User login
Laparoscopic myomectomy: 8 pearls
Myomectomy is the surgery of choice for women who have symptomatic fibroids and who wish to retain their uterus. And laparoscopic myomectomy is preferable to the abdominal approach in many ways, offering: 1-4
- faster recovery
- a shorter hospital stay
- diminished blood loss
- decreased adhesion formation
- a comparable or higher rate of pregnancy.
Nevertheless, laparoscopic myomectomy is a technically challenging procedure with surgeon-specific limitations. The biggest challenge: appropriately suturing the hysterotomy site.
In this article, I share my experience with laparoscopic myomectomy and offer 8 pearls that may contribute to a successful outcome.
1. Don't settle on laparoscopy prematurely
Given its advantages over the abdominal route, laparoscopic myomectomy should be the preferred approach in the treatment of symptomatic uterine fibroids ( FIGURE 1 ). However, not all patients are appropriate candidates for laparoscopy. Several guidelines have recommended a maximum number and size of fibroids for laparoscopic removal, but practice varies widely, and experienced surgeons successfully take on cases that are well beyond the limits set by most published guidelines.5-7
At our institution, we do not have firm guidelines in place for the number and size of fibroids that can be removed laparoscopically. Other variables enter into decision-making and counseling, among them any medical comorbidity or history of uterine surgery the patient may have, as well as her desires in regard to childbearing and uterine retention.
Hysterectomy may be the most straightforward option for women who have symptomatic fibroids and who have completed childbearing. However, myomectomy is also appropriate as long as the patient is aware of the risk that fibroids may recur and the potential for further surgery. When the patient is in her late 40s or early 50s, the likelihood of fibroid recurrence may be lower than it is in the general population.
In my practice, submucosal and intracavitary fibroids smaller than 4 cm and more than 5 mm away from the uterine serosa are generally removed hysteroscopically, an approach beyond the scope of this article. In women who have completed childbearing but who wish to conserve the uterus, we deliberately enter the uterine cavity laparoscopically because this strategy allows for efficient removal of submucosal and intracavitary fibroids.
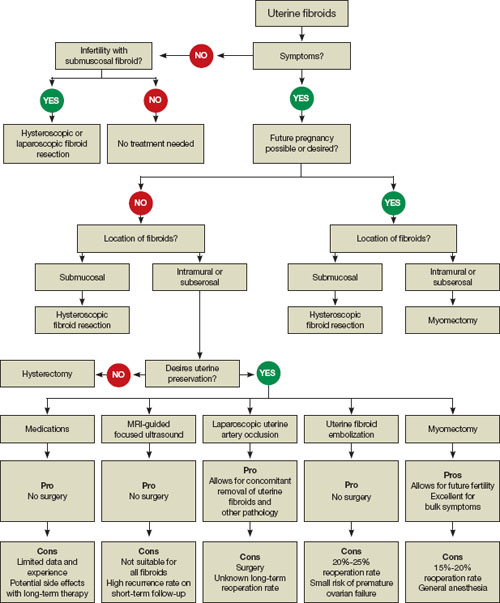
FIGURE 1 When and how to treat uterine fibroids
2. Estimate the duration of surgery
When the patient has fibroids that are intramural or subserosal, our general rule of thumb is to determine her suitability for laparoscopic myomectomy, based on the estimated duration of the operation. A surgeon's ability to calculate the length of the operation for a particular patient increases with experience.
We tend to recommend the laparoscopic approach when the procedure is expected to take less than 3 hours to complete. More than 95% of our patients fall into this category.
When we anticipate a prolonged operating time, we discuss the option of hand-assisted laparoscopic myomectomy. This approach involves two or three 5-mm trocar punctures high on the abdomen in conjunction with a suprapubic incision, 6 to 7 cm in length with a hand port in place. Prospective studies have demonstrated a significantly longer recovery with minilaparotomy than with laparoscopy, but these trials compared uteri of similar size.4,8 We expect the laparoscopic approach to confer fewer advantages when operative time is prolonged significantly.
In our practice, we consider one or more of the following conditions appropriate for hand-assisted laparoscopic myomectomy:
- a very large uterus (i.e., heavier than 1,500 to 2,000 g). In these cases, operating times can be excessive because of the need for extensive suturing and morcellation, and bleeding may increase as a result
- more than 20 fibroids on magnetic resonance imaging (MRI). It can be a challenge to locate all of the fibroids; multiple uterine incisions may be necessary
- a medical comorbidity that renders the patient unable to tolerate prolonged anesthesia. For example, we operated on a patient who had Ehlers-Danlos syndrome and who needed to avoid a prolonged operation due to fragile bones and joint laxity.
Of necessity, these guidelines will vary from practice to practice, and gynecologic surgeons who are just beginning to perform laparoscopic myomectomy should not include multiple fibroids or a large uterus among their initial cases. Instead, perform the first few cases in patients who have not had abdominal surgery and who have a symptomatic intramural or subserosal fibroid that is close to the uterine fundus and no larger than 6 cm in diameter.
3. Consider preoperative MRI
Preoperative imaging greatly supplements the clinical examination and facilitates identification of the number, location, and characteristics of the fibroids. Pelvic ultrasonography (US) is appropriate for most patients. We prefer preoperative MRI of the pelvis in the following scenarios:
- uterus larger than 12 weeks (280 g) on clinical examination
- identification of multiple fibroids via US
- history suggestive of adenomyosis.
MRI facilitates preoperative planning by accurately delineating the size and location of the fibroids, and by distinguishing between an adenomyoma and fibroid in most cases.9 For an example of its utility, see "How MRI can guide treatment: 3 cases."
4. Preoperative medical therapy may be indicated
When given preoperatively, gonadotropin-releasing hormone (GnRH) agonists have been shown to reduce blood loss and shorten operative time. The exception: cases involving hypoechoic fibroids, because the cleavage plane may be more difficult to identify, prolonging operative time.10
We generally prefer to use a GnRH agonist in two clinical scenarios: 1) anemia and 2) a uterus that extends above the umbilicus. In the second scenario, the GnRH agonist helps reduce the uterus to a more manageable size.
Aromatase inhibitors show great promise as preoperative agents because there is no initial flare. In addition, because fibroids have a higher concentration of aromatase activity than the surrounding myometrium, a low dosage of an aromatase inhibitor is effective and does not cause significant menopausal symptoms.
A recent comparative study found that fibroid shrinkage was greater after 3 months of letrozole (2.5 mg/day) than after use of a GnRH agonist.11 Total myoma volume decreased by 45.6% in the letrozole group, compared with 33.2% in the group that received a GnRH agonist (P =.02).11
Aromatase inhibitors have also been successfully used during the initial period of GnRH agonist therapy to prevent the symptoms of flare.12 However, because clinical experience is limited, the long-term efficacy and safety of aromatase inhibitors in premenopausal women is unknown.
Findings A 40-year-old nulliparous woman seeks treatment for menometrorrhagia and dysmenorrhea but wants to conserve her uterus. MRI reveals a 4.5-cm submucosal fibroid (arrow) that extends all the way to the uterine serosa, with no evidence of adenomyosis. Her thyroid-stimulating hormone (TSH) level is normal, as is an endometrial biopsy.

CASE 1
Outcome We decide to proceed with laparoscopic myomectomy because a hysteroscopic approach would carry a risk of uterine rupture.
Findings A 36-year-old nulliparous woman complains of significant "bulk" symptoms (heaviness, urinary frequency, and abdominal bloating). She has a visible mass that extends four finger-breadths above the umbilicus. Pelvic MRI reveals multiple intramural fibroids in a uterus estimated to weigh roughly 2,850 g. The patient is given a 3-month course of a GnRH agonist.
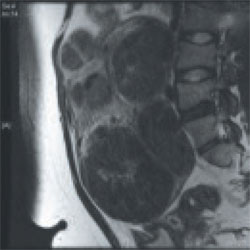
CASE 2
Outcome After treatment with the GnRH agonist, the patient undergoes hand-assisted, laparoscopic, multiple myomectomy. She is discharged home the following day and resumes normal activities within two weeks.
Findings A 32-year-old nulliparous patient seeks treatment for menomenorrhagia and symptoms of bulk and expresses a desire for uterine conservation. Pelvic MRI reveals two distinct intramural fibroids, 6 cm and 9 cm in size.

CASE 3
Outcome The patient undergoes laparoscopic myomectomy without preoperative treatment with a GnRH agonist and is discharged home the same day without postoperative complication. (Although the uterus had two large fibroids, we did not use a GnRH agonist because the uterus was well below the belly button.)
5. Use careful surgical technique
Pay attention to set-up, initial entry
Although we lack definitive data on the practical utility of preoperative, intravenous (IV) antibiotics, we administer cefazolin prophylactically, switching to IV clindamycin if there is a documented allergy to penicillin.
In addition, a uterine manipulator is helpful when the patient has a small or medium-sized uterus. A variety of manipulators are available, but we generally use the VCare manipulator (ConMed Corp) because it is easy to insert and provides excellent uterine mobility.
Initial entry is at the umbilicus, unless the uterus extends above the umbilicus, in which case we enter in the left upper quadrant. We generally place the camera through the umbilical port and use two parallel operative ports on the left side of the patient, where the primary surgeon stands. A detailed description of our laparoscopic entry technique was published recently.13
Place the first trocar two finger-breadths medial and superior to the iliac spine and the second trocar 8 cm cephalad to the first port ( FIGURE 2 ). In a large uterus, trocars may have to be placed higher on the abdomen. A third operative port may be added on the right side, if it is needed.
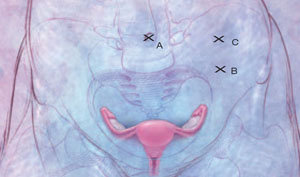
FIGURE 2 Laparoscopic port placement
Place the camera through the umbilical port (A) and operate through two additional ports on the left-hand side of the patient, where the primary surgeon stands. Place the first operative port two finger-breadths medial and superior to the iliac spine (B) and the second port 8 cm cephalad to the first (C).
Incise the uterus
Infiltrate the uterus with dilute vasopressin (20 U in 60 mL of saline), taking care to administer no more than 10 U at a time to minimize the potential for cardiovascular side effects such as bradycardia and hypertension.14 In the past, we periodically encountered episodes of bradycardia when we used 20 U in 40 mL of saline, but we have not had that problem since we changed to a more dilute vasopressin and used no more than 10 U at a time. It may be that an even smaller amount of vasopressin is just as effective, but we do not yet have sufficient data on myomectomy to determine whether that is the case.
Inject the vasopressin subserosally and along the planned hysterotomy. The fibroid itself contains no blood vessels, but the blood supply to the fibroid generally assumes a coronal pattern around it.15 Therefore, it is important to inject the vasopressin into the correct subserosal plane.
We prefer to make a horizontal hysterotomy using the Harmonic Scalpel (Ethicon Endo-Surgery), but other energy sources, such as a monopolar hook or bipolar spatula, are also appropriate.
