User login
CV risk prediction tools: Imperfect, Yes, but are they serviceable?
Prevention of cardiovascular disease (CVD) requires timely identification of people who are at increased risk in order to target effective dietary, lifestyle, or pharmacotherapeutic intervention—or a combination of the 3. Risk factors for CVD are well understood, but the relative impact of each factor on an individual’s overall risk is difficult to accurately quantify, making a validated CVD risk calculator an important clinical tool.
Despite numerous available CVD risk calculators, one best tool has yet to emerge. This state of affairs has limited the ability of front-line providers who are tasked with primary prevention of CVD—including family physicians (FPs)—to provide the best evidence-based recommendations to patients.
Implications of CVD risk assessment
Baseline CVD risk assessment is the cornerstone of recommendations for primary prevention of CVD, including aspirin and statin therapy. Interventions to lower CVD risk are of greatest benefit to those at highest risk at initiation of therapy. Overall, statins reduce the risk of a first cardiovascular event in otherwise healthy people by approximately 25% over 10 years.1 Because relative risk reduction is fairly consistent across different levels of absolute risk, a 25% relative reduction confers more actual benefit if risk starts at, say, 40% than at 10%.2 In that example, the same 25% reduction in relative risk results in 1) an absolute risk reduction of 10% when risk starts at 40%, compared to an absolute risk reduction of 2.5% when risk starts at 10% and 2) a number needed to treat (NNT) of, respectively, 10 and 40 (over 10 years).
Identifying a person with an elevated risk of developing CVD has multiple implications. Ideally, that patient is motivated to pursue positive therapeutic lifestyle modifications and make changes that positively affect long-term CVD risk. Conversely, that asymptomatic person identified as at elevated risk also becomes a patient with a medical problem that might adversely affect insurance premiums and self-esteem, and may trigger the use of medications with cost and potential adverse effects. Although the benefit of preventive therapy is greater for a patient at higher risk of disease, the harm of a therapy is relatively constant across all risk groups. Accurately discriminating high and low risk of CVD is, therefore, imperative.
The venerable Framingham risk score
Cardiovascular risk prediction has its roots in the late 1940s, when primary risk factors for CVD were not well-understood, with the inception of the Framingham Heart Study. (A greater understanding of CVD risk today notwithstanding, coronary artery disease [CAD] remains the leading cause of death among American adults.) In the late 1940s, blood pressure (BP) was recognized as the single most useful variable for identifying people at high risk of CVD; other variables were understood to be predictive as well. A composite score—the Framingham Risk Score (FRS)—was thereby developed to calculate the probability that CVD would occur over 8 years in a person who was initially free of such disease.3
The original FRS included glucose intolerance and left ventricular hypertrophy (LVH) identified by electrocardiography (EKG) in its algorithm.3 Other, older algorithms also include a family history of premature CVD. In each risk calculator, these variables are treated as dichotomous (Yes or No), but actual risk associated with each variable is in fact more along a continuum. It is now well-recognized that the sensitivity of EKG for accurately detecting LVH is relatively low; more recent algorithms no longer include this component. A family history of premature CVD variably contributes to an individual’s CVD risk; however, its true impact is nearly impossible to accurately quantify, so this variable is also not included in more modern risk calculators.
Caution: The FRS has meaningful limitations
Although the original Framingham cohort has been expanded multiple times since its inception, clinicians and researchers continue to express concern that the predominantly white, middle-class Framingham, Massachusetts, population might not be representative of the United States in general—which would limit the accuracy of the FRS predictive tool when it is applied to a more diverse population. Furthermore, cholesterol-lowering medications were not available when the FRS was first developed. The FRS, therefore, might not accurately estimate risk in more modern populations, in whom aggressive modification of CVD risk factors has resulted in a lower overall rate of atherosclerotic CVD than when the FRS was developed.4
Continue to: Although demographic changes have increasingly...
Although demographic changes have increasingly led to an extension of primary prevention strategies for CAD to elderly people, the FRS has been demonstrated to perform less well in patients older than 70 years, particularly men.5 An ideal CAD prediction model for elderly people should take into account that, with growing age and frailty, CAD events may be increasingly preempted by death from competing non-coronary causes. In addition, the predictive association of typical CVD risk factors diminishes with increasing age.6,7 Koller and colleagues developed a CAD risk prediction model that accounted for death from non-coronary causes and was validated specifically in patients 65 years and older. Koller’s prediction model provided well-calibrated risk estimates, but it was still not substantially more accurate than the FRS—illustrating the overall difficulty in predicting CAD risk in elderly people.8
Alternative risk calculators have come on the scene
Over the past 2 decades, numerous models have been developed in an attempt to overcome the perceived shortcomings of the FRS. A recent systematic review identified 363 prediction models described in the medical literature prior to July 2013.9 The usefulness of most models remains unclear, however, owing to:
- methodological shortcomings,
- considerable heterogeneity in the definitions of outcomes, and
- lack of external validation.
Even models that are well-validated for a specific population suffer from lack of applicability to a broad multinational population.
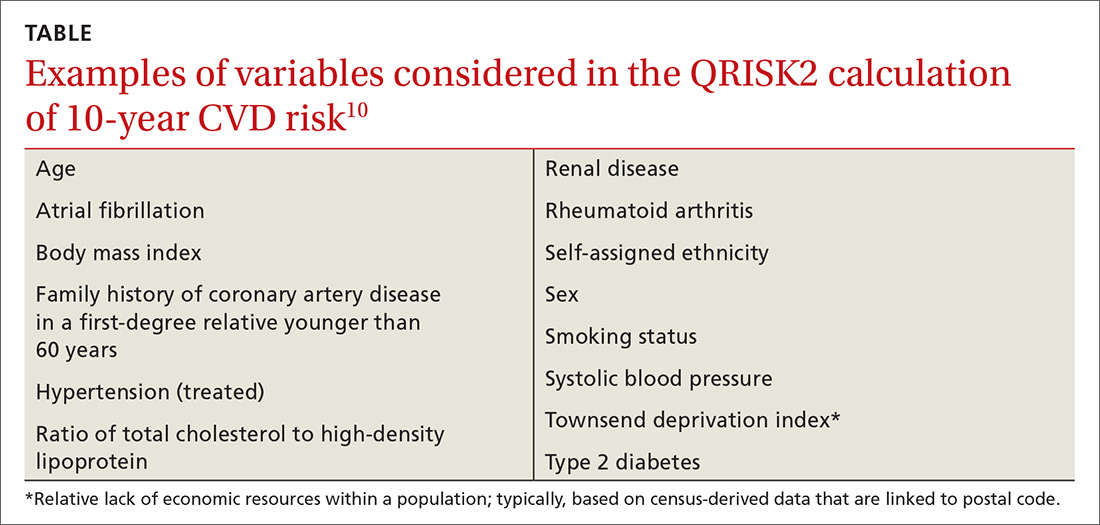
In the United Kingdom (UK), electronic health record systems now have the QRISK2 tool embedded to calculate 10-year CVD risk. This algorithm incorporates multiple traditional and nontraditional risk factors (TABLE10). With the inclusion of additional risk factors and validation performed in a population similar to the one from which the algorithm was derived, QRISK2 predicts CVD risk in the UK population more accurately than the modified FRS does.10 It is not clear, however, whether the same algorithm can be applied to the general US population.
New tool: 2013 ACC/AHA pooled cohort risk equations
In the context of multiple imperfect CVD risk-prediction algorithms, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines published the 2013 Pooled Cohort Risk (PCR) equations to predict 10-year risk of a first atherosclerotic CVD event. The Task Force acknowledged concern that the FRS is based on a cohort that might not accurately represent the general US population. Accordingly, PCR equations were developed from 5 large National Institutes of Health (NIH)-funded cohorts: the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Coronary Artery Risk Development in Young Adults Study.
Continue to: The resulting CVD risk calculator incorporates...
The resulting CVD risk calculator incorporates 4 risk equations: 1 each for African-American and non-Hispanic white males and females.11 Of note, PCR equations are typically used to estimate 10-year CVD risk, but they can be modified to estimate risk over any period. The associated Guideline on the Assessment of Cardiovascular Risk recommends statin therapy for primary prevention of CVD in patients with a predicted 10-year risk ≥7.5% and consideration of statin therapy for patients with a predicted 10-year risk between 5% and 7.5%.12
In late 2016, the US Preventive Services Task Force (USPSTF) recommended low- to moderate-dosage statin therapy in adults 40 to 75 years of age without a history of CVD but with at least 1 CVD risk factor (dyslipidemia, diabetes, hypertension, or smoking), and a PCR-calculated 10-year CVD risk of ≥10%. For people with a PCR-calculated risk of 7.5% to 10%, the USPSTF recommended that clinicians “selectively offer” low- to moderate-dosage statin therapy, noting a smaller likelihood of benefit and uncertainty in an individual’s risk prediction.13
Pooled cohort risk equations have predictive validity
Estimates are that nearly 50% of US adults and as many as 65% of European adults would be candidates for statin therapy if, using PCR equations, the 2013 ACC/AHA guidelines were broadly applied.14 Since PCR equations were released, multiple groups have attempted to evaluate the predictive validity of the algorithm in various populations, with mixed findings.
