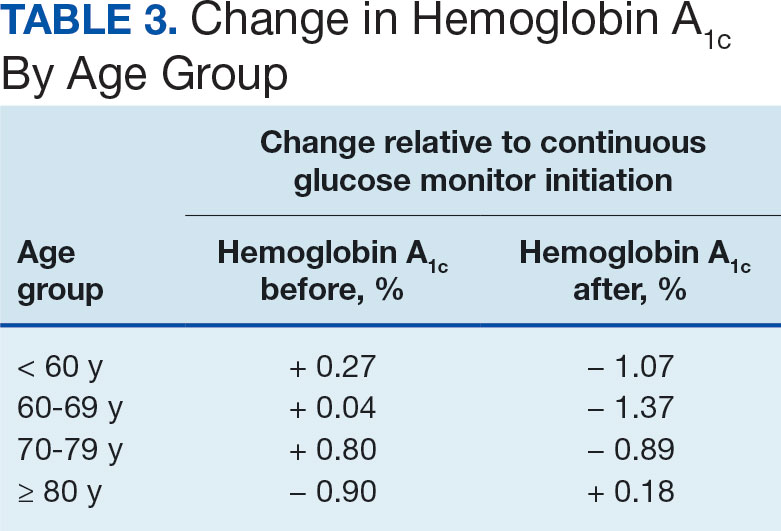
The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.
Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
Weight Gain in Veterans Taking Duloxetine, Pregabalin, or Both for the Treatment of Neuropathy
Neuropathy is the result of damage to the nervous system. This dysfunction generally occurs in peripheral nerves, which are the circuits that transmit signals to the brain and spinal cord. The peripheral nervous system is responsible for controlling motor and autonomic nerves and conduction of sensory information. Injury to the nervous system can lead to changes in nerve fiber sensitivity and malfunctioning of nerve stimuli pathways. Neuropathy may be a sequela of a wide variety of diseases, including diabetes mellitus (DM), autoimmune disorders, infections, and cancer. Also, neuropathy can be caused by medications, trauma, exposure to toxins, classified idiopathic.1-5
Peripheral neuropathy is a common condition with an estimated incidence of > 3 million cases in the United States per year.4 The burden of neuropathy may be greater among veterans, due to a higher prevalence of type 2 DM (T2DM) and an aging population. Manifestations of neuropathy include weakness, numbness, burning or tingling sensations, and lingering pain.3,5 This can lead to limited mobility and decreased quality of life. Neuropathy can be debilitating, but several medications can be used to alleviate symptoms—including duloxetine and pregabalin. The American Diabetes Association recommends either agent as initial treatment for neuropathic pain in patients with DM.2 As with all medication use, the benefits and risks of treatment must be assessed prior to initiation of therapy.
The Centers for Disease Control and Prevention estimates > 70% of adults in the United States are overweight or obese.6 Excessive weight gain causes a higher risk of developing certain comorbidities, such as coronary artery disease, cerebrovascular accident, T2DM, and cancer, and all can lead to premature death. It is important to avoid excessive weight gain whenever possible, especially in patients already at a high risk for developing these diseases.
The correlation of weight gain in patients taking duloxetine, pregabalin, or both is not well studied. Duloxetine has the potential to cause weight gain or weight loss, with reports of > 1% incidence for either effect.7 Clinical significance of weight changes caused by duloxetine is uncertain.Pregabalin is more likely to cause weight gain, with a reported incidence between 2 and 14%.8 Weight gain may be associated with dose and duration; 1 study demonstrated an average weight gain of about 11 lb after 2 years of pregabalin treatment.8 The medical literature lacks information regarding weight gain associated with combination therapy of duloxetine and pregabalin. The objective of this study was to investigate the association of weight gain in veterans taking duloxetine, pregabalin, or both for the treatment of neuropathy.
