User login
2012 Update on Obstetrics
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7
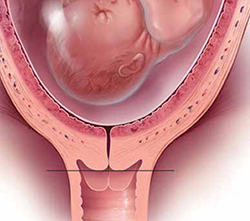
There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7

There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7

There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
Can cerclage prevent preterm birth in women who have a short cervix?
This meta-analysis by Berghella and colleagues adds to the debate about the role of cervical-length measurement in determining candidacy for cerclage in an effort to reduce the rate of preterm birth. The authors are clearly passionate about the prevention of preterm birth—as we all are—but the conclusions they reach must be questioned.
First, it is misleading to report these results as Level-1 evidence. A meta-analysis can never, strictly speaking, be Level-1 evidence, although it may be based on an analysis of Level-1 evidence.
Sound confusing? Let’s take, as an example, the study of the role of calcium supplementation in the prevention of preeclampsia. In JAMA, in 1996, a meta-analysis fairly conclusively demonstrated that calcium supplementation was effective in preventing preeclampsia (odds ratio, 0.38; 95% CI, 0.22–0.65).1 Yet, a subsequent large randomized trial failed to confirm the findings of this meta-analysis.2
The lesson here? Level-1 evidence consists of appropriately powered, large-scale, randomized clinical trials. To date, we lack such trials with respect to cervical-length measurement and indications for cerclage. In fact, two of the authors of this paper are “on the record” as saying this very thing.
A 2009 paper by Owen and coworkers demonstrated only that cerclage for a cervical length below 25 mm reduced previable birth and perinatal death, but did not prevent births before 35 weeks unless the cervical length was less than 15 mm—and that bit of information came from a secondary analysis of the data.3 In a follow-up study, Owen and coworkers concluded that cervical length did not predict preterm birth before 37, 35, or 28 weeks, whether or not cervical length was viewed as a continuum or was stratified.4 And in an earlier meta-analysis reported by Berghella and associates in 2005, the authors conclude that “…cerclage may reduce preterm birth, and a well-powered trial should be carried out on this group of patients.”5
We should continue to rely on clinical assessment and history to make cerclage decisions, a conclusion reached in a recent randomized, controlled trial.6
In the meantime, those of us who practice maternal-fetal medicine would be wise to stop spending time massaging the data (i.e., meta-analysis and secondary analyses) from trials that have already been performed and start spending time, effort, and money to conduct the well-powered trials that I (and Dr. Berghella and colleagues) believe that we need. This is not to say that cervical-length measurement is without value. We simply don’t yet have the strength of association to accurately determine what that value is—most certainly not in the form of a screening tool for low-risk populations.
—John T. Repke, MD
We want to hear from you! Tell us what you think.
1. Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113-1117.
2. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69-76.
3. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
4. Owen J, Szychowski JM, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010;203(4):393.e1-5.
5. Berghella V, Obido AO, To MS, Rust OA, Althiusius SM. Cerclage for short cervix on ultrasound: meta-analysis of trials using individual patient level data. Obstet Gynecol. 2005;106(1):181-189.
6. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-6.
This meta-analysis by Berghella and colleagues adds to the debate about the role of cervical-length measurement in determining candidacy for cerclage in an effort to reduce the rate of preterm birth. The authors are clearly passionate about the prevention of preterm birth—as we all are—but the conclusions they reach must be questioned.
First, it is misleading to report these results as Level-1 evidence. A meta-analysis can never, strictly speaking, be Level-1 evidence, although it may be based on an analysis of Level-1 evidence.
Sound confusing? Let’s take, as an example, the study of the role of calcium supplementation in the prevention of preeclampsia. In JAMA, in 1996, a meta-analysis fairly conclusively demonstrated that calcium supplementation was effective in preventing preeclampsia (odds ratio, 0.38; 95% CI, 0.22–0.65).1 Yet, a subsequent large randomized trial failed to confirm the findings of this meta-analysis.2
The lesson here? Level-1 evidence consists of appropriately powered, large-scale, randomized clinical trials. To date, we lack such trials with respect to cervical-length measurement and indications for cerclage. In fact, two of the authors of this paper are “on the record” as saying this very thing.
A 2009 paper by Owen and coworkers demonstrated only that cerclage for a cervical length below 25 mm reduced previable birth and perinatal death, but did not prevent births before 35 weeks unless the cervical length was less than 15 mm—and that bit of information came from a secondary analysis of the data.3 In a follow-up study, Owen and coworkers concluded that cervical length did not predict preterm birth before 37, 35, or 28 weeks, whether or not cervical length was viewed as a continuum or was stratified.4 And in an earlier meta-analysis reported by Berghella and associates in 2005, the authors conclude that “…cerclage may reduce preterm birth, and a well-powered trial should be carried out on this group of patients.”5
We should continue to rely on clinical assessment and history to make cerclage decisions, a conclusion reached in a recent randomized, controlled trial.6
In the meantime, those of us who practice maternal-fetal medicine would be wise to stop spending time massaging the data (i.e., meta-analysis and secondary analyses) from trials that have already been performed and start spending time, effort, and money to conduct the well-powered trials that I (and Dr. Berghella and colleagues) believe that we need. This is not to say that cervical-length measurement is without value. We simply don’t yet have the strength of association to accurately determine what that value is—most certainly not in the form of a screening tool for low-risk populations.
—John T. Repke, MD
We want to hear from you! Tell us what you think.
This meta-analysis by Berghella and colleagues adds to the debate about the role of cervical-length measurement in determining candidacy for cerclage in an effort to reduce the rate of preterm birth. The authors are clearly passionate about the prevention of preterm birth—as we all are—but the conclusions they reach must be questioned.
First, it is misleading to report these results as Level-1 evidence. A meta-analysis can never, strictly speaking, be Level-1 evidence, although it may be based on an analysis of Level-1 evidence.
Sound confusing? Let’s take, as an example, the study of the role of calcium supplementation in the prevention of preeclampsia. In JAMA, in 1996, a meta-analysis fairly conclusively demonstrated that calcium supplementation was effective in preventing preeclampsia (odds ratio, 0.38; 95% CI, 0.22–0.65).1 Yet, a subsequent large randomized trial failed to confirm the findings of this meta-analysis.2
The lesson here? Level-1 evidence consists of appropriately powered, large-scale, randomized clinical trials. To date, we lack such trials with respect to cervical-length measurement and indications for cerclage. In fact, two of the authors of this paper are “on the record” as saying this very thing.
A 2009 paper by Owen and coworkers demonstrated only that cerclage for a cervical length below 25 mm reduced previable birth and perinatal death, but did not prevent births before 35 weeks unless the cervical length was less than 15 mm—and that bit of information came from a secondary analysis of the data.3 In a follow-up study, Owen and coworkers concluded that cervical length did not predict preterm birth before 37, 35, or 28 weeks, whether or not cervical length was viewed as a continuum or was stratified.4 And in an earlier meta-analysis reported by Berghella and associates in 2005, the authors conclude that “…cerclage may reduce preterm birth, and a well-powered trial should be carried out on this group of patients.”5
We should continue to rely on clinical assessment and history to make cerclage decisions, a conclusion reached in a recent randomized, controlled trial.6
In the meantime, those of us who practice maternal-fetal medicine would be wise to stop spending time massaging the data (i.e., meta-analysis and secondary analyses) from trials that have already been performed and start spending time, effort, and money to conduct the well-powered trials that I (and Dr. Berghella and colleagues) believe that we need. This is not to say that cervical-length measurement is without value. We simply don’t yet have the strength of association to accurately determine what that value is—most certainly not in the form of a screening tool for low-risk populations.
—John T. Repke, MD
We want to hear from you! Tell us what you think.
1. Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113-1117.
2. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69-76.
3. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
4. Owen J, Szychowski JM, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010;203(4):393.e1-5.
5. Berghella V, Obido AO, To MS, Rust OA, Althiusius SM. Cerclage for short cervix on ultrasound: meta-analysis of trials using individual patient level data. Obstet Gynecol. 2005;106(1):181-189.
6. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-6.
1. Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113-1117.
2. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69-76.
3. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
4. Owen J, Szychowski JM, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010;203(4):393.e1-5.
5. Berghella V, Obido AO, To MS, Rust OA, Althiusius SM. Cerclage for short cervix on ultrasound: meta-analysis of trials using individual patient level data. Obstet Gynecol. 2005;106(1):181-189.
6. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-6.
Is the incidence of amniotic fluid embolism rising?
From February 2005 to February 2009, Knight and associates identified a total of 60 cases of AFE in the UK Obstetric Surveillance System. Their analysis of these cases, along with the cases of 1,227 women in the control group, is a valuable contribution to our understanding of AFE—an entity that few obstetricians will have the occasion to manage in their professional careers. One of the strengths of the study is the use of a comprehensive database, which made it possible to exclude 26 additional cases originally diagnosed as AFE but determined to be another entity. Scrutiny of these cases suggests that AFE may be over-reported.
Although the findings of this study are interesting—particularly the association between AFE and induction of labor, twin gestation, cesarean delivery, and the combination of older age and ethnic-minority status—they must be interpreted with caution. The study was an elegant mathematical exercise, but I would hesitate to join the authors in sounding too many alarms. For example, without a biological explanation, I would be reluctant to tell clinicians to look for any increased risk of AFE among ethnic minorities.
I would be just as hesitant to “warn” obstetricians about induction of labor. If the risk of AFE attributable to induction is 35%, as the authors maintain, the elimination of induction altogether would only lower the rate of AFE from 2 cases to 1.3 cases for every 100,000 deliveries. Moreover, some of the variables that contribute to the need for induction could also contribute to an increased risk of AFE.
Postpartum cases that occur after cesarean delivery could actually be air embolism misclassified as AFE, especially if the uterus was exteriorized for repair—a phenomenon that has been reported.2
Recognition of amniotic fluid embolism (AFE) is exceedingly rare. In general, unless maternal hemorrhage is the presenting feature (without coagulopathy or cardiorespiratory compromise), suspect AFE when the mother experiences acute collapse along with one of the following features:
- acute fetal compromise
- cardiac arrest or arrhythmia
- coagulopathy
- hypotension
- hemorrhage
- premonitory symptoms (e.g., agitation)
- seizure.
When AFE is suspected, prompt intervention and initiation of supportive care are essential.
Although there are some risk factors for AFE, most cases of this phenomenon will remain sporadic and unpredictable.—JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
1. Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid emoblism: a population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199(1):49.e1–e8.-
2. Younker D, Rodriguez V, Kavanagh J. Massive air embolism during cesarean section. Anesthesiology. 1986;65(1):77-79.
From February 2005 to February 2009, Knight and associates identified a total of 60 cases of AFE in the UK Obstetric Surveillance System. Their analysis of these cases, along with the cases of 1,227 women in the control group, is a valuable contribution to our understanding of AFE—an entity that few obstetricians will have the occasion to manage in their professional careers. One of the strengths of the study is the use of a comprehensive database, which made it possible to exclude 26 additional cases originally diagnosed as AFE but determined to be another entity. Scrutiny of these cases suggests that AFE may be over-reported.
Although the findings of this study are interesting—particularly the association between AFE and induction of labor, twin gestation, cesarean delivery, and the combination of older age and ethnic-minority status—they must be interpreted with caution. The study was an elegant mathematical exercise, but I would hesitate to join the authors in sounding too many alarms. For example, without a biological explanation, I would be reluctant to tell clinicians to look for any increased risk of AFE among ethnic minorities.
I would be just as hesitant to “warn” obstetricians about induction of labor. If the risk of AFE attributable to induction is 35%, as the authors maintain, the elimination of induction altogether would only lower the rate of AFE from 2 cases to 1.3 cases for every 100,000 deliveries. Moreover, some of the variables that contribute to the need for induction could also contribute to an increased risk of AFE.
Postpartum cases that occur after cesarean delivery could actually be air embolism misclassified as AFE, especially if the uterus was exteriorized for repair—a phenomenon that has been reported.2
Recognition of amniotic fluid embolism (AFE) is exceedingly rare. In general, unless maternal hemorrhage is the presenting feature (without coagulopathy or cardiorespiratory compromise), suspect AFE when the mother experiences acute collapse along with one of the following features:
- acute fetal compromise
- cardiac arrest or arrhythmia
- coagulopathy
- hypotension
- hemorrhage
- premonitory symptoms (e.g., agitation)
- seizure.
When AFE is suspected, prompt intervention and initiation of supportive care are essential.
Although there are some risk factors for AFE, most cases of this phenomenon will remain sporadic and unpredictable.—JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
From February 2005 to February 2009, Knight and associates identified a total of 60 cases of AFE in the UK Obstetric Surveillance System. Their analysis of these cases, along with the cases of 1,227 women in the control group, is a valuable contribution to our understanding of AFE—an entity that few obstetricians will have the occasion to manage in their professional careers. One of the strengths of the study is the use of a comprehensive database, which made it possible to exclude 26 additional cases originally diagnosed as AFE but determined to be another entity. Scrutiny of these cases suggests that AFE may be over-reported.
Although the findings of this study are interesting—particularly the association between AFE and induction of labor, twin gestation, cesarean delivery, and the combination of older age and ethnic-minority status—they must be interpreted with caution. The study was an elegant mathematical exercise, but I would hesitate to join the authors in sounding too many alarms. For example, without a biological explanation, I would be reluctant to tell clinicians to look for any increased risk of AFE among ethnic minorities.
I would be just as hesitant to “warn” obstetricians about induction of labor. If the risk of AFE attributable to induction is 35%, as the authors maintain, the elimination of induction altogether would only lower the rate of AFE from 2 cases to 1.3 cases for every 100,000 deliveries. Moreover, some of the variables that contribute to the need for induction could also contribute to an increased risk of AFE.
Postpartum cases that occur after cesarean delivery could actually be air embolism misclassified as AFE, especially if the uterus was exteriorized for repair—a phenomenon that has been reported.2
Recognition of amniotic fluid embolism (AFE) is exceedingly rare. In general, unless maternal hemorrhage is the presenting feature (without coagulopathy or cardiorespiratory compromise), suspect AFE when the mother experiences acute collapse along with one of the following features:
- acute fetal compromise
- cardiac arrest or arrhythmia
- coagulopathy
- hypotension
- hemorrhage
- premonitory symptoms (e.g., agitation)
- seizure.
When AFE is suspected, prompt intervention and initiation of supportive care are essential.
Although there are some risk factors for AFE, most cases of this phenomenon will remain sporadic and unpredictable.—JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
1. Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid emoblism: a population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199(1):49.e1–e8.-
2. Younker D, Rodriguez V, Kavanagh J. Massive air embolism during cesarean section. Anesthesiology. 1986;65(1):77-79.
1. Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid emoblism: a population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199(1):49.e1–e8.-
2. Younker D, Rodriguez V, Kavanagh J. Massive air embolism during cesarean section. Anesthesiology. 1986;65(1):77-79.
Does vaginal birth after cesarean have a future?
Once again, vaginal birth after cesarean, or VBAC—sometimes referred to as a trial of labor after cesarean, or TOLAC—has arisen as a topic of interest in obstetrics, as demonstrated in this issue of OBG Management.1 I say “once again” because, frankly, I thought that the matter had become irrelevant—reminiscent of a debate over vaginal breech delivery in the 1970s and 1980s now largely resolved in the United States, thanks to evidence-based randomized clinical trials.
I thought the issue was closed when, in 2005, the chair of ACOG’s Committee on Obstetric Practice was quoted in USA Today: “… the VBAC rupture rate may seem quite low but it’s damn high if you’re the one.” And later in the same article: “I think VBAC is dead.”
And I considered VBAC finished when I compared the target VBAC rate established in the US Department of Health and Human Services’s Healthy People 2010 report against the astounding data that we see reported today:
- In 1998, the US primary cesarean delivery rate was 18%; the Healthy People 2010 target was 15%. Today, that rate exceeds 25%.
- In 1998, the repeat cesarean delivery rate was 72%; again, the Healthy People 2010 target was 63%. In 2003, however, the repeat cesarean rate had climbed to 88.7%—and today, that rate exceeds 90%.
Called “reasonable” for many women
Yet, in a recent report, a consensus panel convened by The National Institutes of Health declares that VBAC is a “reasonable option” for many pregnant women. The panel encourages physicians to incorporate evidence-based data into the counseling they provide to patients.2
But even our own College admits to a paucity of high-quality evidence about VBAC. A 2009 ACOG Practice Bulletin says that “despite thousands of citations in the world’s literature there are currently no randomized trials comparing maternal or neonatal outcomes for both repeat cesarean delivery and VBAC.”3
So the question remains: How can medical science help patients and physicians make the best decisions about VBAC? Let me try to provide an answer here. Some of the ideas I draw on are discussed by Dr. Aviva Lee-Parritz in her article beginning on page 17.
What are the risks?
The true risks of VBAC are unknown. However, we do know—all the data are in agreement—that elective repeat cesarean delivery, performed at the appropriate gestational age, is safer for fetus and newborn than a trial of labor.4
We also know that most mothers accept a greater burden of risk for themselves if there is potential benefit for their newborn. (An example is expectant management of severe preeclampsia remote from term, when a delay in delivery offers no maternal benefit but does offer potential benefit to the newborn.) With VBAC, mothers must be willing to accept the risks of the procedure; better ways to assess that risk have been proposed to help them make a decision.5
What are the chances of success?
It amazes me when the quoted VBAC success rate at a given hospital exceeds the likelihood there of successful vaginal delivery of a nullipara. I see such data reported often.
Be certain that your patients know the hospital-specific cesarean delivery rate and VBAC success rate—and if you don’t have those data, then tell the patient that you don’t. It doesn’t make sense to quote an 85% VBAC success rate if your institution’s primary cesarean delivery rate is 25%.
What does VBAC cost?
The data with which to answer this question are hard to obtain cleanly; ultimately, however, the choices we make should be based on proper medical decision-making, not cost. That said, I remain unconvinced that VBAC overall offers significant savings over repeat cesarean delivery when total cost (not just the cost of postpartum care or the cost of post-delivery length of stay) is examined.
Furthermore, the expense of settling malpractice claims of “VBACs gone awry” is never included in estimates of the cost of care.
How are VBACs reimbursed?
The current structure of reimbursement for health care doesn’t favor VBAC. In most regions of the country, 1) physicians’ reimbursement for performing a VBAC is either the same as, or lower than, it is for cesarean delivery and 2) most hospitals enjoy a greater margin on the hospital stay postcesarean than after a vaginal delivery.
Given the increased time involved in managing a VBAC, a change in reimbursement to recognize the greater effort and exposure to liability would be a reasonable step for payers—if there is true interest in reversing the trend away from VBAC that we’re seeing.
How great are concerns over liability?
In every data set that I have reviewed, perinatal morbidity and mortality are clearly higher in the VBAC group than in the repeat cesarean group. In essence, the central issue with VBAC is uterine rupture and all the complications that can flow from that event.6
A problem for small hospitals. ACOG has already issued guidelines for what care should be “readily available” in a hospital that offers VBAC. For the College to retreat from these recommendations in an effort to increase acceptance of VBAC among smaller community hospitals—many of which are without students, residents, fellows, or myriad other support personnel—would, I think, be disingenuous and ill-advised. Add to this recent data suggesting that peripartum hysterectomy (for which VBAC patients are at increased risk) is best done in a high-volume hospital setting7 and you further reduce the likelihood that smaller community hospitals will ever embrace VBAC.
How well do patients accept VBAC?
It’s tough to sell a product that people don’t want. My anecdotal experience (meaning that my conclusions are unencumbered by data) is that informed health care personnel who themselves have had a cesarean delivery almost uniformly select cesarean delivery subsequently. They know the data and they’re aware of the risks. Often, they aren’t planning on having more than two children, so the problem of placenta accreta in the future doesn’t apply.
These observations suggest, to me, that maybe 1) we need to do a better job counseling patients or 2) our society’s value system overwhelmingly favors predictability of delivery and safety of the newborn at the expense of even a slight increase in risk to the mother.
Alas, common sense is the most difficult thing to legislate
VBAC was, and is, a good idea. It’s based on sound principles and good intentions.
Recall that, in 1970, our dictum was “once a section always a section.” The cesarean delivery rate in the United States was 5%, and we didn’t need to worry about VBAC.
VBAC became popular only as the primary cesarean rate began to rise above 15%; at that time, strict rules accompanied the procedure: no oxytocin or epidural anesthesia, and, in many institutions, x-ray pelvimetry was required to document “adequacy” of the pelvis.