We choose a horizontal incision because of the ipsilateral port placement we use for suturing. Surgeons who use a midline or contralateral port for suturing may find it easier to repair a vertical hysterotomy. The pattern of blood vessels along the uterus is heterogeneous and variable, and there is no evidence that blood loss or other outcomes are affected by the direction of the uterine incision.15
Once the uterus has been incised, it is important to work efficiently because bleeding will probably continue until the hysterotomy site is completely closed.
Extract the fibroid ("rock and roll")
Extract the fibroid from the uterus by applying generous traction using a tenaculum, and by applying counter-traction using an atraumatic grasper and the Harmonic Scalpel, as needed. We try to limit the use of thermal energy during this step.
The most important aspect of fibroid extraction is ensuring entry into the correct plane. Appropriate entry makes it possible to remove most fibroids without the need for sharp or thermal dissection.
If you are not sure whether you have entered the correct plane, it is better to cut into the fibroid rather than remain too shallow. If you do not enter all the way into the correct plane, you run the risk of pulling and tearing uterine muscle fibers and causing bleeding.
We describe the technique of fibroid extraction as "rock and roll" because it is generally easier to grab the fibroid near the hysterotomy and roll it out rather than pull on the portion of the fibroid that protrudes from the uterus (see the image, for example).
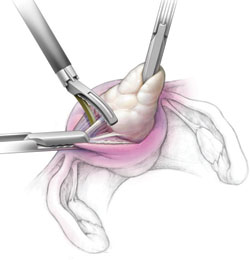
During laparoscopic myomectomy, extract the fibroid by applying generous traction with the tenaculum and counter-traction with an atraumatic grasper and ultrasonic shears. Once you have entered the correct surgical plane, grasp the fibroid near the hysterotomy and simply roll it out of the uterus.
Pregnancy outcomes after laparoscopic myomectomy are generally favorable, with a pregnancy rate that is comparable to or even higher than the rate associated with abdominal myomectomy.1-4
Uneventful vaginal deliveries following laparoscopic myomectomy have been reported in several case series, but so have a number of cases of gravid uterine rupture.5 In a recent study involving 2,050 laparoscopic myomectomies, investigators tracked 386 post-myomectomy pregnancies, 309 deliveries in all, of which 68 were vaginal deliveries.25 It found one case of uterine rupture documented at 33 weeks in a woman who had undergone adenomyomectomy.25
Overall, the literature suggests that uterine rupture after laparoscopic myomectomy is a rare event, occurring in fewer than 1% of pregnancies. Some surgeons use a somewhat arbitrary rule of thumb requiring cesarean delivery if the uterine cavity is entered at myomectomy. This practice is not based on hard evidence, but it does make intuitive sense. If the uterine cavity is entered during myomectomy, it creates a transmural defect that may be more difficult to repair and could carry a higher risk of rupture.
Uterine rupture has also occurred several years after removal of a pedunculated fibroid, suggesting that the use of electrosurgery may weaken the uterine muscle and increase the risk of rupture.
In general—and regardless of the depth of the hysterotomy—it is advisable to counsel patients who have undergone laparoscopic myomectomy that the uterus heals with a scar that may be slightly weaker than the normal myometrium and that elective cesarean delivery may be the optimal strategy. However, a trial of labor is a reasonable alternative, provided the patient receives careful surveillance in a hospital setting.
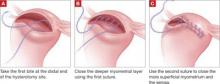
Close the myometrium
In the event of inadvertent entry into the uterine cavity, close the endometrial defect using running 2-0 polyglactin 910 suture, taking care to avoid suture entry into the uterine cavity. Tie this suture using intracorporeal knot-tying.
Close the hysterotomy in layers using 14 × 14 cm bidirectional barbed 0 PDO suture on a 36-mm, half-circle, taper-point needle. If the hysterotomy is longer than 8 cm, we prefer to use 24 × 24 cm suture.
Tack the first needle into the opposite anterior abdominal wall to help prevent tangling of the suture. Close the deepest layer using the first needle and the more superficial layer and serosa using the second needle. Then cut the needles. Because of the uniform tension and bidirectional nature of the barbed suture, no knots are required.
We began using Quill bidirectional barbed suture (Angiotech) in March 2008.16 Since then, we have completed almost 300 laparoscopic cases using this material, including approximately 100 laparoscopic myomectomies. We compiled data on our first year of experience with this material ( TABLE ), during which we had no major complications related to use of the suture, no conversions to laparotomy, and no returns to the OR to address bleeding or complications arising from the use of bidirectional barbed suture.
The original version of barbed suture included a 6-cm segment of regular, smooth suture. If suturing extends to include this segment, apply a LapraTy clip (Ethicon). This use of LapraTy is off-label because the clip is intended for use with Vicryl 2/0, 3/0, and 4/0 (manufacturer). Nevertheless, our clinical experience with this approach has been favorable.16,17
When closing the uterus, use as many layers as necessary to eliminate all dead space within the myometrium. Sometimes, as many as five layers are needed to close a deep myometrial defect, but a two- or three-layer closure is most common.
TABLE
1 year of experience with laparoscopic myomectomy using bidirectional barbed suture
| Variable | Mean ± standard deviation |
|---|---|
| Duration of surgery (min) | 125.47 ± 55.30 |
| Estimated blood loss (mL) | 158.68 ± 252.35 |
| Number of fibroids removed | 4.01 ± 4.21 |
| Weight of fibroids (g) | 252.07 ± 196.43 |
| Hospital stay (days) | 0.73 ± 0.36 |
| Data represent the author's experience with 55 consecutive laparoscopic myomectomy cases between March 2008 and March 2009. | |
Ward off adhesions
We generally cover the hysterotomy site with an adhesion barrier such as Interceed (Gynecare). Although no adhesion barrier is ideal, Interceed has proved to be effective in this clinical scenario.18 Make sure that the hysterotomy site is completely hemostatic at the time the barrier is applied.
Morcellate with caution
We generally use a 12- to 15-mm electronic morcellator for fibroid removal. Morcellation through the umbilicus is often feasible and prevents the need for a large peripheral incision, which may be less cosmetically pleasing to the patient and potentially more painful than a 15-mm umbilical incision.
We place a 5-mm optic through a peripheral port on the ipsilateral side of the surgeon because it allows the surgeon to operate away from the camera, causing less disorientation. Morcellation is inherently dangerous because of the risk of injury to internal organs such as bowel and blood vessels. The best way to prevent such injuries is to:
- keep the rotating blade in view at all times
- stay on the surface of the fibroid during morcellation (avoid coring)
- hold the morcellator steady during morcellation, i.e., do not move it forward while it is active.
6. NSAIDs and few restrictions are the norm postoperatively
We discharge almost all of our patients postoperatively on the day of laparoscopic myomectomy. Patients who have several medical comorbidities may need to stay overnight, however. We have not yet had to readmit a patient after a day-of-procedure discharge, and patients generally recover fairly rapidly.
We are prospectively evaluating our patients' return to daily activities. Most have resumed normal preoperative activities within 10 days. We recommend the scheduled use of nonsteroidal anti-inflammatory medications (NSAIDs), such as 800 mg of ibuprofen every 6 to 8 hours, for the first 3 to 5 days after surgery.
We encourage patients to remain active after surgery, with no weight-lifting restrictions. Instead, we instruct patients to live by the rule, "If it hurts, don't do it." We do prescribe narcotics, but we instruct patients to limit their use as much as possible. We give IV ketorolac perioperatively.
7. Uterine artery occlusion may prevent recurrence
One treatment option that we occasionally use in conjunction with laparoscopic myomectomy is laparoscopic uterine artery occlusion (LUAO) because it can significantly reduce the recurrence rate of uterine fibroids.19 LUAO is especially valuable in the setting of multiple, small uterine fibroids ("bag of marbles") and other cases where it is unlikely that all fibroids will be removed during myomectomy.
At present, we perform LUAO only in patients who have completed childbearing, although early evidence suggests that pregnancy may be relatively safe after uterine artery occlusion.19,20 More data and longer follow-up are required before LUAO should be offered to all women of reproductive age.
8. Consider single-incision laparoscopic myomectomy
This approach has been touted as offering an improved cosmetic outcome and, possibly, less postoperative pain, although these potential benefits have yet to be demonstrated in a well-designed prospective trial.21
We have performed three cases of single-incision myomectomy for an intramural fibroid and have demonstrated this approach to be feasible (article in press). Barbed suture is especially valuable in single-incision surgery because intracorporeal knot-tying can be more challenging when there is only one incision. However, limitations include:
- a lack of triangulation
- instrument crowding at the umbilicus
- difficulty suturing using traditional or barbed suture.
Proper documentation is key
Current Procedural Terminology (CPT) offers two coding options when you've performed a laparoscopic myomectomy:
- 58545 (laparoscopy, surgical, myomectomy, excision; 1 to 4 intramural myomas with total weight of 250 g or less and/or removal of surface myomas)
- 58546 (laparoscopy, surgical, myomectomy, excision; 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 g).
Which code you submit can, of course, make a difference in how much you're reimbursed: 58545 carries 24.21 relative value units (RVU); 58546, 30.59 RVU. The documentation that you present will, therefore, be key in getting paid for the work you've performed.
First, look at the description of 58545. You have two documentation options:
- You removed between one and four intramural myomas (International Classification of Diseases [ICD-9] 218.1; intramural leiomyoma of uterus) whose total weight was =250 g
- You encountered surface myomas (ICD9 218.2; subserous leiomyoma of uterus) and removed all of them, weight aside.
Second, to bill the higher-paying code (58546), you must clearly document removal of intramural myomas only. Again, your work must meet either of two criteria:
- Total weight of all intramural myomas removed is >250 g
- You removed five or more intramural myomas.
You can determine the total weight of the excised tissue 1) in the operating room, if a scale is available, or 2) from the pathology report. A caution: The tissue that you've removed will shrink after it arrives in pathology, and this shrinkage may make a difference when, for example, fewer than five myomas were removed and their total weight is close to 250 g.
Last, estimating the weight of myomas by ultrasonography before surgery is not considered acceptable documentation of weight by most payers.—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American College of Obstetricians and Gynecologists.
Therefore, it may be challenging to apply single-port surgery to more complex pathology, such as very large fibroids and severe pelvic adhesive disease.
Single-incision surgery may offer marginal cosmetic benefit for some patients. When we surveyed our patients informally, however, most of them expressed satisfaction with the cosmetic appearance of peripheral laparoscopic port incisions.
Another potential limitation of single-incision surgery is the cost associated with disposable, articulating instruments and single-port access devices. Although robotic surgery is a feasible approach to both multi-port22,23 and single-port surgery,24 prospective data are lacking, and cost remains an issue. It is possible that future developments in robotic surgery may facilitate suture-intensive, single-incision cases such as myomectomy and sacrocolpopexy. Well-designed prospective trials are urgently needed.
1. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15(12):2663-2668.
2. Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long-term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Hum Reprod. 2001;16(4):770-774.
3. Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomectomies with 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457-464.
4. Palomba S, Zupi E, Falbo A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomes. Fertil Steril. 2007;88(4):933-941.