Methods
A retrospective, single-center, chart review was conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS). Data were collected through manual chart review of US Department of Veterans Affairs (VA) electronic health records (EHRs). Patients included were veterans aged 18 to 89 years who were initiated on duloxetine and/or pregabalin between October 2015 and September 2018.
The primary end point of this study was the change in body weight, expressed in pounds, after 12 to 18 months of treatment. If multiple weights were obtained during the 12- to 18-month period, the weight recorded closest to 12 months was used. The secondary end points included the percent change in body weight and dose effect, which evaluated change in weight at doses of duloxetine > 60 mg/d, and pregabalin at doses > 300 mg/d. Duration of effect was evaluated as a secondary end point; contrary to the primary end point, the weight furthest from 12 months was recorded. The change in hemoglobin A1c (HbA1c) in patients with prediabetes and DM also was investigated as a secondary end point. Last, involvement in the Managing Overweight Veterans Everywhere (MOVE!) weight management program at SFVAHCS and its effect on weight gain was reviewed.
Baseline characteristics were collected to determine the variability between each study group. Data collected during the study included age, sex, race, weight, BMI, HbA1c, eGFR, DM diagnosis, insulin therapy prescription, duration of use, and MOVE! program participation.
Statistical Analysis
The primary and secondary end points were analyzed using an analysis of variance statistical test. Results were considered statistically significant at P < .05.
Results
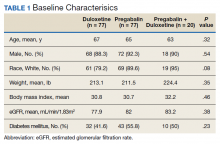
A total of 174 participants were included in this study, with 77 in each monotherapy group, and 22 in the combination therapy group. More than 300 patients were excluded from the study due to prespecified inclusion and exclusion criteria. Baseline characteristics were similar among the 3 groups, with no statistically significant differences identified (Table 1).
Primary End Point
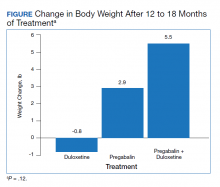
The change in body weight after 12 to 18 months of treatment was –0.8 lb in the duloxetine group, +2.9 lb in the pregabalin group, and +5.5 lb in the pregabalin plus duloxetine group (P = .12) (Figure).
Secondary End Points
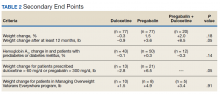
The percent change in body weight after 12 to 18 months of treatment was −0.3% in the duloxetine group, +1.5% in the pregabalin group, and +2.0% in the duloxetine plus pregabalin group (P = .18). The change in body weight beyond 12 months of treatment was −0.9 lb in the duloxetine group, +3.6 lb in the pregabalin group, and +8.5 lb in the duloxetine plus pregabalin group (P = .05). The change in HbA1c in patients with DM and pre-DM was −0.1% in the duloxetine group, +0.3% in the pregabalin group, and −0.3% in the duloxetine plus pregabalin group (P = .14). The change in body weight in patients who received increased doses of the study agents was −2.8 lb in the duloxetine group and +6.5 lb in the pregabalin group (P = .05). Among veterans who participated in MOVE!, change in body weight after 12 to 18 months of treatment was +1.5 lb in the duloxetine group, +4.9 lb in the pregabalin group, and +3.4 lb in the pregabalin plus duloxetine group (P = .91)(Table 2).
Discussion
The purpose of this retrospective chart review was to evaluate the association of weight gain in veterans taking duloxetine and/or pregabalin for the treatment of neuropathy. Although the primary end point, weight gain after 12 to 18 months of therapy, was not statistically significant, we found notable trends and associations worthy of discussion.
The secondary end point of the difference in weight gain in veterans taking duloxetine, pregabalin, or both for a treatment duration > 12 months was statistically significant. For this secondary end point, the weight recorded was when the study agent(s) were discontinued or the most recent weight obtained if participants still had an active prescription; the average duration of treatment in the 3 study groups was about 24 months. These weights differed from the primary end point, in which weight closest to 12 months of therapy was recorded.