Now, we’ve moved to the other end of the spectrum: It seems we offer VBAC to anyone who wants it, regardless of comorbidities.
Can we compromise?
I support a middle-of-the-road position that strongly encourages VBAC for women who:
- have no comorbidities
- have had a prior VBAC or previous vaginal delivery of a term baby and
- who have had no more than one prior cesarean delivery.
On the other hand, VBAC should be discouraged for women who:
- have a body mass index >40
- are post-term
- present at term with premature rupture of the membranes, an unengaged vertex, or an unfavorable cervix or
- have any other condition that might make emergency cesarean delivery more difficult and, therefore, best avoided.
Such risk assessment approaches have already been proposed.5
Applying common sense to the matter, we might be able to agree on a solution that makes VBAC attractive and, more important, safe for our patients and for us. Furthermore, we must diligently keep track of our own data on maternal and neonatal outcomes so that we can most appropriately counsel our patients.
It’s up to us to determine whether VBAC should stay or go
I estimate that we have a window of opportunity of 5 to 10 years to resolve whether VBAC remains part of practice. If we don’t take that opportunity, we’ll be left with a generation of physicians who have little or no experience performing the procedure. VBAC will disappear, in a self-fulfilling prophecy—which, when you think about what happened with vaginal breech delivery, may not be a bad thing.
1. Lee-Parritz A. When is VBAC appropriate? OBG Manage. 2010;22(7):17-24.
2. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010 Obstet Gynecol. 2010;115(6):1279-1295.
3. Vaginal birth after previous cesarean. ACOG Practice Bulletin #54. Obstet Gynecol. 2004;104(1):203-212.
4. Guise JM, Denman MA, Emeis C, et al. Vaginal birth after cesarean; new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
5. Grobman WA, Lai Y, Landon MB, et al. Can a prediction model for vaginal birth after cesarean also predict the probability of morbidity related to a trial of labor? Am J Obstet Gynecol. 2009;200(1):56-e1-e6.
6. Scott JR. Solving the vaginal birth after cesarean dilemma. Obstet Gynecol. 2010;115(6):1112-1113.
7. Wright J, Herzog T, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115(6):1194-1200.
Once again, vaginal birth after cesarean, or VBAC—sometimes referred to as a trial of labor after cesarean, or TOLAC—has arisen as a topic of interest in obstetrics, as demonstrated in this issue of OBG Management.1 I say “once again” because, frankly, I thought that the matter had become irrelevant—reminiscent of a debate over vaginal breech delivery in the 1970s and 1980s now largely resolved in the United States, thanks to evidence-based randomized clinical trials.
I thought the issue was closed when, in 2005, the chair of ACOG’s Committee on Obstetric Practice was quoted in USA Today: “… the VBAC rupture rate may seem quite low but it’s damn high if you’re the one.” And later in the same article: “I think VBAC is dead.”
And I considered VBAC finished when I compared the target VBAC rate established in the US Department of Health and Human Services’s Healthy People 2010 report against the astounding data that we see reported today:
- In 1998, the US primary cesarean delivery rate was 18%; the Healthy People 2010 target was 15%. Today, that rate exceeds 25%.
- In 1998, the repeat cesarean delivery rate was 72%; again, the Healthy People 2010 target was 63%. In 2003, however, the repeat cesarean rate had climbed to 88.7%—and today, that rate exceeds 90%.
Called “reasonable” for many women
Yet, in a recent report, a consensus panel convened by The National Institutes of Health declares that VBAC is a “reasonable option” for many pregnant women. The panel encourages physicians to incorporate evidence-based data into the counseling they provide to patients.2
But even our own College admits to a paucity of high-quality evidence about VBAC. A 2009 ACOG Practice Bulletin says that “despite thousands of citations in the world’s literature there are currently no randomized trials comparing maternal or neonatal outcomes for both repeat cesarean delivery and VBAC.”3
So the question remains: How can medical science help patients and physicians make the best decisions about VBAC? Let me try to provide an answer here. Some of the ideas I draw on are discussed by Dr. Aviva Lee-Parritz in her article beginning on page 17.
What are the risks?
The true risks of VBAC are unknown. However, we do know—all the data are in agreement—that elective repeat cesarean delivery, performed at the appropriate gestational age, is safer for fetus and newborn than a trial of labor.4
We also know that most mothers accept a greater burden of risk for themselves if there is potential benefit for their newborn. (An example is expectant management of severe preeclampsia remote from term, when a delay in delivery offers no maternal benefit but does offer potential benefit to the newborn.) With VBAC, mothers must be willing to accept the risks of the procedure; better ways to assess that risk have been proposed to help them make a decision.5
What are the chances of success?
It amazes me when the quoted VBAC success rate at a given hospital exceeds the likelihood there of successful vaginal delivery of a nullipara. I see such data reported often.
Be certain that your patients know the hospital-specific cesarean delivery rate and VBAC success rate—and if you don’t have those data, then tell the patient that you don’t. It doesn’t make sense to quote an 85% VBAC success rate if your institution’s primary cesarean delivery rate is 25%.
What does VBAC cost?
The data with which to answer this question are hard to obtain cleanly; ultimately, however, the choices we make should be based on proper medical decision-making, not cost. That said, I remain unconvinced that VBAC overall offers significant savings over repeat cesarean delivery when total cost (not just the cost of postpartum care or the cost of post-delivery length of stay) is examined.
Furthermore, the expense of settling malpractice claims of “VBACs gone awry” is never included in estimates of the cost of care.
How are VBACs reimbursed?
The current structure of reimbursement for health care doesn’t favor VBAC. In most regions of the country, 1) physicians’ reimbursement for performing a VBAC is either the same as, or lower than, it is for cesarean delivery and 2) most hospitals enjoy a greater margin on the hospital stay postcesarean than after a vaginal delivery.
Given the increased time involved in managing a VBAC, a change in reimbursement to recognize the greater effort and exposure to liability would be a reasonable step for payers—if there is true interest in reversing the trend away from VBAC that we’re seeing.
How great are concerns over liability?
In every data set that I have reviewed, perinatal morbidity and mortality are clearly higher in the VBAC group than in the repeat cesarean group. In essence, the central issue with VBAC is uterine rupture and all the complications that can flow from that event.6
A problem for small hospitals. ACOG has already issued guidelines for what care should be “readily available” in a hospital that offers VBAC. For the College to retreat from these recommendations in an effort to increase acceptance of VBAC among smaller community hospitals—many of which are without students, residents, fellows, or myriad other support personnel—would, I think, be disingenuous and ill-advised. Add to this recent data suggesting that peripartum hysterectomy (for which VBAC patients are at increased risk) is best done in a high-volume hospital setting7 and you further reduce the likelihood that smaller community hospitals will ever embrace VBAC.
How well do patients accept VBAC?
It’s tough to sell a product that people don’t want. My anecdotal experience (meaning that my conclusions are unencumbered by data) is that informed health care personnel who themselves have had a cesarean delivery almost uniformly select cesarean delivery subsequently. They know the data and they’re aware of the risks. Often, they aren’t planning on having more than two children, so the problem of placenta accreta in the future doesn’t apply.
These observations suggest, to me, that maybe 1) we need to do a better job counseling patients or 2) our society’s value system overwhelmingly favors predictability of delivery and safety of the newborn at the expense of even a slight increase in risk to the mother.
Alas, common sense is the most difficult thing to legislate
VBAC was, and is, a good idea. It’s based on sound principles and good intentions.
Recall that, in 1970, our dictum was “once a section always a section.” The cesarean delivery rate in the United States was 5%, and we didn’t need to worry about VBAC.
VBAC became popular only as the primary cesarean rate began to rise above 15%; at that time, strict rules accompanied the procedure: no oxytocin or epidural anesthesia, and, in many institutions, x-ray pelvimetry was required to document “adequacy” of the pelvis.
Now, we’ve moved to the other end of the spectrum: It seems we offer VBAC to anyone who wants it, regardless of comorbidities.
Can we compromise?
I support a middle-of-the-road position that strongly encourages VBAC for women who:
- have no comorbidities
- have had a prior VBAC or previous vaginal delivery of a term baby and
- who have had no more than one prior cesarean delivery.
On the other hand, VBAC should be discouraged for women who:
- have a body mass index >40
- are post-term
- present at term with premature rupture of the membranes, an unengaged vertex, or an unfavorable cervix or
- have any other condition that might make emergency cesarean delivery more difficult and, therefore, best avoided.
Such risk assessment approaches have already been proposed.5
Applying common sense to the matter, we might be able to agree on a solution that makes VBAC attractive and, more important, safe for our patients and for us. Furthermore, we must diligently keep track of our own data on maternal and neonatal outcomes so that we can most appropriately counsel our patients.
It’s up to us to determine whether VBAC should stay or go
I estimate that we have a window of opportunity of 5 to 10 years to resolve whether VBAC remains part of practice. If we don’t take that opportunity, we’ll be left with a generation of physicians who have little or no experience performing the procedure. VBAC will disappear, in a self-fulfilling prophecy—which, when you think about what happened with vaginal breech delivery, may not be a bad thing.
Once again, vaginal birth after cesarean, or VBAC—sometimes referred to as a trial of labor after cesarean, or TOLAC—has arisen as a topic of interest in obstetrics, as demonstrated in this issue of OBG Management.1 I say “once again” because, frankly, I thought that the matter had become irrelevant—reminiscent of a debate over vaginal breech delivery in the 1970s and 1980s now largely resolved in the United States, thanks to evidence-based randomized clinical trials.
I thought the issue was closed when, in 2005, the chair of ACOG’s Committee on Obstetric Practice was quoted in USA Today: “… the VBAC rupture rate may seem quite low but it’s damn high if you’re the one.” And later in the same article: “I think VBAC is dead.”
And I considered VBAC finished when I compared the target VBAC rate established in the US Department of Health and Human Services’s Healthy People 2010 report against the astounding data that we see reported today:
- In 1998, the US primary cesarean delivery rate was 18%; the Healthy People 2010 target was 15%. Today, that rate exceeds 25%.
- In 1998, the repeat cesarean delivery rate was 72%; again, the Healthy People 2010 target was 63%. In 2003, however, the repeat cesarean rate had climbed to 88.7%—and today, that rate exceeds 90%.
Called “reasonable” for many women
Yet, in a recent report, a consensus panel convened by The National Institutes of Health declares that VBAC is a “reasonable option” for many pregnant women. The panel encourages physicians to incorporate evidence-based data into the counseling they provide to patients.2
But even our own College admits to a paucity of high-quality evidence about VBAC. A 2009 ACOG Practice Bulletin says that “despite thousands of citations in the world’s literature there are currently no randomized trials comparing maternal or neonatal outcomes for both repeat cesarean delivery and VBAC.”3
So the question remains: How can medical science help patients and physicians make the best decisions about VBAC? Let me try to provide an answer here. Some of the ideas I draw on are discussed by Dr. Aviva Lee-Parritz in her article beginning on page 17.
What are the risks?
The true risks of VBAC are unknown. However, we do know—all the data are in agreement—that elective repeat cesarean delivery, performed at the appropriate gestational age, is safer for fetus and newborn than a trial of labor.4
We also know that most mothers accept a greater burden of risk for themselves if there is potential benefit for their newborn. (An example is expectant management of severe preeclampsia remote from term, when a delay in delivery offers no maternal benefit but does offer potential benefit to the newborn.) With VBAC, mothers must be willing to accept the risks of the procedure; better ways to assess that risk have been proposed to help them make a decision.5
What are the chances of success?
It amazes me when the quoted VBAC success rate at a given hospital exceeds the likelihood there of successful vaginal delivery of a nullipara. I see such data reported often.
Be certain that your patients know the hospital-specific cesarean delivery rate and VBAC success rate—and if you don’t have those data, then tell the patient that you don’t. It doesn’t make sense to quote an 85% VBAC success rate if your institution’s primary cesarean delivery rate is 25%.
What does VBAC cost?
The data with which to answer this question are hard to obtain cleanly; ultimately, however, the choices we make should be based on proper medical decision-making, not cost. That said, I remain unconvinced that VBAC overall offers significant savings over repeat cesarean delivery when total cost (not just the cost of postpartum care or the cost of post-delivery length of stay) is examined.
Furthermore, the expense of settling malpractice claims of “VBACs gone awry” is never included in estimates of the cost of care.
How are VBACs reimbursed?
The current structure of reimbursement for health care doesn’t favor VBAC. In most regions of the country, 1) physicians’ reimbursement for performing a VBAC is either the same as, or lower than, it is for cesarean delivery and 2) most hospitals enjoy a greater margin on the hospital stay postcesarean than after a vaginal delivery.
Given the increased time involved in managing a VBAC, a change in reimbursement to recognize the greater effort and exposure to liability would be a reasonable step for payers—if there is true interest in reversing the trend away from VBAC that we’re seeing.
How great are concerns over liability?
In every data set that I have reviewed, perinatal morbidity and mortality are clearly higher in the VBAC group than in the repeat cesarean group. In essence, the central issue with VBAC is uterine rupture and all the complications that can flow from that event.6
A problem for small hospitals. ACOG has already issued guidelines for what care should be “readily available” in a hospital that offers VBAC. For the College to retreat from these recommendations in an effort to increase acceptance of VBAC among smaller community hospitals—many of which are without students, residents, fellows, or myriad other support personnel—would, I think, be disingenuous and ill-advised. Add to this recent data suggesting that peripartum hysterectomy (for which VBAC patients are at increased risk) is best done in a high-volume hospital setting7 and you further reduce the likelihood that smaller community hospitals will ever embrace VBAC.
How well do patients accept VBAC?
It’s tough to sell a product that people don’t want. My anecdotal experience (meaning that my conclusions are unencumbered by data) is that informed health care personnel who themselves have had a cesarean delivery almost uniformly select cesarean delivery subsequently. They know the data and they’re aware of the risks. Often, they aren’t planning on having more than two children, so the problem of placenta accreta in the future doesn’t apply.
These observations suggest, to me, that maybe 1) we need to do a better job counseling patients or 2) our society’s value system overwhelmingly favors predictability of delivery and safety of the newborn at the expense of even a slight increase in risk to the mother.
Alas, common sense is the most difficult thing to legislate
VBAC was, and is, a good idea. It’s based on sound principles and good intentions.
Recall that, in 1970, our dictum was “once a section always a section.” The cesarean delivery rate in the United States was 5%, and we didn’t need to worry about VBAC.
VBAC became popular only as the primary cesarean rate began to rise above 15%; at that time, strict rules accompanied the procedure: no oxytocin or epidural anesthesia, and, in many institutions, x-ray pelvimetry was required to document “adequacy” of the pelvis.
Now, we’ve moved to the other end of the spectrum: It seems we offer VBAC to anyone who wants it, regardless of comorbidities.
Can we compromise?
I support a middle-of-the-road position that strongly encourages VBAC for women who:
- have no comorbidities
- have had a prior VBAC or previous vaginal delivery of a term baby and
- who have had no more than one prior cesarean delivery.
On the other hand, VBAC should be discouraged for women who:
- have a body mass index >40
- are post-term
- present at term with premature rupture of the membranes, an unengaged vertex, or an unfavorable cervix or
- have any other condition that might make emergency cesarean delivery more difficult and, therefore, best avoided.
Such risk assessment approaches have already been proposed.5
Applying common sense to the matter, we might be able to agree on a solution that makes VBAC attractive and, more important, safe for our patients and for us. Furthermore, we must diligently keep track of our own data on maternal and neonatal outcomes so that we can most appropriately counsel our patients.
It’s up to us to determine whether VBAC should stay or go
I estimate that we have a window of opportunity of 5 to 10 years to resolve whether VBAC remains part of practice. If we don’t take that opportunity, we’ll be left with a generation of physicians who have little or no experience performing the procedure. VBAC will disappear, in a self-fulfilling prophecy—which, when you think about what happened with vaginal breech delivery, may not be a bad thing.
1. Lee-Parritz A. When is VBAC appropriate? OBG Manage. 2010;22(7):17-24.
2. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010 Obstet Gynecol. 2010;115(6):1279-1295.
3. Vaginal birth after previous cesarean. ACOG Practice Bulletin #54. Obstet Gynecol. 2004;104(1):203-212.
4. Guise JM, Denman MA, Emeis C, et al. Vaginal birth after cesarean; new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
5. Grobman WA, Lai Y, Landon MB, et al. Can a prediction model for vaginal birth after cesarean also predict the probability of morbidity related to a trial of labor? Am J Obstet Gynecol. 2009;200(1):56-e1-e6.
6. Scott JR. Solving the vaginal birth after cesarean dilemma. Obstet Gynecol. 2010;115(6):1112-1113.
7. Wright J, Herzog T, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115(6):1194-1200.
1. Lee-Parritz A. When is VBAC appropriate? OBG Manage. 2010;22(7):17-24.
2. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010 Obstet Gynecol. 2010;115(6):1279-1295.
3. Vaginal birth after previous cesarean. ACOG Practice Bulletin #54. Obstet Gynecol. 2004;104(1):203-212.
4. Guise JM, Denman MA, Emeis C, et al. Vaginal birth after cesarean; new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
5. Grobman WA, Lai Y, Landon MB, et al. Can a prediction model for vaginal birth after cesarean also predict the probability of morbidity related to a trial of labor? Am J Obstet Gynecol. 2009;200(1):56-e1-e6.
6. Scott JR. Solving the vaginal birth after cesarean dilemma. Obstet Gynecol. 2010;115(6):1112-1113.
7. Wright J, Herzog T, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115(6):1194-1200.
Does US measurement of cervical length to determine the need for cerclage reduce preterm delivery?
In their discussion of the findings, Simcox and colleagues assert that a trial to settle the questions frequently raised in the cerclage debate would require thousands of patients and may not be feasible.
Rigorous trial is impressive,
but doesn’t settle key questions
The authors performed an excellent randomized, controlled trial, and their intention-to-treat analysis is laudable. They are also to be congratulated for remaining focused on the primary outcome of delivery before 34 weeks’ gestation. It is notable that the primary outcome was essentially the same in each group, regardless of the treatment, be it 1) US screening and cerclage for cervical length 2) no screening and cerclage for historical indications. I recall a conference on prematurity from the mid-1980s that included, as one of its conclusions, the observation that as many as 70% of patients who have historical “indications” for cerclage will deliver at term in their next pregnancy if left untreated.
Unresolved questions in regard to cervical cerclage include:
- What is the best way to determine who is a candidate?
- What is the best type of cerclage?
- What is the most appropriate outcome to be measured?
- Is there a place in practice for “universal” screening of cervical length?
- What is the true cost (in terms of both dollars and morbidity) of intervention versus no intervention?
- What are the medicolegal implications of each approach?
High-risk women may benefit from US imaging, but the data from this study do not support that conclusion. Nor is the best type of cerclage defined, though there is ample opinion on this topic.
Is 34 weeks’ gestation the appropriate primary outcome? More and more, we read about late preterm or so-called near-term outcomes being less optimal than they once were thought to be—though delivery at 34 to 37 weeks would seem to be preferable to delivery at less than 34 weeks.
The cost of each approach is unclear. How many “unnecessary” cerclages would be needed to prevent one very-low-birth-weight delivery? And how “risky” is elective cerclage placement in skilled hands?
Finally, not many patients or physicians are likely to want to embrace a wait-and-see approach if they have already had one or more adverse outcomes, and the risk of doing nothing may be considerably greater in medicolegal terms than the risk of proceeding with what may be an unnecessary intervention that ends in a term or near-term delivery.
On the basis of these results, I think the practitioner should rely on history to make a clinical judgment about the need for cerclage. Ultrasonographic imaging may not only be of little help, but it may lead to greater intervention than would otherwise be needed. Perhaps a return to clinical basics, such as detailed history taking and physical examination, is a good message for these economic times.—JOHN T. REPKE, MD
In their discussion of the findings, Simcox and colleagues assert that a trial to settle the questions frequently raised in the cerclage debate would require thousands of patients and may not be feasible.
Rigorous trial is impressive,
but doesn’t settle key questions
The authors performed an excellent randomized, controlled trial, and their intention-to-treat analysis is laudable. They are also to be congratulated for remaining focused on the primary outcome of delivery before 34 weeks’ gestation. It is notable that the primary outcome was essentially the same in each group, regardless of the treatment, be it 1) US screening and cerclage for cervical length 2) no screening and cerclage for historical indications. I recall a conference on prematurity from the mid-1980s that included, as one of its conclusions, the observation that as many as 70% of patients who have historical “indications” for cerclage will deliver at term in their next pregnancy if left untreated.