5. Hurst BS, Matthews ML, Marshburn PB. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83:1-23.
6. Agdi M, Tulandi T. Endoscopic management of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):707-716.
7. Sinha R, Hegde A, Mahajan C, Dubey N, Sundaram M. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15(3):292-300.
8. Malzoni M, Tinelli R, Cosentino F, et al. Laparoscopy versus minilaparotomy in women with symptomatic uterine myomas: short-term and fertility results [published online ahead of print March 12, 2009; corrected proof March 16, 2009]. Fertil Steril. 2009;doi:10.1016/j.fertnstert.2008.12.127.
9. Dudiak CM, Turner DA, Patel SK, Archie JT, Silver B, Norusis M. Uterine leiomyomas in the infertile patient: preoperative localization with MR imaging versus US and hysterosalpingography. Radiology. 1988;167(3):627-630.
10. Zullo F, Pellicano M, De Stefano R, Zupi E, Mastrantonio P. A prospective randomized study to evaluate leuprolide acetate treatment before laparoscopic myomectomy: efficacy and ultrasonographic predictors. Am J Obstet Gynecol. 1998;178(1 Pt 1):108-112.
11. Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93(1):192-198.
12. Bedaiwy MA, Mousa NA, Casper RF. Aromatase inhibitors prevent the estrogen rise associated with the flare effect of gonadotropins in patients treated with GnRH agonists. Fertil Steril. 2009;91(suppl 4):1574-1577.
13. Vellinga TT, De Alwis S, Suzuki Y, Einarsson JI. Laparoscopic entry: the modified Alwis method and more. Rev Obstet Gynecol. 2009;2(3):193-198.
14. Frishman G. Vasopressin: if some is good, is more better? Obstet Gynecol. 2009;113(2 Pt 2):476-477.
15. Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301-1303.
16. Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15(5):621-623.
17. Einarsson JI, Vellinga TT, Twijnstra AR, Suzuki Y, Greenberg JA. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy; an evaluation of safety and clinical outcomes [abstract]. J Minim Invasive Gynecol. 2009;16(6)(suppl 1):S28-S29.Abstract 95.
18. Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod. 1995;10(12):3133-3135.
19. Alborzi S, Ghannadan E, Alborzi S, Alborzi M. A comparison of combined laparoscopic uterine artery ligation and myomectomy versus laparoscopic myomectomy in treatment of symptomatic myoma. Fertil Steril. 2009;92(2):742-747.
20. Holub Z, Mara M, Kuzel D, Jabor A, Maskova J, Eim J. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008;90(5):1886-1891.
21. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single Port Access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
22. Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy—a retrospective matched control study. Fertil Steril. 2009;91(2):556-559.
23. Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201(6):566.e1-5.
24. Escobar PF, Fader AN, Paraiso MF, Kaouk JH, Falcone T. Robotic-assisted laparoendoscopic single-site surgery in gynecology: initial report and technique. J Minim Invasive Gynecol. 2009;16(5):589-591.
25. Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453-462.
Myomectomy is the surgery of choice for women who have symptomatic fibroids and who wish to retain their uterus. And laparoscopic myomectomy is preferable to the abdominal approach in many ways, offering: 1-4
- faster recovery
- a shorter hospital stay
- diminished blood loss
- decreased adhesion formation
- a comparable or higher rate of pregnancy.
Nevertheless, laparoscopic myomectomy is a technically challenging procedure with surgeon-specific limitations. The biggest challenge: appropriately suturing the hysterotomy site.
In this article, I share my experience with laparoscopic myomectomy and offer 8 pearls that may contribute to a successful outcome.
1. Don't settle on laparoscopy prematurely
Given its advantages over the abdominal route, laparoscopic myomectomy should be the preferred approach in the treatment of symptomatic uterine fibroids ( FIGURE 1 ). However, not all patients are appropriate candidates for laparoscopy. Several guidelines have recommended a maximum number and size of fibroids for laparoscopic removal, but practice varies widely, and experienced surgeons successfully take on cases that are well beyond the limits set by most published guidelines.5-7
At our institution, we do not have firm guidelines in place for the number and size of fibroids that can be removed laparoscopically. Other variables enter into decision-making and counseling, among them any medical comorbidity or history of uterine surgery the patient may have, as well as her desires in regard to childbearing and uterine retention.
Hysterectomy may be the most straightforward option for women who have symptomatic fibroids and who have completed childbearing. However, myomectomy is also appropriate as long as the patient is aware of the risk that fibroids may recur and the potential for further surgery. When the patient is in her late 40s or early 50s, the likelihood of fibroid recurrence may be lower than it is in the general population.
In my practice, submucosal and intracavitary fibroids smaller than 4 cm and more than 5 mm away from the uterine serosa are generally removed hysteroscopically, an approach beyond the scope of this article. In women who have completed childbearing but who wish to conserve the uterus, we deliberately enter the uterine cavity laparoscopically because this strategy allows for efficient removal of submucosal and intracavitary fibroids.

FIGURE 1 When and how to treat uterine fibroids
2. Estimate the duration of surgery
When the patient has fibroids that are intramural or subserosal, our general rule of thumb is to determine her suitability for laparoscopic myomectomy, based on the estimated duration of the operation. A surgeon's ability to calculate the length of the operation for a particular patient increases with experience.
We tend to recommend the laparoscopic approach when the procedure is expected to take less than 3 hours to complete. More than 95% of our patients fall into this category.
When we anticipate a prolonged operating time, we discuss the option of hand-assisted laparoscopic myomectomy. This approach involves two or three 5-mm trocar punctures high on the abdomen in conjunction with a suprapubic incision, 6 to 7 cm in length with a hand port in place. Prospective studies have demonstrated a significantly longer recovery with minilaparotomy than with laparoscopy, but these trials compared uteri of similar size.4,8 We expect the laparoscopic approach to confer fewer advantages when operative time is prolonged significantly.
In our practice, we consider one or more of the following conditions appropriate for hand-assisted laparoscopic myomectomy:
- a very large uterus (i.e., heavier than 1,500 to 2,000 g). In these cases, operating times can be excessive because of the need for extensive suturing and morcellation, and bleeding may increase as a result
- more than 20 fibroids on magnetic resonance imaging (MRI). It can be a challenge to locate all of the fibroids; multiple uterine incisions may be necessary
- a medical comorbidity that renders the patient unable to tolerate prolonged anesthesia. For example, we operated on a patient who had Ehlers-Danlos syndrome and who needed to avoid a prolonged operation due to fragile bones and joint laxity.
Of necessity, these guidelines will vary from practice to practice, and gynecologic surgeons who are just beginning to perform laparoscopic myomectomy should not include multiple fibroids or a large uterus among their initial cases. Instead, perform the first few cases in patients who have not had abdominal surgery and who have a symptomatic intramural or subserosal fibroid that is close to the uterine fundus and no larger than 6 cm in diameter.
3. Consider preoperative MRI
Preoperative imaging greatly supplements the clinical examination and facilitates identification of the number, location, and characteristics of the fibroids. Pelvic ultrasonography (US) is appropriate for most patients. We prefer preoperative MRI of the pelvis in the following scenarios:
- uterus larger than 12 weeks (280 g) on clinical examination
- identification of multiple fibroids via US
- history suggestive of adenomyosis.
MRI facilitates preoperative planning by accurately delineating the size and location of the fibroids, and by distinguishing between an adenomyoma and fibroid in most cases.9 For an example of its utility, see "How MRI can guide treatment: 3 cases."
4. Preoperative medical therapy may be indicated
When given preoperatively, gonadotropin-releasing hormone (GnRH) agonists have been shown to reduce blood loss and shorten operative time. The exception: cases involving hypoechoic fibroids, because the cleavage plane may be more difficult to identify, prolonging operative time.10
We generally prefer to use a GnRH agonist in two clinical scenarios: 1) anemia and 2) a uterus that extends above the umbilicus. In the second scenario, the GnRH agonist helps reduce the uterus to a more manageable size.
Aromatase inhibitors show great promise as preoperative agents because there is no initial flare. In addition, because fibroids have a higher concentration of aromatase activity than the surrounding myometrium, a low dosage of an aromatase inhibitor is effective and does not cause significant menopausal symptoms.
A recent comparative study found that fibroid shrinkage was greater after 3 months of letrozole (2.5 mg/day) than after use of a GnRH agonist.11 Total myoma volume decreased by 45.6% in the letrozole group, compared with 33.2% in the group that received a GnRH agonist (P =.02).11
Aromatase inhibitors have also been successfully used during the initial period of GnRH agonist therapy to prevent the symptoms of flare.12 However, because clinical experience is limited, the long-term efficacy and safety of aromatase inhibitors in premenopausal women is unknown.
Findings A 40-year-old nulliparous woman seeks treatment for menometrorrhagia and dysmenorrhea but wants to conserve her uterus. MRI reveals a 4.5-cm submucosal fibroid (arrow) that extends all the way to the uterine serosa, with no evidence of adenomyosis. Her thyroid-stimulating hormone (TSH) level is normal, as is an endometrial biopsy.

CASE 1
Outcome We decide to proceed with laparoscopic myomectomy because a hysteroscopic approach would carry a risk of uterine rupture.
Findings A 36-year-old nulliparous woman complains of significant "bulk" symptoms (heaviness, urinary frequency, and abdominal bloating). She has a visible mass that extends four finger-breadths above the umbilicus. Pelvic MRI reveals multiple intramural fibroids in a uterus estimated to weigh roughly 2,850 g. The patient is given a 3-month course of a GnRH agonist.

CASE 2
Outcome After treatment with the GnRH agonist, the patient undergoes hand-assisted, laparoscopic, multiple myomectomy. She is discharged home the following day and resumes normal activities within two weeks.
Findings A 32-year-old nulliparous patient seeks treatment for menomenorrhagia and symptoms of bulk and expresses a desire for uterine conservation. Pelvic MRI reveals two distinct intramural fibroids, 6 cm and 9 cm in size.

CASE 3
Outcome The patient undergoes laparoscopic myomectomy without preoperative treatment with a GnRH agonist and is discharged home the same day without postoperative complication. (Although the uterus had two large fibroids, we did not use a GnRH agonist because the uterus was well below the belly button.)
5. Use careful surgical technique
Pay attention to set-up, initial entry
Although we lack definitive data on the practical utility of preoperative, intravenous (IV) antibiotics, we administer cefazolin prophylactically, switching to IV clindamycin if there is a documented allergy to penicillin.
In addition, a uterine manipulator is helpful when the patient has a small or medium-sized uterus. A variety of manipulators are available, but we generally use the VCare manipulator (ConMed Corp) because it is easy to insert and provides excellent uterine mobility.
Initial entry is at the umbilicus, unless the uterus extends above the umbilicus, in which case we enter in the left upper quadrant. We generally place the camera through the umbilical port and use two parallel operative ports on the left side of the patient, where the primary surgeon stands. A detailed description of our laparoscopic entry technique was published recently.13
Place the first trocar two finger-breadths medial and superior to the iliac spine and the second trocar 8 cm cephalad to the first port ( FIGURE 2 ). In a large uterus, trocars may have to be placed higher on the abdomen. A third operative port may be added on the right side, if it is needed.

FIGURE 2 Laparoscopic port placement
Place the camera through the umbilical port (A) and operate through two additional ports on the left-hand side of the patient, where the primary surgeon stands. Place the first operative port two finger-breadths medial and superior to the iliac spine (B) and the second port 8 cm cephalad to the first (C).