The other secondary end point that was statistically significant was the difference in weight gain in patients who were on higher doses of duloxetine or pregabalin. This specifically examined participants who were on doses of duloxetine > 60 mg/d and pregabalin > 300 mg/d. Duloxetine was associated with weight loss, whereas pregabalin was associated with weight gain, with a difference of about 10 lb between the groups. The significance of this secondary end point demonstrates that increased doses of duloxetine and pregabalin are more associated with changes in weight compared with standard doses.
The secondary end points of percent change in body weight, change in HBA1c in patients with DM and prediabetes, and weight gain in patients who participated in the MOVE! weight management program were not statistically significant among the 3 study groups. Given the relatively small sample sizes, more significant differences in the evaluation of the primary and secondary end points may have been observed with a larger patient population.
Study investigators made additional observations beyond the primary and secondary end points. Most notably, > 300 patients were excluded from this study because they did not continue treatment beyond 12 months. The investigators found this number staggering, as it may imply that veterans were not satisfied with treatment agent(s) within 1 year of initiation, which could be due to lack of efficacy or intolerable adverse effects.
The mechanism of why combination therapy of duloxetine and pregabalin may be more associated with weight gain compared with either agent alone is unknown. Since this study found duloxetine to be more associated with weight loss, the mechanism does not seem to be an additive effect. The alternative hypothesis proposed prior to the completion of this study stemmed from an observation seen by health care providers at SFVAHCS.
Limitations
The retrospective nature of the study does not provide proof of causation but does demonstrate association. There was no control group, and the study design did not allow for randomization of participants. Additionally, since the study was completed at a single center, there was potential for selection bias. Future studies could benefit from pursuing a multicenter study design, which may provide a higher level of external validity. There are several confounding factors that have the potential to influence changes in weight, all of which cannot feasibly be accounted for. Since participants were ambulatory veterans, medication adherence could not be confirmed.
Conclusions
There was no difference in weight gain in veterans who took duloxetine, pregabalin, or both for treatment of neuropathy after 12 to 18 months of therapy. However, there was a difference in weight gain between the 3 groups when therapy lasted > 12 months. The combination therapy of pregabalin and duloxetine was associated with the most amount of weight gain, followed by pregabalin alone. Duloxetine monotherapy had minimal impact on weight.
In veterans who took increased doses of duloxetine or pregabalin, there was a statistically significant difference in weight between the monotherapy groups, with pregabalin associated with weight gain and duloxetine associated with weight loss.
For patients in which weight gain may be a concern, it would be reasonable to prefer duloxetine rather than pregabalin for initial treatment of neuropathy. Pregabalin should be used at the lowest effective dose to minimize risk of weight gain. Combination therapy of duloxetine and pregabalin for the treatment of neuropathy seems to be associated with the most amount of weight gain compared with either therapy alone. Association of changes in weight is greater as treatment duration lasts beyond 12 months.
1. Onakpoya IJ, Thomas ET, Lee JJ, Goldacre B, Heneghan CJ. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9(1):e023600. Published 2019 Jan 21. doi:10.1136/bmjopen-2018-023600
2. American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S124-S138. doi:10.2337/dc19-S011
3. Baumann TJ, Herndon CM, Strickland JM. Pain Management. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:925.
4. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. Updated March 16, 2020. Accessed March 10, 2021. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet
5. Feldman EL. Patient education: diabetic neuropathy (beyond the basics). Updated January 20, 2021. Accessed April 21, 2021. https://www.uptodate.com/contents/diabetic-neuropathy-beyond-the-basics
6. Centers for Disease Control and Prevention. Overweight and obesity. Updated October 29, 2020. Accessed March 10, 2021. https://www.cdc.gov/obesity/index.html