Unresolved questions in regard to cervical cerclage include:
- What is the best way to determine who is a candidate?
- What is the best type of cerclage?
- What is the most appropriate outcome to be measured?
- Is there a place in practice for “universal” screening of cervical length?
- What is the true cost (in terms of both dollars and morbidity) of intervention versus no intervention?
- What are the medicolegal implications of each approach?
High-risk women may benefit from US imaging, but the data from this study do not support that conclusion. Nor is the best type of cerclage defined, though there is ample opinion on this topic.
Is 34 weeks’ gestation the appropriate primary outcome? More and more, we read about late preterm or so-called near-term outcomes being less optimal than they once were thought to be—though delivery at 34 to 37 weeks would seem to be preferable to delivery at less than 34 weeks.
The cost of each approach is unclear. How many “unnecessary” cerclages would be needed to prevent one very-low-birth-weight delivery? And how “risky” is elective cerclage placement in skilled hands?
Finally, not many patients or physicians are likely to want to embrace a wait-and-see approach if they have already had one or more adverse outcomes, and the risk of doing nothing may be considerably greater in medicolegal terms than the risk of proceeding with what may be an unnecessary intervention that ends in a term or near-term delivery.
On the basis of these results, I think the practitioner should rely on history to make a clinical judgment about the need for cerclage. Ultrasonographic imaging may not only be of little help, but it may lead to greater intervention than would otherwise be needed. Perhaps a return to clinical basics, such as detailed history taking and physical examination, is a good message for these economic times.—JOHN T. REPKE, MD
In their discussion of the findings, Simcox and colleagues assert that a trial to settle the questions frequently raised in the cerclage debate would require thousands of patients and may not be feasible.
Rigorous trial is impressive,
but doesn’t settle key questions
The authors performed an excellent randomized, controlled trial, and their intention-to-treat analysis is laudable. They are also to be congratulated for remaining focused on the primary outcome of delivery before 34 weeks’ gestation. It is notable that the primary outcome was essentially the same in each group, regardless of the treatment, be it 1) US screening and cerclage for cervical length 2) no screening and cerclage for historical indications. I recall a conference on prematurity from the mid-1980s that included, as one of its conclusions, the observation that as many as 70% of patients who have historical “indications” for cerclage will deliver at term in their next pregnancy if left untreated.
Unresolved questions in regard to cervical cerclage include:
- What is the best way to determine who is a candidate?
- What is the best type of cerclage?
- What is the most appropriate outcome to be measured?
- Is there a place in practice for “universal” screening of cervical length?
- What is the true cost (in terms of both dollars and morbidity) of intervention versus no intervention?
- What are the medicolegal implications of each approach?
High-risk women may benefit from US imaging, but the data from this study do not support that conclusion. Nor is the best type of cerclage defined, though there is ample opinion on this topic.
Is 34 weeks’ gestation the appropriate primary outcome? More and more, we read about late preterm or so-called near-term outcomes being less optimal than they once were thought to be—though delivery at 34 to 37 weeks would seem to be preferable to delivery at less than 34 weeks.
The cost of each approach is unclear. How many “unnecessary” cerclages would be needed to prevent one very-low-birth-weight delivery? And how “risky” is elective cerclage placement in skilled hands?
Finally, not many patients or physicians are likely to want to embrace a wait-and-see approach if they have already had one or more adverse outcomes, and the risk of doing nothing may be considerably greater in medicolegal terms than the risk of proceeding with what may be an unnecessary intervention that ends in a term or near-term delivery.
On the basis of these results, I think the practitioner should rely on history to make a clinical judgment about the need for cerclage. Ultrasonographic imaging may not only be of little help, but it may lead to greater intervention than would otherwise be needed. Perhaps a return to clinical basics, such as detailed history taking and physical examination, is a good message for these economic times.—JOHN T. REPKE, MD
Preeclampsia and eclampsia: 7 management challenges (and zero shortcuts)
CASE: At risk, or just very pregnant?
At her first prenatal visit, a 31-year-old gravida has blood pressure (BP) of 100/60 mm Hg, no proteinuria, and normal weight for her gestational age. As she enters the third trimester, however, her BP rises to 138/86 mm Hg, she now has proteinuria of 1+, and she has gained 10 lb in the past 2 weeks.
Does she have preeclampsia, or do these findings reflect normal development in the last trimester?
These findings, in and of themselves, may not indicate preeclampsia—but they do suggest a serious risk of developing the disease.
Preeclampsia complicates approximately 3% to 7% of nulliparous pregnancies in the United States, and about 0.8% to 5% of multiparous pregnancies.
Although severe preeclampsia represents only a fraction of those amounts, and eclampsia an even lower percentage, they are potentially catastrophic complications of pregnancy and one of the leading causes of maternal death. They also are responsible for a large percentage of infants born prematurely as a result of a worsening maternal or fetal condition.
Preeclampsia and eclampsia are obstetric diseases, and obstetricians are the group best equipped to diagnose, evaluate, and manage them. In this article, we highlight seven challenges that obstetricians face when managing preeclampsia and eclampsia, and offer useful strategies to help minimize morbidity and mortality in both mother and infant.
CHALLENGE NO. 1: Making the diagnosis
Good prenatal care is a prerequisite
We can’t overemphasize the importance of early and adequate prenatal care! Although the diagnostic criteria for preeclampsia have been widely established—persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period—the condition does not always play by the rules. With close monitoring of weight, urine protein, and BP, the clinician can identify and follow potentially worrisome trends.
Earlier diagnostic criteria—which included a rise in systolic BP of 30 mm Hg or a rise in diastolic BP of 15 mm Hg above initial baseline BP, as well as the presence of pathologic edema—may have been revised, but it remains important for clinicians to put all pieces of clinical information together at each visit. For example, given her rising BP, proteinuria, and weight gain, the patient in the opening case must be considered at risk for preeclampsia. Suspicion also is justified if the patient has any of the risk factors for preeclampsia in TABLE 1.
TABLE 1
Risk factors for preeclampsia
|
|
|
Early detection is critical
Early identification of preeclampsia may allow for interventions, including delivery, that will lessen the risk of progression to severe preeclampsia and eclampsia and reduce fetal and maternal morbidity and mortality. It is, therefore, essential for the clinician to ask specifically about signs and symptoms of preeclampsia and to listen carefully to the answers.
Signs and symptoms may sometimes be typical:
- weight gain
- increasing edema
- persistent headache
- blurred vision.
- malaise
- nausea
- epigastric discomfort
- right upper-quadrant discomfort.
Diagnostic criteria
The diagnosis of preeclampsia is based on persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period.1 Other criteria have been applied, such as a rise in systolic or diastolic BP above baseline and urine dipstick criteria for proteinuria, but BP above 140/90 mm Hg and proteinuria above 300 mg are most frequently used in medical centers in the United States.2
Gestational hypertension and chronic hypertension do sometimes coexist with superimposed preeclampsia, but should not be confused with preeclampsia or lead to management decisions that should apply only to patients with preeclampsia.3
Before severe preeclampsia can be diagnosed, the initial criteria for preeclampsia should have been fulfilled, along with one or more of the findings listed in TABLE 2.
Attempts to predict preeclampsia have met with poor results. Measurement of the ratio of uterine artery systolic to diastolic flow has not been informative in the general healthy population of pregnant women. Nor has uric acid determination been useful; it generally has very poor predictive value and should be interpreted with caution.
TABLE 2
13 criteria for establishing severe preeclampsia
|
|
|
Hospitalization is essential for severe disease
Mild preeclampsia can be managed expectantly until fetal maturity or 37 weeks’ gestation. Severe preeclampsia can be managed expectantly in the mid trimester or early third trimester if both mother and fetus are stable, but hospitalization is necessary in a tertiary care facility that has critical-care OB expertise, an ICU facility, and a NICU facility and personnel on site.
Distinguish an existing condition from superimposed preeclampsia
One of the most difficult management challenges is the diagnosis of superimposed preeclampsia. Patients who have chronic hypertension often have underlying renal disease as well; in these patients, it may be difficult, if not impossible, to distinguish a worsening underlying medical condition from superimposed preeclampsia.
Our advice is not to agonize about this difference too much in the patient at or near term, as delivery may be indicated and the patient’s postpartum course may help resolve the question, with rapid resolution tending to favor a diagnosis of superimposed preeclampsia.
It also is important to note whether these patients are receiving antihypertensive therapy. If they are, hospitalization is recommended until delivery once the diagnosis of superimposed preeclampsia is made.
Given that the use of antihypertensive agents removes one of the major indicators of disease progression (i.e., rising BP), it is our practice to deliver these patients according to our severe preeclampsia management protocol and not to carry such pregnancies beyond 34 weeks. In carefully selected cases, the pregnancy can be continued to 37 weeks, but the decision to do so should be weighed carefully—ideally, with input from a maternal–fetal medicine specialist.
CHALLENGE NO. 2: Forgoing shortcuts
Evaluation and management of preeclampsia are relatively straightforward, but there are no shortcuts. Many patients who feel well initially may push for outpatient evaluation, but once a diagnosis of preeclampsia is established, in-hospital evaluation is preferable, at least until the degree of illness can be determined, fetal well-being can be established, and the patient’s candidacy for subsequent outpatient management can be more fully determined.
In-hospital management may be particularly useful for patients who have any of the risk factors for preeclampsia listed in TABLE 1.
Initial evaluation consists of:
- fetal nonstress testing
- amniotic fluid index
- serial BP determination
- 24-hour urine collection
- initial laboratory evaluation comprising a complete blood count with platelets and aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels.
If fetal and maternal evaluations are reassuring, and if the patient has remained stable, then outpatient management may be considered. In general, if proteinuria exceeds 1 g in 24 hours, in-hospital management is recommended, regardless of other parameters.
If outpatient management is considered, the level of care and surveillance must mirror what could be provided in the hospital. Hospitalization alone will not prevent all cases from progressing to severe preeclampsia or eclampsia, but daily and diligent observation and evaluation may minimize the risk.
CHALLENGE NO. 3: Treating the disease
Appropriate treatment of preeclampsia requires not only that the patient show up for prenatal care, but also that we:
- are certain of the diagnosis
- recognize the potential seriousness of the disease
- are thorough (remember, no shortcuts!).
Many experts in the field of preeclampsia have stated, on numerous occasions, that preeclampsia is more than simple hypertension. It is almost never advisable to initiate antihypertensive therapy for a patient in the third trimester when she was previously normotensive, because one runs the risk of masking a key clinical parameter used to assess disease progression.
In our institutions, any patient who is taking antihypertensive medication and in whom we are entertaining a diagnosis of preeclampsia is recommended for hospitalization for the duration of her pregnancy or until a diagnosis of preeclampsia can be ruled out with reasonable certainty.
Expectant management beyond 37 weeks does not benefit mother or fetus
Because preeclampsia is a multisystem disease, it has maternal, placental, and fetal consequences. The cure for preeclampsia remains delivery of the placenta. Expectant management offers no maternal benefit, but does offer some potential neonatal benefits if prematurity is a concern. Once concerns about prematurity have been largely eliminated, generally by achieving a gestational age of 37 weeks, further expectant management is not indicated, offers little or no additional benefit to the fetus, and leaves both mother and fetus at risk.
Therefore, once the pregnancy reaches 37 weeks, delivery is recommended.
When preeclampsia is severe, and when it is superimposed in a patient who is taking antihypertensive medication, we generally do not continue the pregnancy beyond 34 weeks.4 In our institutions, most patients who are being expectantly managed for severe preeclampsia remote from term—and who have remained stable—are delivered between 32 and 34 weeks’ gestation, depending on the specific clinical circumstances.
CHALLENGE NO. 4: Controlling blood pressure
Cerebrovascular accident (stroke) is the leading cause of maternal mortality from preeclampsia in the United States. Not all cases can be prevented, but one suggested preventive strategy is adequate BP control. Some cases of stroke in the setting of preeclampsia will occur despite systemic BP readings that are not considered to be in a dangerous range. One reason may be an override of normal cerebral blood flow autoregulatory mechanisms, resulting in increased cerebral blood flow, rising cerebral perfusion pressures, and vessel rupture. Such occurrences may sometimes, but not always, be related to coagulopathy.
When a patient has elevated BP, generally defined as persistent systolic pressures above 160 to 170 mm Hg and persistent diastolic pressures above 105 to 110 mm Hg, antihypertensive therapy is indicated and should be administered in a timely fashion.
Labetalol, nifedipine, and hydralazine have all been used effectively in such acute settings, when administered parenterally (except nifedipine, which may be given orally) and when given in proper dosages (TABLE 3).
Avoid oral use of labetalol or hydralazine to treat acute hypertensive emergencies.
TABLE 3
Pharmacotherapy of acute hypertension
| Drug | Dosage | Directions |
|---|---|---|
| Hydralazine* | 5 mg IV | Repeat in 10 min, then give 10 mg IV every 20 min until BP stabilizes (140–150/90–100 mm Hg) |
| Labetalol* | 10–20 mg IV push | Repeat every 10-20 min, doubling the dosage each time until a maximum total cumulative dosage of 300 mg has been given |
| Nifedipine* | 10 mg | Repeat in 20 min for four doses (maximum 40 mg); then give 10–20 mg orally every 4–6 h to achieve a stable BP of 140–150/90–100 mm Hg |
| * If target blood pressure is not reached after the maximum dosage of an agent is given, then additional or alternative pharmacotherapy must be utilized. | ||
Goals for treatment
In the antepartum patient, the goal is to maintain systolic BP at 140 to 150 mm Hg and diastolic pressure at 90 to 100 mm Hg to keep from inadvertently inducing uteroplacental insufficiency secondary to reduced uterine blood flow.
In the delivered patient, the risk of mild hypotension is not quite as great, although an attempt to rapidly return the patient to her previous normal BP profile may cause symptomatic hypotension.
If a patient develops a true hypertensive crisis with hypertensive encephalopathy (which generally occurs at BPs exceeding 240/140 mm Hg), then emergent intervention with a rapidly acting agent such as sodium nitroprusside is necessary and should be managed by someone skilled in critical care and the use of such drugs.
CHALLENGE NO. 5: Preventing seizures
Magnesium sulfate is the drug of choice to prevent both initial and recurrent eclamptic seizures.5 Two large clinical trials ended any doubts about its efficacy, demonstrating its superiority over both phenytoin and diazepam in the settings of preeclampsia and eclampsia.
Magnesium sulfate is best administered intravenously (IV) via continuous infusion pump. An initial bolus of 4 to 6 g is given over 15 to 30 minutes; this amount does not need to be adjusted to the patient’s level of renal function. A continuous infusion of magnesium sulfate is usually initiated at a rate of 2 g/hour. It is this infusion dosage that may need to be altered, based on the patient’s urine output and renal function.
Evidence of magnesium toxicity includes:
- loss of deep-tendon reflexes
- respiratory depression
- blurred vision
- cardiotoxicity.
There is no debate about the utility of magnesium sulfate in severe preeclampsia, but when it comes to intrapartum management of mild preeclampsia or cases in which preeclampsia first manifests in the postpartum period, data are not so clear. This debate will not be resolved to anyone’s satisfaction in the course of this article. Historically, the practice has been to use magnesium in these circumstances, but the pendulum has begun to shift based on a few arguments:
- Eclampsia is a rare event (about 1 case for every 300 to 1,000 deliveries).
- Most cases occur outside of the hospital.
- Some women experience seizures before preeclampsia has been diagnosed.
- Some patients experience seizures while taking magnesium sulfate.
Regardless of one’s position on this debate, there is broad consensus that regular careful clinical assessment of the patient who has preeclampsia is essential to minimize morbidity and mortality. This disease can progress from mild to severe rapidly. Only through regular careful assessment can a physician observe this change soon enough to alter management as necessary.
Treatment of magnesium toxicity
Most often, an ampule of 10% calcium gluconate (1 g) is administered IV to reverse the effects of suspected magnesium toxicity.
In addition, because magnesium freely crosses the placenta, we recommend that a newborn resuscitation team be present at all deliveries during which the mother was receiving magnesium sulfate because neonatal respiratory and cardiac depression have been reported in this setting.
CHALLENGE NO. 6: Delivering the patient
Preeclampsia, severe preeclampsia, and eclampsia present a dilemma for the managing clinician: subject her to the rigors of labor, or to the heightened risk of cesarean delivery? Overall, a properly managed vaginal delivery is less hemodynamically stressful than cesarean delivery for the mother. To accomplish vaginal delivery, it is necessary to provide optimal anesthesia and analgesia.
Risks of regional anesthesia
Women who have preeclampsia are volume-depleted. As such, they are prone to hypotension after administration of regional anesthesia if the block sets up too rapidly. For this reason, epidural anesthesia or some of the newer combined techniques offer optimal analgesia by allowing for slower implementation of the regional block.
Women who have preeclampsia, especially severe preeclampsia, are usually candidates for regional analgesia and anesthesia. Some requisites for regional anesthesia under these conditions include the following:
- The patient can tolerate preblock hydration.
- She has adequate IV access.
- There is a reproducible means of determining BP.
- The patient has a normal coagulation profile. (A normal platelet count with normal transaminase should be sufficient to confirm this; women who have preeclampsia are not at increased risk of having altered prothrombin time, partial thromboplastin time, or fibrinogen levels, provided there are no other mitigating clinical circumstances.)
- The anesthesiology team is skilled in the administration of regional anesthesia.
If eclampsia occurs
Do not proceed to emergent cesarean section. Rather, stabilize the mother, protect her from injury during the seizure, protect her airway, and allow the seizure to take its course.
Begin magnesium at once. If it was being infused before the seizure, consider giving an additional 2-g bolus over several minutes. As the mother stabilizes, the fetal heart rate will recover and she can be reassessed to determine optimal timing and route of delivery.
Continue magnesium after delivery?
Yes, but how long remains unclear. Most authorities have recommended 24 hours, based on the observation that most eclamptic seizures that occur in the first 48 hours postpartum actually occur in the first 24 hours.
Clinical assessment can guide management to some degree. The most reliable sign of disease resolution is spontaneous, brisk diuresis, so some clinicians use this finding as an indication to discontinue magnesium.
Regardless of clinical preference, if magnesium sulfate is being used postpartum, continue it until there is evidence of disease resolution, such as the diuresis noted above.
When HELLP syndrome arises
If HELLP [Hemolysis, Elevated Liver en zymes, Low Platelets] syndrome is present, continue magnesium sulfate until there is laboratory evidence of improvement in the platelet count and transaminase. Because a return to normal levels can take several days, it is not required before discontinuation of magnesium in cases of HELLP syndrome. However, at the time of discontinuation, it should be clear that there is no longer evidence of a worsening laboratory or clinical trend.
CHALLENGE NO. 7: Managing HELLP, atypical eclampsia
These two diagnoses pose daunting clinical challenges too numerous to cover in detail in this article, but a few key points merit consideration. When HELLP syndrome is diagnosed (using established criteria, TABLE 4), follow guidelines for severe preeclampsia. Use of dexamethasone remains somewhat controversial, as randomized clinical trials so far do not support it.6
Atypical eclampsia has been defined as eclampsia that occurs before 20 weeks’ gestation or from 48 hours to 14 days after delivery. Its management is similar to the management of eclampsia, with BP control and magnesium sulfate being the mainstays of therapy.
Because of the relative rarity of atypical eclampsia, we recommend neurologic consultation in these cases to evaluate for other possible causes of seizure.
TABLE 4
HELLP syndrome—Sibai criteria
|
CASE RESOLVED
After initial hospitalization, the patient is monitored as an outpatient until 35 weeks’ gestation, when more labile BP and increased proteinuria necessitate hospitalization. However, her preeclampsia remains mild by definition and, after continued reassuring fetal testing, she undergoes labor induction at 37 weeks.
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
3. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
4. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514-519.
5. Altman D, Carroli G, Duley L, et al:. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
6. Fonseca JE, Méndez F, Cataño C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193:1591-1598.
CASE: At risk, or just very pregnant?
At her first prenatal visit, a 31-year-old gravida has blood pressure (BP) of 100/60 mm Hg, no proteinuria, and normal weight for her gestational age. As she enters the third trimester, however, her BP rises to 138/86 mm Hg, she now has proteinuria of 1+, and she has gained 10 lb in the past 2 weeks.
Does she have preeclampsia, or do these findings reflect normal development in the last trimester?
These findings, in and of themselves, may not indicate preeclampsia—but they do suggest a serious risk of developing the disease.