Incise the uterus
Infiltrate the uterus with dilute vasopressin (20 U in 60 mL of saline), taking care to administer no more than 10 U at a time to minimize the potential for cardiovascular side effects such as bradycardia and hypertension.14 In the past, we periodically encountered episodes of bradycardia when we used 20 U in 40 mL of saline, but we have not had that problem since we changed to a more dilute vasopressin and used no more than 10 U at a time. It may be that an even smaller amount of vasopressin is just as effective, but we do not yet have sufficient data on myomectomy to determine whether that is the case.
Inject the vasopressin subserosally and along the planned hysterotomy. The fibroid itself contains no blood vessels, but the blood supply to the fibroid generally assumes a coronal pattern around it.15 Therefore, it is important to inject the vasopressin into the correct subserosal plane.
We prefer to make a horizontal hysterotomy using the Harmonic Scalpel (Ethicon Endo-Surgery), but other energy sources, such as a monopolar hook or bipolar spatula, are also appropriate.
We choose a horizontal incision because of the ipsilateral port placement we use for suturing. Surgeons who use a midline or contralateral port for suturing may find it easier to repair a vertical hysterotomy. The pattern of blood vessels along the uterus is heterogeneous and variable, and there is no evidence that blood loss or other outcomes are affected by the direction of the uterine incision.15
Once the uterus has been incised, it is important to work efficiently because bleeding will probably continue until the hysterotomy site is completely closed.
Extract the fibroid ("rock and roll")
Extract the fibroid from the uterus by applying generous traction using a tenaculum, and by applying counter-traction using an atraumatic grasper and the Harmonic Scalpel, as needed. We try to limit the use of thermal energy during this step.
The most important aspect of fibroid extraction is ensuring entry into the correct plane. Appropriate entry makes it possible to remove most fibroids without the need for sharp or thermal dissection.
If you are not sure whether you have entered the correct plane, it is better to cut into the fibroid rather than remain too shallow. If you do not enter all the way into the correct plane, you run the risk of pulling and tearing uterine muscle fibers and causing bleeding.
We describe the technique of fibroid extraction as "rock and roll" because it is generally easier to grab the fibroid near the hysterotomy and roll it out rather than pull on the portion of the fibroid that protrudes from the uterus (see the image, for example).

During laparoscopic myomectomy, extract the fibroid by applying generous traction with the tenaculum and counter-traction with an atraumatic grasper and ultrasonic shears. Once you have entered the correct surgical plane, grasp the fibroid near the hysterotomy and simply roll it out of the uterus.
Pregnancy outcomes after laparoscopic myomectomy are generally favorable, with a pregnancy rate that is comparable to or even higher than the rate associated with abdominal myomectomy.1-4
Uneventful vaginal deliveries following laparoscopic myomectomy have been reported in several case series, but so have a number of cases of gravid uterine rupture.5 In a recent study involving 2,050 laparoscopic myomectomies, investigators tracked 386 post-myomectomy pregnancies, 309 deliveries in all, of which 68 were vaginal deliveries.25 It found one case of uterine rupture documented at 33 weeks in a woman who had undergone adenomyomectomy.25
Overall, the literature suggests that uterine rupture after laparoscopic myomectomy is a rare event, occurring in fewer than 1% of pregnancies. Some surgeons use a somewhat arbitrary rule of thumb requiring cesarean delivery if the uterine cavity is entered at myomectomy. This practice is not based on hard evidence, but it does make intuitive sense. If the uterine cavity is entered during myomectomy, it creates a transmural defect that may be more difficult to repair and could carry a higher risk of rupture.
Uterine rupture has also occurred several years after removal of a pedunculated fibroid, suggesting that the use of electrosurgery may weaken the uterine muscle and increase the risk of rupture.
In general—and regardless of the depth of the hysterotomy—it is advisable to counsel patients who have undergone laparoscopic myomectomy that the uterus heals with a scar that may be slightly weaker than the normal myometrium and that elective cesarean delivery may be the optimal strategy. However, a trial of labor is a reasonable alternative, provided the patient receives careful surveillance in a hospital setting.
Close the myometrium
In the event of inadvertent entry into the uterine cavity, close the endometrial defect using running 2-0 polyglactin 910 suture, taking care to avoid suture entry into the uterine cavity. Tie this suture using intracorporeal knot-tying.
Close the hysterotomy in layers using 14 × 14 cm bidirectional barbed 0 PDO suture on a 36-mm, half-circle, taper-point needle. If the hysterotomy is longer than 8 cm, we prefer to use 24 × 24 cm suture.
Tack the first needle into the opposite anterior abdominal wall to help prevent tangling of the suture. Close the deepest layer using the first needle and the more superficial layer and serosa using the second needle. Then cut the needles. Because of the uniform tension and bidirectional nature of the barbed suture, no knots are required.
We began using Quill bidirectional barbed suture (Angiotech) in March 2008.16 Since then, we have completed almost 300 laparoscopic cases using this material, including approximately 100 laparoscopic myomectomies. We compiled data on our first year of experience with this material ( TABLE ), during which we had no major complications related to use of the suture, no conversions to laparotomy, and no returns to the OR to address bleeding or complications arising from the use of bidirectional barbed suture.
The original version of barbed suture included a 6-cm segment of regular, smooth suture. If suturing extends to include this segment, apply a LapraTy clip (Ethicon). This use of LapraTy is off-label because the clip is intended for use with Vicryl 2/0, 3/0, and 4/0 (manufacturer). Nevertheless, our clinical experience with this approach has been favorable.16,17
When closing the uterus, use as many layers as necessary to eliminate all dead space within the myometrium. Sometimes, as many as five layers are needed to close a deep myometrial defect, but a two- or three-layer closure is most common.
TABLE
1 year of experience with laparoscopic myomectomy using bidirectional barbed suture
| Variable | Mean ± standard deviation |
|---|---|
| Duration of surgery (min) | 125.47 ± 55.30 |
| Estimated blood loss (mL) | 158.68 ± 252.35 |
| Number of fibroids removed | 4.01 ± 4.21 |
| Weight of fibroids (g) | 252.07 ± 196.43 |
| Hospital stay (days) | 0.73 ± 0.36 |
| Data represent the author's experience with 55 consecutive laparoscopic myomectomy cases between March 2008 and March 2009. | |
Ward off adhesions
We generally cover the hysterotomy site with an adhesion barrier such as Interceed (Gynecare). Although no adhesion barrier is ideal, Interceed has proved to be effective in this clinical scenario.18 Make sure that the hysterotomy site is completely hemostatic at the time the barrier is applied.
Morcellate with caution
We generally use a 12- to 15-mm electronic morcellator for fibroid removal. Morcellation through the umbilicus is often feasible and prevents the need for a large peripheral incision, which may be less cosmetically pleasing to the patient and potentially more painful than a 15-mm umbilical incision.
We place a 5-mm optic through a peripheral port on the ipsilateral side of the surgeon because it allows the surgeon to operate away from the camera, causing less disorientation. Morcellation is inherently dangerous because of the risk of injury to internal organs such as bowel and blood vessels. The best way to prevent such injuries is to:
- keep the rotating blade in view at all times
- stay on the surface of the fibroid during morcellation (avoid coring)
- hold the morcellator steady during morcellation, i.e., do not move it forward while it is active.
6. NSAIDs and few restrictions are the norm postoperatively
We discharge almost all of our patients postoperatively on the day of laparoscopic myomectomy. Patients who have several medical comorbidities may need to stay overnight, however. We have not yet had to readmit a patient after a day-of-procedure discharge, and patients generally recover fairly rapidly.
We are prospectively evaluating our patients' return to daily activities. Most have resumed normal preoperative activities within 10 days. We recommend the scheduled use of nonsteroidal anti-inflammatory medications (NSAIDs), such as 800 mg of ibuprofen every 6 to 8 hours, for the first 3 to 5 days after surgery.
We encourage patients to remain active after surgery, with no weight-lifting restrictions. Instead, we instruct patients to live by the rule, "If it hurts, don't do it." We do prescribe narcotics, but we instruct patients to limit their use as much as possible. We give IV ketorolac perioperatively.
7. Uterine artery occlusion may prevent recurrence
One treatment option that we occasionally use in conjunction with laparoscopic myomectomy is laparoscopic uterine artery occlusion (LUAO) because it can significantly reduce the recurrence rate of uterine fibroids.19 LUAO is especially valuable in the setting of multiple, small uterine fibroids ("bag of marbles") and other cases where it is unlikely that all fibroids will be removed during myomectomy.
At present, we perform LUAO only in patients who have completed childbearing, although early evidence suggests that pregnancy may be relatively safe after uterine artery occlusion.19,20 More data and longer follow-up are required before LUAO should be offered to all women of reproductive age.
8. Consider single-incision laparoscopic myomectomy
This approach has been touted as offering an improved cosmetic outcome and, possibly, less postoperative pain, although these potential benefits have yet to be demonstrated in a well-designed prospective trial.21
We have performed three cases of single-incision myomectomy for an intramural fibroid and have demonstrated this approach to be feasible (article in press). Barbed suture is especially valuable in single-incision surgery because intracorporeal knot-tying can be more challenging when there is only one incision. However, limitations include:
- a lack of triangulation
- instrument crowding at the umbilicus
- difficulty suturing using traditional or barbed suture.
Proper documentation is key
Current Procedural Terminology (CPT) offers two coding options when you've performed a laparoscopic myomectomy:
- 58545 (laparoscopy, surgical, myomectomy, excision; 1 to 4 intramural myomas with total weight of 250 g or less and/or removal of surface myomas)
- 58546 (laparoscopy, surgical, myomectomy, excision; 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 g).
Which code you submit can, of course, make a difference in how much you're reimbursed: 58545 carries 24.21 relative value units (RVU); 58546, 30.59 RVU. The documentation that you present will, therefore, be key in getting paid for the work you've performed.
First, look at the description of 58545. You have two documentation options:
- You removed between one and four intramural myomas (International Classification of Diseases [ICD-9] 218.1; intramural leiomyoma of uterus) whose total weight was =250 g
- You encountered surface myomas (ICD9 218.2; subserous leiomyoma of uterus) and removed all of them, weight aside.
Second, to bill the higher-paying code (58546), you must clearly document removal of intramural myomas only. Again, your work must meet either of two criteria:
- Total weight of all intramural myomas removed is >250 g
- You removed five or more intramural myomas.
You can determine the total weight of the excised tissue 1) in the operating room, if a scale is available, or 2) from the pathology report. A caution: The tissue that you've removed will shrink after it arrives in pathology, and this shrinkage may make a difference when, for example, fewer than five myomas were removed and their total weight is close to 250 g.
Last, estimating the weight of myomas by ultrasonography before surgery is not considered acceptable documentation of weight by most payers.—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American College of Obstetricians and Gynecologists.
Therefore, it may be challenging to apply single-port surgery to more complex pathology, such as very large fibroids and severe pelvic adhesive disease.
Single-incision surgery may offer marginal cosmetic benefit for some patients. When we surveyed our patients informally, however, most of them expressed satisfaction with the cosmetic appearance of peripheral laparoscopic port incisions.