7. Cymbalta (duloxetine) [prescribing information]. Eli Lilly and Company; April 2020.
8. Lyrica (pregabalin) [prescribing information]. Parke-Davis, Division of Pfizer Inc; June 2020.
Neuropathy is the result of damage to the nervous system. This dysfunction generally occurs in peripheral nerves, which are the circuits that transmit signals to the brain and spinal cord. The peripheral nervous system is responsible for controlling motor and autonomic nerves and conduction of sensory information. Injury to the nervous system can lead to changes in nerve fiber sensitivity and malfunctioning of nerve stimuli pathways. Neuropathy may be a sequela of a wide variety of diseases, including diabetes mellitus (DM), autoimmune disorders, infections, and cancer. Also, neuropathy can be caused by medications, trauma, exposure to toxins, classified idiopathic.1-5
Peripheral neuropathy is a common condition with an estimated incidence of > 3 million cases in the United States per year.4 The burden of neuropathy may be greater among veterans, due to a higher prevalence of type 2 DM (T2DM) and an aging population. Manifestations of neuropathy include weakness, numbness, burning or tingling sensations, and lingering pain.3,5 This can lead to limited mobility and decreased quality of life. Neuropathy can be debilitating, but several medications can be used to alleviate symptoms—including duloxetine and pregabalin. The American Diabetes Association recommends either agent as initial treatment for neuropathic pain in patients with DM.2 As with all medication use, the benefits and risks of treatment must be assessed prior to initiation of therapy.
The Centers for Disease Control and Prevention estimates > 70% of adults in the United States are overweight or obese.6 Excessive weight gain causes a higher risk of developing certain comorbidities, such as coronary artery disease, cerebrovascular accident, T2DM, and cancer, and all can lead to premature death. It is important to avoid excessive weight gain whenever possible, especially in patients already at a high risk for developing these diseases.
The correlation of weight gain in patients taking duloxetine, pregabalin, or both is not well studied. Duloxetine has the potential to cause weight gain or weight loss, with reports of > 1% incidence for either effect.7 Clinical significance of weight changes caused by duloxetine is uncertain.Pregabalin is more likely to cause weight gain, with a reported incidence between 2 and 14%.8 Weight gain may be associated with dose and duration; 1 study demonstrated an average weight gain of about 11 lb after 2 years of pregabalin treatment.8 The medical literature lacks information regarding weight gain associated with combination therapy of duloxetine and pregabalin. The objective of this study was to investigate the association of weight gain in veterans taking duloxetine, pregabalin, or both for the treatment of neuropathy.
Methods
A retrospective, single-center, chart review was conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS). Data were collected through manual chart review of US Department of Veterans Affairs (VA) electronic health records (EHRs). Patients included were veterans aged 18 to 89 years who were initiated on duloxetine and/or pregabalin between October 2015 and September 2018.
The primary end point of this study was the change in body weight, expressed in pounds, after 12 to 18 months of treatment. If multiple weights were obtained during the 12- to 18-month period, the weight recorded closest to 12 months was used. The secondary end points included the percent change in body weight and dose effect, which evaluated change in weight at doses of duloxetine > 60 mg/d, and pregabalin at doses > 300 mg/d. Duration of effect was evaluated as a secondary end point; contrary to the primary end point, the weight furthest from 12 months was recorded. The change in hemoglobin A1c (HbA1c) in patients with prediabetes and DM also was investigated as a secondary end point. Last, involvement in the Managing Overweight Veterans Everywhere (MOVE!) weight management program at SFVAHCS and its effect on weight gain was reviewed.
Baseline characteristics were collected to determine the variability between each study group. Data collected during the study included age, sex, race, weight, BMI, HbA1c, eGFR, DM diagnosis, insulin therapy prescription, duration of use, and MOVE! program participation.
Statistical Analysis
The primary and secondary end points were analyzed using an analysis of variance statistical test. Results were considered statistically significant at P < .05.
Results
A total of 174 participants were included in this study, with 77 in each monotherapy group, and 22 in the combination therapy group. More than 300 patients were excluded from the study due to prespecified inclusion and exclusion criteria. Baseline characteristics were similar among the 3 groups, with no statistically significant differences identified (Table 1).