Data from the 1999-2010 NHANES—the National Health and Nutrition Examination Survey—were used to calculate estimated CVD risk for patients free of atherosclerotic CVD at baseline. Risk prediction using PCR equations was compared to true all-cause and CVD mortality using the National Center for Health Statistics National Death Index. In this large, US adult population without CVD at baseline, PCR-estimated CVD risk was significantly associated with all-cause and CVD-specific mortality risk.15
In a community-based primary prevention cohort, 39% of participants were found statin-eligible—ie, they had an estimated 10-year CVD risk ≥7.5%—by ACC/AHA guidelines, compared with 14% found statin-eligible by the guidelines of the National Cholesterol Education Program’s 2004 updated “Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).” Despite the larger percentage, participants who were statin-eligible by ACC/AHA guidelines had an increased hazard ratio for incident CVD compared with those who were statin-eligible by ATP III; investigators concluded that ACC/AHA guidelines using PCR equations were associated with greater accuracy and efficiency in identifying increased risk of incident CVD.16
Continue to: Pooled cohort risk equations might overestimate CVD risk
Pooled cohort risk equations might overestimate CVD risk
In contrast, a more recent study followed a large, integrated US health-care delivery system population over 5 years, starting in 2008.17 In this group of adults without diabetes, PCR equations substantially overestimated actual 5-year risk of CVD in both sexes and across multiple socioeconomic strata. Similar overestimation of CVD risk was demonstrated in non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic subjects. The latter 2 ethnic groups are considered “white or other” in the atherosclerotic CVD risk equation, raising additional concern that PCR equations may not be accurate for broad, multiethnic application.17 The ACC/AHA Cardiovascular Risk Assessment guideline recognizes this concern, as well, noting that PCR equations may overestimate risk for Hispanic and Asian Americans.12
Predicted 10-year CVD risk using PCR equations was compared with observed event rates in 3 large-scale primary prevention cohorts: the Women’s Health Study, the Physicians’ Health Study, and the Women’s Health Initiative Observational Study.18 In each cohort, the ACC/AHA risk prediction algorithm overestimated observed risk by 75% to 150%. The authors concluded that 40% to 50% of the 33 million middle-aged Americans deemed statin-eligible by ACC/AHA guidelines may not have actual CVD risk that exceeds the 7.5% threshold recommended for statin treatment.18
Therefore, the discrimination of PCR equations—their ability to differentiate between individuals who are more or less likely to develop clinical CVD—is good. The calibration of the equations—the difference between predicted and observed risk—is not as good, however: PCR equations appear to overestimate actual risk in many groups.15
Additional limitations to pooled cohort risk equations
The predictive value of PCR equations is hampered by several factors:
- Despite expansion of the studied cohorts beyond the original Framingham population, the groups still include people screened for study participation or enrolled in clinical trials. The generalizability of this study population to the diverse population treated in a typical clinical practice is, potentially, limited.
- Use of strategies for primary prevention of CVD (eg, statin therapy, antiplatelet therapy, BP control, blood glucose control) continues to increase. Lowering the risk of CVD in the general population with a broad primary prevention approach effectively widens the gap between observed and equation-predicted CVD risk—and thus strengthens the impression of overestimation of risk by PCR equations.
- Lack of comprehensive surveillance in some studies may result in underassessment of CVD events. In this case, PCR equations would, again, appear to overestimate risk.19
Novel tools are available; their use is qualified
First, newer risk markers offer additional options for improving risk prediction offered by the ACC/AHA PCR equations: Coronary artery calcium, ankle-brachial index, high-sensitivity C-reactive protein, and a family history of CAD are all independently associated with incident CAD. ACC/AHA guidelines suggest that assessment of 1 or more of these variables might be considered an adjunct when risk assessment using PCR equations alone does not offer information for making a clear treatment decision.12
Continue to: Of the 4 risk markers...
Of the 4 risk markers, coronary artery calcium provides the most significant increase in discrimination compared to the FRS alone; comparative data using PCR equations is unavailable.20 ACC/AHA guidelines specifically recommend against routine measurement of carotid intima-media thickness for assessment of risk of a first atherosclerotic event.12
Second, a revised set of PCR equations offers improved discrimination and calibration compared to the 2013 PCR equations. A National Institutes of Health (NIH)-sponsored group updated the equations’ cohort by 1) eliminating the original Framingham Heart Study (FHS) data, which was first collected in 1948, and 2) adding data from the Jackson Heart Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Both new cohorts include patient data from 2000 to 2012. Additionally, the NIH group modified the statistical methods used to derive PCR equations. Although these revised PCR equations offer a substantially more accurate estimate of CVD risk, they have not yet been validated for routine clinical use.21
Bottom line: In prediction there persists imperfection
It is widely held that CVD risk prediction, with subsequent treatment to reduce identified risk, is an important component of an overall strategy to reduce the burden of CVD. Cardiovascular risk factors, such as BP and lipid values, do show limited improvement among populations in which systematic screening is practiced, but the true impact of systematic CVD risk assessment alone for healthy people has yet to be demonstrated in terms of hard clinical outcomes.22
CVD risk prediction is most widely used to inform recommendations for statin treatment. However, ACC/AHA PCR equations might substantially overestimate CVD risk and lead to expanded use of statins in patient populations for which such treatment has less potential benefit. Nonetheless, PCR equations do offer good discrimination between higher-risk and lower-risk people.
CVD risk prediction remains an imperfect science—science that is best used as an adjunct to discussion of comprehensive CVD risk factor modification with the individual patient.
CORRESPONDENCE
Jonathon M. Firnhaber, MD, Brody School of Medicine, East Carolina University, 101 Heart Drive, Greenville, NC 27834; firnhaberj@ecu.edu.
1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD004816.
2. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
3. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51.
4. Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can J Cardiol. 2015;31:613-619.
5. Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87-93.
6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245-1249.
7. Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372.
8. Koller MT, Leening MJ, Wolbers M, et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389-397.
9. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Amer Coll Cardiol. 2014;63:2889-2934.
12. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
13. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007.
14. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422-1431.
15. Loprinzi PD, Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease–specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763-769.
16. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134-141.
17. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130.
18. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765.
19. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
20. Yeboah J, McClelland RJ, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788-795.
21. Yadlowsky S, Hayward RA, Sussman JB, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20-29.
22. Dyakova M, Shantikumar S, Colquitt J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016 Jan 29;(1):CD010411.
Prevention of cardiovascular disease (CVD) requires timely identification of people who are at increased risk in order to target effective dietary, lifestyle, or pharmacotherapeutic intervention—or a combination of the 3. Risk factors for CVD are well understood, but the relative impact of each factor on an individual’s overall risk is difficult to accurately quantify, making a validated CVD risk calculator an important clinical tool.
Despite numerous available CVD risk calculators, one best tool has yet to emerge. This state of affairs has limited the ability of front-line providers who are tasked with primary prevention of CVD—including family physicians (FPs)—to provide the best evidence-based recommendations to patients.
Implications of CVD risk assessment
Baseline CVD risk assessment is the cornerstone of recommendations for primary prevention of CVD, including aspirin and statin therapy. Interventions to lower CVD risk are of greatest benefit to those at highest risk at initiation of therapy. Overall, statins reduce the risk of a first cardiovascular event in otherwise healthy people by approximately 25% over 10 years.1 Because relative risk reduction is fairly consistent across different levels of absolute risk, a 25% relative reduction confers more actual benefit if risk starts at, say, 40% than at 10%.2 In that example, the same 25% reduction in relative risk results in 1) an absolute risk reduction of 10% when risk starts at 40%, compared to an absolute risk reduction of 2.5% when risk starts at 10% and 2) a number needed to treat (NNT) of, respectively, 10 and 40 (over 10 years).
Identifying a person with an elevated risk of developing CVD has multiple implications. Ideally, that patient is motivated to pursue positive therapeutic lifestyle modifications and make changes that positively affect long-term CVD risk. Conversely, that asymptomatic person identified as at elevated risk also becomes a patient with a medical problem that might adversely affect insurance premiums and self-esteem, and may trigger the use of medications with cost and potential adverse effects. Although the benefit of preventive therapy is greater for a patient at higher risk of disease, the harm of a therapy is relatively constant across all risk groups. Accurately discriminating high and low risk of CVD is, therefore, imperative.
The venerable Framingham risk score
Cardiovascular risk prediction has its roots in the late 1940s, when primary risk factors for CVD were not well-understood, with the inception of the Framingham Heart Study. (A greater understanding of CVD risk today notwithstanding, coronary artery disease [CAD] remains the leading cause of death among American adults.) In the late 1940s, blood pressure (BP) was recognized as the single most useful variable for identifying people at high risk of CVD; other variables were understood to be predictive as well. A composite score—the Framingham Risk Score (FRS)—was thereby developed to calculate the probability that CVD would occur over 8 years in a person who was initially free of such disease.3
The original FRS included glucose intolerance and left ventricular hypertrophy (LVH) identified by electrocardiography (EKG) in its algorithm.3 Other, older algorithms also include a family history of premature CVD. In each risk calculator, these variables are treated as dichotomous (Yes or No), but actual risk associated with each variable is in fact more along a continuum. It is now well-recognized that the sensitivity of EKG for accurately detecting LVH is relatively low; more recent algorithms no longer include this component. A family history of premature CVD variably contributes to an individual’s CVD risk; however, its true impact is nearly impossible to accurately quantify, so this variable is also not included in more modern risk calculators.
Caution: The FRS has meaningful limitations
Although the original Framingham cohort has been expanded multiple times since its inception, clinicians and researchers continue to express concern that the predominantly white, middle-class Framingham, Massachusetts, population might not be representative of the United States in general—which would limit the accuracy of the FRS predictive tool when it is applied to a more diverse population. Furthermore, cholesterol-lowering medications were not available when the FRS was first developed. The FRS, therefore, might not accurately estimate risk in more modern populations, in whom aggressive modification of CVD risk factors has resulted in a lower overall rate of atherosclerotic CVD than when the FRS was developed.4
Continue to: Although demographic changes have increasingly...
Although demographic changes have increasingly led to an extension of primary prevention strategies for CAD to elderly people, the FRS has been demonstrated to perform less well in patients older than 70 years, particularly men.5 An ideal CAD prediction model for elderly people should take into account that, with growing age and frailty, CAD events may be increasingly preempted by death from competing non-coronary causes. In addition, the predictive association of typical CVD risk factors diminishes with increasing age.6,7 Koller and colleagues developed a CAD risk prediction model that accounted for death from non-coronary causes and was validated specifically in patients 65 years and older. Koller’s prediction model provided well-calibrated risk estimates, but it was still not substantially more accurate than the FRS—illustrating the overall difficulty in predicting CAD risk in elderly people.8
Alternative risk calculators have come on the scene
Over the past 2 decades, numerous models have been developed in an attempt to overcome the perceived shortcomings of the FRS. A recent systematic review identified 363 prediction models described in the medical literature prior to July 2013.9 The usefulness of most models remains unclear, however, owing to:
- methodological shortcomings,
- considerable heterogeneity in the definitions of outcomes, and
- lack of external validation.
Even models that are well-validated for a specific population suffer from lack of applicability to a broad multinational population.
In the United Kingdom (UK), electronic health record systems now have the QRISK2 tool embedded to calculate 10-year CVD risk. This algorithm incorporates multiple traditional and nontraditional risk factors (TABLE10). With the inclusion of additional risk factors and validation performed in a population similar to the one from which the algorithm was derived, QRISK2 predicts CVD risk in the UK population more accurately than the modified FRS does.10 It is not clear, however, whether the same algorithm can be applied to the general US population.