Another potential limitation of single-incision surgery is the cost associated with disposable, articulating instruments and single-port access devices. Although robotic surgery is a feasible approach to both multi-port22,23 and single-port surgery,24 prospective data are lacking, and cost remains an issue. It is possible that future developments in robotic surgery may facilitate suture-intensive, single-incision cases such as myomectomy and sacrocolpopexy. Well-designed prospective trials are urgently needed.
Myomectomy is the surgery of choice for women who have symptomatic fibroids and who wish to retain their uterus. And laparoscopic myomectomy is preferable to the abdominal approach in many ways, offering: 1-4
- faster recovery
- a shorter hospital stay
- diminished blood loss
- decreased adhesion formation
- a comparable or higher rate of pregnancy.
Nevertheless, laparoscopic myomectomy is a technically challenging procedure with surgeon-specific limitations. The biggest challenge: appropriately suturing the hysterotomy site.
In this article, I share my experience with laparoscopic myomectomy and offer 8 pearls that may contribute to a successful outcome.
1. Don't settle on laparoscopy prematurely
Given its advantages over the abdominal route, laparoscopic myomectomy should be the preferred approach in the treatment of symptomatic uterine fibroids ( FIGURE 1 ). However, not all patients are appropriate candidates for laparoscopy. Several guidelines have recommended a maximum number and size of fibroids for laparoscopic removal, but practice varies widely, and experienced surgeons successfully take on cases that are well beyond the limits set by most published guidelines.5-7
At our institution, we do not have firm guidelines in place for the number and size of fibroids that can be removed laparoscopically. Other variables enter into decision-making and counseling, among them any medical comorbidity or history of uterine surgery the patient may have, as well as her desires in regard to childbearing and uterine retention.
Hysterectomy may be the most straightforward option for women who have symptomatic fibroids and who have completed childbearing. However, myomectomy is also appropriate as long as the patient is aware of the risk that fibroids may recur and the potential for further surgery. When the patient is in her late 40s or early 50s, the likelihood of fibroid recurrence may be lower than it is in the general population.
In my practice, submucosal and intracavitary fibroids smaller than 4 cm and more than 5 mm away from the uterine serosa are generally removed hysteroscopically, an approach beyond the scope of this article. In women who have completed childbearing but who wish to conserve the uterus, we deliberately enter the uterine cavity laparoscopically because this strategy allows for efficient removal of submucosal and intracavitary fibroids.

FIGURE 1 When and how to treat uterine fibroids
2. Estimate the duration of surgery
When the patient has fibroids that are intramural or subserosal, our general rule of thumb is to determine her suitability for laparoscopic myomectomy, based on the estimated duration of the operation. A surgeon's ability to calculate the length of the operation for a particular patient increases with experience.
We tend to recommend the laparoscopic approach when the procedure is expected to take less than 3 hours to complete. More than 95% of our patients fall into this category.
When we anticipate a prolonged operating time, we discuss the option of hand-assisted laparoscopic myomectomy. This approach involves two or three 5-mm trocar punctures high on the abdomen in conjunction with a suprapubic incision, 6 to 7 cm in length with a hand port in place. Prospective studies have demonstrated a significantly longer recovery with minilaparotomy than with laparoscopy, but these trials compared uteri of similar size.4,8 We expect the laparoscopic approach to confer fewer advantages when operative time is prolonged significantly.
In our practice, we consider one or more of the following conditions appropriate for hand-assisted laparoscopic myomectomy:
- a very large uterus (i.e., heavier than 1,500 to 2,000 g). In these cases, operating times can be excessive because of the need for extensive suturing and morcellation, and bleeding may increase as a result
- more than 20 fibroids on magnetic resonance imaging (MRI). It can be a challenge to locate all of the fibroids; multiple uterine incisions may be necessary
- a medical comorbidity that renders the patient unable to tolerate prolonged anesthesia. For example, we operated on a patient who had Ehlers-Danlos syndrome and who needed to avoid a prolonged operation due to fragile bones and joint laxity.
Of necessity, these guidelines will vary from practice to practice, and gynecologic surgeons who are just beginning to perform laparoscopic myomectomy should not include multiple fibroids or a large uterus among their initial cases. Instead, perform the first few cases in patients who have not had abdominal surgery and who have a symptomatic intramural or subserosal fibroid that is close to the uterine fundus and no larger than 6 cm in diameter.
3. Consider preoperative MRI
Preoperative imaging greatly supplements the clinical examination and facilitates identification of the number, location, and characteristics of the fibroids. Pelvic ultrasonography (US) is appropriate for most patients. We prefer preoperative MRI of the pelvis in the following scenarios:
- uterus larger than 12 weeks (280 g) on clinical examination
- identification of multiple fibroids via US
- history suggestive of adenomyosis.
MRI facilitates preoperative planning by accurately delineating the size and location of the fibroids, and by distinguishing between an adenomyoma and fibroid in most cases.9 For an example of its utility, see "How MRI can guide treatment: 3 cases."
4. Preoperative medical therapy may be indicated
When given preoperatively, gonadotropin-releasing hormone (GnRH) agonists have been shown to reduce blood loss and shorten operative time. The exception: cases involving hypoechoic fibroids, because the cleavage plane may be more difficult to identify, prolonging operative time.10
We generally prefer to use a GnRH agonist in two clinical scenarios: 1) anemia and 2) a uterus that extends above the umbilicus. In the second scenario, the GnRH agonist helps reduce the uterus to a more manageable size.
Aromatase inhibitors show great promise as preoperative agents because there is no initial flare. In addition, because fibroids have a higher concentration of aromatase activity than the surrounding myometrium, a low dosage of an aromatase inhibitor is effective and does not cause significant menopausal symptoms.
A recent comparative study found that fibroid shrinkage was greater after 3 months of letrozole (2.5 mg/day) than after use of a GnRH agonist.11 Total myoma volume decreased by 45.6% in the letrozole group, compared with 33.2% in the group that received a GnRH agonist (P =.02).11
Aromatase inhibitors have also been successfully used during the initial period of GnRH agonist therapy to prevent the symptoms of flare.12 However, because clinical experience is limited, the long-term efficacy and safety of aromatase inhibitors in premenopausal women is unknown.
Findings A 40-year-old nulliparous woman seeks treatment for menometrorrhagia and dysmenorrhea but wants to conserve her uterus. MRI reveals a 4.5-cm submucosal fibroid (arrow) that extends all the way to the uterine serosa, with no evidence of adenomyosis. Her thyroid-stimulating hormone (TSH) level is normal, as is an endometrial biopsy.

CASE 1
Outcome We decide to proceed with laparoscopic myomectomy because a hysteroscopic approach would carry a risk of uterine rupture.
Findings A 36-year-old nulliparous woman complains of significant "bulk" symptoms (heaviness, urinary frequency, and abdominal bloating). She has a visible mass that extends four finger-breadths above the umbilicus. Pelvic MRI reveals multiple intramural fibroids in a uterus estimated to weigh roughly 2,850 g. The patient is given a 3-month course of a GnRH agonist.

CASE 2
Outcome After treatment with the GnRH agonist, the patient undergoes hand-assisted, laparoscopic, multiple myomectomy. She is discharged home the following day and resumes normal activities within two weeks.
Findings A 32-year-old nulliparous patient seeks treatment for menomenorrhagia and symptoms of bulk and expresses a desire for uterine conservation. Pelvic MRI reveals two distinct intramural fibroids, 6 cm and 9 cm in size.

CASE 3
Outcome The patient undergoes laparoscopic myomectomy without preoperative treatment with a GnRH agonist and is discharged home the same day without postoperative complication. (Although the uterus had two large fibroids, we did not use a GnRH agonist because the uterus was well below the belly button.)
5. Use careful surgical technique
Pay attention to set-up, initial entry
Although we lack definitive data on the practical utility of preoperative, intravenous (IV) antibiotics, we administer cefazolin prophylactically, switching to IV clindamycin if there is a documented allergy to penicillin.
In addition, a uterine manipulator is helpful when the patient has a small or medium-sized uterus. A variety of manipulators are available, but we generally use the VCare manipulator (ConMed Corp) because it is easy to insert and provides excellent uterine mobility.
Initial entry is at the umbilicus, unless the uterus extends above the umbilicus, in which case we enter in the left upper quadrant. We generally place the camera through the umbilical port and use two parallel operative ports on the left side of the patient, where the primary surgeon stands. A detailed description of our laparoscopic entry technique was published recently.13
Place the first trocar two finger-breadths medial and superior to the iliac spine and the second trocar 8 cm cephalad to the first port ( FIGURE 2 ). In a large uterus, trocars may have to be placed higher on the abdomen. A third operative port may be added on the right side, if it is needed.

FIGURE 2 Laparoscopic port placement
Place the camera through the umbilical port (A) and operate through two additional ports on the left-hand side of the patient, where the primary surgeon stands. Place the first operative port two finger-breadths medial and superior to the iliac spine (B) and the second port 8 cm cephalad to the first (C).
Incise the uterus
Infiltrate the uterus with dilute vasopressin (20 U in 60 mL of saline), taking care to administer no more than 10 U at a time to minimize the potential for cardiovascular side effects such as bradycardia and hypertension.14 In the past, we periodically encountered episodes of bradycardia when we used 20 U in 40 mL of saline, but we have not had that problem since we changed to a more dilute vasopressin and used no more than 10 U at a time. It may be that an even smaller amount of vasopressin is just as effective, but we do not yet have sufficient data on myomectomy to determine whether that is the case.
Inject the vasopressin subserosally and along the planned hysterotomy. The fibroid itself contains no blood vessels, but the blood supply to the fibroid generally assumes a coronal pattern around it.15 Therefore, it is important to inject the vasopressin into the correct subserosal plane.
We prefer to make a horizontal hysterotomy using the Harmonic Scalpel (Ethicon Endo-Surgery), but other energy sources, such as a monopolar hook or bipolar spatula, are also appropriate.
We choose a horizontal incision because of the ipsilateral port placement we use for suturing. Surgeons who use a midline or contralateral port for suturing may find it easier to repair a vertical hysterotomy. The pattern of blood vessels along the uterus is heterogeneous and variable, and there is no evidence that blood loss or other outcomes are affected by the direction of the uterine incision.15
Once the uterus has been incised, it is important to work efficiently because bleeding will probably continue until the hysterotomy site is completely closed.
Extract the fibroid ("rock and roll")
Extract the fibroid from the uterus by applying generous traction using a tenaculum, and by applying counter-traction using an atraumatic grasper and the Harmonic Scalpel, as needed. We try to limit the use of thermal energy during this step.
The most important aspect of fibroid extraction is ensuring entry into the correct plane. Appropriate entry makes it possible to remove most fibroids without the need for sharp or thermal dissection.
If you are not sure whether you have entered the correct plane, it is better to cut into the fibroid rather than remain too shallow. If you do not enter all the way into the correct plane, you run the risk of pulling and tearing uterine muscle fibers and causing bleeding.
We describe the technique of fibroid extraction as "rock and roll" because it is generally easier to grab the fibroid near the hysterotomy and roll it out rather than pull on the portion of the fibroid that protrudes from the uterus (see the image, for example).