Primary End Point
The change in body weight after 12 to 18 months of treatment was –0.8 lb in the duloxetine group, +2.9 lb in the pregabalin group, and +5.5 lb in the pregabalin plus duloxetine group (P = .12) (Figure).
Secondary End Points
The percent change in body weight after 12 to 18 months of treatment was −0.3% in the duloxetine group, +1.5% in the pregabalin group, and +2.0% in the duloxetine plus pregabalin group (P = .18). The change in body weight beyond 12 months of treatment was −0.9 lb in the duloxetine group, +3.6 lb in the pregabalin group, and +8.5 lb in the duloxetine plus pregabalin group (P = .05). The change in HbA1c in patients with DM and pre-DM was −0.1% in the duloxetine group, +0.3% in the pregabalin group, and −0.3% in the duloxetine plus pregabalin group (P = .14). The change in body weight in patients who received increased doses of the study agents was −2.8 lb in the duloxetine group and +6.5 lb in the pregabalin group (P = .05). Among veterans who participated in MOVE!, change in body weight after 12 to 18 months of treatment was +1.5 lb in the duloxetine group, +4.9 lb in the pregabalin group, and +3.4 lb in the pregabalin plus duloxetine group (P = .91)(Table 2).
Discussion
The purpose of this retrospective chart review was to evaluate the association of weight gain in veterans taking duloxetine and/or pregabalin for the treatment of neuropathy. Although the primary end point, weight gain after 12 to 18 months of therapy, was not statistically significant, we found notable trends and associations worthy of discussion.
The secondary end point of the difference in weight gain in veterans taking duloxetine, pregabalin, or both for a treatment duration > 12 months was statistically significant. For this secondary end point, the weight recorded was when the study agent(s) were discontinued or the most recent weight obtained if participants still had an active prescription; the average duration of treatment in the 3 study groups was about 24 months. These weights differed from the primary end point, in which weight closest to 12 months of therapy was recorded.
The other secondary end point that was statistically significant was the difference in weight gain in patients who were on higher doses of duloxetine or pregabalin. This specifically examined participants who were on doses of duloxetine > 60 mg/d and pregabalin > 300 mg/d. Duloxetine was associated with weight loss, whereas pregabalin was associated with weight gain, with a difference of about 10 lb between the groups. The significance of this secondary end point demonstrates that increased doses of duloxetine and pregabalin are more associated with changes in weight compared with standard doses.
The secondary end points of percent change in body weight, change in HBA1c in patients with DM and prediabetes, and weight gain in patients who participated in the MOVE! weight management program were not statistically significant among the 3 study groups. Given the relatively small sample sizes, more significant differences in the evaluation of the primary and secondary end points may have been observed with a larger patient population.
Study investigators made additional observations beyond the primary and secondary end points. Most notably, > 300 patients were excluded from this study because they did not continue treatment beyond 12 months. The investigators found this number staggering, as it may imply that veterans were not satisfied with treatment agent(s) within 1 year of initiation, which could be due to lack of efficacy or intolerable adverse effects.
The mechanism of why combination therapy of duloxetine and pregabalin may be more associated with weight gain compared with either agent alone is unknown. Since this study found duloxetine to be more associated with weight loss, the mechanism does not seem to be an additive effect. The alternative hypothesis proposed prior to the completion of this study stemmed from an observation seen by health care providers at SFVAHCS.
Limitations
The retrospective nature of the study does not provide proof of causation but does demonstrate association. There was no control group, and the study design did not allow for randomization of participants. Additionally, since the study was completed at a single center, there was potential for selection bias. Future studies could benefit from pursuing a multicenter study design, which may provide a higher level of external validity. There are several confounding factors that have the potential to influence changes in weight, all of which cannot feasibly be accounted for. Since participants were ambulatory veterans, medication adherence could not be confirmed.