New tool: 2013 ACC/AHA pooled cohort risk equations
In the context of multiple imperfect CVD risk-prediction algorithms, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines published the 2013 Pooled Cohort Risk (PCR) equations to predict 10-year risk of a first atherosclerotic CVD event. The Task Force acknowledged concern that the FRS is based on a cohort that might not accurately represent the general US population. Accordingly, PCR equations were developed from 5 large National Institutes of Health (NIH)-funded cohorts: the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Coronary Artery Risk Development in Young Adults Study.
Continue to: The resulting CVD risk calculator incorporates...
The resulting CVD risk calculator incorporates 4 risk equations: 1 each for African-American and non-Hispanic white males and females.11 Of note, PCR equations are typically used to estimate 10-year CVD risk, but they can be modified to estimate risk over any period. The associated Guideline on the Assessment of Cardiovascular Risk recommends statin therapy for primary prevention of CVD in patients with a predicted 10-year risk ≥7.5% and consideration of statin therapy for patients with a predicted 10-year risk between 5% and 7.5%.12
In late 2016, the US Preventive Services Task Force (USPSTF) recommended low- to moderate-dosage statin therapy in adults 40 to 75 years of age without a history of CVD but with at least 1 CVD risk factor (dyslipidemia, diabetes, hypertension, or smoking), and a PCR-calculated 10-year CVD risk of ≥10%. For people with a PCR-calculated risk of 7.5% to 10%, the USPSTF recommended that clinicians “selectively offer” low- to moderate-dosage statin therapy, noting a smaller likelihood of benefit and uncertainty in an individual’s risk prediction.13
Pooled cohort risk equations have predictive validity
Estimates are that nearly 50% of US adults and as many as 65% of European adults would be candidates for statin therapy if, using PCR equations, the 2013 ACC/AHA guidelines were broadly applied.14 Since PCR equations were released, multiple groups have attempted to evaluate the predictive validity of the algorithm in various populations, with mixed findings.
Data from the 1999-2010 NHANES—the National Health and Nutrition Examination Survey—were used to calculate estimated CVD risk for patients free of atherosclerotic CVD at baseline. Risk prediction using PCR equations was compared to true all-cause and CVD mortality using the National Center for Health Statistics National Death Index. In this large, US adult population without CVD at baseline, PCR-estimated CVD risk was significantly associated with all-cause and CVD-specific mortality risk.15
In a community-based primary prevention cohort, 39% of participants were found statin-eligible—ie, they had an estimated 10-year CVD risk ≥7.5%—by ACC/AHA guidelines, compared with 14% found statin-eligible by the guidelines of the National Cholesterol Education Program’s 2004 updated “Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).” Despite the larger percentage, participants who were statin-eligible by ACC/AHA guidelines had an increased hazard ratio for incident CVD compared with those who were statin-eligible by ATP III; investigators concluded that ACC/AHA guidelines using PCR equations were associated with greater accuracy and efficiency in identifying increased risk of incident CVD.16
Continue to: Pooled cohort risk equations might overestimate CVD risk
Pooled cohort risk equations might overestimate CVD risk
In contrast, a more recent study followed a large, integrated US health-care delivery system population over 5 years, starting in 2008.17 In this group of adults without diabetes, PCR equations substantially overestimated actual 5-year risk of CVD in both sexes and across multiple socioeconomic strata. Similar overestimation of CVD risk was demonstrated in non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic subjects. The latter 2 ethnic groups are considered “white or other” in the atherosclerotic CVD risk equation, raising additional concern that PCR equations may not be accurate for broad, multiethnic application.17 The ACC/AHA Cardiovascular Risk Assessment guideline recognizes this concern, as well, noting that PCR equations may overestimate risk for Hispanic and Asian Americans.12
Predicted 10-year CVD risk using PCR equations was compared with observed event rates in 3 large-scale primary prevention cohorts: the Women’s Health Study, the Physicians’ Health Study, and the Women’s Health Initiative Observational Study.18 In each cohort, the ACC/AHA risk prediction algorithm overestimated observed risk by 75% to 150%. The authors concluded that 40% to 50% of the 33 million middle-aged Americans deemed statin-eligible by ACC/AHA guidelines may not have actual CVD risk that exceeds the 7.5% threshold recommended for statin treatment.18
Therefore, the discrimination of PCR equations—their ability to differentiate between individuals who are more or less likely to develop clinical CVD—is good. The calibration of the equations—the difference between predicted and observed risk—is not as good, however: PCR equations appear to overestimate actual risk in many groups.15
Additional limitations to pooled cohort risk equations
The predictive value of PCR equations is hampered by several factors:
- Despite expansion of the studied cohorts beyond the original Framingham population, the groups still include people screened for study participation or enrolled in clinical trials. The generalizability of this study population to the diverse population treated in a typical clinical practice is, potentially, limited.
- Use of strategies for primary prevention of CVD (eg, statin therapy, antiplatelet therapy, BP control, blood glucose control) continues to increase. Lowering the risk of CVD in the general population with a broad primary prevention approach effectively widens the gap between observed and equation-predicted CVD risk—and thus strengthens the impression of overestimation of risk by PCR equations.
- Lack of comprehensive surveillance in some studies may result in underassessment of CVD events. In this case, PCR equations would, again, appear to overestimate risk.19
Novel tools are available; their use is qualified
First, newer risk markers offer additional options for improving risk prediction offered by the ACC/AHA PCR equations: Coronary artery calcium, ankle-brachial index, high-sensitivity C-reactive protein, and a family history of CAD are all independently associated with incident CAD. ACC/AHA guidelines suggest that assessment of 1 or more of these variables might be considered an adjunct when risk assessment using PCR equations alone does not offer information for making a clear treatment decision.12
Continue to: Of the 4 risk markers...
Of the 4 risk markers, coronary artery calcium provides the most significant increase in discrimination compared to the FRS alone; comparative data using PCR equations is unavailable.20 ACC/AHA guidelines specifically recommend against routine measurement of carotid intima-media thickness for assessment of risk of a first atherosclerotic event.12
Second, a revised set of PCR equations offers improved discrimination and calibration compared to the 2013 PCR equations. A National Institutes of Health (NIH)-sponsored group updated the equations’ cohort by 1) eliminating the original Framingham Heart Study (FHS) data, which was first collected in 1948, and 2) adding data from the Jackson Heart Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Both new cohorts include patient data from 2000 to 2012. Additionally, the NIH group modified the statistical methods used to derive PCR equations. Although these revised PCR equations offer a substantially more accurate estimate of CVD risk, they have not yet been validated for routine clinical use.21
Bottom line: In prediction there persists imperfection
It is widely held that CVD risk prediction, with subsequent treatment to reduce identified risk, is an important component of an overall strategy to reduce the burden of CVD. Cardiovascular risk factors, such as BP and lipid values, do show limited improvement among populations in which systematic screening is practiced, but the true impact of systematic CVD risk assessment alone for healthy people has yet to be demonstrated in terms of hard clinical outcomes.22
CVD risk prediction is most widely used to inform recommendations for statin treatment. However, ACC/AHA PCR equations might substantially overestimate CVD risk and lead to expanded use of statins in patient populations for which such treatment has less potential benefit. Nonetheless, PCR equations do offer good discrimination between higher-risk and lower-risk people.
CVD risk prediction remains an imperfect science—science that is best used as an adjunct to discussion of comprehensive CVD risk factor modification with the individual patient.
CORRESPONDENCE
Jonathon M. Firnhaber, MD, Brody School of Medicine, East Carolina University, 101 Heart Drive, Greenville, NC 27834; firnhaberj@ecu.edu.
Prevention of cardiovascular disease (CVD) requires timely identification of people who are at increased risk in order to target effective dietary, lifestyle, or pharmacotherapeutic intervention—or a combination of the 3. Risk factors for CVD are well understood, but the relative impact of each factor on an individual’s overall risk is difficult to accurately quantify, making a validated CVD risk calculator an important clinical tool.
Despite numerous available CVD risk calculators, one best tool has yet to emerge. This state of affairs has limited the ability of front-line providers who are tasked with primary prevention of CVD—including family physicians (FPs)—to provide the best evidence-based recommendations to patients.
Implications of CVD risk assessment
Baseline CVD risk assessment is the cornerstone of recommendations for primary prevention of CVD, including aspirin and statin therapy. Interventions to lower CVD risk are of greatest benefit to those at highest risk at initiation of therapy. Overall, statins reduce the risk of a first cardiovascular event in otherwise healthy people by approximately 25% over 10 years.1 Because relative risk reduction is fairly consistent across different levels of absolute risk, a 25% relative reduction confers more actual benefit if risk starts at, say, 40% than at 10%.2 In that example, the same 25% reduction in relative risk results in 1) an absolute risk reduction of 10% when risk starts at 40%, compared to an absolute risk reduction of 2.5% when risk starts at 10% and 2) a number needed to treat (NNT) of, respectively, 10 and 40 (over 10 years).
Identifying a person with an elevated risk of developing CVD has multiple implications. Ideally, that patient is motivated to pursue positive therapeutic lifestyle modifications and make changes that positively affect long-term CVD risk. Conversely, that asymptomatic person identified as at elevated risk also becomes a patient with a medical problem that might adversely affect insurance premiums and self-esteem, and may trigger the use of medications with cost and potential adverse effects. Although the benefit of preventive therapy is greater for a patient at higher risk of disease, the harm of a therapy is relatively constant across all risk groups. Accurately discriminating high and low risk of CVD is, therefore, imperative.
The venerable Framingham risk score
Cardiovascular risk prediction has its roots in the late 1940s, when primary risk factors for CVD were not well-understood, with the inception of the Framingham Heart Study. (A greater understanding of CVD risk today notwithstanding, coronary artery disease [CAD] remains the leading cause of death among American adults.) In the late 1940s, blood pressure (BP) was recognized as the single most useful variable for identifying people at high risk of CVD; other variables were understood to be predictive as well. A composite score—the Framingham Risk Score (FRS)—was thereby developed to calculate the probability that CVD would occur over 8 years in a person who was initially free of such disease.3
The original FRS included glucose intolerance and left ventricular hypertrophy (LVH) identified by electrocardiography (EKG) in its algorithm.3 Other, older algorithms also include a family history of premature CVD. In each risk calculator, these variables are treated as dichotomous (Yes or No), but actual risk associated with each variable is in fact more along a continuum. It is now well-recognized that the sensitivity of EKG for accurately detecting LVH is relatively low; more recent algorithms no longer include this component. A family history of premature CVD variably contributes to an individual’s CVD risk; however, its true impact is nearly impossible to accurately quantify, so this variable is also not included in more modern risk calculators.