Preeclampsia complicates approximately 3% to 7% of nulliparous pregnancies in the United States, and about 0.8% to 5% of multiparous pregnancies.
Although severe preeclampsia represents only a fraction of those amounts, and eclampsia an even lower percentage, they are potentially catastrophic complications of pregnancy and one of the leading causes of maternal death. They also are responsible for a large percentage of infants born prematurely as a result of a worsening maternal or fetal condition.
Preeclampsia and eclampsia are obstetric diseases, and obstetricians are the group best equipped to diagnose, evaluate, and manage them. In this article, we highlight seven challenges that obstetricians face when managing preeclampsia and eclampsia, and offer useful strategies to help minimize morbidity and mortality in both mother and infant.
CHALLENGE NO. 1: Making the diagnosis
Good prenatal care is a prerequisite
We can’t overemphasize the importance of early and adequate prenatal care! Although the diagnostic criteria for preeclampsia have been widely established—persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period—the condition does not always play by the rules. With close monitoring of weight, urine protein, and BP, the clinician can identify and follow potentially worrisome trends.
Earlier diagnostic criteria—which included a rise in systolic BP of 30 mm Hg or a rise in diastolic BP of 15 mm Hg above initial baseline BP, as well as the presence of pathologic edema—may have been revised, but it remains important for clinicians to put all pieces of clinical information together at each visit. For example, given her rising BP, proteinuria, and weight gain, the patient in the opening case must be considered at risk for preeclampsia. Suspicion also is justified if the patient has any of the risk factors for preeclampsia in TABLE 1.
TABLE 1
Risk factors for preeclampsia
|
|
|
Early detection is critical
Early identification of preeclampsia may allow for interventions, including delivery, that will lessen the risk of progression to severe preeclampsia and eclampsia and reduce fetal and maternal morbidity and mortality. It is, therefore, essential for the clinician to ask specifically about signs and symptoms of preeclampsia and to listen carefully to the answers.
Signs and symptoms may sometimes be typical:
- weight gain
- increasing edema
- persistent headache
- blurred vision.
- malaise
- nausea
- epigastric discomfort
- right upper-quadrant discomfort.
Diagnostic criteria
The diagnosis of preeclampsia is based on persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period.1 Other criteria have been applied, such as a rise in systolic or diastolic BP above baseline and urine dipstick criteria for proteinuria, but BP above 140/90 mm Hg and proteinuria above 300 mg are most frequently used in medical centers in the United States.2
Gestational hypertension and chronic hypertension do sometimes coexist with superimposed preeclampsia, but should not be confused with preeclampsia or lead to management decisions that should apply only to patients with preeclampsia.3
Before severe preeclampsia can be diagnosed, the initial criteria for preeclampsia should have been fulfilled, along with one or more of the findings listed in TABLE 2.
Attempts to predict preeclampsia have met with poor results. Measurement of the ratio of uterine artery systolic to diastolic flow has not been informative in the general healthy population of pregnant women. Nor has uric acid determination been useful; it generally has very poor predictive value and should be interpreted with caution.
TABLE 2
13 criteria for establishing severe preeclampsia
|
|
|
Hospitalization is essential for severe disease
Mild preeclampsia can be managed expectantly until fetal maturity or 37 weeks’ gestation. Severe preeclampsia can be managed expectantly in the mid trimester or early third trimester if both mother and fetus are stable, but hospitalization is necessary in a tertiary care facility that has critical-care OB expertise, an ICU facility, and a NICU facility and personnel on site.
Distinguish an existing condition from superimposed preeclampsia
One of the most difficult management challenges is the diagnosis of superimposed preeclampsia. Patients who have chronic hypertension often have underlying renal disease as well; in these patients, it may be difficult, if not impossible, to distinguish a worsening underlying medical condition from superimposed preeclampsia.
Our advice is not to agonize about this difference too much in the patient at or near term, as delivery may be indicated and the patient’s postpartum course may help resolve the question, with rapid resolution tending to favor a diagnosis of superimposed preeclampsia.
It also is important to note whether these patients are receiving antihypertensive therapy. If they are, hospitalization is recommended until delivery once the diagnosis of superimposed preeclampsia is made.
Given that the use of antihypertensive agents removes one of the major indicators of disease progression (i.e., rising BP), it is our practice to deliver these patients according to our severe preeclampsia management protocol and not to carry such pregnancies beyond 34 weeks. In carefully selected cases, the pregnancy can be continued to 37 weeks, but the decision to do so should be weighed carefully—ideally, with input from a maternal–fetal medicine specialist.
CHALLENGE NO. 2: Forgoing shortcuts
Evaluation and management of preeclampsia are relatively straightforward, but there are no shortcuts. Many patients who feel well initially may push for outpatient evaluation, but once a diagnosis of preeclampsia is established, in-hospital evaluation is preferable, at least until the degree of illness can be determined, fetal well-being can be established, and the patient’s candidacy for subsequent outpatient management can be more fully determined.
In-hospital management may be particularly useful for patients who have any of the risk factors for preeclampsia listed in TABLE 1.
Initial evaluation consists of:
- fetal nonstress testing
- amniotic fluid index
- serial BP determination
- 24-hour urine collection
- initial laboratory evaluation comprising a complete blood count with platelets and aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels.
If fetal and maternal evaluations are reassuring, and if the patient has remained stable, then outpatient management may be considered. In general, if proteinuria exceeds 1 g in 24 hours, in-hospital management is recommended, regardless of other parameters.
If outpatient management is considered, the level of care and surveillance must mirror what could be provided in the hospital. Hospitalization alone will not prevent all cases from progressing to severe preeclampsia or eclampsia, but daily and diligent observation and evaluation may minimize the risk.
CHALLENGE NO. 3: Treating the disease
Appropriate treatment of preeclampsia requires not only that the patient show up for prenatal care, but also that we:
- are certain of the diagnosis
- recognize the potential seriousness of the disease
- are thorough (remember, no shortcuts!).
Many experts in the field of preeclampsia have stated, on numerous occasions, that preeclampsia is more than simple hypertension. It is almost never advisable to initiate antihypertensive therapy for a patient in the third trimester when she was previously normotensive, because one runs the risk of masking a key clinical parameter used to assess disease progression.
In our institutions, any patient who is taking antihypertensive medication and in whom we are entertaining a diagnosis of preeclampsia is recommended for hospitalization for the duration of her pregnancy or until a diagnosis of preeclampsia can be ruled out with reasonable certainty.
Expectant management beyond 37 weeks does not benefit mother or fetus
Because preeclampsia is a multisystem disease, it has maternal, placental, and fetal consequences. The cure for preeclampsia remains delivery of the placenta. Expectant management offers no maternal benefit, but does offer some potential neonatal benefits if prematurity is a concern. Once concerns about prematurity have been largely eliminated, generally by achieving a gestational age of 37 weeks, further expectant management is not indicated, offers little or no additional benefit to the fetus, and leaves both mother and fetus at risk.
Therefore, once the pregnancy reaches 37 weeks, delivery is recommended.
When preeclampsia is severe, and when it is superimposed in a patient who is taking antihypertensive medication, we generally do not continue the pregnancy beyond 34 weeks.4 In our institutions, most patients who are being expectantly managed for severe preeclampsia remote from term—and who have remained stable—are delivered between 32 and 34 weeks’ gestation, depending on the specific clinical circumstances.
CHALLENGE NO. 4: Controlling blood pressure
Cerebrovascular accident (stroke) is the leading cause of maternal mortality from preeclampsia in the United States. Not all cases can be prevented, but one suggested preventive strategy is adequate BP control. Some cases of stroke in the setting of preeclampsia will occur despite systemic BP readings that are not considered to be in a dangerous range. One reason may be an override of normal cerebral blood flow autoregulatory mechanisms, resulting in increased cerebral blood flow, rising cerebral perfusion pressures, and vessel rupture. Such occurrences may sometimes, but not always, be related to coagulopathy.
When a patient has elevated BP, generally defined as persistent systolic pressures above 160 to 170 mm Hg and persistent diastolic pressures above 105 to 110 mm Hg, antihypertensive therapy is indicated and should be administered in a timely fashion.
Labetalol, nifedipine, and hydralazine have all been used effectively in such acute settings, when administered parenterally (except nifedipine, which may be given orally) and when given in proper dosages (TABLE 3).
Avoid oral use of labetalol or hydralazine to treat acute hypertensive emergencies.
TABLE 3
Pharmacotherapy of acute hypertension
| Drug | Dosage | Directions |
|---|---|---|
| Hydralazine* | 5 mg IV | Repeat in 10 min, then give 10 mg IV every 20 min until BP stabilizes (140–150/90–100 mm Hg) |
| Labetalol* | 10–20 mg IV push | Repeat every 10-20 min, doubling the dosage each time until a maximum total cumulative dosage of 300 mg has been given |
| Nifedipine* | 10 mg | Repeat in 20 min for four doses (maximum 40 mg); then give 10–20 mg orally every 4–6 h to achieve a stable BP of 140–150/90–100 mm Hg |
| * If target blood pressure is not reached after the maximum dosage of an agent is given, then additional or alternative pharmacotherapy must be utilized. | ||
Goals for treatment
In the antepartum patient, the goal is to maintain systolic BP at 140 to 150 mm Hg and diastolic pressure at 90 to 100 mm Hg to keep from inadvertently inducing uteroplacental insufficiency secondary to reduced uterine blood flow.
In the delivered patient, the risk of mild hypotension is not quite as great, although an attempt to rapidly return the patient to her previous normal BP profile may cause symptomatic hypotension.
If a patient develops a true hypertensive crisis with hypertensive encephalopathy (which generally occurs at BPs exceeding 240/140 mm Hg), then emergent intervention with a rapidly acting agent such as sodium nitroprusside is necessary and should be managed by someone skilled in critical care and the use of such drugs.
CHALLENGE NO. 5: Preventing seizures
Magnesium sulfate is the drug of choice to prevent both initial and recurrent eclamptic seizures.5 Two large clinical trials ended any doubts about its efficacy, demonstrating its superiority over both phenytoin and diazepam in the settings of preeclampsia and eclampsia.
Magnesium sulfate is best administered intravenously (IV) via continuous infusion pump. An initial bolus of 4 to 6 g is given over 15 to 30 minutes; this amount does not need to be adjusted to the patient’s level of renal function. A continuous infusion of magnesium sulfate is usually initiated at a rate of 2 g/hour. It is this infusion dosage that may need to be altered, based on the patient’s urine output and renal function.
Evidence of magnesium toxicity includes:
- loss of deep-tendon reflexes
- respiratory depression
- blurred vision
- cardiotoxicity.
There is no debate about the utility of magnesium sulfate in severe preeclampsia, but when it comes to intrapartum management of mild preeclampsia or cases in which preeclampsia first manifests in the postpartum period, data are not so clear. This debate will not be resolved to anyone’s satisfaction in the course of this article. Historically, the practice has been to use magnesium in these circumstances, but the pendulum has begun to shift based on a few arguments:
- Eclampsia is a rare event (about 1 case for every 300 to 1,000 deliveries).
- Most cases occur outside of the hospital.
- Some women experience seizures before preeclampsia has been diagnosed.
- Some patients experience seizures while taking magnesium sulfate.
Regardless of one’s position on this debate, there is broad consensus that regular careful clinical assessment of the patient who has preeclampsia is essential to minimize morbidity and mortality. This disease can progress from mild to severe rapidly. Only through regular careful assessment can a physician observe this change soon enough to alter management as necessary.
Treatment of magnesium toxicity
Most often, an ampule of 10% calcium gluconate (1 g) is administered IV to reverse the effects of suspected magnesium toxicity.
In addition, because magnesium freely crosses the placenta, we recommend that a newborn resuscitation team be present at all deliveries during which the mother was receiving magnesium sulfate because neonatal respiratory and cardiac depression have been reported in this setting.
CHALLENGE NO. 6: Delivering the patient
Preeclampsia, severe preeclampsia, and eclampsia present a dilemma for the managing clinician: subject her to the rigors of labor, or to the heightened risk of cesarean delivery? Overall, a properly managed vaginal delivery is less hemodynamically stressful than cesarean delivery for the mother. To accomplish vaginal delivery, it is necessary to provide optimal anesthesia and analgesia.
Risks of regional anesthesia
Women who have preeclampsia are volume-depleted. As such, they are prone to hypotension after administration of regional anesthesia if the block sets up too rapidly. For this reason, epidural anesthesia or some of the newer combined techniques offer optimal analgesia by allowing for slower implementation of the regional block.
Women who have preeclampsia, especially severe preeclampsia, are usually candidates for regional analgesia and anesthesia. Some requisites for regional anesthesia under these conditions include the following:
- The patient can tolerate preblock hydration.
- She has adequate IV access.
- There is a reproducible means of determining BP.
- The patient has a normal coagulation profile. (A normal platelet count with normal transaminase should be sufficient to confirm this; women who have preeclampsia are not at increased risk of having altered prothrombin time, partial thromboplastin time, or fibrinogen levels, provided there are no other mitigating clinical circumstances.)
- The anesthesiology team is skilled in the administration of regional anesthesia.
If eclampsia occurs
Do not proceed to emergent cesarean section. Rather, stabilize the mother, protect her from injury during the seizure, protect her airway, and allow the seizure to take its course.
Begin magnesium at once. If it was being infused before the seizure, consider giving an additional 2-g bolus over several minutes. As the mother stabilizes, the fetal heart rate will recover and she can be reassessed to determine optimal timing and route of delivery.
Continue magnesium after delivery?
Yes, but how long remains unclear. Most authorities have recommended 24 hours, based on the observation that most eclamptic seizures that occur in the first 48 hours postpartum actually occur in the first 24 hours.
Clinical assessment can guide management to some degree. The most reliable sign of disease resolution is spontaneous, brisk diuresis, so some clinicians use this finding as an indication to discontinue magnesium.
Regardless of clinical preference, if magnesium sulfate is being used postpartum, continue it until there is evidence of disease resolution, such as the diuresis noted above.
When HELLP syndrome arises
If HELLP [Hemolysis, Elevated Liver en zymes, Low Platelets] syndrome is present, continue magnesium sulfate until there is laboratory evidence of improvement in the platelet count and transaminase. Because a return to normal levels can take several days, it is not required before discontinuation of magnesium in cases of HELLP syndrome. However, at the time of discontinuation, it should be clear that there is no longer evidence of a worsening laboratory or clinical trend.
CHALLENGE NO. 7: Managing HELLP, atypical eclampsia
These two diagnoses pose daunting clinical challenges too numerous to cover in detail in this article, but a few key points merit consideration. When HELLP syndrome is diagnosed (using established criteria, TABLE 4), follow guidelines for severe preeclampsia. Use of dexamethasone remains somewhat controversial, as randomized clinical trials so far do not support it.6
Atypical eclampsia has been defined as eclampsia that occurs before 20 weeks’ gestation or from 48 hours to 14 days after delivery. Its management is similar to the management of eclampsia, with BP control and magnesium sulfate being the mainstays of therapy.
Because of the relative rarity of atypical eclampsia, we recommend neurologic consultation in these cases to evaluate for other possible causes of seizure.
TABLE 4
HELLP syndrome—Sibai criteria
|
CASE RESOLVED
After initial hospitalization, the patient is monitored as an outpatient until 35 weeks’ gestation, when more labile BP and increased proteinuria necessitate hospitalization. However, her preeclampsia remains mild by definition and, after continued reassuring fetal testing, she undergoes labor induction at 37 weeks.
CASE: At risk, or just very pregnant?
At her first prenatal visit, a 31-year-old gravida has blood pressure (BP) of 100/60 mm Hg, no proteinuria, and normal weight for her gestational age. As she enters the third trimester, however, her BP rises to 138/86 mm Hg, she now has proteinuria of 1+, and she has gained 10 lb in the past 2 weeks.
Does she have preeclampsia, or do these findings reflect normal development in the last trimester?
These findings, in and of themselves, may not indicate preeclampsia—but they do suggest a serious risk of developing the disease.
Preeclampsia complicates approximately 3% to 7% of nulliparous pregnancies in the United States, and about 0.8% to 5% of multiparous pregnancies.
Although severe preeclampsia represents only a fraction of those amounts, and eclampsia an even lower percentage, they are potentially catastrophic complications of pregnancy and one of the leading causes of maternal death. They also are responsible for a large percentage of infants born prematurely as a result of a worsening maternal or fetal condition.
Preeclampsia and eclampsia are obstetric diseases, and obstetricians are the group best equipped to diagnose, evaluate, and manage them. In this article, we highlight seven challenges that obstetricians face when managing preeclampsia and eclampsia, and offer useful strategies to help minimize morbidity and mortality in both mother and infant.
CHALLENGE NO. 1: Making the diagnosis
Good prenatal care is a prerequisite
We can’t overemphasize the importance of early and adequate prenatal care! Although the diagnostic criteria for preeclampsia have been widely established—persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period—the condition does not always play by the rules. With close monitoring of weight, urine protein, and BP, the clinician can identify and follow potentially worrisome trends.
Earlier diagnostic criteria—which included a rise in systolic BP of 30 mm Hg or a rise in diastolic BP of 15 mm Hg above initial baseline BP, as well as the presence of pathologic edema—may have been revised, but it remains important for clinicians to put all pieces of clinical information together at each visit. For example, given her rising BP, proteinuria, and weight gain, the patient in the opening case must be considered at risk for preeclampsia. Suspicion also is justified if the patient has any of the risk factors for preeclampsia in TABLE 1.
TABLE 1
Risk factors for preeclampsia
|
|
|
Early detection is critical
Early identification of preeclampsia may allow for interventions, including delivery, that will lessen the risk of progression to severe preeclampsia and eclampsia and reduce fetal and maternal morbidity and mortality. It is, therefore, essential for the clinician to ask specifically about signs and symptoms of preeclampsia and to listen carefully to the answers.
Signs and symptoms may sometimes be typical:
- weight gain
- increasing edema
- persistent headache
- blurred vision.
- malaise
- nausea
- epigastric discomfort
- right upper-quadrant discomfort.
Diagnostic criteria
The diagnosis of preeclampsia is based on persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period.1 Other criteria have been applied, such as a rise in systolic or diastolic BP above baseline and urine dipstick criteria for proteinuria, but BP above 140/90 mm Hg and proteinuria above 300 mg are most frequently used in medical centers in the United States.2
Gestational hypertension and chronic hypertension do sometimes coexist with superimposed preeclampsia, but should not be confused with preeclampsia or lead to management decisions that should apply only to patients with preeclampsia.3
Before severe preeclampsia can be diagnosed, the initial criteria for preeclampsia should have been fulfilled, along with one or more of the findings listed in TABLE 2.
Attempts to predict preeclampsia have met with poor results. Measurement of the ratio of uterine artery systolic to diastolic flow has not been informative in the general healthy population of pregnant women. Nor has uric acid determination been useful; it generally has very poor predictive value and should be interpreted with caution.
TABLE 2
13 criteria for establishing severe preeclampsia
|
|
|
Hospitalization is essential for severe disease
Mild preeclampsia can be managed expectantly until fetal maturity or 37 weeks’ gestation. Severe preeclampsia can be managed expectantly in the mid trimester or early third trimester if both mother and fetus are stable, but hospitalization is necessary in a tertiary care facility that has critical-care OB expertise, an ICU facility, and a NICU facility and personnel on site.
Distinguish an existing condition from superimposed preeclampsia
One of the most difficult management challenges is the diagnosis of superimposed preeclampsia. Patients who have chronic hypertension often have underlying renal disease as well; in these patients, it may be difficult, if not impossible, to distinguish a worsening underlying medical condition from superimposed preeclampsia.
Our advice is not to agonize about this difference too much in the patient at or near term, as delivery may be indicated and the patient’s postpartum course may help resolve the question, with rapid resolution tending to favor a diagnosis of superimposed preeclampsia.
It also is important to note whether these patients are receiving antihypertensive therapy. If they are, hospitalization is recommended until delivery once the diagnosis of superimposed preeclampsia is made.
Given that the use of antihypertensive agents removes one of the major indicators of disease progression (i.e., rising BP), it is our practice to deliver these patients according to our severe preeclampsia management protocol and not to carry such pregnancies beyond 34 weeks. In carefully selected cases, the pregnancy can be continued to 37 weeks, but the decision to do so should be weighed carefully—ideally, with input from a maternal–fetal medicine specialist.