Conclusions
There was no difference in weight gain in veterans who took duloxetine, pregabalin, or both for treatment of neuropathy after 12 to 18 months of therapy. However, there was a difference in weight gain between the 3 groups when therapy lasted > 12 months. The combination therapy of pregabalin and duloxetine was associated with the most amount of weight gain, followed by pregabalin alone. Duloxetine monotherapy had minimal impact on weight.
In veterans who took increased doses of duloxetine or pregabalin, there was a statistically significant difference in weight between the monotherapy groups, with pregabalin associated with weight gain and duloxetine associated with weight loss.
For patients in which weight gain may be a concern, it would be reasonable to prefer duloxetine rather than pregabalin for initial treatment of neuropathy. Pregabalin should be used at the lowest effective dose to minimize risk of weight gain. Combination therapy of duloxetine and pregabalin for the treatment of neuropathy seems to be associated with the most amount of weight gain compared with either therapy alone. Association of changes in weight is greater as treatment duration lasts beyond 12 months.
Neuropathy is the result of damage to the nervous system. This dysfunction generally occurs in peripheral nerves, which are the circuits that transmit signals to the brain and spinal cord. The peripheral nervous system is responsible for controlling motor and autonomic nerves and conduction of sensory information. Injury to the nervous system can lead to changes in nerve fiber sensitivity and malfunctioning of nerve stimuli pathways. Neuropathy may be a sequela of a wide variety of diseases, including diabetes mellitus (DM), autoimmune disorders, infections, and cancer. Also, neuropathy can be caused by medications, trauma, exposure to toxins, classified idiopathic.1-5
Peripheral neuropathy is a common condition with an estimated incidence of > 3 million cases in the United States per year.4 The burden of neuropathy may be greater among veterans, due to a higher prevalence of type 2 DM (T2DM) and an aging population. Manifestations of neuropathy include weakness, numbness, burning or tingling sensations, and lingering pain.3,5 This can lead to limited mobility and decreased quality of life. Neuropathy can be debilitating, but several medications can be used to alleviate symptoms—including duloxetine and pregabalin. The American Diabetes Association recommends either agent as initial treatment for neuropathic pain in patients with DM.2 As with all medication use, the benefits and risks of treatment must be assessed prior to initiation of therapy.
The Centers for Disease Control and Prevention estimates > 70% of adults in the United States are overweight or obese.6 Excessive weight gain causes a higher risk of developing certain comorbidities, such as coronary artery disease, cerebrovascular accident, T2DM, and cancer, and all can lead to premature death. It is important to avoid excessive weight gain whenever possible, especially in patients already at a high risk for developing these diseases.
The correlation of weight gain in patients taking duloxetine, pregabalin, or both is not well studied. Duloxetine has the potential to cause weight gain or weight loss, with reports of > 1% incidence for either effect.7 Clinical significance of weight changes caused by duloxetine is uncertain.Pregabalin is more likely to cause weight gain, with a reported incidence between 2 and 14%.8 Weight gain may be associated with dose and duration; 1 study demonstrated an average weight gain of about 11 lb after 2 years of pregabalin treatment.8 The medical literature lacks information regarding weight gain associated with combination therapy of duloxetine and pregabalin. The objective of this study was to investigate the association of weight gain in veterans taking duloxetine, pregabalin, or both for the treatment of neuropathy.
Methods
A retrospective, single-center, chart review was conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS). Data were collected through manual chart review of US Department of Veterans Affairs (VA) electronic health records (EHRs). Patients included were veterans aged 18 to 89 years who were initiated on duloxetine and/or pregabalin between October 2015 and September 2018.
The primary end point of this study was the change in body weight, expressed in pounds, after 12 to 18 months of treatment. If multiple weights were obtained during the 12- to 18-month period, the weight recorded closest to 12 months was used. The secondary end points included the percent change in body weight and dose effect, which evaluated change in weight at doses of duloxetine > 60 mg/d, and pregabalin at doses > 300 mg/d. Duration of effect was evaluated as a secondary end point; contrary to the primary end point, the weight furthest from 12 months was recorded. The change in hemoglobin A1c (HbA1c) in patients with prediabetes and DM also was investigated as a secondary end point. Last, involvement in the Managing Overweight Veterans Everywhere (MOVE!) weight management program at SFVAHCS and its effect on weight gain was reviewed.