Caution: The FRS has meaningful limitations
Although the original Framingham cohort has been expanded multiple times since its inception, clinicians and researchers continue to express concern that the predominantly white, middle-class Framingham, Massachusetts, population might not be representative of the United States in general—which would limit the accuracy of the FRS predictive tool when it is applied to a more diverse population. Furthermore, cholesterol-lowering medications were not available when the FRS was first developed. The FRS, therefore, might not accurately estimate risk in more modern populations, in whom aggressive modification of CVD risk factors has resulted in a lower overall rate of atherosclerotic CVD than when the FRS was developed.4
Continue to: Although demographic changes have increasingly...
Although demographic changes have increasingly led to an extension of primary prevention strategies for CAD to elderly people, the FRS has been demonstrated to perform less well in patients older than 70 years, particularly men.5 An ideal CAD prediction model for elderly people should take into account that, with growing age and frailty, CAD events may be increasingly preempted by death from competing non-coronary causes. In addition, the predictive association of typical CVD risk factors diminishes with increasing age.6,7 Koller and colleagues developed a CAD risk prediction model that accounted for death from non-coronary causes and was validated specifically in patients 65 years and older. Koller’s prediction model provided well-calibrated risk estimates, but it was still not substantially more accurate than the FRS—illustrating the overall difficulty in predicting CAD risk in elderly people.8
Alternative risk calculators have come on the scene
Over the past 2 decades, numerous models have been developed in an attempt to overcome the perceived shortcomings of the FRS. A recent systematic review identified 363 prediction models described in the medical literature prior to July 2013.9 The usefulness of most models remains unclear, however, owing to:
- methodological shortcomings,
- considerable heterogeneity in the definitions of outcomes, and
- lack of external validation.
Even models that are well-validated for a specific population suffer from lack of applicability to a broad multinational population.
In the United Kingdom (UK), electronic health record systems now have the QRISK2 tool embedded to calculate 10-year CVD risk. This algorithm incorporates multiple traditional and nontraditional risk factors (TABLE10). With the inclusion of additional risk factors and validation performed in a population similar to the one from which the algorithm was derived, QRISK2 predicts CVD risk in the UK population more accurately than the modified FRS does.10 It is not clear, however, whether the same algorithm can be applied to the general US population.
New tool: 2013 ACC/AHA pooled cohort risk equations
In the context of multiple imperfect CVD risk-prediction algorithms, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines published the 2013 Pooled Cohort Risk (PCR) equations to predict 10-year risk of a first atherosclerotic CVD event. The Task Force acknowledged concern that the FRS is based on a cohort that might not accurately represent the general US population. Accordingly, PCR equations were developed from 5 large National Institutes of Health (NIH)-funded cohorts: the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Coronary Artery Risk Development in Young Adults Study.
Continue to: The resulting CVD risk calculator incorporates...
The resulting CVD risk calculator incorporates 4 risk equations: 1 each for African-American and non-Hispanic white males and females.11 Of note, PCR equations are typically used to estimate 10-year CVD risk, but they can be modified to estimate risk over any period. The associated Guideline on the Assessment of Cardiovascular Risk recommends statin therapy for primary prevention of CVD in patients with a predicted 10-year risk ≥7.5% and consideration of statin therapy for patients with a predicted 10-year risk between 5% and 7.5%.12
In late 2016, the US Preventive Services Task Force (USPSTF) recommended low- to moderate-dosage statin therapy in adults 40 to 75 years of age without a history of CVD but with at least 1 CVD risk factor (dyslipidemia, diabetes, hypertension, or smoking), and a PCR-calculated 10-year CVD risk of ≥10%. For people with a PCR-calculated risk of 7.5% to 10%, the USPSTF recommended that clinicians “selectively offer” low- to moderate-dosage statin therapy, noting a smaller likelihood of benefit and uncertainty in an individual’s risk prediction.13
Pooled cohort risk equations have predictive validity
Estimates are that nearly 50% of US adults and as many as 65% of European adults would be candidates for statin therapy if, using PCR equations, the 2013 ACC/AHA guidelines were broadly applied.14 Since PCR equations were released, multiple groups have attempted to evaluate the predictive validity of the algorithm in various populations, with mixed findings.
Data from the 1999-2010 NHANES—the National Health and Nutrition Examination Survey—were used to calculate estimated CVD risk for patients free of atherosclerotic CVD at baseline. Risk prediction using PCR equations was compared to true all-cause and CVD mortality using the National Center for Health Statistics National Death Index. In this large, US adult population without CVD at baseline, PCR-estimated CVD risk was significantly associated with all-cause and CVD-specific mortality risk.15
In a community-based primary prevention cohort, 39% of participants were found statin-eligible—ie, they had an estimated 10-year CVD risk ≥7.5%—by ACC/AHA guidelines, compared with 14% found statin-eligible by the guidelines of the National Cholesterol Education Program’s 2004 updated “Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).” Despite the larger percentage, participants who were statin-eligible by ACC/AHA guidelines had an increased hazard ratio for incident CVD compared with those who were statin-eligible by ATP III; investigators concluded that ACC/AHA guidelines using PCR equations were associated with greater accuracy and efficiency in identifying increased risk of incident CVD.16
Continue to: Pooled cohort risk equations might overestimate CVD risk
Pooled cohort risk equations might overestimate CVD risk
In contrast, a more recent study followed a large, integrated US health-care delivery system population over 5 years, starting in 2008.17 In this group of adults without diabetes, PCR equations substantially overestimated actual 5-year risk of CVD in both sexes and across multiple socioeconomic strata. Similar overestimation of CVD risk was demonstrated in non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic subjects. The latter 2 ethnic groups are considered “white or other” in the atherosclerotic CVD risk equation, raising additional concern that PCR equations may not be accurate for broad, multiethnic application.17 The ACC/AHA Cardiovascular Risk Assessment guideline recognizes this concern, as well, noting that PCR equations may overestimate risk for Hispanic and Asian Americans.12
Predicted 10-year CVD risk using PCR equations was compared with observed event rates in 3 large-scale primary prevention cohorts: the Women’s Health Study, the Physicians’ Health Study, and the Women’s Health Initiative Observational Study.18 In each cohort, the ACC/AHA risk prediction algorithm overestimated observed risk by 75% to 150%. The authors concluded that 40% to 50% of the 33 million middle-aged Americans deemed statin-eligible by ACC/AHA guidelines may not have actual CVD risk that exceeds the 7.5% threshold recommended for statin treatment.18
Therefore, the discrimination of PCR equations—their ability to differentiate between individuals who are more or less likely to develop clinical CVD—is good. The calibration of the equations—the difference between predicted and observed risk—is not as good, however: PCR equations appear to overestimate actual risk in many groups.15
Additional limitations to pooled cohort risk equations
The predictive value of PCR equations is hampered by several factors:
- Despite expansion of the studied cohorts beyond the original Framingham population, the groups still include people screened for study participation or enrolled in clinical trials. The generalizability of this study population to the diverse population treated in a typical clinical practice is, potentially, limited.
- Use of strategies for primary prevention of CVD (eg, statin therapy, antiplatelet therapy, BP control, blood glucose control) continues to increase. Lowering the risk of CVD in the general population with a broad primary prevention approach effectively widens the gap between observed and equation-predicted CVD risk—and thus strengthens the impression of overestimation of risk by PCR equations.
- Lack of comprehensive surveillance in some studies may result in underassessment of CVD events. In this case, PCR equations would, again, appear to overestimate risk.19
Novel tools are available; their use is qualified
First, newer risk markers offer additional options for improving risk prediction offered by the ACC/AHA PCR equations: Coronary artery calcium, ankle-brachial index, high-sensitivity C-reactive protein, and a family history of CAD are all independently associated with incident CAD. ACC/AHA guidelines suggest that assessment of 1 or more of these variables might be considered an adjunct when risk assessment using PCR equations alone does not offer information for making a clear treatment decision.12
Continue to: Of the 4 risk markers...
Of the 4 risk markers, coronary artery calcium provides the most significant increase in discrimination compared to the FRS alone; comparative data using PCR equations is unavailable.20 ACC/AHA guidelines specifically recommend against routine measurement of carotid intima-media thickness for assessment of risk of a first atherosclerotic event.12
Second, a revised set of PCR equations offers improved discrimination and calibration compared to the 2013 PCR equations. A National Institutes of Health (NIH)-sponsored group updated the equations’ cohort by 1) eliminating the original Framingham Heart Study (FHS) data, which was first collected in 1948, and 2) adding data from the Jackson Heart Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Both new cohorts include patient data from 2000 to 2012. Additionally, the NIH group modified the statistical methods used to derive PCR equations. Although these revised PCR equations offer a substantially more accurate estimate of CVD risk, they have not yet been validated for routine clinical use.21
Bottom line: In prediction there persists imperfection
It is widely held that CVD risk prediction, with subsequent treatment to reduce identified risk, is an important component of an overall strategy to reduce the burden of CVD. Cardiovascular risk factors, such as BP and lipid values, do show limited improvement among populations in which systematic screening is practiced, but the true impact of systematic CVD risk assessment alone for healthy people has yet to be demonstrated in terms of hard clinical outcomes.22
CVD risk prediction is most widely used to inform recommendations for statin treatment. However, ACC/AHA PCR equations might substantially overestimate CVD risk and lead to expanded use of statins in patient populations for which such treatment has less potential benefit. Nonetheless, PCR equations do offer good discrimination between higher-risk and lower-risk people.
CVD risk prediction remains an imperfect science—science that is best used as an adjunct to discussion of comprehensive CVD risk factor modification with the individual patient.
CORRESPONDENCE
Jonathon M. Firnhaber, MD, Brody School of Medicine, East Carolina University, 101 Heart Drive, Greenville, NC 27834; firnhaberj@ecu.edu.
1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD004816.
2. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
3. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51.
4. Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can J Cardiol. 2015;31:613-619.
5. Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87-93.
6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245-1249.
7. Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372.
8. Koller MT, Leening MJ, Wolbers M, et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389-397.
9. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Amer Coll Cardiol. 2014;63:2889-2934.
12. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
13. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007.
14. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422-1431.
15. Loprinzi PD, Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease–specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763-769.
16. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134-141.
17. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130.
18. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765.
19. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
20. Yeboah J, McClelland RJ, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788-795.
21. Yadlowsky S, Hayward RA, Sussman JB, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20-29.
22. Dyakova M, Shantikumar S, Colquitt J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016 Jan 29;(1):CD010411.
1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD004816.
2. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
3. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51.
4. Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can J Cardiol. 2015;31:613-619.
5. Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87-93.
6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245-1249.
7. Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372.
8. Koller MT, Leening MJ, Wolbers M, et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389-397.
9. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Amer Coll Cardiol. 2014;63:2889-2934.
12. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
13. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007.
14. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422-1431.
15. Loprinzi PD, Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease–specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763-769.
16. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134-141.
17. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130.
18. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765.
19. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
20. Yeboah J, McClelland RJ, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788-795.
21. Yadlowsky S, Hayward RA, Sussman JB, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20-29.
22. Dyakova M, Shantikumar S, Colquitt J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016 Jan 29;(1):CD010411.
PRACTICE RECOMMENDATIONS
› Avoid the inclination to think that there is 1 best tool for accurately estimating an asymptomatic patient’s risk of cardiovascular disease (CVD). C
› Be mindful that 2013 ACC/AHA Pooled Cohort Risk equations can overestimate CVD risk depending on multiple factors, including the population being evaluated (even though the equations might be the most generalizable of available CVD risk calculators). C
› Consider using one of the newer CVD risk markers to further inform treatment recommendations when quantitative risk assessment does not offer information for making a clear treatment decision. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Newer cholesterol-lowering agents: What you must know
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
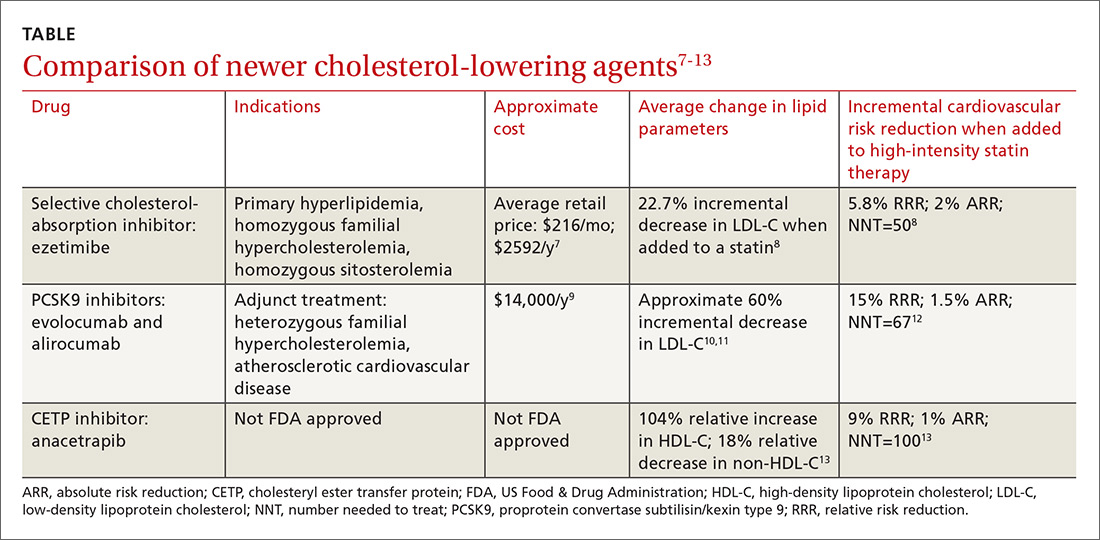
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; firnhaberj@ecu.edu.
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; firnhaberj@ecu.edu.
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; firnhaberj@ecu.edu.
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
From The Journal of Family Practice | 2018;67(6):339-341,344-345.
PRACTICE RECOMMENDATIONS
› Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting low-density lipoprotein cholesterol (LDL-C) goals with a statin alone. B
› Limit consideration of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors to adults at highest risk: those with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who must further lower LDL-C levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How should we use the coronary artery calcium score to predict cardiovascular risk?
THE CORONARY ARTERY CALCIUM (CAC) SCORE—an independent predictor of cardiovascular events (strength of recommendation [SOR]: C, systematic review of disease-oriented outcomes)—can be used, in addition to traditional risk factor assessment, to further stratify the risk of coronary heart disease (CHD) in asymptomatic patients (SOR: C, multiple large observational studies with disease-oriented outcomes).
Although a high CAC score is associated with a greater risk of cardiovascular disease, no studies have evaluated cardiovascular outcomes of CAC-guided treatment, so its value remains theoretical.
Evidence summary
Most atherosclerotic lesions are calcified. The degree of calcification is proportional to the severity of atherosclerosis and can be quantified by the CAC score as measured by electron beam computed tomography (EBCT).1
As the CAC score rises, so does risk
A systematic review of 9 studies evaluated the relationship between CAC scores and coronary artery disease (CAD) in asymptomatic patients (TABLE). CAD was measured by such clinical outcomes as unstable angina, myocardial infarction, stroke, coronary death, all-cause mortality, or need for revascularization. The relative risk of CAD for a moderate CAC score compared with a low score was 3.5 (95% confidence interval [CI], 2.4-5.1) and for a high score compared with a low score was 9.9 (95% CI, 5.3-17.6). Some of the studies were of poor quality, including self-referred patients, for example.2
A subsequent study found similar associations. The observational study of 25,253 asymptomatic individuals referred for CAC testing to assess cardiovascular risk demonstrated that CAC was an independent predictor of all-cause mortality. After a mean of 6.8 years, the adjusted relative risk increased incrementally from 1.48 (95% CI, 0.71-3.07) for a CAC score of 1 to 10, to 9.36 (95% CI, 5.36-16.33) for a score ≥1,000, compared with a score of 0.3
Table
What does that CAC score mean?1
| Score | Severity of disease |
|---|---|
| <1 | No identifiable disease |
| 1-10 | Mild |
| 11-100 | Moderate |
| 101-400 | Moderate to high |
| >400 | Severe |
| CAC, coronary artery calcium. | |
CAC predicts risk, but does it improve treatment or outcomes?
Analysis of 3201 women followed for an average of 3.75 years in the Multi-Ethnic Study of Atherosclerosis cohort revealed that women at low Framingham risk, but with detectable CAC, had an increased risk of CHD (hazard ratio [HR]=6.5; 95% CI, 2.6-16.4) and cardiovascular disease events (HR=5.2; 95% CI, 2.5-10.8).4
The St. Francis Heart Study prospectively compared CAC with standard CHD risk factors for predicting atherosclerotic cardiovascular disease (ASCVD) events in 4903 asymptomatic people between 50 and 70 years of age. For CAC scores ≥100 compared with scores <100, relative risk was 9.6 (95% CI, 6.7-13.9) for all ASCVD events, 11.1 (95% CI, 7.3-16.7) for all CHD events, and 9.2 (95% CI, 4.9-17.3) for nonfatal myocardial infarction and death. The CAC score predicted CHD events more accurately than standard risk factors and reclassified 24% of intermediate-risk women and 17% of intermediate-risk men into a higher-risk group.5
Despite studies that correlate higher CAC score with increased cardiovascular risk, we found no evidence that testing leads to improved treatment for preventing CHD or better cardiovascular outcomes.
Recommendations
The American College of Cardiology suggests that measuring CAC may be reasonable for asymptomatic patients with intermediate CHD risk (10%-20% 10-year risk of CHD events), for whom elevated CAC scores could lead to a higher-risk classification and subsequent modification of management.6 The College doesn’t recommend evaluating CAC in patients with low CHD risk, because it would constitute screening the general population, or asymptomatic patients with high CHD risk, who are already candidates for intensive risk modification.
The US Preventive Services Task Force conducted its own review and concluded that although the CAC score is an independent predictor of major CHD events, insufficient evidence exists to support its use to further stratify risk in intermediate-risk patients.7
1. Rumberger JA, Brundage BH, Rader DJ, et al. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243-252.
2. Dendukuri N, Chiu K, Brophy JM. Validity of electron beam computed tomography for coronary artery disease: a systematic review and meta-analysis. BMC Med. 2007;5:35.-
3. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860-1870.
4. Lakoski SG, Greenland P, Wong N, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167:2437-2442.
5. Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, creactive protein, and atherosclerotic cardiovascular disease events. J Am Coll Cardiol. 2005;46:158-165.
6. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document of Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378-402.
7. US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
THE CORONARY ARTERY CALCIUM (CAC) SCORE—an independent predictor of cardiovascular events (strength of recommendation [SOR]: C, systematic review of disease-oriented outcomes)—can be used, in addition to traditional risk factor assessment, to further stratify the risk of coronary heart disease (CHD) in asymptomatic patients (SOR: C, multiple large observational studies with disease-oriented outcomes).
Although a high CAC score is associated with a greater risk of cardiovascular disease, no studies have evaluated cardiovascular outcomes of CAC-guided treatment, so its value remains theoretical.
Evidence summary
Most atherosclerotic lesions are calcified. The degree of calcification is proportional to the severity of atherosclerosis and can be quantified by the CAC score as measured by electron beam computed tomography (EBCT).1
As the CAC score rises, so does risk
A systematic review of 9 studies evaluated the relationship between CAC scores and coronary artery disease (CAD) in asymptomatic patients (TABLE). CAD was measured by such clinical outcomes as unstable angina, myocardial infarction, stroke, coronary death, all-cause mortality, or need for revascularization. The relative risk of CAD for a moderate CAC score compared with a low score was 3.5 (95% confidence interval [CI], 2.4-5.1) and for a high score compared with a low score was 9.9 (95% CI, 5.3-17.6). Some of the studies were of poor quality, including self-referred patients, for example.2
A subsequent study found similar associations. The observational study of 25,253 asymptomatic individuals referred for CAC testing to assess cardiovascular risk demonstrated that CAC was an independent predictor of all-cause mortality. After a mean of 6.8 years, the adjusted relative risk increased incrementally from 1.48 (95% CI, 0.71-3.07) for a CAC score of 1 to 10, to 9.36 (95% CI, 5.36-16.33) for a score ≥1,000, compared with a score of 0.3
Table
What does that CAC score mean?1
| Score | Severity of disease |
|---|---|
| <1 | No identifiable disease |
| 1-10 | Mild |
| 11-100 | Moderate |
| 101-400 | Moderate to high |
| >400 | Severe |
| CAC, coronary artery calcium. | |
CAC predicts risk, but does it improve treatment or outcomes?