During laparoscopic myomectomy, extract the fibroid by applying generous traction with the tenaculum and counter-traction with an atraumatic grasper and ultrasonic shears. Once you have entered the correct surgical plane, grasp the fibroid near the hysterotomy and simply roll it out of the uterus.
Pregnancy outcomes after laparoscopic myomectomy are generally favorable, with a pregnancy rate that is comparable to or even higher than the rate associated with abdominal myomectomy.1-4
Uneventful vaginal deliveries following laparoscopic myomectomy have been reported in several case series, but so have a number of cases of gravid uterine rupture.5 In a recent study involving 2,050 laparoscopic myomectomies, investigators tracked 386 post-myomectomy pregnancies, 309 deliveries in all, of which 68 were vaginal deliveries.25 It found one case of uterine rupture documented at 33 weeks in a woman who had undergone adenomyomectomy.25
Overall, the literature suggests that uterine rupture after laparoscopic myomectomy is a rare event, occurring in fewer than 1% of pregnancies. Some surgeons use a somewhat arbitrary rule of thumb requiring cesarean delivery if the uterine cavity is entered at myomectomy. This practice is not based on hard evidence, but it does make intuitive sense. If the uterine cavity is entered during myomectomy, it creates a transmural defect that may be more difficult to repair and could carry a higher risk of rupture.
Uterine rupture has also occurred several years after removal of a pedunculated fibroid, suggesting that the use of electrosurgery may weaken the uterine muscle and increase the risk of rupture.
In general—and regardless of the depth of the hysterotomy—it is advisable to counsel patients who have undergone laparoscopic myomectomy that the uterus heals with a scar that may be slightly weaker than the normal myometrium and that elective cesarean delivery may be the optimal strategy. However, a trial of labor is a reasonable alternative, provided the patient receives careful surveillance in a hospital setting.
Close the myometrium
In the event of inadvertent entry into the uterine cavity, close the endometrial defect using running 2-0 polyglactin 910 suture, taking care to avoid suture entry into the uterine cavity. Tie this suture using intracorporeal knot-tying.
Close the hysterotomy in layers using 14 × 14 cm bidirectional barbed 0 PDO suture on a 36-mm, half-circle, taper-point needle. If the hysterotomy is longer than 8 cm, we prefer to use 24 × 24 cm suture.
Tack the first needle into the opposite anterior abdominal wall to help prevent tangling of the suture. Close the deepest layer using the first needle and the more superficial layer and serosa using the second needle. Then cut the needles. Because of the uniform tension and bidirectional nature of the barbed suture, no knots are required.
We began using Quill bidirectional barbed suture (Angiotech) in March 2008.16 Since then, we have completed almost 300 laparoscopic cases using this material, including approximately 100 laparoscopic myomectomies. We compiled data on our first year of experience with this material ( TABLE ), during which we had no major complications related to use of the suture, no conversions to laparotomy, and no returns to the OR to address bleeding or complications arising from the use of bidirectional barbed suture.
The original version of barbed suture included a 6-cm segment of regular, smooth suture. If suturing extends to include this segment, apply a LapraTy clip (Ethicon). This use of LapraTy is off-label because the clip is intended for use with Vicryl 2/0, 3/0, and 4/0 (manufacturer). Nevertheless, our clinical experience with this approach has been favorable.16,17
When closing the uterus, use as many layers as necessary to eliminate all dead space within the myometrium. Sometimes, as many as five layers are needed to close a deep myometrial defect, but a two- or three-layer closure is most common.
TABLE
1 year of experience with laparoscopic myomectomy using bidirectional barbed suture
| Variable | Mean ± standard deviation |
|---|---|
| Duration of surgery (min) | 125.47 ± 55.30 |
| Estimated blood loss (mL) | 158.68 ± 252.35 |
| Number of fibroids removed | 4.01 ± 4.21 |
| Weight of fibroids (g) | 252.07 ± 196.43 |
| Hospital stay (days) | 0.73 ± 0.36 |
| Data represent the author's experience with 55 consecutive laparoscopic myomectomy cases between March 2008 and March 2009. | |
Ward off adhesions
We generally cover the hysterotomy site with an adhesion barrier such as Interceed (Gynecare). Although no adhesion barrier is ideal, Interceed has proved to be effective in this clinical scenario.18 Make sure that the hysterotomy site is completely hemostatic at the time the barrier is applied.
Morcellate with caution
We generally use a 12- to 15-mm electronic morcellator for fibroid removal. Morcellation through the umbilicus is often feasible and prevents the need for a large peripheral incision, which may be less cosmetically pleasing to the patient and potentially more painful than a 15-mm umbilical incision.
We place a 5-mm optic through a peripheral port on the ipsilateral side of the surgeon because it allows the surgeon to operate away from the camera, causing less disorientation. Morcellation is inherently dangerous because of the risk of injury to internal organs such as bowel and blood vessels. The best way to prevent such injuries is to:
- keep the rotating blade in view at all times
- stay on the surface of the fibroid during morcellation (avoid coring)
- hold the morcellator steady during morcellation, i.e., do not move it forward while it is active.
6. NSAIDs and few restrictions are the norm postoperatively
We discharge almost all of our patients postoperatively on the day of laparoscopic myomectomy. Patients who have several medical comorbidities may need to stay overnight, however. We have not yet had to readmit a patient after a day-of-procedure discharge, and patients generally recover fairly rapidly.
We are prospectively evaluating our patients' return to daily activities. Most have resumed normal preoperative activities within 10 days. We recommend the scheduled use of nonsteroidal anti-inflammatory medications (NSAIDs), such as 800 mg of ibuprofen every 6 to 8 hours, for the first 3 to 5 days after surgery.
We encourage patients to remain active after surgery, with no weight-lifting restrictions. Instead, we instruct patients to live by the rule, "If it hurts, don't do it." We do prescribe narcotics, but we instruct patients to limit their use as much as possible. We give IV ketorolac perioperatively.
7. Uterine artery occlusion may prevent recurrence
One treatment option that we occasionally use in conjunction with laparoscopic myomectomy is laparoscopic uterine artery occlusion (LUAO) because it can significantly reduce the recurrence rate of uterine fibroids.19 LUAO is especially valuable in the setting of multiple, small uterine fibroids ("bag of marbles") and other cases where it is unlikely that all fibroids will be removed during myomectomy.
At present, we perform LUAO only in patients who have completed childbearing, although early evidence suggests that pregnancy may be relatively safe after uterine artery occlusion.19,20 More data and longer follow-up are required before LUAO should be offered to all women of reproductive age.
8. Consider single-incision laparoscopic myomectomy
This approach has been touted as offering an improved cosmetic outcome and, possibly, less postoperative pain, although these potential benefits have yet to be demonstrated in a well-designed prospective trial.21
We have performed three cases of single-incision myomectomy for an intramural fibroid and have demonstrated this approach to be feasible (article in press). Barbed suture is especially valuable in single-incision surgery because intracorporeal knot-tying can be more challenging when there is only one incision. However, limitations include:
- a lack of triangulation
- instrument crowding at the umbilicus
- difficulty suturing using traditional or barbed suture.
Proper documentation is key
Current Procedural Terminology (CPT) offers two coding options when you've performed a laparoscopic myomectomy:
- 58545 (laparoscopy, surgical, myomectomy, excision; 1 to 4 intramural myomas with total weight of 250 g or less and/or removal of surface myomas)
- 58546 (laparoscopy, surgical, myomectomy, excision; 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 g).
Which code you submit can, of course, make a difference in how much you're reimbursed: 58545 carries 24.21 relative value units (RVU); 58546, 30.59 RVU. The documentation that you present will, therefore, be key in getting paid for the work you've performed.
First, look at the description of 58545. You have two documentation options:
- You removed between one and four intramural myomas (International Classification of Diseases [ICD-9] 218.1; intramural leiomyoma of uterus) whose total weight was =250 g
- You encountered surface myomas (ICD9 218.2; subserous leiomyoma of uterus) and removed all of them, weight aside.
Second, to bill the higher-paying code (58546), you must clearly document removal of intramural myomas only. Again, your work must meet either of two criteria:
- Total weight of all intramural myomas removed is >250 g
- You removed five or more intramural myomas.
You can determine the total weight of the excised tissue 1) in the operating room, if a scale is available, or 2) from the pathology report. A caution: The tissue that you've removed will shrink after it arrives in pathology, and this shrinkage may make a difference when, for example, fewer than five myomas were removed and their total weight is close to 250 g.
Last, estimating the weight of myomas by ultrasonography before surgery is not considered acceptable documentation of weight by most payers.—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American College of Obstetricians and Gynecologists.
Therefore, it may be challenging to apply single-port surgery to more complex pathology, such as very large fibroids and severe pelvic adhesive disease.
Single-incision surgery may offer marginal cosmetic benefit for some patients. When we surveyed our patients informally, however, most of them expressed satisfaction with the cosmetic appearance of peripheral laparoscopic port incisions.
Another potential limitation of single-incision surgery is the cost associated with disposable, articulating instruments and single-port access devices. Although robotic surgery is a feasible approach to both multi-port22,23 and single-port surgery,24 prospective data are lacking, and cost remains an issue. It is possible that future developments in robotic surgery may facilitate suture-intensive, single-incision cases such as myomectomy and sacrocolpopexy. Well-designed prospective trials are urgently needed.
1. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15(12):2663-2668.
2. Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long-term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Hum Reprod. 2001;16(4):770-774.
3. Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomectomies with 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457-464.
4. Palomba S, Zupi E, Falbo A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomes. Fertil Steril. 2007;88(4):933-941.
5. Hurst BS, Matthews ML, Marshburn PB. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83:1-23.
6. Agdi M, Tulandi T. Endoscopic management of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):707-716.
7. Sinha R, Hegde A, Mahajan C, Dubey N, Sundaram M. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15(3):292-300.
8. Malzoni M, Tinelli R, Cosentino F, et al. Laparoscopy versus minilaparotomy in women with symptomatic uterine myomas: short-term and fertility results [published online ahead of print March 12, 2009; corrected proof March 16, 2009]. Fertil Steril. 2009;doi:10.1016/j.fertnstert.2008.12.127.
9. Dudiak CM, Turner DA, Patel SK, Archie JT, Silver B, Norusis M. Uterine leiomyomas in the infertile patient: preoperative localization with MR imaging versus US and hysterosalpingography. Radiology. 1988;167(3):627-630.
10. Zullo F, Pellicano M, De Stefano R, Zupi E, Mastrantonio P. A prospective randomized study to evaluate leuprolide acetate treatment before laparoscopic myomectomy: efficacy and ultrasonographic predictors. Am J Obstet Gynecol. 1998;178(1 Pt 1):108-112.
11. Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93(1):192-198.
12. Bedaiwy MA, Mousa NA, Casper RF. Aromatase inhibitors prevent the estrogen rise associated with the flare effect of gonadotropins in patients treated with GnRH agonists. Fertil Steril. 2009;91(suppl 4):1574-1577.
13. Vellinga TT, De Alwis S, Suzuki Y, Einarsson JI. Laparoscopic entry: the modified Alwis method and more. Rev Obstet Gynecol. 2009;2(3):193-198.
