User login
Exophytic Papule on the Chin of a Child
Exophytic Papule on the Chin of a Child
THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
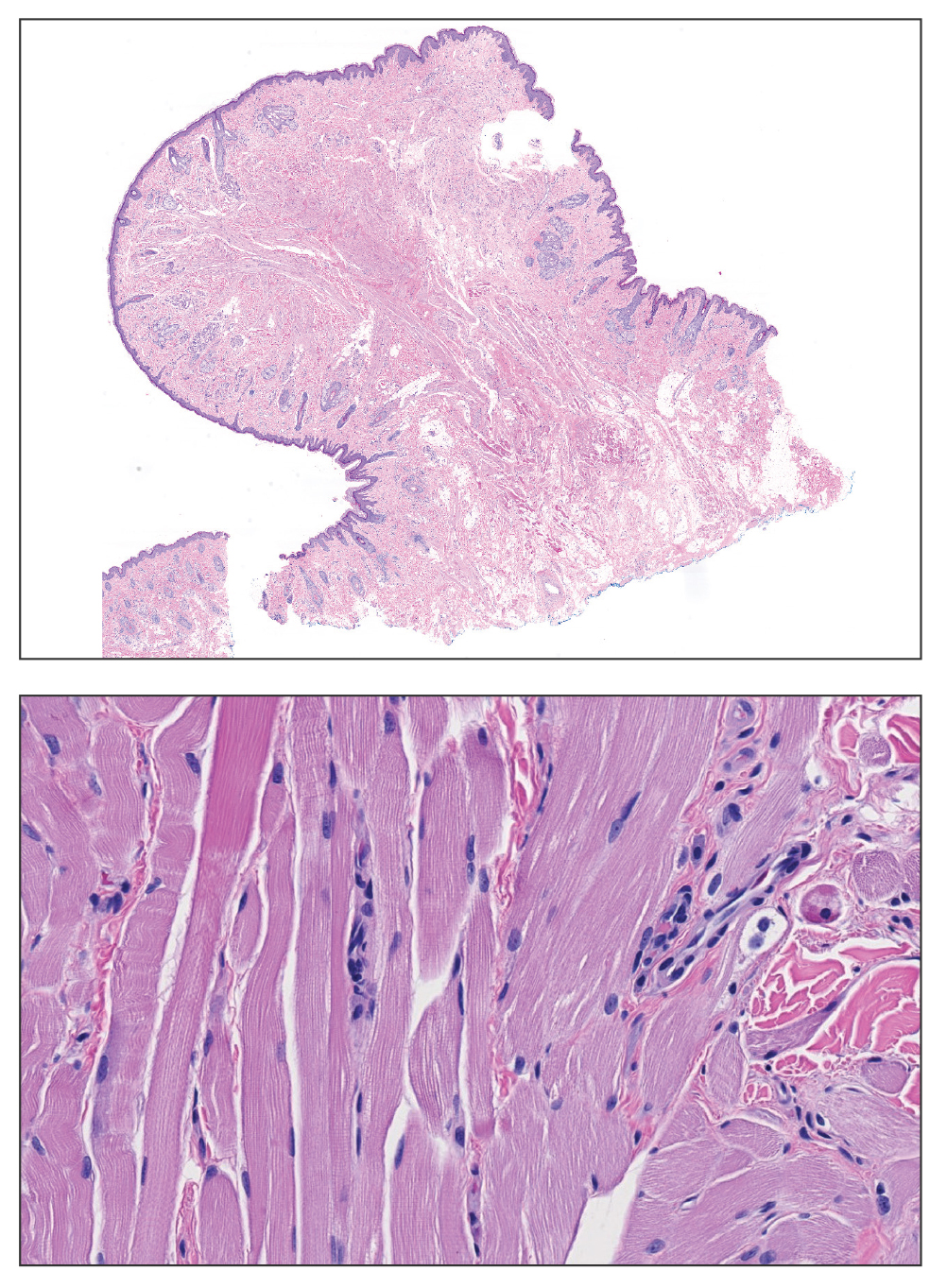
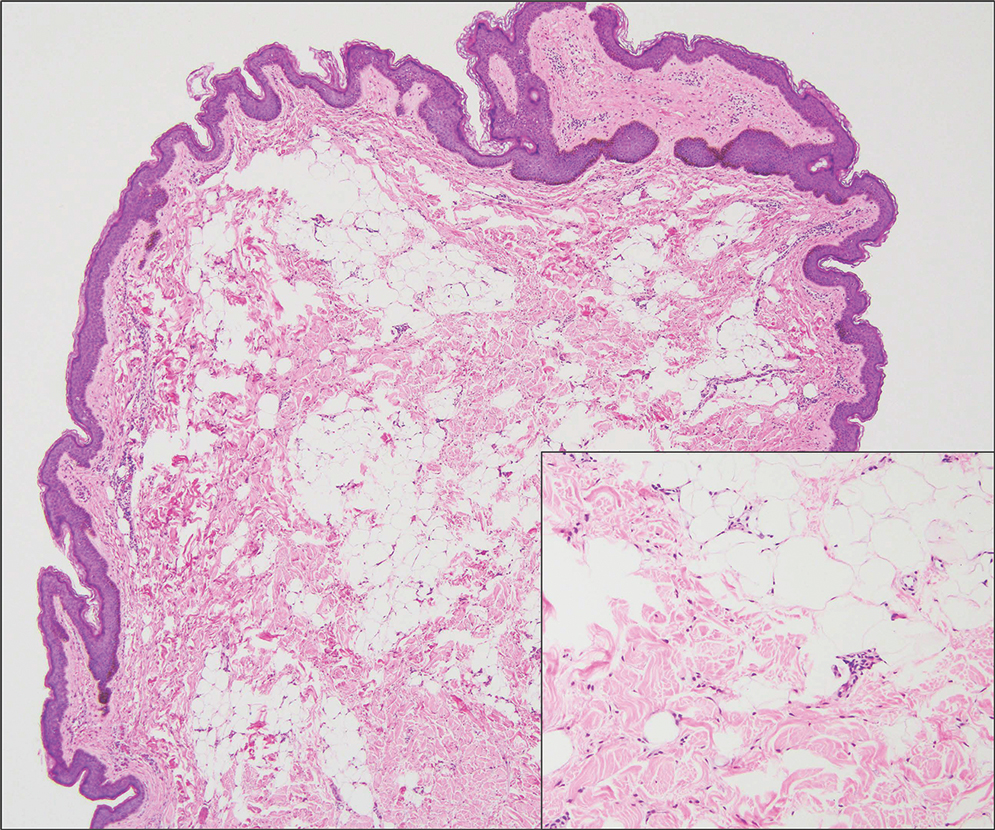
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
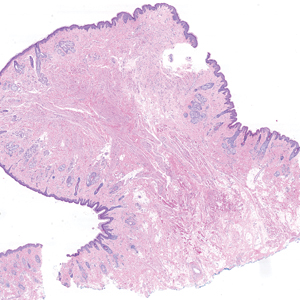
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.
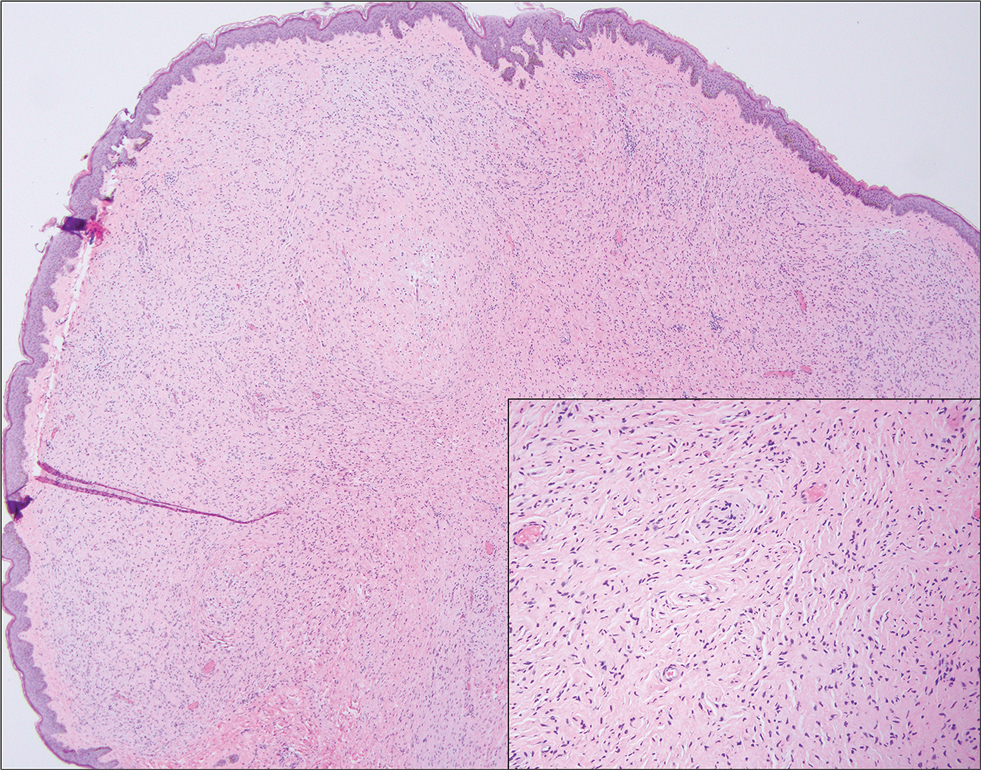
Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.
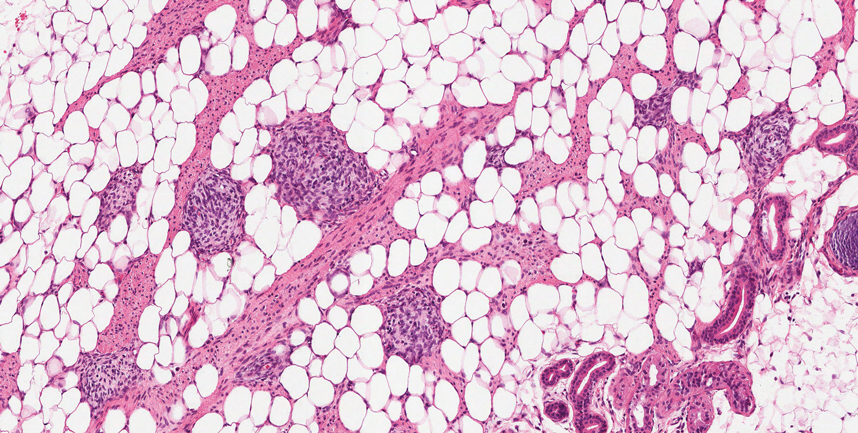
Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.
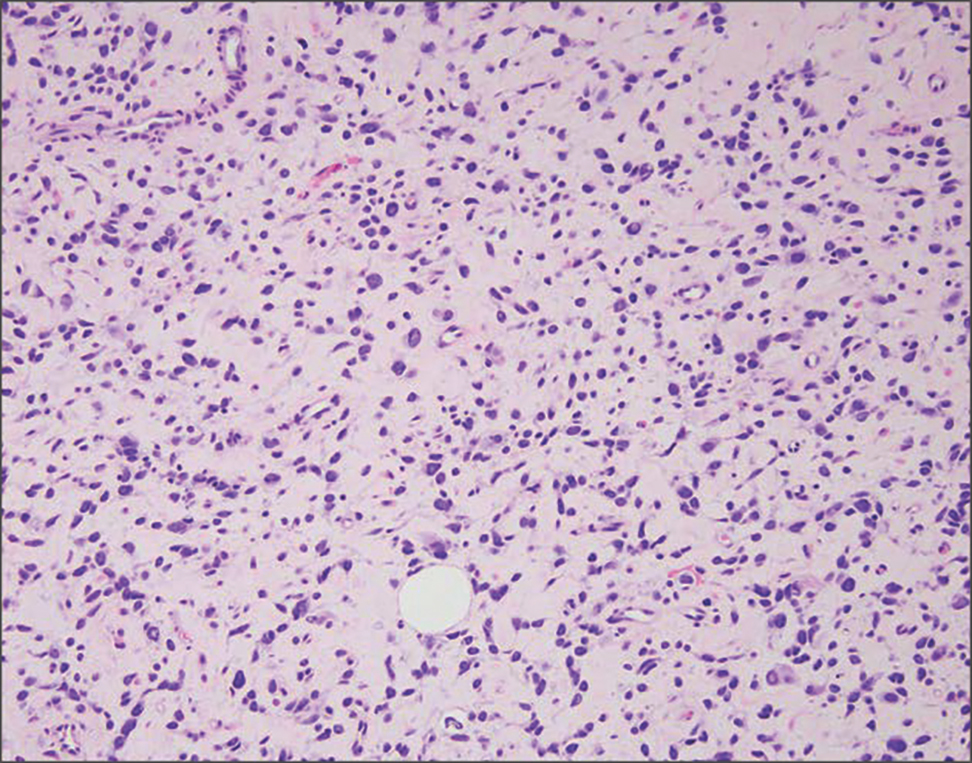
It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14
- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.

Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.

Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.

It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14

THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.

Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.

Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.

It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14

- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
Exophytic Papule on the Chin of a Child
Exophytic Papule on the Chin of a Child
A 3-year-old boy presented to the dermatology department for evaluation of an asymptomatic papule on the chin that had been present since birth. His medical history was otherwise unremarkable. Physical examination revealed a 4×2-mm, flesh-colored, exophytic papule on the midline chin. An excisional biopsy was performed.