Analysis of 3201 women followed for an average of 3.75 years in the Multi-Ethnic Study of Atherosclerosis cohort revealed that women at low Framingham risk, but with detectable CAC, had an increased risk of CHD (hazard ratio [HR]=6.5; 95% CI, 2.6-16.4) and cardiovascular disease events (HR=5.2; 95% CI, 2.5-10.8).4
The St. Francis Heart Study prospectively compared CAC with standard CHD risk factors for predicting atherosclerotic cardiovascular disease (ASCVD) events in 4903 asymptomatic people between 50 and 70 years of age. For CAC scores ≥100 compared with scores <100, relative risk was 9.6 (95% CI, 6.7-13.9) for all ASCVD events, 11.1 (95% CI, 7.3-16.7) for all CHD events, and 9.2 (95% CI, 4.9-17.3) for nonfatal myocardial infarction and death. The CAC score predicted CHD events more accurately than standard risk factors and reclassified 24% of intermediate-risk women and 17% of intermediate-risk men into a higher-risk group.5
Despite studies that correlate higher CAC score with increased cardiovascular risk, we found no evidence that testing leads to improved treatment for preventing CHD or better cardiovascular outcomes.
Recommendations
The American College of Cardiology suggests that measuring CAC may be reasonable for asymptomatic patients with intermediate CHD risk (10%-20% 10-year risk of CHD events), for whom elevated CAC scores could lead to a higher-risk classification and subsequent modification of management.6 The College doesn’t recommend evaluating CAC in patients with low CHD risk, because it would constitute screening the general population, or asymptomatic patients with high CHD risk, who are already candidates for intensive risk modification.
The US Preventive Services Task Force conducted its own review and concluded that although the CAC score is an independent predictor of major CHD events, insufficient evidence exists to support its use to further stratify risk in intermediate-risk patients.7
THE CORONARY ARTERY CALCIUM (CAC) SCORE—an independent predictor of cardiovascular events (strength of recommendation [SOR]: C, systematic review of disease-oriented outcomes)—can be used, in addition to traditional risk factor assessment, to further stratify the risk of coronary heart disease (CHD) in asymptomatic patients (SOR: C, multiple large observational studies with disease-oriented outcomes).
Although a high CAC score is associated with a greater risk of cardiovascular disease, no studies have evaluated cardiovascular outcomes of CAC-guided treatment, so its value remains theoretical.
Evidence summary
Most atherosclerotic lesions are calcified. The degree of calcification is proportional to the severity of atherosclerosis and can be quantified by the CAC score as measured by electron beam computed tomography (EBCT).1
As the CAC score rises, so does risk
A systematic review of 9 studies evaluated the relationship between CAC scores and coronary artery disease (CAD) in asymptomatic patients (TABLE). CAD was measured by such clinical outcomes as unstable angina, myocardial infarction, stroke, coronary death, all-cause mortality, or need for revascularization. The relative risk of CAD for a moderate CAC score compared with a low score was 3.5 (95% confidence interval [CI], 2.4-5.1) and for a high score compared with a low score was 9.9 (95% CI, 5.3-17.6). Some of the studies were of poor quality, including self-referred patients, for example.2
A subsequent study found similar associations. The observational study of 25,253 asymptomatic individuals referred for CAC testing to assess cardiovascular risk demonstrated that CAC was an independent predictor of all-cause mortality. After a mean of 6.8 years, the adjusted relative risk increased incrementally from 1.48 (95% CI, 0.71-3.07) for a CAC score of 1 to 10, to 9.36 (95% CI, 5.36-16.33) for a score ≥1,000, compared with a score of 0.3
Table
What does that CAC score mean?1
| Score | Severity of disease |
|---|---|
| <1 | No identifiable disease |
| 1-10 | Mild |
| 11-100 | Moderate |
| 101-400 | Moderate to high |
| >400 | Severe |
| CAC, coronary artery calcium. | |
CAC predicts risk, but does it improve treatment or outcomes?
Analysis of 3201 women followed for an average of 3.75 years in the Multi-Ethnic Study of Atherosclerosis cohort revealed that women at low Framingham risk, but with detectable CAC, had an increased risk of CHD (hazard ratio [HR]=6.5; 95% CI, 2.6-16.4) and cardiovascular disease events (HR=5.2; 95% CI, 2.5-10.8).4
The St. Francis Heart Study prospectively compared CAC with standard CHD risk factors for predicting atherosclerotic cardiovascular disease (ASCVD) events in 4903 asymptomatic people between 50 and 70 years of age. For CAC scores ≥100 compared with scores <100, relative risk was 9.6 (95% CI, 6.7-13.9) for all ASCVD events, 11.1 (95% CI, 7.3-16.7) for all CHD events, and 9.2 (95% CI, 4.9-17.3) for nonfatal myocardial infarction and death. The CAC score predicted CHD events more accurately than standard risk factors and reclassified 24% of intermediate-risk women and 17% of intermediate-risk men into a higher-risk group.5
Despite studies that correlate higher CAC score with increased cardiovascular risk, we found no evidence that testing leads to improved treatment for preventing CHD or better cardiovascular outcomes.
Recommendations
The American College of Cardiology suggests that measuring CAC may be reasonable for asymptomatic patients with intermediate CHD risk (10%-20% 10-year risk of CHD events), for whom elevated CAC scores could lead to a higher-risk classification and subsequent modification of management.6 The College doesn’t recommend evaluating CAC in patients with low CHD risk, because it would constitute screening the general population, or asymptomatic patients with high CHD risk, who are already candidates for intensive risk modification.
The US Preventive Services Task Force conducted its own review and concluded that although the CAC score is an independent predictor of major CHD events, insufficient evidence exists to support its use to further stratify risk in intermediate-risk patients.7
1. Rumberger JA, Brundage BH, Rader DJ, et al. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243-252.
2. Dendukuri N, Chiu K, Brophy JM. Validity of electron beam computed tomography for coronary artery disease: a systematic review and meta-analysis. BMC Med. 2007;5:35.-
3. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860-1870.
4. Lakoski SG, Greenland P, Wong N, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167:2437-2442.
5. Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, creactive protein, and atherosclerotic cardiovascular disease events. J Am Coll Cardiol. 2005;46:158-165.
6. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document of Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378-402.
7. US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
1. Rumberger JA, Brundage BH, Rader DJ, et al. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243-252.
2. Dendukuri N, Chiu K, Brophy JM. Validity of electron beam computed tomography for coronary artery disease: a systematic review and meta-analysis. BMC Med. 2007;5:35.-
3. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860-1870.
4. Lakoski SG, Greenland P, Wong N, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167:2437-2442.
5. Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, creactive protein, and atherosclerotic cardiovascular disease events. J Am Coll Cardiol. 2005;46:158-165.
6. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document of Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378-402.
7. US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
Evidence-based answers from the Family Physicians Inquiries Network
What are the best prophylactic drugs for migraine?
BETA-BLOCKERS without intrinsic sympathomimetic activity, amitriptyline, divalproex sodium/sodium valproate, and topiramate are the most effective drugs for preventing episodic migraine (strength of recommendation: A, multiple, well-designed, randomized controlled trials [RCTs]).
Evidence summary
Many medications have been evaluated for migraine prophylaxis. However, very few head-to-head trials of more than 2 drugs have been published, and no recent meta-analyses of available drug classes have been performed. The most commonly evaluated outcome is a 50% reduction in headache frequency.
Propranolol and timolol offer consistent prevention
Propranolol and timolol have consistently demonstrated efficacy for preventing episodic migraine. In a 1991 meta-analysis, propranolol resulted in a 44% reduction in the headache index—a composite score that takes into account both intensity and duration—compared with a 14% reduction for placebo.1
Less evidence supports other beta-blockers
Atenolol, metoprolol, and nadolol have demonstrated a moderate effect, but less evidence exists to support their use.2 A recent trial comparing metoprolol and nebivolol demonstrated a positive response—defined as a 50% reduction in headache frequency—to each drug at 14 weeks (57% of metoprolol-treated and 50% of nebivolol-treated patients), but noted that nebivolol was better tolerated.3
Beta-blockers with intrinsic sympathomimetic activity (acebutolol, alprenolol, oxprenolol, pindolol) appear to be ineffective for migraine prevention.4
Amitriptyline works better than propranolol for some migraines
Amitriptyline is the most often studied antidepressant and the only one with consistent support for efficacy in preventing migraine. A 1981 trial found amitriptyline to be more effective than propranolol in mixed migraine-tension-type headache, whereas propranolol was more effective for migraine alone.5
Some support for fluoxetine, none for similar drugs
Limited evidence exists for the use of fluoxetine, 20 mg daily. A small 1999 study of patients with migraine without aura found a 57% reduction in total pain index—a value based on pain intensity and hours of headache per month—with fluoxetine compared with an insignificant 31% reduction with placebo.6
No evidence from controlled trials supports the use of fluvoxamine, paroxetine, sertraline, phenelzine, venlafaxine, mirtazapine, trazodone, or bupropion.4
Divalproex sodium, sodium valproate are effective
Divalproex sodium and sodium valproate show strong, consistent evidence of efficacy; they may be particularly useful for patients with prolonged or atypical migraine aura.4 Initial studies of delayed-release divalproex at doses ranging from 500 to 1500 mg daily found that 44% of divalproex-treated patients reported a 50% reduction in migraine frequency, compared with 21% in the placebo group (number needed to treat [NNT]=4).7
A more recent study of the extended-release form of divalproex sodium demonstrated a 4-week reduction in headache rate to 1.2 from a baseline of 4.4, compared with a decrease of 0.6 for placebo (95% confidence interval [CI] of treatment difference, 0.2-1.2).8
TABLE
Recommended drugs for migraine prophylaxis13
| Drug | Dose | Comments |
|---|---|---|
| Propranolol | 80-240 mg/d | May cause fatigue. When used in combination with rizatriptan, give a lower dose of rizatriptan. |
| Timolol | 20-30 mg/d | As with propranolol, may cause fatigue. Avoid β-blockers in patients with asthma or Raynaud’s disease. |
| Amitriptyline | 25-150 mg/d | Drowsiness, weight gain, and significant anticholinergic adverse events are common. |
| Divalproex sodium; Sodium valproate | 500-1500 mg/d; 800-1500 mg/d | Side effects include nausea, drowsiness, weight gain, hair loss, and tremor. Hepatotoxicity, pancreatitis, and hyperammonemia have been reported rarely. Pregnancy category D. |
| Topiramate | 100-200 mg/d | Paresthesia is the most common adverse event; fatigue, nausea, anorexia, and cognitive symptoms are less common. Carbonic anhydrase inhibition may cause metabolic acidosis. Acute myopia and angle closure glaucoma are rare events. |
Topiramate may decrease frequency as much as propranolol
Topiramate has significantly reduced the mean frequency of episodic migraine at doses of 100 to 200 mg daily and also improved secondary end points, including number of migraine days per month, use of acute medication, and daily activity.9 One study found that topiramate 100 mg daily had comparable efficacy to propranolol 160 mg daily; both drugs decreased monthly migraine frequency to 1.6 from a baseline of 4.9 with topiramate and 5.1 with propranolol (95% CI for the pair-wise difference of topiramate minus propranolol,-0.58 to 0.60).10
Anticonvulsants also reduce migraine frequency
A 2004 Cochrane review of anticonvulsant drugs for migraine prophylaxis found that anticonvulsants, as a class, reduce migraine frequency by about 1.3 attacks per 28 days when compared with placebo (based on 10 trials [N=902]). When analyzing data on relative frequency of migraines, data from 13 trials (N=1773) were combined and showed that anticonvulsants more than doubled the number of patients with a 50% or greater decrease in migraine frequency relative to placebo (relative risk=2.25; 95% CI, 1.79-2.84; NNT=3.9; 95% CI, 3.4-4.7).11
Other drugs to keep on your radar
Agents available in the United States that have at least limited evidence supporting their use to prevent episodic migraine include gabapentin, lisinopril, candesartan, memantine, riboflavin, magnesium, feverfew, coenzyme Q10, butterbur, and melatonin.