14. Frishman G. Vasopressin: if some is good, is more better? Obstet Gynecol. 2009;113(2 Pt 2):476-477.
15. Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301-1303.
16. Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15(5):621-623.
17. Einarsson JI, Vellinga TT, Twijnstra AR, Suzuki Y, Greenberg JA. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy; an evaluation of safety and clinical outcomes [abstract]. J Minim Invasive Gynecol. 2009;16(6)(suppl 1):S28-S29.Abstract 95.
18. Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod. 1995;10(12):3133-3135.
19. Alborzi S, Ghannadan E, Alborzi S, Alborzi M. A comparison of combined laparoscopic uterine artery ligation and myomectomy versus laparoscopic myomectomy in treatment of symptomatic myoma. Fertil Steril. 2009;92(2):742-747.
20. Holub Z, Mara M, Kuzel D, Jabor A, Maskova J, Eim J. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008;90(5):1886-1891.
21. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single Port Access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
22. Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy—a retrospective matched control study. Fertil Steril. 2009;91(2):556-559.
23. Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201(6):566.e1-5.
24. Escobar PF, Fader AN, Paraiso MF, Kaouk JH, Falcone T. Robotic-assisted laparoendoscopic single-site surgery in gynecology: initial report and technique. J Minim Invasive Gynecol. 2009;16(5):589-591.
25. Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453-462.
1. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15(12):2663-2668.
2. Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long-term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Hum Reprod. 2001;16(4):770-774.
3. Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomectomies with 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457-464.
4. Palomba S, Zupi E, Falbo A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomes. Fertil Steril. 2007;88(4):933-941.
5. Hurst BS, Matthews ML, Marshburn PB. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83:1-23.
6. Agdi M, Tulandi T. Endoscopic management of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):707-716.
7. Sinha R, Hegde A, Mahajan C, Dubey N, Sundaram M. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15(3):292-300.
8. Malzoni M, Tinelli R, Cosentino F, et al. Laparoscopy versus minilaparotomy in women with symptomatic uterine myomas: short-term and fertility results [published online ahead of print March 12, 2009; corrected proof March 16, 2009]. Fertil Steril. 2009;doi:10.1016/j.fertnstert.2008.12.127.
9. Dudiak CM, Turner DA, Patel SK, Archie JT, Silver B, Norusis M. Uterine leiomyomas in the infertile patient: preoperative localization with MR imaging versus US and hysterosalpingography. Radiology. 1988;167(3):627-630.
10. Zullo F, Pellicano M, De Stefano R, Zupi E, Mastrantonio P. A prospective randomized study to evaluate leuprolide acetate treatment before laparoscopic myomectomy: efficacy and ultrasonographic predictors. Am J Obstet Gynecol. 1998;178(1 Pt 1):108-112.
11. Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93(1):192-198.
12. Bedaiwy MA, Mousa NA, Casper RF. Aromatase inhibitors prevent the estrogen rise associated with the flare effect of gonadotropins in patients treated with GnRH agonists. Fertil Steril. 2009;91(suppl 4):1574-1577.
13. Vellinga TT, De Alwis S, Suzuki Y, Einarsson JI. Laparoscopic entry: the modified Alwis method and more. Rev Obstet Gynecol. 2009;2(3):193-198.
14. Frishman G. Vasopressin: if some is good, is more better? Obstet Gynecol. 2009;113(2 Pt 2):476-477.
15. Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301-1303.
16. Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15(5):621-623.
17. Einarsson JI, Vellinga TT, Twijnstra AR, Suzuki Y, Greenberg JA. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy; an evaluation of safety and clinical outcomes [abstract]. J Minim Invasive Gynecol. 2009;16(6)(suppl 1):S28-S29.Abstract 95.
18. Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod. 1995;10(12):3133-3135.
19. Alborzi S, Ghannadan E, Alborzi S, Alborzi M. A comparison of combined laparoscopic uterine artery ligation and myomectomy versus laparoscopic myomectomy in treatment of symptomatic myoma. Fertil Steril. 2009;92(2):742-747.
20. Holub Z, Mara M, Kuzel D, Jabor A, Maskova J, Eim J. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008;90(5):1886-1891.
21. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single Port Access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
22. Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy—a retrospective matched control study. Fertil Steril. 2009;91(2):556-559.
23. Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201(6):566.e1-5.
24. Escobar PF, Fader AN, Paraiso MF, Kaouk JH, Falcone T. Robotic-assisted laparoendoscopic single-site surgery in gynecology: initial report and technique. J Minim Invasive Gynecol. 2009;16(5):589-591.
25. Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453-462.
Barbed suture, now in the toolbox of minimally invasive gyn surgery
The authors report no financial relationships relevant to this article.
Barbed suture is a relatively new technology that has the potential to greatly facilitate laparoscopic suturing. Two barbed sutures, each a different type, are available, or soon will be:
- the Quill bidirectional barbed suture (Angiotech Pharmaceuticals) was approved by the US Food and Drug Administration (FDA) in 2004 for soft-tissue approximation and has been sold in the United States since 2007 ( FIGURE 1A )1
- the V-Loc 180 unidirectional barbed suture (Covidien) won FDA approval in March for soft-tissue approximation. It should be available in October ( FIGURE 1B ).
(Note: We have performed laparoscopic surgery using the Quill suture, but not the V-Loc 180, both as part of clinical research and in practice.)
In the bidirectional suture, barbs are cut into suture in a direction opposite to the needle; the barbs change direction at the midpoint of the suture, and needles are swaged onto both ends ( FIGURE 1A ).2
The anchoring of bidirectional barbed suture resists migration and can be conceptualized as a “continuous interrupted” suture without knots; it has been shown to have tissue-holding performance that is at least equal to knot-anchored suture.3,4
A suture with bidirectional barbs offers several advantages:
- Because it self-anchors and is balanced by the countervailing barbs, no knots are required
- It self-anchors every 1 mm of tissue, yielding more consistent wound opposition; this may result in a more “watertight” seal
- Because it is knotless, it can securely reapproximate tissues in less time, at less cost, and with less aggravation.5,6
Note: Whether these characteristics apply to unidirectional barbed suture remains to be determined.
FIGURE 1 Two types of barbed suture
Bidirectional suture, in which barbs are cut into suture and change direction at the midpoint.
Unidirectional suture has a loop at the distal end to facilitate initial suture fastening.
Specifications
Because the effective diameter of suture is decreased when barbs are cut into it, barbed suture is rated one suture size smaller than smooth suture. A 0 barbed suture, for example, equals a 2-0 smooth suture.
In the current version of the Quill suture, there is a segment of approximately 7 cm of smooth suture proximal to each needle. A second-generation Quill will be released this year, with barbs extending all the way to the needles. Bidirectional barbed suture materials currently include polydioxanone (PDO), poliglecaprone 25 (Monoderm), nylon, and polypropylene.
The V-Loc 180 is a unidirectional barbed polyglyconate (Maxon) suture with a loop at the distal end to facilitate initial fastening.
We have performed more than 200 laparoscopic surgeries using Quill suture since March 2008; most involved total laparoscopic hysterectomy and laparoscopic myomectomy. We have also used Quill suture for laparoscopic sacrocolpopexy and uterosacral ligament suspension.
We have had outstanding clinical results: no cuff evisceration and only minor cases of vaginal bleeding or spotting in the immediate postoperative period.7 In one case, reoperation was necessary after laparoscopic myomectomy, but the repeat surgery was performed to treat small-bowel obstruction that was not directly related to the hysterotomy repair.
Technique
For vaginal-cuff closure, we use 0 PDO suture on a 36-mm half-circle taper-point needle. We initially used half of a 14×14-cm suture with a LapraTy clip on the distal end. We began the repair at the distal end of the vaginal cuff, taking care to incorporate the uterosacral ligament into the initial bite, and continuing proximally until the other uterosacral ligament was incorporated into the repair.
More recently, we have been using the 7×7-cm 0 PDO because it is now available with the 36-mm half-circle taper-point needle. Regardless of suture material, it is important to obtain a full-thickness bite, with a 1-cm margin on the vaginal mucosa on each bite.
With the shorter suture, we begin closure in the middle of the cuff and take each needle to the opposite end of the cuff ( FIGURE 2 ). We incorporate the uterosacral ligament into the final bite on each side, and we cut the suture without a knot or a LapraTy clip.
FIGURE 2 Closing the vaginal cuff with bidirectional barbed suture For myomectomy closure, we use 14×14-cm 0 PDO suture on a 36-mm half-circle taper-point needle. If the hysterotomy is longer than 8 cm, we prefer to use 24×24-cm suture. We tack the first needle to the opposite anterior abdominal wall to avoid having the suture become entangled. We close the deepest layer using the first needle; we use the second needle to close the more superficial layer and the serosa, if possible ( FIGURE 3 ). If the suture is used beyond the barbed portion, we cut the needles and apply a LapraTy clip. Sometimes, three or four layers are needed to close a deep myometrial defect. 2/0 Monoderm can also be used for the serosa, continuously or as a baseball stitch.
FIGURE 3 Myomectomy closure with bidirectional barbed suture
1. Department of Health and Human Services letter approving Section 510(k) premarket notification for Quill suture. Available at http://www.accessdata.fda.gov/cdrh_docs/pdf4/k042075.pdf. Accessed August 13, 2009.
2. Leung JC. Barbed suture technology: recent advances. In: Medical Textiles 2004, Conference Proceedings; Oct. 26–27, 2004; pp. 62–80; Pittsburgh.
3. Rashid RM, Sartori M, White LE, Villa MT, Yoo SS, Alam M. Breaking strength of barbed polypropylene sutures: rater-blinded, controlled comparison with nonbarbed sutures of various calibers. Arch Dermatol. 2007;143:869-872.
4. Rodeheaver GT, Piñeros-Fernandez A, Salopek LS, et al. Barbed sutures for wound closure: in vivo wound security, tissue compatibility and cosmesis measurements. In: Society for Biomaterials 30th Annual Meeting Transactions; 2004; transaction 229, p. 232.
5. Greenberg JA, Einarsson JI. Use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15:621-623.
6. Moran ME, Marsh C, Perrotti M. Bidirectional-barbed sutured knotless running anastomosis v classic Van Velthoven suturing in a model system. J Endourol. 2007;21:1175-1178.
7. Einarsson JI, Vellinga T, Twijnstra A, Suzuki Y, Greenberg JA. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy; an evaluation of safety and clinical outcomes. Submitted as an abstract to the 38th Global Congress in Minimally Invasive Gynecology, 2009.
The authors report no financial relationships relevant to this article.
Barbed suture is a relatively new technology that has the potential to greatly facilitate laparoscopic suturing. Two barbed sutures, each a different type, are available, or soon will be:
- the Quill bidirectional barbed suture (Angiotech Pharmaceuticals) was approved by the US Food and Drug Administration (FDA) in 2004 for soft-tissue approximation and has been sold in the United States since 2007 ( FIGURE 1A )1
- the V-Loc 180 unidirectional barbed suture (Covidien) won FDA approval in March for soft-tissue approximation. It should be available in October ( FIGURE 1B ).
(Note: We have performed laparoscopic surgery using the Quill suture, but not the V-Loc 180, both as part of clinical research and in practice.)