CHALLENGE NO. 2: Forgoing shortcuts
Evaluation and management of preeclampsia are relatively straightforward, but there are no shortcuts. Many patients who feel well initially may push for outpatient evaluation, but once a diagnosis of preeclampsia is established, in-hospital evaluation is preferable, at least until the degree of illness can be determined, fetal well-being can be established, and the patient’s candidacy for subsequent outpatient management can be more fully determined.
In-hospital management may be particularly useful for patients who have any of the risk factors for preeclampsia listed in TABLE 1.
Initial evaluation consists of:
- fetal nonstress testing
- amniotic fluid index
- serial BP determination
- 24-hour urine collection
- initial laboratory evaluation comprising a complete blood count with platelets and aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels.
If fetal and maternal evaluations are reassuring, and if the patient has remained stable, then outpatient management may be considered. In general, if proteinuria exceeds 1 g in 24 hours, in-hospital management is recommended, regardless of other parameters.
If outpatient management is considered, the level of care and surveillance must mirror what could be provided in the hospital. Hospitalization alone will not prevent all cases from progressing to severe preeclampsia or eclampsia, but daily and diligent observation and evaluation may minimize the risk.
CHALLENGE NO. 3: Treating the disease
Appropriate treatment of preeclampsia requires not only that the patient show up for prenatal care, but also that we:
- are certain of the diagnosis
- recognize the potential seriousness of the disease
- are thorough (remember, no shortcuts!).
Many experts in the field of preeclampsia have stated, on numerous occasions, that preeclampsia is more than simple hypertension. It is almost never advisable to initiate antihypertensive therapy for a patient in the third trimester when she was previously normotensive, because one runs the risk of masking a key clinical parameter used to assess disease progression.
In our institutions, any patient who is taking antihypertensive medication and in whom we are entertaining a diagnosis of preeclampsia is recommended for hospitalization for the duration of her pregnancy or until a diagnosis of preeclampsia can be ruled out with reasonable certainty.
Expectant management beyond 37 weeks does not benefit mother or fetus
Because preeclampsia is a multisystem disease, it has maternal, placental, and fetal consequences. The cure for preeclampsia remains delivery of the placenta. Expectant management offers no maternal benefit, but does offer some potential neonatal benefits if prematurity is a concern. Once concerns about prematurity have been largely eliminated, generally by achieving a gestational age of 37 weeks, further expectant management is not indicated, offers little or no additional benefit to the fetus, and leaves both mother and fetus at risk.
Therefore, once the pregnancy reaches 37 weeks, delivery is recommended.
When preeclampsia is severe, and when it is superimposed in a patient who is taking antihypertensive medication, we generally do not continue the pregnancy beyond 34 weeks.4 In our institutions, most patients who are being expectantly managed for severe preeclampsia remote from term—and who have remained stable—are delivered between 32 and 34 weeks’ gestation, depending on the specific clinical circumstances.
CHALLENGE NO. 4: Controlling blood pressure
Cerebrovascular accident (stroke) is the leading cause of maternal mortality from preeclampsia in the United States. Not all cases can be prevented, but one suggested preventive strategy is adequate BP control. Some cases of stroke in the setting of preeclampsia will occur despite systemic BP readings that are not considered to be in a dangerous range. One reason may be an override of normal cerebral blood flow autoregulatory mechanisms, resulting in increased cerebral blood flow, rising cerebral perfusion pressures, and vessel rupture. Such occurrences may sometimes, but not always, be related to coagulopathy.
When a patient has elevated BP, generally defined as persistent systolic pressures above 160 to 170 mm Hg and persistent diastolic pressures above 105 to 110 mm Hg, antihypertensive therapy is indicated and should be administered in a timely fashion.
Labetalol, nifedipine, and hydralazine have all been used effectively in such acute settings, when administered parenterally (except nifedipine, which may be given orally) and when given in proper dosages (TABLE 3).
Avoid oral use of labetalol or hydralazine to treat acute hypertensive emergencies.
TABLE 3
Pharmacotherapy of acute hypertension
| Drug | Dosage | Directions |
|---|---|---|
| Hydralazine* | 5 mg IV | Repeat in 10 min, then give 10 mg IV every 20 min until BP stabilizes (140–150/90–100 mm Hg) |
| Labetalol* | 10–20 mg IV push | Repeat every 10-20 min, doubling the dosage each time until a maximum total cumulative dosage of 300 mg has been given |
| Nifedipine* | 10 mg | Repeat in 20 min for four doses (maximum 40 mg); then give 10–20 mg orally every 4–6 h to achieve a stable BP of 140–150/90–100 mm Hg |
| * If target blood pressure is not reached after the maximum dosage of an agent is given, then additional or alternative pharmacotherapy must be utilized. | ||
Goals for treatment
In the antepartum patient, the goal is to maintain systolic BP at 140 to 150 mm Hg and diastolic pressure at 90 to 100 mm Hg to keep from inadvertently inducing uteroplacental insufficiency secondary to reduced uterine blood flow.
In the delivered patient, the risk of mild hypotension is not quite as great, although an attempt to rapidly return the patient to her previous normal BP profile may cause symptomatic hypotension.
If a patient develops a true hypertensive crisis with hypertensive encephalopathy (which generally occurs at BPs exceeding 240/140 mm Hg), then emergent intervention with a rapidly acting agent such as sodium nitroprusside is necessary and should be managed by someone skilled in critical care and the use of such drugs.
CHALLENGE NO. 5: Preventing seizures
Magnesium sulfate is the drug of choice to prevent both initial and recurrent eclamptic seizures.5 Two large clinical trials ended any doubts about its efficacy, demonstrating its superiority over both phenytoin and diazepam in the settings of preeclampsia and eclampsia.
Magnesium sulfate is best administered intravenously (IV) via continuous infusion pump. An initial bolus of 4 to 6 g is given over 15 to 30 minutes; this amount does not need to be adjusted to the patient’s level of renal function. A continuous infusion of magnesium sulfate is usually initiated at a rate of 2 g/hour. It is this infusion dosage that may need to be altered, based on the patient’s urine output and renal function.
Evidence of magnesium toxicity includes:
- loss of deep-tendon reflexes
- respiratory depression
- blurred vision
- cardiotoxicity.
There is no debate about the utility of magnesium sulfate in severe preeclampsia, but when it comes to intrapartum management of mild preeclampsia or cases in which preeclampsia first manifests in the postpartum period, data are not so clear. This debate will not be resolved to anyone’s satisfaction in the course of this article. Historically, the practice has been to use magnesium in these circumstances, but the pendulum has begun to shift based on a few arguments:
- Eclampsia is a rare event (about 1 case for every 300 to 1,000 deliveries).
- Most cases occur outside of the hospital.
- Some women experience seizures before preeclampsia has been diagnosed.
- Some patients experience seizures while taking magnesium sulfate.
Regardless of one’s position on this debate, there is broad consensus that regular careful clinical assessment of the patient who has preeclampsia is essential to minimize morbidity and mortality. This disease can progress from mild to severe rapidly. Only through regular careful assessment can a physician observe this change soon enough to alter management as necessary.
Treatment of magnesium toxicity
Most often, an ampule of 10% calcium gluconate (1 g) is administered IV to reverse the effects of suspected magnesium toxicity.
In addition, because magnesium freely crosses the placenta, we recommend that a newborn resuscitation team be present at all deliveries during which the mother was receiving magnesium sulfate because neonatal respiratory and cardiac depression have been reported in this setting.
CHALLENGE NO. 6: Delivering the patient
Preeclampsia, severe preeclampsia, and eclampsia present a dilemma for the managing clinician: subject her to the rigors of labor, or to the heightened risk of cesarean delivery? Overall, a properly managed vaginal delivery is less hemodynamically stressful than cesarean delivery for the mother. To accomplish vaginal delivery, it is necessary to provide optimal anesthesia and analgesia.
Risks of regional anesthesia
Women who have preeclampsia are volume-depleted. As such, they are prone to hypotension after administration of regional anesthesia if the block sets up too rapidly. For this reason, epidural anesthesia or some of the newer combined techniques offer optimal analgesia by allowing for slower implementation of the regional block.
Women who have preeclampsia, especially severe preeclampsia, are usually candidates for regional analgesia and anesthesia. Some requisites for regional anesthesia under these conditions include the following:
- The patient can tolerate preblock hydration.
- She has adequate IV access.
- There is a reproducible means of determining BP.
- The patient has a normal coagulation profile. (A normal platelet count with normal transaminase should be sufficient to confirm this; women who have preeclampsia are not at increased risk of having altered prothrombin time, partial thromboplastin time, or fibrinogen levels, provided there are no other mitigating clinical circumstances.)
- The anesthesiology team is skilled in the administration of regional anesthesia.
If eclampsia occurs
Do not proceed to emergent cesarean section. Rather, stabilize the mother, protect her from injury during the seizure, protect her airway, and allow the seizure to take its course.
Begin magnesium at once. If it was being infused before the seizure, consider giving an additional 2-g bolus over several minutes. As the mother stabilizes, the fetal heart rate will recover and she can be reassessed to determine optimal timing and route of delivery.
Continue magnesium after delivery?
Yes, but how long remains unclear. Most authorities have recommended 24 hours, based on the observation that most eclamptic seizures that occur in the first 48 hours postpartum actually occur in the first 24 hours.
Clinical assessment can guide management to some degree. The most reliable sign of disease resolution is spontaneous, brisk diuresis, so some clinicians use this finding as an indication to discontinue magnesium.
Regardless of clinical preference, if magnesium sulfate is being used postpartum, continue it until there is evidence of disease resolution, such as the diuresis noted above.
When HELLP syndrome arises
If HELLP [Hemolysis, Elevated Liver en zymes, Low Platelets] syndrome is present, continue magnesium sulfate until there is laboratory evidence of improvement in the platelet count and transaminase. Because a return to normal levels can take several days, it is not required before discontinuation of magnesium in cases of HELLP syndrome. However, at the time of discontinuation, it should be clear that there is no longer evidence of a worsening laboratory or clinical trend.
CHALLENGE NO. 7: Managing HELLP, atypical eclampsia
These two diagnoses pose daunting clinical challenges too numerous to cover in detail in this article, but a few key points merit consideration. When HELLP syndrome is diagnosed (using established criteria, TABLE 4), follow guidelines for severe preeclampsia. Use of dexamethasone remains somewhat controversial, as randomized clinical trials so far do not support it.6
Atypical eclampsia has been defined as eclampsia that occurs before 20 weeks’ gestation or from 48 hours to 14 days after delivery. Its management is similar to the management of eclampsia, with BP control and magnesium sulfate being the mainstays of therapy.
Because of the relative rarity of atypical eclampsia, we recommend neurologic consultation in these cases to evaluate for other possible causes of seizure.
TABLE 4
HELLP syndrome—Sibai criteria
|
CASE RESOLVED
After initial hospitalization, the patient is monitored as an outpatient until 35 weeks’ gestation, when more labile BP and increased proteinuria necessitate hospitalization. However, her preeclampsia remains mild by definition and, after continued reassuring fetal testing, she undergoes labor induction at 37 weeks.
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
3. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
4. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514-519.
5. Altman D, Carroli G, Duley L, et al:. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
6. Fonseca JE, Méndez F, Cataño C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193:1591-1598.
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
3. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
4. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514-519.
5. Altman D, Carroli G, Duley L, et al:. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
6. Fonseca JE, Méndez F, Cataño C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193:1591-1598.
What percentage of cerebral palsy cases might be associated with intrapartum asphyxia?
In his editorial, Freeman acknowledges the complexity of the issue and allows that some cases of CP are clearly caused by substandard intrapartum care, but he leaves many essential questions unanswered.1 Similarly, the meta-analysis itself offers multiple explanations of the possible causes of CP but relatively few conclusions that can be applied to any specific case of CP, when the cause in that case is unclear.
Authors focus on cord pH below 7.0
To be sure, an umbilical artery pH of less than 7.0 at birth is concerning, but even at this level of acidosis, results are conflicting. Combining data from multiple studies, the authors concluded that the incidence of significant neonatal neurologic morbidity and mortality was significant among nonanomalous infants who had such a pH level (23.1%). However, the remaining 76.9% of infants were neurologically normal at the time of hospital discharge.
Nor did Apgar scores predict asphyxial complications when cord pH was less than 7.0. However, the combination of an Apgar score of 3 or less and cord pH below 7.0 was a sensitive predictor of serious neonatal morbidity.
Questions abound—but not answers
It was thought that electronic fetal monitoring would eliminate intrapartum stillbirth and reduce the incidence of CP—but neither goal has been achieved. Moreover, the presence of meconium, long associated with nonreassuring fetal status, was found in one study to have no association with CP.2
As for the role of infection, inflammation, and intrapartum fever, Eastman and DeLeon suggested as early as 1955 that intrapartum fever was seven times more likely in mothers of children who were later diagnosed with CP than in mothers of normal children—and intrapartum fever, infection, and neonatal fever remain prime suspects in the CP mystery.3
How does the average clinical obstetrician interpret and use these results? How does our legal system use these results?
It depends. The overwhelming majority of nonanomalous term infants do well. For the few who develop CP, there often is an accepted reason for the diagnosis.
Graham and colleagues conclude that only 14.5% of CP cases are associated with intrapartum asphyxia. The dilemma? For that 14.5%—or even the remaining 85.5%—our ability to determine the true cause of CP in any given case is unreliable. Who or what test can conclusively eliminate intrapartum asphyxia as a medically probable cause?
The answers are disheartening.
- Recognize that prematurity and infection are the leading risk factors for cerebral palsy (CP) in nonanomalous infants.
- Be cognizant of the lack of predictive value—both positive and negative—of current methodologies, such as Apgar score and pH level, in regard to CP.
- Remember that neither the introduction of electronic fetal monitoring nor the increase in the cesarean delivery rate from 5.5% in 1970 to 31.1% in 2006 has appreciably altered the rate of CP in the United States.
- Don’t discount the importance of the first 20 minutes of postnatal life. They are perhaps at least as important as the final 20 minutes of fetal life. When delivering a patient who has a risk factor for CP, do not hesitate to request the presence of a skilled neonatologist for assistance with newborn resuscitation.—JOHN T. REPKE, MD
1. Freeman RK. Medical and legal implications for necessary requirements to diagnose damaging hypoxic–ischemic encephalopathy leading to later cerebral palsy. Am J Obstet Gynecol. 2008;199:585-586.
2. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
3. Eastman NJ, DeLeon M. The etiology of cerebral palsy. Am J Obstet Gynecol. 1955;69:950-961.
In his editorial, Freeman acknowledges the complexity of the issue and allows that some cases of CP are clearly caused by substandard intrapartum care, but he leaves many essential questions unanswered.1 Similarly, the meta-analysis itself offers multiple explanations of the possible causes of CP but relatively few conclusions that can be applied to any specific case of CP, when the cause in that case is unclear.
Authors focus on cord pH below 7.0
To be sure, an umbilical artery pH of less than 7.0 at birth is concerning, but even at this level of acidosis, results are conflicting. Combining data from multiple studies, the authors concluded that the incidence of significant neonatal neurologic morbidity and mortality was significant among nonanomalous infants who had such a pH level (23.1%). However, the remaining 76.9% of infants were neurologically normal at the time of hospital discharge.
Nor did Apgar scores predict asphyxial complications when cord pH was less than 7.0. However, the combination of an Apgar score of 3 or less and cord pH below 7.0 was a sensitive predictor of serious neonatal morbidity.
Questions abound—but not answers
It was thought that electronic fetal monitoring would eliminate intrapartum stillbirth and reduce the incidence of CP—but neither goal has been achieved. Moreover, the presence of meconium, long associated with nonreassuring fetal status, was found in one study to have no association with CP.2
As for the role of infection, inflammation, and intrapartum fever, Eastman and DeLeon suggested as early as 1955 that intrapartum fever was seven times more likely in mothers of children who were later diagnosed with CP than in mothers of normal children—and intrapartum fever, infection, and neonatal fever remain prime suspects in the CP mystery.3
How does the average clinical obstetrician interpret and use these results? How does our legal system use these results?
It depends. The overwhelming majority of nonanomalous term infants do well. For the few who develop CP, there often is an accepted reason for the diagnosis.
Graham and colleagues conclude that only 14.5% of CP cases are associated with intrapartum asphyxia. The dilemma? For that 14.5%—or even the remaining 85.5%—our ability to determine the true cause of CP in any given case is unreliable. Who or what test can conclusively eliminate intrapartum asphyxia as a medically probable cause?
The answers are disheartening.
- Recognize that prematurity and infection are the leading risk factors for cerebral palsy (CP) in nonanomalous infants.
- Be cognizant of the lack of predictive value—both positive and negative—of current methodologies, such as Apgar score and pH level, in regard to CP.
- Remember that neither the introduction of electronic fetal monitoring nor the increase in the cesarean delivery rate from 5.5% in 1970 to 31.1% in 2006 has appreciably altered the rate of CP in the United States.
- Don’t discount the importance of the first 20 minutes of postnatal life. They are perhaps at least as important as the final 20 minutes of fetal life. When delivering a patient who has a risk factor for CP, do not hesitate to request the presence of a skilled neonatologist for assistance with newborn resuscitation.—JOHN T. REPKE, MD
In his editorial, Freeman acknowledges the complexity of the issue and allows that some cases of CP are clearly caused by substandard intrapartum care, but he leaves many essential questions unanswered.1 Similarly, the meta-analysis itself offers multiple explanations of the possible causes of CP but relatively few conclusions that can be applied to any specific case of CP, when the cause in that case is unclear.
Authors focus on cord pH below 7.0
To be sure, an umbilical artery pH of less than 7.0 at birth is concerning, but even at this level of acidosis, results are conflicting. Combining data from multiple studies, the authors concluded that the incidence of significant neonatal neurologic morbidity and mortality was significant among nonanomalous infants who had such a pH level (23.1%). However, the remaining 76.9% of infants were neurologically normal at the time of hospital discharge.
Nor did Apgar scores predict asphyxial complications when cord pH was less than 7.0. However, the combination of an Apgar score of 3 or less and cord pH below 7.0 was a sensitive predictor of serious neonatal morbidity.
Questions abound—but not answers
It was thought that electronic fetal monitoring would eliminate intrapartum stillbirth and reduce the incidence of CP—but neither goal has been achieved. Moreover, the presence of meconium, long associated with nonreassuring fetal status, was found in one study to have no association with CP.2
As for the role of infection, inflammation, and intrapartum fever, Eastman and DeLeon suggested as early as 1955 that intrapartum fever was seven times more likely in mothers of children who were later diagnosed with CP than in mothers of normal children—and intrapartum fever, infection, and neonatal fever remain prime suspects in the CP mystery.3
How does the average clinical obstetrician interpret and use these results? How does our legal system use these results?
It depends. The overwhelming majority of nonanomalous term infants do well. For the few who develop CP, there often is an accepted reason for the diagnosis.
Graham and colleagues conclude that only 14.5% of CP cases are associated with intrapartum asphyxia. The dilemma? For that 14.5%—or even the remaining 85.5%—our ability to determine the true cause of CP in any given case is unreliable. Who or what test can conclusively eliminate intrapartum asphyxia as a medically probable cause?
The answers are disheartening.
- Recognize that prematurity and infection are the leading risk factors for cerebral palsy (CP) in nonanomalous infants.
- Be cognizant of the lack of predictive value—both positive and negative—of current methodologies, such as Apgar score and pH level, in regard to CP.
- Remember that neither the introduction of electronic fetal monitoring nor the increase in the cesarean delivery rate from 5.5% in 1970 to 31.1% in 2006 has appreciably altered the rate of CP in the United States.
- Don’t discount the importance of the first 20 minutes of postnatal life. They are perhaps at least as important as the final 20 minutes of fetal life. When delivering a patient who has a risk factor for CP, do not hesitate to request the presence of a skilled neonatologist for assistance with newborn resuscitation.—JOHN T. REPKE, MD
1. Freeman RK. Medical and legal implications for necessary requirements to diagnose damaging hypoxic–ischemic encephalopathy leading to later cerebral palsy. Am J Obstet Gynecol. 2008;199:585-586.
2. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
3. Eastman NJ, DeLeon M. The etiology of cerebral palsy. Am J Obstet Gynecol. 1955;69:950-961.
1. Freeman RK. Medical and legal implications for necessary requirements to diagnose damaging hypoxic–ischemic encephalopathy leading to later cerebral palsy. Am J Obstet Gynecol. 2008;199:585-586.
2. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
3. Eastman NJ, DeLeon M. The etiology of cerebral palsy. Am J Obstet Gynecol. 1955;69:950-961.
Metformin for gestational diabetes: As safe and as effective as insulin?
EXPERT COMMENTARY
Rowan and colleagues add to the data on the potential benefits of oral hypoglycemic agents, compared with insulin, in managing gestational diabetes. The presumption was that dietary treatment alone would not result in adequate glycemic control.
In the study, women assigned to metformin were given a starting dosage of 500 mg once or twice daily, which was then increased to a maximum daily dosage of 2,500 mg. According to the authors, women assigned to insulin were prescribed the drug “according to usual practice,” although that practice was never defined. In addition, if adequate glycemic control was not achieved in the metformin group, insulin was added.
Overall, 363 of the women who received metformin completed the study, with 195 receiving metformin alone and 168 ultimately receiving metformin plus insulin. In the other arm, 370 of the women assigned to insulin completed the study. Maternal baseline characteristics were the same for both groups, except that a statistically greater number of patients in the metformin group had had three or more pregnancy terminations or miscarriages.
The primary outcome of this study was a composite of various neonatal outcomes. Of the variables analyzed, significant differences were found only for prematurity (delivery
A variety of secondary outcomes were also analyzed, with no meaningful differences. The authors conclude that metformin with or without supplemental insulin is “effective and safe” for women with gestational diabetes. In the next sentence, however, they observe that “follow-up data are needed to establish long-term safety.”
All the attention to gestational diabetes has yet to significantly improve obstetric outcomes such as birth injury, C-section, or serious short-term neonatal morbidity. Nor is it any surprise that women in this study preferred metformin to insulin; most people would prefer a pill to a “shot.” However, nearly half of the pill group ended up needing a shot anyway.
Metformin is pregnancy category B and should not be used by nursing women. Rowan and colleagues acknowledge that long-term safety data are insufficient to recommend the use of oral hypoglycemic agents to manage diabetes in pregnancy.
This trial was well designed and executed, but insulin remains, in my opinion, the standard of care. Oral hypoglycemic agents just are not “ready for prime time” when it comes to gestational diabetes.—JOHN T. REPKE, MD
EXPERT COMMENTARY
Rowan and colleagues add to the data on the potential benefits of oral hypoglycemic agents, compared with insulin, in managing gestational diabetes. The presumption was that dietary treatment alone would not result in adequate glycemic control.
In the study, women assigned to metformin were given a starting dosage of 500 mg once or twice daily, which was then increased to a maximum daily dosage of 2,500 mg. According to the authors, women assigned to insulin were prescribed the drug “according to usual practice,” although that practice was never defined. In addition, if adequate glycemic control was not achieved in the metformin group, insulin was added.
Overall, 363 of the women who received metformin completed the study, with 195 receiving metformin alone and 168 ultimately receiving metformin plus insulin. In the other arm, 370 of the women assigned to insulin completed the study. Maternal baseline characteristics were the same for both groups, except that a statistically greater number of patients in the metformin group had had three or more pregnancy terminations or miscarriages.
The primary outcome of this study was a composite of various neonatal outcomes. Of the variables analyzed, significant differences were found only for prematurity (delivery
A variety of secondary outcomes were also analyzed, with no meaningful differences. The authors conclude that metformin with or without supplemental insulin is “effective and safe” for women with gestational diabetes. In the next sentence, however, they observe that “follow-up data are needed to establish long-term safety.”
All the attention to gestational diabetes has yet to significantly improve obstetric outcomes such as birth injury, C-section, or serious short-term neonatal morbidity. Nor is it any surprise that women in this study preferred metformin to insulin; most people would prefer a pill to a “shot.” However, nearly half of the pill group ended up needing a shot anyway.
Metformin is pregnancy category B and should not be used by nursing women. Rowan and colleagues acknowledge that long-term safety data are insufficient to recommend the use of oral hypoglycemic agents to manage diabetes in pregnancy.
This trial was well designed and executed, but insulin remains, in my opinion, the standard of care. Oral hypoglycemic agents just are not “ready for prime time” when it comes to gestational diabetes.—JOHN T. REPKE, MD
EXPERT COMMENTARY
Rowan and colleagues add to the data on the potential benefits of oral hypoglycemic agents, compared with insulin, in managing gestational diabetes. The presumption was that dietary treatment alone would not result in adequate glycemic control.
In the study, women assigned to metformin were given a starting dosage of 500 mg once or twice daily, which was then increased to a maximum daily dosage of 2,500 mg. According to the authors, women assigned to insulin were prescribed the drug “according to usual practice,” although that practice was never defined. In addition, if adequate glycemic control was not achieved in the metformin group, insulin was added.
Overall, 363 of the women who received metformin completed the study, with 195 receiving metformin alone and 168 ultimately receiving metformin plus insulin. In the other arm, 370 of the women assigned to insulin completed the study. Maternal baseline characteristics were the same for both groups, except that a statistically greater number of patients in the metformin group had had three or more pregnancy terminations or miscarriages.
The primary outcome of this study was a composite of various neonatal outcomes. Of the variables analyzed, significant differences were found only for prematurity (delivery
A variety of secondary outcomes were also analyzed, with no meaningful differences. The authors conclude that metformin with or without supplemental insulin is “effective and safe” for women with gestational diabetes. In the next sentence, however, they observe that “follow-up data are needed to establish long-term safety.”
All the attention to gestational diabetes has yet to significantly improve obstetric outcomes such as birth injury, C-section, or serious short-term neonatal morbidity. Nor is it any surprise that women in this study preferred metformin to insulin; most people would prefer a pill to a “shot.” However, nearly half of the pill group ended up needing a shot anyway.
Metformin is pregnancy category B and should not be used by nursing women. Rowan and colleagues acknowledge that long-term safety data are insufficient to recommend the use of oral hypoglycemic agents to manage diabetes in pregnancy.
This trial was well designed and executed, but insulin remains, in my opinion, the standard of care. Oral hypoglycemic agents just are not “ready for prime time” when it comes to gestational diabetes.—JOHN T. REPKE, MD
Q. Does progesterone reduce the risk of preterm birth among women with a short cervix?
The trial included both singleton and twin gestations.
Expert Commentary
The study by Fonseca and colleagues is an important contribution to the ever-expanding body of literature on ways to reduce preterm birth. It immediately follows a paper in the same issue of the New England Journal of Medicine declaring the failure of intramuscular 17α-hydroxyprogesterone caproate (17P) to prevent preterm birth in twin gestations.1 In contrast, an earlier study from 2003 found 17P to be more effective than no treatment in preventing spontaneous preterm delivery in singleton pregnancies of women with a history of premature delivery.2
The trial by Fonseca and colleagues capitalizes on two unique aspects of the prematurity debate. The first is that history is probably not any more useful in screening for risk of prematurity than it is for screening for gestational diabetes. This is not to say that history is unimportant, but rather to emphasize that history alone may be insufficient to identify populations that may be at risk for preterm delivery and therefore might possibly benefit from intervention.
The second is the suggestion, based on earlier work by Iams and associates,3 that decreased cervical length may distinguish a group of women at high risk for preterm delivery.
What to make of equivocal findings?
Is an intervention worthwhile if it has no effect on key outcomes such as morbidity and mortality? And what are we to make of the fact that intramuscular 17P is effective in singleton but not twin gestations?
The current trial was insufficiently powered to affirm the null hypothesis with respect to perinatal mortality and neonatal morbidity—ie, there were not enough patients in the study to determine whether the lack of difference in these variables was real or due to insufficient sample size. However, intravaginal progesterone seems more attractive than intramuscular injection—at least intuitively. It may be that a local anti-inflammatory response is what reduced spontaneous preterm delivery—and that may be why intramuscular injection of synthetic progestin at a remote site was less effective, depending on whether the pregnancy was a singleton or twin gestation.
We must also be concerned about the yet-unexplained observation of higher—though statistically insignificant—rates of miscarriage and intrauterine fetal death among women receiving intramuscular 17P, compared with placebo.2
In my own practice, I estimate that fewer than 20% of patients who might be eligible for progesterone accept the treatment after I review all the data with them. Two illustrative cases highlight the conundrum a clinician faces with respect to “measurable outcome differences.”
Patient #1 is a healthy 30-year-old gravida 3 para 1102 whose obstetric history includes:
- a full-term delivery in 2002 that was complicated by premature onset of contractions but never required tocolytic therapy
- a delivery at 29 weeks in 2005 that was complicated by preterm premature rupture of membranes and chorioamnionitis.
Her current pregnancy is managed with cervical-length assessment (all measurements exceed 35 mm) and 17α-hydroxyprogesterone caproate. She delivers a healthy 3,500-g infant at term without complication.
Patient #2 is a healthy 31-year-old gravida 3 para 0202 who delivered at 33 weeks in 2002 and at 35 weeks in 2003. Both deliveries were secondary to idiopathic preterm labor. Her current pregnancy is followed routinely, with no cervical-length assessment. After counseling, she decides against progesterone therapy and goes on to deliver a 3,300-g healthy infant at 39 weeks’ gestation.
The dilemma
These two cases are medically similar; both were eligible for progesterone treatment. One patient chose it and one did not—yet their obstetric outcomes were identical.—John T. Repke, MD
Too early to elevate either regimen to “standard of care”
In my opinion, it is still too early to elevate either intravaginal or intramuscular progesterone to the level of “standard of care.” What may be reasonable in this age of increasing use of first- and mid-trimester ultrasonographic screening is to make certain that information on cervical length is provided. Also important is a candid discussion with the patient about the range of options for management of a short cervix in pregnancy. In the meantime, more than a dozen trials on the prevention of prematurity are in progress. The hope is that more definitive answers to the prematurity riddle are “just around the corner”—which, I suspect, was also the hope in 1975.4
1. Rouse DJ, Caritis SN, Peaceman AM, et al. For the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454-461.
2. Meis PJ, Klebanoff M, Thom E, et al. For the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
3. Iams JD, Goldenberg RL, Meis PL, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567-572.
4. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675-680.
The trial included both singleton and twin gestations.
Expert Commentary
The study by Fonseca and colleagues is an important contribution to the ever-expanding body of literature on ways to reduce preterm birth. It immediately follows a paper in the same issue of the New England Journal of Medicine declaring the failure of intramuscular 17α-hydroxyprogesterone caproate (17P) to prevent preterm birth in twin gestations.1 In contrast, an earlier study from 2003 found 17P to be more effective than no treatment in preventing spontaneous preterm delivery in singleton pregnancies of women with a history of premature delivery.2
The trial by Fonseca and colleagues capitalizes on two unique aspects of the prematurity debate. The first is that history is probably not any more useful in screening for risk of prematurity than it is for screening for gestational diabetes. This is not to say that history is unimportant, but rather to emphasize that history alone may be insufficient to identify populations that may be at risk for preterm delivery and therefore might possibly benefit from intervention.
The second is the suggestion, based on earlier work by Iams and associates,3 that decreased cervical length may distinguish a group of women at high risk for preterm delivery.
What to make of equivocal findings?
Is an intervention worthwhile if it has no effect on key outcomes such as morbidity and mortality? And what are we to make of the fact that intramuscular 17P is effective in singleton but not twin gestations?
The current trial was insufficiently powered to affirm the null hypothesis with respect to perinatal mortality and neonatal morbidity—ie, there were not enough patients in the study to determine whether the lack of difference in these variables was real or due to insufficient sample size. However, intravaginal progesterone seems more attractive than intramuscular injection—at least intuitively. It may be that a local anti-inflammatory response is what reduced spontaneous preterm delivery—and that may be why intramuscular injection of synthetic progestin at a remote site was less effective, depending on whether the pregnancy was a singleton or twin gestation.
We must also be concerned about the yet-unexplained observation of higher—though statistically insignificant—rates of miscarriage and intrauterine fetal death among women receiving intramuscular 17P, compared with placebo.2
In my own practice, I estimate that fewer than 20% of patients who might be eligible for progesterone accept the treatment after I review all the data with them. Two illustrative cases highlight the conundrum a clinician faces with respect to “measurable outcome differences.”
Patient #1 is a healthy 30-year-old gravida 3 para 1102 whose obstetric history includes:
- a full-term delivery in 2002 that was complicated by premature onset of contractions but never required tocolytic therapy
- a delivery at 29 weeks in 2005 that was complicated by preterm premature rupture of membranes and chorioamnionitis.
Her current pregnancy is managed with cervical-length assessment (all measurements exceed 35 mm) and 17α-hydroxyprogesterone caproate. She delivers a healthy 3,500-g infant at term without complication.
Patient #2 is a healthy 31-year-old gravida 3 para 0202 who delivered at 33 weeks in 2002 and at 35 weeks in 2003. Both deliveries were secondary to idiopathic preterm labor. Her current pregnancy is followed routinely, with no cervical-length assessment. After counseling, she decides against progesterone therapy and goes on to deliver a 3,300-g healthy infant at 39 weeks’ gestation.
The dilemma
These two cases are medically similar; both were eligible for progesterone treatment. One patient chose it and one did not—yet their obstetric outcomes were identical.—John T. Repke, MD
Too early to elevate either regimen to “standard of care”
In my opinion, it is still too early to elevate either intravaginal or intramuscular progesterone to the level of “standard of care.” What may be reasonable in this age of increasing use of first- and mid-trimester ultrasonographic screening is to make certain that information on cervical length is provided. Also important is a candid discussion with the patient about the range of options for management of a short cervix in pregnancy. In the meantime, more than a dozen trials on the prevention of prematurity are in progress. The hope is that more definitive answers to the prematurity riddle are “just around the corner”—which, I suspect, was also the hope in 1975.4
The trial included both singleton and twin gestations.
Expert Commentary
The study by Fonseca and colleagues is an important contribution to the ever-expanding body of literature on ways to reduce preterm birth. It immediately follows a paper in the same issue of the New England Journal of Medicine declaring the failure of intramuscular 17α-hydroxyprogesterone caproate (17P) to prevent preterm birth in twin gestations.1 In contrast, an earlier study from 2003 found 17P to be more effective than no treatment in preventing spontaneous preterm delivery in singleton pregnancies of women with a history of premature delivery.2
The trial by Fonseca and colleagues capitalizes on two unique aspects of the prematurity debate. The first is that history is probably not any more useful in screening for risk of prematurity than it is for screening for gestational diabetes. This is not to say that history is unimportant, but rather to emphasize that history alone may be insufficient to identify populations that may be at risk for preterm delivery and therefore might possibly benefit from intervention.
The second is the suggestion, based on earlier work by Iams and associates,3 that decreased cervical length may distinguish a group of women at high risk for preterm delivery.
What to make of equivocal findings?
Is an intervention worthwhile if it has no effect on key outcomes such as morbidity and mortality? And what are we to make of the fact that intramuscular 17P is effective in singleton but not twin gestations?
The current trial was insufficiently powered to affirm the null hypothesis with respect to perinatal mortality and neonatal morbidity—ie, there were not enough patients in the study to determine whether the lack of difference in these variables was real or due to insufficient sample size. However, intravaginal progesterone seems more attractive than intramuscular injection—at least intuitively. It may be that a local anti-inflammatory response is what reduced spontaneous preterm delivery—and that may be why intramuscular injection of synthetic progestin at a remote site was less effective, depending on whether the pregnancy was a singleton or twin gestation.
We must also be concerned about the yet-unexplained observation of higher—though statistically insignificant—rates of miscarriage and intrauterine fetal death among women receiving intramuscular 17P, compared with placebo.2
In my own practice, I estimate that fewer than 20% of patients who might be eligible for progesterone accept the treatment after I review all the data with them. Two illustrative cases highlight the conundrum a clinician faces with respect to “measurable outcome differences.”
Patient #1 is a healthy 30-year-old gravida 3 para 1102 whose obstetric history includes:
- a full-term delivery in 2002 that was complicated by premature onset of contractions but never required tocolytic therapy
- a delivery at 29 weeks in 2005 that was complicated by preterm premature rupture of membranes and chorioamnionitis.
Her current pregnancy is managed with cervical-length assessment (all measurements exceed 35 mm) and 17α-hydroxyprogesterone caproate. She delivers a healthy 3,500-g infant at term without complication.
Patient #2 is a healthy 31-year-old gravida 3 para 0202 who delivered at 33 weeks in 2002 and at 35 weeks in 2003. Both deliveries were secondary to idiopathic preterm labor. Her current pregnancy is followed routinely, with no cervical-length assessment. After counseling, she decides against progesterone therapy and goes on to deliver a 3,300-g healthy infant at 39 weeks’ gestation.
The dilemma
These two cases are medically similar; both were eligible for progesterone treatment. One patient chose it and one did not—yet their obstetric outcomes were identical.—John T. Repke, MD
Too early to elevate either regimen to “standard of care”
In my opinion, it is still too early to elevate either intravaginal or intramuscular progesterone to the level of “standard of care.” What may be reasonable in this age of increasing use of first- and mid-trimester ultrasonographic screening is to make certain that information on cervical length is provided. Also important is a candid discussion with the patient about the range of options for management of a short cervix in pregnancy. In the meantime, more than a dozen trials on the prevention of prematurity are in progress. The hope is that more definitive answers to the prematurity riddle are “just around the corner”—which, I suspect, was also the hope in 1975.4
1. Rouse DJ, Caritis SN, Peaceman AM, et al. For the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454-461.
2. Meis PJ, Klebanoff M, Thom E, et al. For the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
3. Iams JD, Goldenberg RL, Meis PL, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567-572.
4. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675-680.
1. Rouse DJ, Caritis SN, Peaceman AM, et al. For the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454-461.
2. Meis PJ, Klebanoff M, Thom E, et al. For the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
3. Iams JD, Goldenberg RL, Meis PL, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567-572.
4. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675-680.
Management of lupus flare
Still, lupus flare during pregnancy is a medical and obstetric emergency, and a persistent obstetric dilemma. The most difficult dilemma is how to differentiate a lupus flare from preeclampsia, as both may present with worsening blood pressure, proteinuria and deteriorating renal function, and edema.1
If anticipated and handled quickly, most outcomes will be good for mother and fetus, but occasional severe consequences of lupus flare resulting in loss of mother, fetus, or both, are not always avoidable.
90% of lupus cases are in reproductive-age women
SLE is an autoimmune disease that affects virtually all organ systems. Specific clinical and laboratory criteria must be met to establish the diagnosis. About 90% of all cases are in women in the reproductive age range, with an overrepresentation of African Americans. The overall prevalence of lupus is approximately 15 to 50 per 100,000 population (both sexes, all ages).
Counsel the patient, gauge the risks
The most important first step is the preconception visit. While early prenatal care is better than late presentation, the best option is a preconception visit so that the relative risks of pregnancy may be assessed and discussed, and alterations to medication regimens can be made prior to establishment of a pregnancy (TABLE 1).2
As lupus patients are at increased risk for early pregnancy loss, the preconception visit may also allow for identification of risk factors and risk assessment. A recent study3 proposed the acronym PATH to help identify high-risk patients:
Proteinuria
Antiphospholipid syndrome
Thrombocytopenia
Hypertension
TABLE 1
Factors that increase the likelihood of a good outcome
|
Disease quiescence is not an infallible sign
One of the better indicators of a favorable prognosis for pregnancy is disease quiescence for at least 6 months, and preferably more than 12 months, prior to conception. A number of factors go into the definition of “disease quiescence” including blood pressure control, need for immunosuppressive medication, renal function, and overall physician global assessment, to name but a few, and these factors will be briefly reviewed.
Renal disease and hypertension
Nephritis
Patients with SLE not infrequently have hypertension secondary to renal involvement, specifically lupus nephritis. Nephritis is generally the most serious of lupus manifestations, and if not aggressively treated can lead to nephrotic syndrome, edema and end-stage renal disease in more than 50% of patients within 2 to 3 years.4 Patients with this complication, especially in the setting of proliferative glomerulonephritis, have a poorer prognosis for pregnancy.
Accelerated atherosclerotic vascular disease may also affect these patients—in addition to nephritis—and may herald poor placental function and fetal growth.
Hypertension
When there is coexisting hypertension, antihypertensive agents that are safe for use in pregnancy are preferred, such as beta-blockers, calcium channel blockers, and alpha methyldopa. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should be avoided during the second and third trimester due to adverse effects on fetal renal function.