Drugs so far proved ineffective in preventing episodic migraine include clonidine, carbamazepine, clonazepam, vigabatrin, oxcarbazepine, zonisamide, lamotrigine, nifedipine, and acetazolamide. Botulinum toxin type A given by intramuscular injection in the head and neck region has demonstrated limited efficacy in chronic headache disorders, but doesn’t prevent episodic migraine.12
Recommendations
The 2000 guidelines of the American Association of Neurology address Group 1 (first-line) drugs and Group 2 drugs:
Group 1 drugs (medium to high efficacy, good strength of evidence, and a range of severity [mild to moderate] and frequency [infrequent to frequent] of side effects) include amitriptyline, divalproex sodium, propranolol, and timolol.
Group 2 drugs (lower efficacy than Group 1, or limited strength of evidence, and mild to moderate side effects) include aspirin (but not combination products), atenolol, fenoprofen, feverfew, flurbiprofen, fluoxetine, gabapentin, guanfacine, ketoprofen, magnesium, mefenamic acid, metoprolol, nadolol, naproxen, nimodipine, verapamil, and vitamin B2.13
Topiramate was still under study when the guidelines were released and wasn’t approved by the US Food and Drug Administration for migraine prophylaxis until 2004. The 2000 guidelines are undergoing revision.
1. Holroyd KA, Penzien DB, Cordingley GE. Propranolol in the management of recurrent migraine: a meta-analytic review. Headache. 1991;31:333-340.
2. Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia. 2002;22:491-512.
3. Schellenberg R, Lichtenthal A, Wöhling H, et al. Nebivolol and metoprolol for treating migraine: an advance on β-blocker treatment? Headache. 2008;48:118-125.
4. Snow V, Weiss K, Wall EM, et al. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med. 2002;137:840-849.
5. Mathew NT. Prophylaxis of migraine and mixed headache: a randomized controlled study. Headache. 1981;21:105-109.
6. d’Amato CC, Pizza V, Marmolo T, et al. Fluoxetine for migraine prophylaxis: a double-blind trial. Headache. 1999;39:716-719.
7. Klapper J. Divalproex sodium in migraine prophylaxis: a dose-controlled study [published correction appears in Cephalalgia. 1997;17:798]. Cephalalgia. 1997;17:103-108.
8. Freitag FG, Collins SD, Carlson HA, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58:1652-1659.
9. Kaniecki R. Neuromodulators for migraine prevention. Headache. 2008;48:586-600.
10. Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis—results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943-950.
11. Chronicle EP, Mulleners WM. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(3):CD003226.
12. Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache. 2008;48:210-220.
13. Ramadan NM, Silberstein SD, Freitag FG, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. 2000. Available at: www.aan.com/professionals/practice/pdfs/gl0090.pdf. Accessed March 26, 2008.
BETA-BLOCKERS without intrinsic sympathomimetic activity, amitriptyline, divalproex sodium/sodium valproate, and topiramate are the most effective drugs for preventing episodic migraine (strength of recommendation: A, multiple, well-designed, randomized controlled trials [RCTs]).
Evidence summary
Many medications have been evaluated for migraine prophylaxis. However, very few head-to-head trials of more than 2 drugs have been published, and no recent meta-analyses of available drug classes have been performed. The most commonly evaluated outcome is a 50% reduction in headache frequency.
Propranolol and timolol offer consistent prevention
Propranolol and timolol have consistently demonstrated efficacy for preventing episodic migraine. In a 1991 meta-analysis, propranolol resulted in a 44% reduction in the headache index—a composite score that takes into account both intensity and duration—compared with a 14% reduction for placebo.1
Less evidence supports other beta-blockers
Atenolol, metoprolol, and nadolol have demonstrated a moderate effect, but less evidence exists to support their use.2 A recent trial comparing metoprolol and nebivolol demonstrated a positive response—defined as a 50% reduction in headache frequency—to each drug at 14 weeks (57% of metoprolol-treated and 50% of nebivolol-treated patients), but noted that nebivolol was better tolerated.3
Beta-blockers with intrinsic sympathomimetic activity (acebutolol, alprenolol, oxprenolol, pindolol) appear to be ineffective for migraine prevention.4
Amitriptyline works better than propranolol for some migraines
Amitriptyline is the most often studied antidepressant and the only one with consistent support for efficacy in preventing migraine. A 1981 trial found amitriptyline to be more effective than propranolol in mixed migraine-tension-type headache, whereas propranolol was more effective for migraine alone.5
Some support for fluoxetine, none for similar drugs
Limited evidence exists for the use of fluoxetine, 20 mg daily. A small 1999 study of patients with migraine without aura found a 57% reduction in total pain index—a value based on pain intensity and hours of headache per month—with fluoxetine compared with an insignificant 31% reduction with placebo.6
No evidence from controlled trials supports the use of fluvoxamine, paroxetine, sertraline, phenelzine, venlafaxine, mirtazapine, trazodone, or bupropion.4
Divalproex sodium, sodium valproate are effective
Divalproex sodium and sodium valproate show strong, consistent evidence of efficacy; they may be particularly useful for patients with prolonged or atypical migraine aura.4 Initial studies of delayed-release divalproex at doses ranging from 500 to 1500 mg daily found that 44% of divalproex-treated patients reported a 50% reduction in migraine frequency, compared with 21% in the placebo group (number needed to treat [NNT]=4).7
A more recent study of the extended-release form of divalproex sodium demonstrated a 4-week reduction in headache rate to 1.2 from a baseline of 4.4, compared with a decrease of 0.6 for placebo (95% confidence interval [CI] of treatment difference, 0.2-1.2).8
TABLE
Recommended drugs for migraine prophylaxis13
| Drug | Dose | Comments |
|---|---|---|
| Propranolol | 80-240 mg/d | May cause fatigue. When used in combination with rizatriptan, give a lower dose of rizatriptan. |
| Timolol | 20-30 mg/d | As with propranolol, may cause fatigue. Avoid β-blockers in patients with asthma or Raynaud’s disease. |
| Amitriptyline | 25-150 mg/d | Drowsiness, weight gain, and significant anticholinergic adverse events are common. |
| Divalproex sodium; Sodium valproate | 500-1500 mg/d; 800-1500 mg/d | Side effects include nausea, drowsiness, weight gain, hair loss, and tremor. Hepatotoxicity, pancreatitis, and hyperammonemia have been reported rarely. Pregnancy category D. |
| Topiramate | 100-200 mg/d | Paresthesia is the most common adverse event; fatigue, nausea, anorexia, and cognitive symptoms are less common. Carbonic anhydrase inhibition may cause metabolic acidosis. Acute myopia and angle closure glaucoma are rare events. |
Topiramate may decrease frequency as much as propranolol
Topiramate has significantly reduced the mean frequency of episodic migraine at doses of 100 to 200 mg daily and also improved secondary end points, including number of migraine days per month, use of acute medication, and daily activity.9 One study found that topiramate 100 mg daily had comparable efficacy to propranolol 160 mg daily; both drugs decreased monthly migraine frequency to 1.6 from a baseline of 4.9 with topiramate and 5.1 with propranolol (95% CI for the pair-wise difference of topiramate minus propranolol,-0.58 to 0.60).10
Anticonvulsants also reduce migraine frequency
A 2004 Cochrane review of anticonvulsant drugs for migraine prophylaxis found that anticonvulsants, as a class, reduce migraine frequency by about 1.3 attacks per 28 days when compared with placebo (based on 10 trials [N=902]). When analyzing data on relative frequency of migraines, data from 13 trials (N=1773) were combined and showed that anticonvulsants more than doubled the number of patients with a 50% or greater decrease in migraine frequency relative to placebo (relative risk=2.25; 95% CI, 1.79-2.84; NNT=3.9; 95% CI, 3.4-4.7).11
Other drugs to keep on your radar
Agents available in the United States that have at least limited evidence supporting their use to prevent episodic migraine include gabapentin, lisinopril, candesartan, memantine, riboflavin, magnesium, feverfew, coenzyme Q10, butterbur, and melatonin.
Drugs so far proved ineffective in preventing episodic migraine include clonidine, carbamazepine, clonazepam, vigabatrin, oxcarbazepine, zonisamide, lamotrigine, nifedipine, and acetazolamide. Botulinum toxin type A given by intramuscular injection in the head and neck region has demonstrated limited efficacy in chronic headache disorders, but doesn’t prevent episodic migraine.12
Recommendations
The 2000 guidelines of the American Association of Neurology address Group 1 (first-line) drugs and Group 2 drugs:
Group 1 drugs (medium to high efficacy, good strength of evidence, and a range of severity [mild to moderate] and frequency [infrequent to frequent] of side effects) include amitriptyline, divalproex sodium, propranolol, and timolol.
