User login
A Road Map for Creating a CPRS Template for a Cancer Survivorship Treatment Summary and Care Plan (FULL)
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
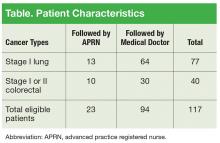
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.