Diagnosis of antiphospholipid syndrome
Patients with SLE may have associated antiphospholipid antibodies. Screening tests such as antinuclear antibodies (ANA) and activated partial thromboplastin times (aPTT) are not very reliable and have relatively poor positive predictive value, although in the case of ANA, when the diagnosis of lupus is suspected, repetitive negative ANA titers make SLE unlikely. Anti-double stranded DNA is quite specific to SLE, and elevations in the Anti-ds-DNA titers correlate well with SLE disease activity, and can be helpful in making the diagnosis of a lupus flare.
Other antibodies such as Anti-Ro (SS-A) and Anti-La (SS-B) may be useful for predicting and managing for neonatal lupus syndromes, but are not very useful in maternal management.
Additional tests for anticardiolipin, lupus anticoagulant (Russell viper venom test), and beta-2-glycoprotein are also of use.
Diagnosis of APLS requires positive serologies (at least twice, separated by a minimum of 2 weeks), thrombosis, and/or recurrent early pregnancy loss.
Does pregnancy bring on lupus flare?
Whether or not pregnancy increases the incidence of lupus flare is a continuing controversy, stemming from variable definitions of “flare.” Universally accepted criteria have been lacking in published studies.5 Consensus indicates, however, that lupus flares are more common in pregnancy than in nonpregnant controls.
Some studies suggest that SLE flares are more common in the second and third trimesters, but the data are not clear on this point.6 This variability is due in part to differences in disease activity of the patients when they entered the studies.
One may conclude that for any given patient it is impossible to accurately predict whether she will experience a lupus flare, and if she does, when it will occur, and to what level of severity it will rise.
The mainstay of management: is to aggressively treat the lupus flare before irreparable maternal harm occurs
Nephritis is the most serious of lupus manifestations, and if not aggressively treated, can lead to nephrotic syndrome, edema, and end-stage renal disease in more than 50% of patients within 2 to 3 years
Potential adverse outcomes
Predicting who will have a lupus flare and its degree of severity may be difficult if not impossible, but there is little doubt that a significant percentage of women with SLE will experience a flare of some degree. How a lupus flare will affect the pregnancy is uncertain, as well. SLE activity in pregnancy has been linked to adverse outcomes ranging from increased risk of miscarriage to preterm delivery.
Diagnosis and initial management
Preventive treatments
Steroids. SLE flares being somewhat more common in pregnancy than in the nonpregnant patient has led to the belief in some centers that prophylactic administration of steroids to prevent flares would have beneficial pregnancy effects. To date, however, no conclusive evidence supports this approach. In fact, steroid use in particular has been associated in some series with higher rates of premature rupture of membranes (both term and preterm), preeclampsia, and gestational diabetes.
Hydroxychloroquine. It has been suggested that SLE patients whose disease has been controlled with hydroxychloroquine need not discontinue this therapy due to the pregnancy.
The risks of this agent in pregnancy—which are not thought to be significant—are far outweighed by the potential maternal and fetal benefits of averting a lupus flare.
The differential diagnosis
It is imperative, before starting a management strategy, to determine if in fact a lupus flare is the correct diagnosis. Many features of a lupus flare can be confused with features of normal pregnancy, or pregnancy associated complications such as preeclampsia (TABLE 2). The differential diagnosis includes most commonly preeclampsia, but diagnoses such as immune thrombocytopenia, poststreptococcal glomerulonephritis, and hemolyticuremic syndrome must also be considered.
TABLE 2
Clinical features of preeclampsia vs lupus flare*
| FEATURE | PREECLAMPSIA | LUPUS FLARE |
|---|---|---|
| Hypertension | Present | Present |
| Proteinuria | Present | Present |
| Edema | Present | Present |
| Malar rash | Absent | Present |
| Arthritis | Absent | Present |
| Photophobia | Absent | Present |
| Oral ulcers | Absent | Present |
| Serositis | Absent | Present |
| Seizures | Present | Present |
| *Denoting presence or absence does not suggest absolute presence or absence, but rather, the likely compatibility with the diagnosis. | ||
Is it lupus flare or preeclampsia?
The most frequent cause for uncertainty is whether the diagnosis is lupus flare or preeclampsia. It is important to find their distinguishing features, because therapy for these 2 conditions is radically different.
A few easy tests can help (TABLE 3), but the most important are:
- positive ANA screen,
- active urinary sediment,
- hypocomplementemia (C3, C4, or CH 50—the latter is an assay of total serum hemolytic complement), and
- high titers of anti-ds-DNA.
TABLE 3
Tests that help tell lupus flare from preeclampsia
| TEST | PREECLAMPSIA | LUPUS FLARE |
|---|---|---|
| PTT | Usually normal | May be abnormal |
| Platelet count | Normal or reduced | Normal or reduced |
| Urinalysis | Normal sediment | Active sediment |
| ANA | Usually negative | Usually positive |
| Anti-ds-DNA | Usually negative | Usually positive |
| AST | May be abnormal | May be abnormal |
| ALT | May be abnormal | May be abnormal |
| Lupus anticoagulant | Negative | May be positive |
| Hemolysis | May be present | Usually absent |
| Complement levels | Usually normal | Usually reduced |
| WBC | Increased | Decreased |
| SFlt-1* | Increased | Normal |
| *Investigational and not widely available for clinical use. | ||
Aggressive drug therapy is imperative
Management of lupus flare depends on aggressive drug therapy. The choice of therapy is determined by whether the patient is currently on an immunosuppressive regimen, and if so, the types and doses of medications, and whether this is her first flare during the pregnancy.
The usual initial therapy is glucocorticoids, or the so-called steroid “pulses,” typically consisting of very high doses of steroids administered either orally or intravenously over a 3-day period. This strategy has had some success in ameliorating lupus flares, particularly lupus nephritis.
Dosage. Methylprednisolone, 1,000 mg/day intravenously, for 3 days followed by oral prednisone, 0.5 to 1.0 mg/kg per day, provides better survival than lower steroid doses in patients with diffuse proliferative glomerulonephritis.
The intravenous route is preferred because of its rapid response, though long-term outcome is probably not altered.
Even a healthy lupus patient who fulfills all the accepted criteria for a safe pregnancy can take a disastrous turn
A 28-year-old G0 with SLE since age 11 presented for preconception consultation. She was on no medications, with normal blood pressure and no evidence of disease activity for more than 2 years. Physical examination and laboratory findings were normal, including serum creatinine 0.7 mg/dL; less than 30 mg protein in a 24-hour urine collection; creatinine clearance 110 mL/min; and lupus anticoagulant, anti-cardiolipin antibodies, anti-Ro, and anti-La were negative.
Green light
One year later, she returned for follow-up and to inform her obstetrician that she was getting married and wished to conceive. She had no SLE activity since her last visit. Repeat laboratory studies were unchanged. She was given medical clearance to attempt conception, and told that she met all the criteria that would make her a suitable candidate for pregnancy.
7 weeks All findings normal
Three months later, a single intrauterine gestation of approximately 7 weeks was confirmed. Laboratory studies and physical examination were normal, and she reported no SLE-related symptoms.
11 weeks Lupus flare
Four weeks later, at her next prenatal visit, a 3+ proteinuria and blood pressure of 140/90 mm Hg were noted. Her rheumatologist made a diagnosis of lupus flare with probable nephritis. Oral prednisone was begun, with rapid taper. Clinical response was good. She remained on prednisone, 10 mg/day.
14 weeks Recurrence
A recurrence of lupus flare with probable nephritis was diagnosed and her oral prednisone dose was increased. One week later the patient seemed to worsen. She was admitted for steroid pulse therapy. Initially, she improved, but then continued to worsen.
16 weeks Cyclophosphamide therapy
After counseling, she was begun on cyclophosphamide, but her condition continued to deteriorate. Renal function worsened and the patient, now with nephrotic proteinuria, was profoundly edematous and hypoalbuminemic with a rising serum creatinine.
18 weeks Dilatation and evacuation
Ultrasound evaluation of the fetus revealed evidence of early growth restriction. After much discussion, the patient underwent dilatation and evacuation.
Cerebral infarct and anticoagulation
Her lupus flare did not abate. More aggressive medical therapy ensued. Transfer to the intensive care unit with intubation was necessary. She subsequently had an ischemic cerebral infarct requiring anticoagulation.
The next 7 weeks Lupus remission
Over the next several weeks, the patient improved. She had some residual sequelae from the cerebral infarct, but was doing well, with her lupus flare in remission. She was responding well to rehabilitation therapy.
Fatal cerebrovascular accident
One day before anticipated discharge to home, she had a massive cerebrovascular accident and died.
A vivid reminder
This case vividly illustrates the difficulties we must be prepared to manage in lupus pregnancies.
The foremost concern is that even the best candidates for pregnancy can have unfavorable outcomes when this highly unpredictable disease flares.
These women can become severely ill. Ideally, their care should be provided at a facility where expertise in maternal-fetal medicine, anesthesiology, rheumatology, neonatology, and critical care medicine can be readily mobilized to deal with the occasionally catastrophic complications. Even with all of this expertise available, maternal and fetal mortality will not be preventable in all cases.
Pregnancy termination does not necessarily result in amelioration of the lupus flare or its sequelae.
Patients with SLE must be informed of the unpredictability of this disease during pregnancy. The entire family, where appropriate and desired, should be included in the information-sharing process.
A team approach, both pre- and postconception, will help to maximize (but not guarantee) the likelihood of a successful outcome for mother and child.
Refractory flares: Focus on damage control
In pregnancy, most lupus flares involve nephritis and the systemic effects of nephritis, such as hypertension, proteinuria, and renal failure. In some cases, however, steroid pulse therapy fails to suppress these sequelae, or recurrent flares seem to become refractory to steroid pulse therapy.
Any evidence for pregnancy termination? In such cases it is essential that appropriate medical decisions be made on behalf of the mother. No conclusive data suggest that pregnancy termination ameliorates a lupus flare, although some anecdotes suggest this possibility.
The mainstay of management is to aggressively treat the lupus flare before irreparable maternal harm occurs.8
Early delivery: When and how
In advanced pregnancies it may be worthwhile considering early delivery so that the fetus may be spared any adverse consequences of maternal cytotoxic therapy, which would be the next step in management.
Amniocentesis for gestations earlier than 34 weeks may assist in guiding the decision for betamethasone administration for the purpose of accelerating maturation of fetal lungs.
Tertiary care facilities are needed. Generally, if aggressive cytotoxic therapy is indicated, delivery of the fetus is indicated after 32 weeks. Such deliveries should occur at tertiary or quaternary care facilities where both adult and neonatal intensivists are available.
Cesarean section may be reserved for accepted obstetric indications.
Cytotoxic therapy
Remote from term, it may be necessary to commence cytotoxic therapy while allowing gestation to progress.
Cyclophosphamide
This is the preferred agent with respect to efficacy, especially for management of glomerulonephritis.9 Unlike steroid therapy, which may show effects within 24 hours, cyclophosphamide therapy may take from 2 to 3 weeks to several months to achieve a satisfactory clinical response.
Warn of potential ovarian failure. It is important that the patient be informed that prolonged therapy with cyclophosphamide might lead to premature ovarian failure and subsequent infertility.
Azathioprine
An alternative, less toxic immunosuppressive agent that can be used is azathioprine. However, it is also less efficacious in treating severe nephritis. In pregnancy, the preferred role for azathioprine may be in the management of an initial, mild flare. Like cyclophosphamide, azathioprine may take several weeks to be effective.
The combination of glucocorticoids and azathioprine may be more effective than glucocorticoids alone in preventing recurrence of lupus flares, data indicate.
Methotrexate
Although this agent has also been used to manage lupus flares, it is generally effective in treating symptoms of arthritis and dermatitis, with little or no efficacy for life-threatening forms of SLE flares.
Thrombosis requires swift anticoagulation
In patients with SLE and antiphospholipid antibodies, the risk of thrombosis is increased. The ideal management during pregnancy is debatable, if the patient has no history of thrombosis. But in the setting of a lupus flare with either arterial or venous thrombosis, there is no debate. These patients require swift anticoagulation with either unfractionated or low molecular weight heparin. (Long-term therapy with a combination of heparin and glucocorticoids increases the risk of maternal osteoporosis.10)
1. Repke JT. Hypertensive disorders of pregnancy: differentiating preeclampsia from active systemic lupus erythematosus. J Reprod Med. 1998;43:350-354.
2. Petri M. Hopkins Lupus Pregnancy Center: 1987–1996. Rheum Dis Clin North Am. 1997;23:1-13.
3. Clowse MEB, Magder LS, Witter F, Petri M. Early risk factors for pregnancy loss in lupus. Obstet Gynecol. 2006;107:293-299.
4. Moroni G, Quaglini S, Banfi G, et al. Pregnancy in lupus nephritis. Am J Kid Dis. 2002;40:713-720.
5. Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy: the Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991;34:1538-1545.
6. Khamashta MA, Ruiz-Irastorza G, Hughes GRV. Systemic lupus erythematosus flares during pregnancy. Rheum Dis Clin North Am. 1997;23:15-30.
7. Mascola MA, Repke JT. Obstetric management of the high risk lupus pregnancy. Rheum Dis Clin North Am. 1997;23:119-132.
8. Williams WW, Ecker JL, Thadhani RI, Rahemtullah A. Case 38-2005: a 29-year-old pregnant woman with the nephritic syndrome and hypertension. N Engl J Med. 2005;353:2590-2600.
9. Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-2131.
10. Hahn BH. Systemic lupus erythematosus. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005:1960-1967.
The author reports no financial relationships relevant to this article.
Still, lupus flare during pregnancy is a medical and obstetric emergency, and a persistent obstetric dilemma. The most difficult dilemma is how to differentiate a lupus flare from preeclampsia, as both may present with worsening blood pressure, proteinuria and deteriorating renal function, and edema.1
If anticipated and handled quickly, most outcomes will be good for mother and fetus, but occasional severe consequences of lupus flare resulting in loss of mother, fetus, or both, are not always avoidable.
90% of lupus cases are in reproductive-age women
SLE is an autoimmune disease that affects virtually all organ systems. Specific clinical and laboratory criteria must be met to establish the diagnosis. About 90% of all cases are in women in the reproductive age range, with an overrepresentation of African Americans. The overall prevalence of lupus is approximately 15 to 50 per 100,000 population (both sexes, all ages).
Counsel the patient, gauge the risks
The most important first step is the preconception visit. While early prenatal care is better than late presentation, the best option is a preconception visit so that the relative risks of pregnancy may be assessed and discussed, and alterations to medication regimens can be made prior to establishment of a pregnancy (TABLE 1).2
As lupus patients are at increased risk for early pregnancy loss, the preconception visit may also allow for identification of risk factors and risk assessment. A recent study3 proposed the acronym PATH to help identify high-risk patients:
Proteinuria
Antiphospholipid syndrome
Thrombocytopenia
Hypertension
TABLE 1
Factors that increase the likelihood of a good outcome
|
Disease quiescence is not an infallible sign
One of the better indicators of a favorable prognosis for pregnancy is disease quiescence for at least 6 months, and preferably more than 12 months, prior to conception. A number of factors go into the definition of “disease quiescence” including blood pressure control, need for immunosuppressive medication, renal function, and overall physician global assessment, to name but a few, and these factors will be briefly reviewed.
Renal disease and hypertension
Nephritis
Patients with SLE not infrequently have hypertension secondary to renal involvement, specifically lupus nephritis. Nephritis is generally the most serious of lupus manifestations, and if not aggressively treated can lead to nephrotic syndrome, edema and end-stage renal disease in more than 50% of patients within 2 to 3 years.4 Patients with this complication, especially in the setting of proliferative glomerulonephritis, have a poorer prognosis for pregnancy.
Accelerated atherosclerotic vascular disease may also affect these patients—in addition to nephritis—and may herald poor placental function and fetal growth.
Hypertension
When there is coexisting hypertension, antihypertensive agents that are safe for use in pregnancy are preferred, such as beta-blockers, calcium channel blockers, and alpha methyldopa. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should be avoided during the second and third trimester due to adverse effects on fetal renal function.
Diagnosis of antiphospholipid syndrome
Patients with SLE may have associated antiphospholipid antibodies. Screening tests such as antinuclear antibodies (ANA) and activated partial thromboplastin times (aPTT) are not very reliable and have relatively poor positive predictive value, although in the case of ANA, when the diagnosis of lupus is suspected, repetitive negative ANA titers make SLE unlikely. Anti-double stranded DNA is quite specific to SLE, and elevations in the Anti-ds-DNA titers correlate well with SLE disease activity, and can be helpful in making the diagnosis of a lupus flare.
Other antibodies such as Anti-Ro (SS-A) and Anti-La (SS-B) may be useful for predicting and managing for neonatal lupus syndromes, but are not very useful in maternal management.
Additional tests for anticardiolipin, lupus anticoagulant (Russell viper venom test), and beta-2-glycoprotein are also of use.
Diagnosis of APLS requires positive serologies (at least twice, separated by a minimum of 2 weeks), thrombosis, and/or recurrent early pregnancy loss.
Does pregnancy bring on lupus flare?
Whether or not pregnancy increases the incidence of lupus flare is a continuing controversy, stemming from variable definitions of “flare.” Universally accepted criteria have been lacking in published studies.5 Consensus indicates, however, that lupus flares are more common in pregnancy than in nonpregnant controls.
Some studies suggest that SLE flares are more common in the second and third trimesters, but the data are not clear on this point.6 This variability is due in part to differences in disease activity of the patients when they entered the studies.
One may conclude that for any given patient it is impossible to accurately predict whether she will experience a lupus flare, and if she does, when it will occur, and to what level of severity it will rise.
The mainstay of management: is to aggressively treat the lupus flare before irreparable maternal harm occurs
Nephritis is the most serious of lupus manifestations, and if not aggressively treated, can lead to nephrotic syndrome, edema, and end-stage renal disease in more than 50% of patients within 2 to 3 years
Potential adverse outcomes
Predicting who will have a lupus flare and its degree of severity may be difficult if not impossible, but there is little doubt that a significant percentage of women with SLE will experience a flare of some degree. How a lupus flare will affect the pregnancy is uncertain, as well. SLE activity in pregnancy has been linked to adverse outcomes ranging from increased risk of miscarriage to preterm delivery.
Diagnosis and initial management
Preventive treatments
Steroids. SLE flares being somewhat more common in pregnancy than in the nonpregnant patient has led to the belief in some centers that prophylactic administration of steroids to prevent flares would have beneficial pregnancy effects. To date, however, no conclusive evidence supports this approach. In fact, steroid use in particular has been associated in some series with higher rates of premature rupture of membranes (both term and preterm), preeclampsia, and gestational diabetes.
Hydroxychloroquine. It has been suggested that SLE patients whose disease has been controlled with hydroxychloroquine need not discontinue this therapy due to the pregnancy.
The risks of this agent in pregnancy—which are not thought to be significant—are far outweighed by the potential maternal and fetal benefits of averting a lupus flare.
The differential diagnosis
It is imperative, before starting a management strategy, to determine if in fact a lupus flare is the correct diagnosis. Many features of a lupus flare can be confused with features of normal pregnancy, or pregnancy associated complications such as preeclampsia (TABLE 2). The differential diagnosis includes most commonly preeclampsia, but diagnoses such as immune thrombocytopenia, poststreptococcal glomerulonephritis, and hemolyticuremic syndrome must also be considered.
TABLE 2
Clinical features of preeclampsia vs lupus flare*
| FEATURE | PREECLAMPSIA | LUPUS FLARE |
|---|---|---|
| Hypertension | Present | Present |
| Proteinuria | Present | Present |
| Edema | Present | Present |
| Malar rash | Absent | Present |
| Arthritis | Absent | Present |
| Photophobia | Absent | Present |
| Oral ulcers | Absent | Present |
| Serositis | Absent | Present |
| Seizures | Present | Present |
| *Denoting presence or absence does not suggest absolute presence or absence, but rather, the likely compatibility with the diagnosis. | ||
Is it lupus flare or preeclampsia?
The most frequent cause for uncertainty is whether the diagnosis is lupus flare or preeclampsia. It is important to find their distinguishing features, because therapy for these 2 conditions is radically different.
A few easy tests can help (TABLE 3), but the most important are:
- positive ANA screen,
- active urinary sediment,
- hypocomplementemia (C3, C4, or CH 50—the latter is an assay of total serum hemolytic complement), and
- high titers of anti-ds-DNA.