Group 2 drugs (lower efficacy than Group 1, or limited strength of evidence, and mild to moderate side effects) include aspirin (but not combination products), atenolol, fenoprofen, feverfew, flurbiprofen, fluoxetine, gabapentin, guanfacine, ketoprofen, magnesium, mefenamic acid, metoprolol, nadolol, naproxen, nimodipine, verapamil, and vitamin B2.13
Topiramate was still under study when the guidelines were released and wasn’t approved by the US Food and Drug Administration for migraine prophylaxis until 2004. The 2000 guidelines are undergoing revision.
BETA-BLOCKERS without intrinsic sympathomimetic activity, amitriptyline, divalproex sodium/sodium valproate, and topiramate are the most effective drugs for preventing episodic migraine (strength of recommendation: A, multiple, well-designed, randomized controlled trials [RCTs]).
Evidence summary
Many medications have been evaluated for migraine prophylaxis. However, very few head-to-head trials of more than 2 drugs have been published, and no recent meta-analyses of available drug classes have been performed. The most commonly evaluated outcome is a 50% reduction in headache frequency.
Propranolol and timolol offer consistent prevention
Propranolol and timolol have consistently demonstrated efficacy for preventing episodic migraine. In a 1991 meta-analysis, propranolol resulted in a 44% reduction in the headache index—a composite score that takes into account both intensity and duration—compared with a 14% reduction for placebo.1
Less evidence supports other beta-blockers
Atenolol, metoprolol, and nadolol have demonstrated a moderate effect, but less evidence exists to support their use.2 A recent trial comparing metoprolol and nebivolol demonstrated a positive response—defined as a 50% reduction in headache frequency—to each drug at 14 weeks (57% of metoprolol-treated and 50% of nebivolol-treated patients), but noted that nebivolol was better tolerated.3
Beta-blockers with intrinsic sympathomimetic activity (acebutolol, alprenolol, oxprenolol, pindolol) appear to be ineffective for migraine prevention.4
Amitriptyline works better than propranolol for some migraines
Amitriptyline is the most often studied antidepressant and the only one with consistent support for efficacy in preventing migraine. A 1981 trial found amitriptyline to be more effective than propranolol in mixed migraine-tension-type headache, whereas propranolol was more effective for migraine alone.5
Some support for fluoxetine, none for similar drugs
Limited evidence exists for the use of fluoxetine, 20 mg daily. A small 1999 study of patients with migraine without aura found a 57% reduction in total pain index—a value based on pain intensity and hours of headache per month—with fluoxetine compared with an insignificant 31% reduction with placebo.6
No evidence from controlled trials supports the use of fluvoxamine, paroxetine, sertraline, phenelzine, venlafaxine, mirtazapine, trazodone, or bupropion.4
Divalproex sodium, sodium valproate are effective
Divalproex sodium and sodium valproate show strong, consistent evidence of efficacy; they may be particularly useful for patients with prolonged or atypical migraine aura.4 Initial studies of delayed-release divalproex at doses ranging from 500 to 1500 mg daily found that 44% of divalproex-treated patients reported a 50% reduction in migraine frequency, compared with 21% in the placebo group (number needed to treat [NNT]=4).7
A more recent study of the extended-release form of divalproex sodium demonstrated a 4-week reduction in headache rate to 1.2 from a baseline of 4.4, compared with a decrease of 0.6 for placebo (95% confidence interval [CI] of treatment difference, 0.2-1.2).8
TABLE
Recommended drugs for migraine prophylaxis13
| Drug | Dose | Comments |
|---|---|---|
| Propranolol | 80-240 mg/d | May cause fatigue. When used in combination with rizatriptan, give a lower dose of rizatriptan. |
| Timolol | 20-30 mg/d | As with propranolol, may cause fatigue. Avoid β-blockers in patients with asthma or Raynaud’s disease. |
| Amitriptyline | 25-150 mg/d | Drowsiness, weight gain, and significant anticholinergic adverse events are common. |
| Divalproex sodium; Sodium valproate | 500-1500 mg/d; 800-1500 mg/d | Side effects include nausea, drowsiness, weight gain, hair loss, and tremor. Hepatotoxicity, pancreatitis, and hyperammonemia have been reported rarely. Pregnancy category D. |
| Topiramate | 100-200 mg/d | Paresthesia is the most common adverse event; fatigue, nausea, anorexia, and cognitive symptoms are less common. Carbonic anhydrase inhibition may cause metabolic acidosis. Acute myopia and angle closure glaucoma are rare events. |
Topiramate may decrease frequency as much as propranolol
Topiramate has significantly reduced the mean frequency of episodic migraine at doses of 100 to 200 mg daily and also improved secondary end points, including number of migraine days per month, use of acute medication, and daily activity.9 One study found that topiramate 100 mg daily had comparable efficacy to propranolol 160 mg daily; both drugs decreased monthly migraine frequency to 1.6 from a baseline of 4.9 with topiramate and 5.1 with propranolol (95% CI for the pair-wise difference of topiramate minus propranolol,-0.58 to 0.60).10
Anticonvulsants also reduce migraine frequency
A 2004 Cochrane review of anticonvulsant drugs for migraine prophylaxis found that anticonvulsants, as a class, reduce migraine frequency by about 1.3 attacks per 28 days when compared with placebo (based on 10 trials [N=902]). When analyzing data on relative frequency of migraines, data from 13 trials (N=1773) were combined and showed that anticonvulsants more than doubled the number of patients with a 50% or greater decrease in migraine frequency relative to placebo (relative risk=2.25; 95% CI, 1.79-2.84; NNT=3.9; 95% CI, 3.4-4.7).11
Other drugs to keep on your radar
Agents available in the United States that have at least limited evidence supporting their use to prevent episodic migraine include gabapentin, lisinopril, candesartan, memantine, riboflavin, magnesium, feverfew, coenzyme Q10, butterbur, and melatonin.
Drugs so far proved ineffective in preventing episodic migraine include clonidine, carbamazepine, clonazepam, vigabatrin, oxcarbazepine, zonisamide, lamotrigine, nifedipine, and acetazolamide. Botulinum toxin type A given by intramuscular injection in the head and neck region has demonstrated limited efficacy in chronic headache disorders, but doesn’t prevent episodic migraine.12
Recommendations
The 2000 guidelines of the American Association of Neurology address Group 1 (first-line) drugs and Group 2 drugs:
Group 1 drugs (medium to high efficacy, good strength of evidence, and a range of severity [mild to moderate] and frequency [infrequent to frequent] of side effects) include amitriptyline, divalproex sodium, propranolol, and timolol.
Group 2 drugs (lower efficacy than Group 1, or limited strength of evidence, and mild to moderate side effects) include aspirin (but not combination products), atenolol, fenoprofen, feverfew, flurbiprofen, fluoxetine, gabapentin, guanfacine, ketoprofen, magnesium, mefenamic acid, metoprolol, nadolol, naproxen, nimodipine, verapamil, and vitamin B2.13
Topiramate was still under study when the guidelines were released and wasn’t approved by the US Food and Drug Administration for migraine prophylaxis until 2004. The 2000 guidelines are undergoing revision.
1. Holroyd KA, Penzien DB, Cordingley GE. Propranolol in the management of recurrent migraine: a meta-analytic review. Headache. 1991;31:333-340.
2. Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia. 2002;22:491-512.
3. Schellenberg R, Lichtenthal A, Wöhling H, et al. Nebivolol and metoprolol for treating migraine: an advance on β-blocker treatment? Headache. 2008;48:118-125.
4. Snow V, Weiss K, Wall EM, et al. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med. 2002;137:840-849.
5. Mathew NT. Prophylaxis of migraine and mixed headache: a randomized controlled study. Headache. 1981;21:105-109.
6. d’Amato CC, Pizza V, Marmolo T, et al. Fluoxetine for migraine prophylaxis: a double-blind trial. Headache. 1999;39:716-719.
7. Klapper J. Divalproex sodium in migraine prophylaxis: a dose-controlled study [published correction appears in Cephalalgia. 1997;17:798]. Cephalalgia. 1997;17:103-108.
8. Freitag FG, Collins SD, Carlson HA, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58:1652-1659.
9. Kaniecki R. Neuromodulators for migraine prevention. Headache. 2008;48:586-600.
10. Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis—results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943-950.
11. Chronicle EP, Mulleners WM. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(3):CD003226.
12. Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache. 2008;48:210-220.
13. Ramadan NM, Silberstein SD, Freitag FG, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. 2000. Available at: www.aan.com/professionals/practice/pdfs/gl0090.pdf. Accessed March 26, 2008.
1. Holroyd KA, Penzien DB, Cordingley GE. Propranolol in the management of recurrent migraine: a meta-analytic review. Headache. 1991;31:333-340.
2. Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia. 2002;22:491-512.
3. Schellenberg R, Lichtenthal A, Wöhling H, et al. Nebivolol and metoprolol for treating migraine: an advance on β-blocker treatment? Headache. 2008;48:118-125.
4. Snow V, Weiss K, Wall EM, et al. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med. 2002;137:840-849.
5. Mathew NT. Prophylaxis of migraine and mixed headache: a randomized controlled study. Headache. 1981;21:105-109.
6. d’Amato CC, Pizza V, Marmolo T, et al. Fluoxetine for migraine prophylaxis: a double-blind trial. Headache. 1999;39:716-719.
7. Klapper J. Divalproex sodium in migraine prophylaxis: a dose-controlled study [published correction appears in Cephalalgia. 1997;17:798]. Cephalalgia. 1997;17:103-108.
8. Freitag FG, Collins SD, Carlson HA, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58:1652-1659.
9. Kaniecki R. Neuromodulators for migraine prevention. Headache. 2008;48:586-600.
10. Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis—results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943-950.
11. Chronicle EP, Mulleners WM. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(3):CD003226.
12. Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache. 2008;48:210-220.
13. Ramadan NM, Silberstein SD, Freitag FG, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. 2000. Available at: www.aan.com/professionals/practice/pdfs/gl0090.pdf. Accessed March 26, 2008.
Evidence-based answers from the Family Physicians Inquiries Network