In the bidirectional suture, barbs are cut into suture in a direction opposite to the needle; the barbs change direction at the midpoint of the suture, and needles are swaged onto both ends ( FIGURE 1A ).2
The anchoring of bidirectional barbed suture resists migration and can be conceptualized as a “continuous interrupted” suture without knots; it has been shown to have tissue-holding performance that is at least equal to knot-anchored suture.3,4
A suture with bidirectional barbs offers several advantages:
- Because it self-anchors and is balanced by the countervailing barbs, no knots are required
- It self-anchors every 1 mm of tissue, yielding more consistent wound opposition; this may result in a more “watertight” seal
- Because it is knotless, it can securely reapproximate tissues in less time, at less cost, and with less aggravation.5,6
Note: Whether these characteristics apply to unidirectional barbed suture remains to be determined.
FIGURE 1 Two types of barbed suture
Bidirectional suture, in which barbs are cut into suture and change direction at the midpoint.
Unidirectional suture has a loop at the distal end to facilitate initial suture fastening.
Specifications
Because the effective diameter of suture is decreased when barbs are cut into it, barbed suture is rated one suture size smaller than smooth suture. A 0 barbed suture, for example, equals a 2-0 smooth suture.
In the current version of the Quill suture, there is a segment of approximately 7 cm of smooth suture proximal to each needle. A second-generation Quill will be released this year, with barbs extending all the way to the needles. Bidirectional barbed suture materials currently include polydioxanone (PDO), poliglecaprone 25 (Monoderm), nylon, and polypropylene.
The V-Loc 180 is a unidirectional barbed polyglyconate (Maxon) suture with a loop at the distal end to facilitate initial fastening.
We have performed more than 200 laparoscopic surgeries using Quill suture since March 2008; most involved total laparoscopic hysterectomy and laparoscopic myomectomy. We have also used Quill suture for laparoscopic sacrocolpopexy and uterosacral ligament suspension.
We have had outstanding clinical results: no cuff evisceration and only minor cases of vaginal bleeding or spotting in the immediate postoperative period.7 In one case, reoperation was necessary after laparoscopic myomectomy, but the repeat surgery was performed to treat small-bowel obstruction that was not directly related to the hysterotomy repair.
Technique
For vaginal-cuff closure, we use 0 PDO suture on a 36-mm half-circle taper-point needle. We initially used half of a 14×14-cm suture with a LapraTy clip on the distal end. We began the repair at the distal end of the vaginal cuff, taking care to incorporate the uterosacral ligament into the initial bite, and continuing proximally until the other uterosacral ligament was incorporated into the repair.
More recently, we have been using the 7×7-cm 0 PDO because it is now available with the 36-mm half-circle taper-point needle. Regardless of suture material, it is important to obtain a full-thickness bite, with a 1-cm margin on the vaginal mucosa on each bite.
With the shorter suture, we begin closure in the middle of the cuff and take each needle to the opposite end of the cuff ( FIGURE 2 ). We incorporate the uterosacral ligament into the final bite on each side, and we cut the suture without a knot or a LapraTy clip.
FIGURE 2 Closing the vaginal cuff with bidirectional barbed suture For myomectomy closure, we use 14×14-cm 0 PDO suture on a 36-mm half-circle taper-point needle. If the hysterotomy is longer than 8 cm, we prefer to use 24×24-cm suture. We tack the first needle to the opposite anterior abdominal wall to avoid having the suture become entangled. We close the deepest layer using the first needle; we use the second needle to close the more superficial layer and the serosa, if possible ( FIGURE 3 ). If the suture is used beyond the barbed portion, we cut the needles and apply a LapraTy clip. Sometimes, three or four layers are needed to close a deep myometrial defect. 2/0 Monoderm can also be used for the serosa, continuously or as a baseball stitch.
FIGURE 3 Myomectomy closure with bidirectional barbed suture
The authors report no financial relationships relevant to this article.
Barbed suture is a relatively new technology that has the potential to greatly facilitate laparoscopic suturing. Two barbed sutures, each a different type, are available, or soon will be:
- the Quill bidirectional barbed suture (Angiotech Pharmaceuticals) was approved by the US Food and Drug Administration (FDA) in 2004 for soft-tissue approximation and has been sold in the United States since 2007 ( FIGURE 1A )1
- the V-Loc 180 unidirectional barbed suture (Covidien) won FDA approval in March for soft-tissue approximation. It should be available in October ( FIGURE 1B ).
(Note: We have performed laparoscopic surgery using the Quill suture, but not the V-Loc 180, both as part of clinical research and in practice.)
In the bidirectional suture, barbs are cut into suture in a direction opposite to the needle; the barbs change direction at the midpoint of the suture, and needles are swaged onto both ends ( FIGURE 1A ).2
The anchoring of bidirectional barbed suture resists migration and can be conceptualized as a “continuous interrupted” suture without knots; it has been shown to have tissue-holding performance that is at least equal to knot-anchored suture.3,4
A suture with bidirectional barbs offers several advantages:
- Because it self-anchors and is balanced by the countervailing barbs, no knots are required
- It self-anchors every 1 mm of tissue, yielding more consistent wound opposition; this may result in a more “watertight” seal
- Because it is knotless, it can securely reapproximate tissues in less time, at less cost, and with less aggravation.5,6
Note: Whether these characteristics apply to unidirectional barbed suture remains to be determined.
FIGURE 1 Two types of barbed suture
Bidirectional suture, in which barbs are cut into suture and change direction at the midpoint.
Unidirectional suture has a loop at the distal end to facilitate initial suture fastening.
Specifications
Because the effective diameter of suture is decreased when barbs are cut into it, barbed suture is rated one suture size smaller than smooth suture. A 0 barbed suture, for example, equals a 2-0 smooth suture.
In the current version of the Quill suture, there is a segment of approximately 7 cm of smooth suture proximal to each needle. A second-generation Quill will be released this year, with barbs extending all the way to the needles. Bidirectional barbed suture materials currently include polydioxanone (PDO), poliglecaprone 25 (Monoderm), nylon, and polypropylene.
The V-Loc 180 is a unidirectional barbed polyglyconate (Maxon) suture with a loop at the distal end to facilitate initial fastening.
We have performed more than 200 laparoscopic surgeries using Quill suture since March 2008; most involved total laparoscopic hysterectomy and laparoscopic myomectomy. We have also used Quill suture for laparoscopic sacrocolpopexy and uterosacral ligament suspension.
We have had outstanding clinical results: no cuff evisceration and only minor cases of vaginal bleeding or spotting in the immediate postoperative period.7 In one case, reoperation was necessary after laparoscopic myomectomy, but the repeat surgery was performed to treat small-bowel obstruction that was not directly related to the hysterotomy repair.
Technique
For vaginal-cuff closure, we use 0 PDO suture on a 36-mm half-circle taper-point needle. We initially used half of a 14×14-cm suture with a LapraTy clip on the distal end. We began the repair at the distal end of the vaginal cuff, taking care to incorporate the uterosacral ligament into the initial bite, and continuing proximally until the other uterosacral ligament was incorporated into the repair.
More recently, we have been using the 7×7-cm 0 PDO because it is now available with the 36-mm half-circle taper-point needle. Regardless of suture material, it is important to obtain a full-thickness bite, with a 1-cm margin on the vaginal mucosa on each bite.
With the shorter suture, we begin closure in the middle of the cuff and take each needle to the opposite end of the cuff ( FIGURE 2 ). We incorporate the uterosacral ligament into the final bite on each side, and we cut the suture without a knot or a LapraTy clip.
FIGURE 2 Closing the vaginal cuff with bidirectional barbed suture For myomectomy closure, we use 14×14-cm 0 PDO suture on a 36-mm half-circle taper-point needle. If the hysterotomy is longer than 8 cm, we prefer to use 24×24-cm suture. We tack the first needle to the opposite anterior abdominal wall to avoid having the suture become entangled. We close the deepest layer using the first needle; we use the second needle to close the more superficial layer and the serosa, if possible ( FIGURE 3 ). If the suture is used beyond the barbed portion, we cut the needles and apply a LapraTy clip. Sometimes, three or four layers are needed to close a deep myometrial defect. 2/0 Monoderm can also be used for the serosa, continuously or as a baseball stitch.
FIGURE 3 Myomectomy closure with bidirectional barbed suture
1. Department of Health and Human Services letter approving Section 510(k) premarket notification for Quill suture. Available at http://www.accessdata.fda.gov/cdrh_docs/pdf4/k042075.pdf. Accessed August 13, 2009.
2. Leung JC. Barbed suture technology: recent advances. In: Medical Textiles 2004, Conference Proceedings; Oct. 26–27, 2004; pp. 62–80; Pittsburgh.
3. Rashid RM, Sartori M, White LE, Villa MT, Yoo SS, Alam M. Breaking strength of barbed polypropylene sutures: rater-blinded, controlled comparison with nonbarbed sutures of various calibers. Arch Dermatol. 2007;143:869-872.
4. Rodeheaver GT, Piñeros-Fernandez A, Salopek LS, et al. Barbed sutures for wound closure: in vivo wound security, tissue compatibility and cosmesis measurements. In: Society for Biomaterials 30th Annual Meeting Transactions; 2004; transaction 229, p. 232.
5. Greenberg JA, Einarsson JI. Use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15:621-623.
6. Moran ME, Marsh C, Perrotti M. Bidirectional-barbed sutured knotless running anastomosis v classic Van Velthoven suturing in a model system. J Endourol. 2007;21:1175-1178.
7. Einarsson JI, Vellinga T, Twijnstra A, Suzuki Y, Greenberg JA. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy; an evaluation of safety and clinical outcomes. Submitted as an abstract to the 38th Global Congress in Minimally Invasive Gynecology, 2009.
1. Department of Health and Human Services letter approving Section 510(k) premarket notification for Quill suture. Available at http://www.accessdata.fda.gov/cdrh_docs/pdf4/k042075.pdf. Accessed August 13, 2009.
2. Leung JC. Barbed suture technology: recent advances. In: Medical Textiles 2004, Conference Proceedings; Oct. 26–27, 2004; pp. 62–80; Pittsburgh.
3. Rashid RM, Sartori M, White LE, Villa MT, Yoo SS, Alam M. Breaking strength of barbed polypropylene sutures: rater-blinded, controlled comparison with nonbarbed sutures of various calibers. Arch Dermatol. 2007;143:869-872.
4. Rodeheaver GT, Piñeros-Fernandez A, Salopek LS, et al. Barbed sutures for wound closure: in vivo wound security, tissue compatibility and cosmesis measurements. In: Society for Biomaterials 30th Annual Meeting Transactions; 2004; transaction 229, p. 232.
5. Greenberg JA, Einarsson JI. Use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15:621-623.
6. Moran ME, Marsh C, Perrotti M. Bidirectional-barbed sutured knotless running anastomosis v classic Van Velthoven suturing in a model system. J Endourol. 2007;21:1175-1178.
7. Einarsson JI, Vellinga T, Twijnstra A, Suzuki Y, Greenberg JA. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy; an evaluation of safety and clinical outcomes. Submitted as an abstract to the 38th Global Congress in Minimally Invasive Gynecology, 2009.