Baseline characteristics were collected to determine the variability between each study group. Data collected during the study included age, sex, race, weight, BMI, HbA1c, eGFR, DM diagnosis, insulin therapy prescription, duration of use, and MOVE! program participation.
Statistical Analysis
The primary and secondary end points were analyzed using an analysis of variance statistical test. Results were considered statistically significant at P < .05.
Results
A total of 174 participants were included in this study, with 77 in each monotherapy group, and 22 in the combination therapy group. More than 300 patients were excluded from the study due to prespecified inclusion and exclusion criteria. Baseline characteristics were similar among the 3 groups, with no statistically significant differences identified (Table 1).
Primary End Point
The change in body weight after 12 to 18 months of treatment was –0.8 lb in the duloxetine group, +2.9 lb in the pregabalin group, and +5.5 lb in the pregabalin plus duloxetine group (P = .12) (Figure).
Secondary End Points
The percent change in body weight after 12 to 18 months of treatment was −0.3% in the duloxetine group, +1.5% in the pregabalin group, and +2.0% in the duloxetine plus pregabalin group (P = .18). The change in body weight beyond 12 months of treatment was −0.9 lb in the duloxetine group, +3.6 lb in the pregabalin group, and +8.5 lb in the duloxetine plus pregabalin group (P = .05). The change in HbA1c in patients with DM and pre-DM was −0.1% in the duloxetine group, +0.3% in the pregabalin group, and −0.3% in the duloxetine plus pregabalin group (P = .14). The change in body weight in patients who received increased doses of the study agents was −2.8 lb in the duloxetine group and +6.5 lb in the pregabalin group (P = .05). Among veterans who participated in MOVE!, change in body weight after 12 to 18 months of treatment was +1.5 lb in the duloxetine group, +4.9 lb in the pregabalin group, and +3.4 lb in the pregabalin plus duloxetine group (P = .91)(Table 2).
Discussion
The purpose of this retrospective chart review was to evaluate the association of weight gain in veterans taking duloxetine and/or pregabalin for the treatment of neuropathy. Although the primary end point, weight gain after 12 to 18 months of therapy, was not statistically significant, we found notable trends and associations worthy of discussion.
The secondary end point of the difference in weight gain in veterans taking duloxetine, pregabalin, or both for a treatment duration > 12 months was statistically significant. For this secondary end point, the weight recorded was when the study agent(s) were discontinued or the most recent weight obtained if participants still had an active prescription; the average duration of treatment in the 3 study groups was about 24 months. These weights differed from the primary end point, in which weight closest to 12 months of therapy was recorded.
The other secondary end point that was statistically significant was the difference in weight gain in patients who were on higher doses of duloxetine or pregabalin. This specifically examined participants who were on doses of duloxetine > 60 mg/d and pregabalin > 300 mg/d. Duloxetine was associated with weight loss, whereas pregabalin was associated with weight gain, with a difference of about 10 lb between the groups. The significance of this secondary end point demonstrates that increased doses of duloxetine and pregabalin are more associated with changes in weight compared with standard doses.
The secondary end points of percent change in body weight, change in HBA1c in patients with DM and prediabetes, and weight gain in patients who participated in the MOVE! weight management program were not statistically significant among the 3 study groups. Given the relatively small sample sizes, more significant differences in the evaluation of the primary and secondary end points may have been observed with a larger patient population.
Study investigators made additional observations beyond the primary and secondary end points. Most notably, > 300 patients were excluded from this study because they did not continue treatment beyond 12 months. The investigators found this number staggering, as it may imply that veterans were not satisfied with treatment agent(s) within 1 year of initiation, which could be due to lack of efficacy or intolerable adverse effects.
The mechanism of why combination therapy of duloxetine and pregabalin may be more associated with weight gain compared with either agent alone is unknown. Since this study found duloxetine to be more associated with weight loss, the mechanism does not seem to be an additive effect. The alternative hypothesis proposed prior to the completion of this study stemmed from an observation seen by health care providers at SFVAHCS.
Limitations
The retrospective nature of the study does not provide proof of causation but does demonstrate association. There was no control group, and the study design did not allow for randomization of participants. Additionally, since the study was completed at a single center, there was potential for selection bias. Future studies could benefit from pursuing a multicenter study design, which may provide a higher level of external validity. There are several confounding factors that have the potential to influence changes in weight, all of which cannot feasibly be accounted for. Since participants were ambulatory veterans, medication adherence could not be confirmed.
Conclusions
There was no difference in weight gain in veterans who took duloxetine, pregabalin, or both for treatment of neuropathy after 12 to 18 months of therapy. However, there was a difference in weight gain between the 3 groups when therapy lasted > 12 months. The combination therapy of pregabalin and duloxetine was associated with the most amount of weight gain, followed by pregabalin alone. Duloxetine monotherapy had minimal impact on weight.
In veterans who took increased doses of duloxetine or pregabalin, there was a statistically significant difference in weight between the monotherapy groups, with pregabalin associated with weight gain and duloxetine associated with weight loss.
For patients in which weight gain may be a concern, it would be reasonable to prefer duloxetine rather than pregabalin for initial treatment of neuropathy. Pregabalin should be used at the lowest effective dose to minimize risk of weight gain. Combination therapy of duloxetine and pregabalin for the treatment of neuropathy seems to be associated with the most amount of weight gain compared with either therapy alone. Association of changes in weight is greater as treatment duration lasts beyond 12 months.
1. Onakpoya IJ, Thomas ET, Lee JJ, Goldacre B, Heneghan CJ. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9(1):e023600. Published 2019 Jan 21. doi:10.1136/bmjopen-2018-023600
2. American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S124-S138. doi:10.2337/dc19-S011
3. Baumann TJ, Herndon CM, Strickland JM. Pain Management. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:925.
4. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. Updated March 16, 2020. Accessed March 10, 2021. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet
5. Feldman EL. Patient education: diabetic neuropathy (beyond the basics). Updated January 20, 2021. Accessed April 21, 2021. https://www.uptodate.com/contents/diabetic-neuropathy-beyond-the-basics
6. Centers for Disease Control and Prevention. Overweight and obesity. Updated October 29, 2020. Accessed March 10, 2021. https://www.cdc.gov/obesity/index.html
7. Cymbalta (duloxetine) [prescribing information]. Eli Lilly and Company; April 2020.
8. Lyrica (pregabalin) [prescribing information]. Parke-Davis, Division of Pfizer Inc; June 2020.
1. Onakpoya IJ, Thomas ET, Lee JJ, Goldacre B, Heneghan CJ. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9(1):e023600. Published 2019 Jan 21. doi:10.1136/bmjopen-2018-023600
2. American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S124-S138. doi:10.2337/dc19-S011
3. Baumann TJ, Herndon CM, Strickland JM. Pain Management. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:925.
4. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. Updated March 16, 2020. Accessed March 10, 2021. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet
5. Feldman EL. Patient education: diabetic neuropathy (beyond the basics). Updated January 20, 2021. Accessed April 21, 2021. https://www.uptodate.com/contents/diabetic-neuropathy-beyond-the-basics
6. Centers for Disease Control and Prevention. Overweight and obesity. Updated October 29, 2020. Accessed March 10, 2021. https://www.cdc.gov/obesity/index.html
7. Cymbalta (duloxetine) [prescribing information]. Eli Lilly and Company; April 2020.
8. Lyrica (pregabalin) [prescribing information]. Parke-Davis, Division of Pfizer Inc; June 2020.