TABLE 3
Tests that help tell lupus flare from preeclampsia
| TEST | PREECLAMPSIA | LUPUS FLARE |
|---|---|---|
| PTT | Usually normal | May be abnormal |
| Platelet count | Normal or reduced | Normal or reduced |
| Urinalysis | Normal sediment | Active sediment |
| ANA | Usually negative | Usually positive |
| Anti-ds-DNA | Usually negative | Usually positive |
| AST | May be abnormal | May be abnormal |
| ALT | May be abnormal | May be abnormal |
| Lupus anticoagulant | Negative | May be positive |
| Hemolysis | May be present | Usually absent |
| Complement levels | Usually normal | Usually reduced |
| WBC | Increased | Decreased |
| SFlt-1* | Increased | Normal |
| *Investigational and not widely available for clinical use. | ||
Aggressive drug therapy is imperative
Management of lupus flare depends on aggressive drug therapy. The choice of therapy is determined by whether the patient is currently on an immunosuppressive regimen, and if so, the types and doses of medications, and whether this is her first flare during the pregnancy.
The usual initial therapy is glucocorticoids, or the so-called steroid “pulses,” typically consisting of very high doses of steroids administered either orally or intravenously over a 3-day period. This strategy has had some success in ameliorating lupus flares, particularly lupus nephritis.
Dosage. Methylprednisolone, 1,000 mg/day intravenously, for 3 days followed by oral prednisone, 0.5 to 1.0 mg/kg per day, provides better survival than lower steroid doses in patients with diffuse proliferative glomerulonephritis.
The intravenous route is preferred because of its rapid response, though long-term outcome is probably not altered.
Even a healthy lupus patient who fulfills all the accepted criteria for a safe pregnancy can take a disastrous turn
A 28-year-old G0 with SLE since age 11 presented for preconception consultation. She was on no medications, with normal blood pressure and no evidence of disease activity for more than 2 years. Physical examination and laboratory findings were normal, including serum creatinine 0.7 mg/dL; less than 30 mg protein in a 24-hour urine collection; creatinine clearance 110 mL/min; and lupus anticoagulant, anti-cardiolipin antibodies, anti-Ro, and anti-La were negative.
Green light
One year later, she returned for follow-up and to inform her obstetrician that she was getting married and wished to conceive. She had no SLE activity since her last visit. Repeat laboratory studies were unchanged. She was given medical clearance to attempt conception, and told that she met all the criteria that would make her a suitable candidate for pregnancy.
7 weeks All findings normal
Three months later, a single intrauterine gestation of approximately 7 weeks was confirmed. Laboratory studies and physical examination were normal, and she reported no SLE-related symptoms.
11 weeks Lupus flare
Four weeks later, at her next prenatal visit, a 3+ proteinuria and blood pressure of 140/90 mm Hg were noted. Her rheumatologist made a diagnosis of lupus flare with probable nephritis. Oral prednisone was begun, with rapid taper. Clinical response was good. She remained on prednisone, 10 mg/day.
14 weeks Recurrence
A recurrence of lupus flare with probable nephritis was diagnosed and her oral prednisone dose was increased. One week later the patient seemed to worsen. She was admitted for steroid pulse therapy. Initially, she improved, but then continued to worsen.
16 weeks Cyclophosphamide therapy
After counseling, she was begun on cyclophosphamide, but her condition continued to deteriorate. Renal function worsened and the patient, now with nephrotic proteinuria, was profoundly edematous and hypoalbuminemic with a rising serum creatinine.
18 weeks Dilatation and evacuation
Ultrasound evaluation of the fetus revealed evidence of early growth restriction. After much discussion, the patient underwent dilatation and evacuation.
Cerebral infarct and anticoagulation
Her lupus flare did not abate. More aggressive medical therapy ensued. Transfer to the intensive care unit with intubation was necessary. She subsequently had an ischemic cerebral infarct requiring anticoagulation.
The next 7 weeks Lupus remission
Over the next several weeks, the patient improved. She had some residual sequelae from the cerebral infarct, but was doing well, with her lupus flare in remission. She was responding well to rehabilitation therapy.
Fatal cerebrovascular accident
One day before anticipated discharge to home, she had a massive cerebrovascular accident and died.
A vivid reminder
This case vividly illustrates the difficulties we must be prepared to manage in lupus pregnancies.
The foremost concern is that even the best candidates for pregnancy can have unfavorable outcomes when this highly unpredictable disease flares.
These women can become severely ill. Ideally, their care should be provided at a facility where expertise in maternal-fetal medicine, anesthesiology, rheumatology, neonatology, and critical care medicine can be readily mobilized to deal with the occasionally catastrophic complications. Even with all of this expertise available, maternal and fetal mortality will not be preventable in all cases.
Pregnancy termination does not necessarily result in amelioration of the lupus flare or its sequelae.
Patients with SLE must be informed of the unpredictability of this disease during pregnancy. The entire family, where appropriate and desired, should be included in the information-sharing process.
A team approach, both pre- and postconception, will help to maximize (but not guarantee) the likelihood of a successful outcome for mother and child.
Refractory flares: Focus on damage control
In pregnancy, most lupus flares involve nephritis and the systemic effects of nephritis, such as hypertension, proteinuria, and renal failure. In some cases, however, steroid pulse therapy fails to suppress these sequelae, or recurrent flares seem to become refractory to steroid pulse therapy.
Any evidence for pregnancy termination? In such cases it is essential that appropriate medical decisions be made on behalf of the mother. No conclusive data suggest that pregnancy termination ameliorates a lupus flare, although some anecdotes suggest this possibility.
The mainstay of management is to aggressively treat the lupus flare before irreparable maternal harm occurs.8
Early delivery: When and how
In advanced pregnancies it may be worthwhile considering early delivery so that the fetus may be spared any adverse consequences of maternal cytotoxic therapy, which would be the next step in management.
Amniocentesis for gestations earlier than 34 weeks may assist in guiding the decision for betamethasone administration for the purpose of accelerating maturation of fetal lungs.
Tertiary care facilities are needed. Generally, if aggressive cytotoxic therapy is indicated, delivery of the fetus is indicated after 32 weeks. Such deliveries should occur at tertiary or quaternary care facilities where both adult and neonatal intensivists are available.
Cesarean section may be reserved for accepted obstetric indications.
Cytotoxic therapy
Remote from term, it may be necessary to commence cytotoxic therapy while allowing gestation to progress.
Cyclophosphamide
This is the preferred agent with respect to efficacy, especially for management of glomerulonephritis.9 Unlike steroid therapy, which may show effects within 24 hours, cyclophosphamide therapy may take from 2 to 3 weeks to several months to achieve a satisfactory clinical response.
Warn of potential ovarian failure. It is important that the patient be informed that prolonged therapy with cyclophosphamide might lead to premature ovarian failure and subsequent infertility.
Azathioprine
An alternative, less toxic immunosuppressive agent that can be used is azathioprine. However, it is also less efficacious in treating severe nephritis. In pregnancy, the preferred role for azathioprine may be in the management of an initial, mild flare. Like cyclophosphamide, azathioprine may take several weeks to be effective.
The combination of glucocorticoids and azathioprine may be more effective than glucocorticoids alone in preventing recurrence of lupus flares, data indicate.
Methotrexate
Although this agent has also been used to manage lupus flares, it is generally effective in treating symptoms of arthritis and dermatitis, with little or no efficacy for life-threatening forms of SLE flares.
Thrombosis requires swift anticoagulation
In patients with SLE and antiphospholipid antibodies, the risk of thrombosis is increased. The ideal management during pregnancy is debatable, if the patient has no history of thrombosis. But in the setting of a lupus flare with either arterial or venous thrombosis, there is no debate. These patients require swift anticoagulation with either unfractionated or low molecular weight heparin. (Long-term therapy with a combination of heparin and glucocorticoids increases the risk of maternal osteoporosis.10)
Still, lupus flare during pregnancy is a medical and obstetric emergency, and a persistent obstetric dilemma. The most difficult dilemma is how to differentiate a lupus flare from preeclampsia, as both may present with worsening blood pressure, proteinuria and deteriorating renal function, and edema.1
If anticipated and handled quickly, most outcomes will be good for mother and fetus, but occasional severe consequences of lupus flare resulting in loss of mother, fetus, or both, are not always avoidable.
90% of lupus cases are in reproductive-age women
SLE is an autoimmune disease that affects virtually all organ systems. Specific clinical and laboratory criteria must be met to establish the diagnosis. About 90% of all cases are in women in the reproductive age range, with an overrepresentation of African Americans. The overall prevalence of lupus is approximately 15 to 50 per 100,000 population (both sexes, all ages).
Counsel the patient, gauge the risks
The most important first step is the preconception visit. While early prenatal care is better than late presentation, the best option is a preconception visit so that the relative risks of pregnancy may be assessed and discussed, and alterations to medication regimens can be made prior to establishment of a pregnancy (TABLE 1).2
As lupus patients are at increased risk for early pregnancy loss, the preconception visit may also allow for identification of risk factors and risk assessment. A recent study3 proposed the acronym PATH to help identify high-risk patients:
Proteinuria
Antiphospholipid syndrome
Thrombocytopenia
Hypertension
TABLE 1
Factors that increase the likelihood of a good outcome
|
Disease quiescence is not an infallible sign
One of the better indicators of a favorable prognosis for pregnancy is disease quiescence for at least 6 months, and preferably more than 12 months, prior to conception. A number of factors go into the definition of “disease quiescence” including blood pressure control, need for immunosuppressive medication, renal function, and overall physician global assessment, to name but a few, and these factors will be briefly reviewed.
Renal disease and hypertension
Nephritis
Patients with SLE not infrequently have hypertension secondary to renal involvement, specifically lupus nephritis. Nephritis is generally the most serious of lupus manifestations, and if not aggressively treated can lead to nephrotic syndrome, edema and end-stage renal disease in more than 50% of patients within 2 to 3 years.4 Patients with this complication, especially in the setting of proliferative glomerulonephritis, have a poorer prognosis for pregnancy.
Accelerated atherosclerotic vascular disease may also affect these patients—in addition to nephritis—and may herald poor placental function and fetal growth.
Hypertension
When there is coexisting hypertension, antihypertensive agents that are safe for use in pregnancy are preferred, such as beta-blockers, calcium channel blockers, and alpha methyldopa. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should be avoided during the second and third trimester due to adverse effects on fetal renal function.
Diagnosis of antiphospholipid syndrome
Patients with SLE may have associated antiphospholipid antibodies. Screening tests such as antinuclear antibodies (ANA) and activated partial thromboplastin times (aPTT) are not very reliable and have relatively poor positive predictive value, although in the case of ANA, when the diagnosis of lupus is suspected, repetitive negative ANA titers make SLE unlikely. Anti-double stranded DNA is quite specific to SLE, and elevations in the Anti-ds-DNA titers correlate well with SLE disease activity, and can be helpful in making the diagnosis of a lupus flare.
Other antibodies such as Anti-Ro (SS-A) and Anti-La (SS-B) may be useful for predicting and managing for neonatal lupus syndromes, but are not very useful in maternal management.
Additional tests for anticardiolipin, lupus anticoagulant (Russell viper venom test), and beta-2-glycoprotein are also of use.
Diagnosis of APLS requires positive serologies (at least twice, separated by a minimum of 2 weeks), thrombosis, and/or recurrent early pregnancy loss.
Does pregnancy bring on lupus flare?
Whether or not pregnancy increases the incidence of lupus flare is a continuing controversy, stemming from variable definitions of “flare.” Universally accepted criteria have been lacking in published studies.5 Consensus indicates, however, that lupus flares are more common in pregnancy than in nonpregnant controls.
Some studies suggest that SLE flares are more common in the second and third trimesters, but the data are not clear on this point.6 This variability is due in part to differences in disease activity of the patients when they entered the studies.
One may conclude that for any given patient it is impossible to accurately predict whether she will experience a lupus flare, and if she does, when it will occur, and to what level of severity it will rise.
The mainstay of management: is to aggressively treat the lupus flare before irreparable maternal harm occurs
Nephritis is the most serious of lupus manifestations, and if not aggressively treated, can lead to nephrotic syndrome, edema, and end-stage renal disease in more than 50% of patients within 2 to 3 years
Potential adverse outcomes
Predicting who will have a lupus flare and its degree of severity may be difficult if not impossible, but there is little doubt that a significant percentage of women with SLE will experience a flare of some degree. How a lupus flare will affect the pregnancy is uncertain, as well. SLE activity in pregnancy has been linked to adverse outcomes ranging from increased risk of miscarriage to preterm delivery.
Diagnosis and initial management
Preventive treatments
Steroids. SLE flares being somewhat more common in pregnancy than in the nonpregnant patient has led to the belief in some centers that prophylactic administration of steroids to prevent flares would have beneficial pregnancy effects. To date, however, no conclusive evidence supports this approach. In fact, steroid use in particular has been associated in some series with higher rates of premature rupture of membranes (both term and preterm), preeclampsia, and gestational diabetes.
Hydroxychloroquine. It has been suggested that SLE patients whose disease has been controlled with hydroxychloroquine need not discontinue this therapy due to the pregnancy.
The risks of this agent in pregnancy—which are not thought to be significant—are far outweighed by the potential maternal and fetal benefits of averting a lupus flare.
The differential diagnosis
It is imperative, before starting a management strategy, to determine if in fact a lupus flare is the correct diagnosis. Many features of a lupus flare can be confused with features of normal pregnancy, or pregnancy associated complications such as preeclampsia (TABLE 2). The differential diagnosis includes most commonly preeclampsia, but diagnoses such as immune thrombocytopenia, poststreptococcal glomerulonephritis, and hemolyticuremic syndrome must also be considered.
TABLE 2
Clinical features of preeclampsia vs lupus flare*
| FEATURE | PREECLAMPSIA | LUPUS FLARE |
|---|---|---|
| Hypertension | Present | Present |
| Proteinuria | Present | Present |
| Edema | Present | Present |
| Malar rash | Absent | Present |
| Arthritis | Absent | Present |
| Photophobia | Absent | Present |
| Oral ulcers | Absent | Present |
| Serositis | Absent | Present |
| Seizures | Present | Present |
| *Denoting presence or absence does not suggest absolute presence or absence, but rather, the likely compatibility with the diagnosis. | ||
Is it lupus flare or preeclampsia?
The most frequent cause for uncertainty is whether the diagnosis is lupus flare or preeclampsia. It is important to find their distinguishing features, because therapy for these 2 conditions is radically different.
A few easy tests can help (TABLE 3), but the most important are:
- positive ANA screen,
- active urinary sediment,
- hypocomplementemia (C3, C4, or CH 50—the latter is an assay of total serum hemolytic complement), and
- high titers of anti-ds-DNA.
TABLE 3
Tests that help tell lupus flare from preeclampsia
| TEST | PREECLAMPSIA | LUPUS FLARE |
|---|---|---|
| PTT | Usually normal | May be abnormal |
| Platelet count | Normal or reduced | Normal or reduced |
| Urinalysis | Normal sediment | Active sediment |
| ANA | Usually negative | Usually positive |
| Anti-ds-DNA | Usually negative | Usually positive |
| AST | May be abnormal | May be abnormal |
| ALT | May be abnormal | May be abnormal |
| Lupus anticoagulant | Negative | May be positive |
| Hemolysis | May be present | Usually absent |
| Complement levels | Usually normal | Usually reduced |
| WBC | Increased | Decreased |
| SFlt-1* | Increased | Normal |
| *Investigational and not widely available for clinical use. | ||
Aggressive drug therapy is imperative
Management of lupus flare depends on aggressive drug therapy. The choice of therapy is determined by whether the patient is currently on an immunosuppressive regimen, and if so, the types and doses of medications, and whether this is her first flare during the pregnancy.
The usual initial therapy is glucocorticoids, or the so-called steroid “pulses,” typically consisting of very high doses of steroids administered either orally or intravenously over a 3-day period. This strategy has had some success in ameliorating lupus flares, particularly lupus nephritis.
Dosage. Methylprednisolone, 1,000 mg/day intravenously, for 3 days followed by oral prednisone, 0.5 to 1.0 mg/kg per day, provides better survival than lower steroid doses in patients with diffuse proliferative glomerulonephritis.
The intravenous route is preferred because of its rapid response, though long-term outcome is probably not altered.
Even a healthy lupus patient who fulfills all the accepted criteria for a safe pregnancy can take a disastrous turn
A 28-year-old G0 with SLE since age 11 presented for preconception consultation. She was on no medications, with normal blood pressure and no evidence of disease activity for more than 2 years. Physical examination and laboratory findings were normal, including serum creatinine 0.7 mg/dL; less than 30 mg protein in a 24-hour urine collection; creatinine clearance 110 mL/min; and lupus anticoagulant, anti-cardiolipin antibodies, anti-Ro, and anti-La were negative.
Green light
One year later, she returned for follow-up and to inform her obstetrician that she was getting married and wished to conceive. She had no SLE activity since her last visit. Repeat laboratory studies were unchanged. She was given medical clearance to attempt conception, and told that she met all the criteria that would make her a suitable candidate for pregnancy.
7 weeks All findings normal
Three months later, a single intrauterine gestation of approximately 7 weeks was confirmed. Laboratory studies and physical examination were normal, and she reported no SLE-related symptoms.
11 weeks Lupus flare
Four weeks later, at her next prenatal visit, a 3+ proteinuria and blood pressure of 140/90 mm Hg were noted. Her rheumatologist made a diagnosis of lupus flare with probable nephritis. Oral prednisone was begun, with rapid taper. Clinical response was good. She remained on prednisone, 10 mg/day.
14 weeks Recurrence
A recurrence of lupus flare with probable nephritis was diagnosed and her oral prednisone dose was increased. One week later the patient seemed to worsen. She was admitted for steroid pulse therapy. Initially, she improved, but then continued to worsen.
16 weeks Cyclophosphamide therapy
After counseling, she was begun on cyclophosphamide, but her condition continued to deteriorate. Renal function worsened and the patient, now with nephrotic proteinuria, was profoundly edematous and hypoalbuminemic with a rising serum creatinine.
18 weeks Dilatation and evacuation
Ultrasound evaluation of the fetus revealed evidence of early growth restriction. After much discussion, the patient underwent dilatation and evacuation.
Cerebral infarct and anticoagulation
Her lupus flare did not abate. More aggressive medical therapy ensued. Transfer to the intensive care unit with intubation was necessary. She subsequently had an ischemic cerebral infarct requiring anticoagulation.
The next 7 weeks Lupus remission
Over the next several weeks, the patient improved. She had some residual sequelae from the cerebral infarct, but was doing well, with her lupus flare in remission. She was responding well to rehabilitation therapy.
Fatal cerebrovascular accident
One day before anticipated discharge to home, she had a massive cerebrovascular accident and died.
A vivid reminder
This case vividly illustrates the difficulties we must be prepared to manage in lupus pregnancies.
The foremost concern is that even the best candidates for pregnancy can have unfavorable outcomes when this highly unpredictable disease flares.
These women can become severely ill. Ideally, their care should be provided at a facility where expertise in maternal-fetal medicine, anesthesiology, rheumatology, neonatology, and critical care medicine can be readily mobilized to deal with the occasionally catastrophic complications. Even with all of this expertise available, maternal and fetal mortality will not be preventable in all cases.
Pregnancy termination does not necessarily result in amelioration of the lupus flare or its sequelae.
Patients with SLE must be informed of the unpredictability of this disease during pregnancy. The entire family, where appropriate and desired, should be included in the information-sharing process.
A team approach, both pre- and postconception, will help to maximize (but not guarantee) the likelihood of a successful outcome for mother and child.
Refractory flares: Focus on damage control
In pregnancy, most lupus flares involve nephritis and the systemic effects of nephritis, such as hypertension, proteinuria, and renal failure. In some cases, however, steroid pulse therapy fails to suppress these sequelae, or recurrent flares seem to become refractory to steroid pulse therapy.
Any evidence for pregnancy termination? In such cases it is essential that appropriate medical decisions be made on behalf of the mother. No conclusive data suggest that pregnancy termination ameliorates a lupus flare, although some anecdotes suggest this possibility.
The mainstay of management is to aggressively treat the lupus flare before irreparable maternal harm occurs.8
Early delivery: When and how
In advanced pregnancies it may be worthwhile considering early delivery so that the fetus may be spared any adverse consequences of maternal cytotoxic therapy, which would be the next step in management.
Amniocentesis for gestations earlier than 34 weeks may assist in guiding the decision for betamethasone administration for the purpose of accelerating maturation of fetal lungs.
Tertiary care facilities are needed. Generally, if aggressive cytotoxic therapy is indicated, delivery of the fetus is indicated after 32 weeks. Such deliveries should occur at tertiary or quaternary care facilities where both adult and neonatal intensivists are available.
Cesarean section may be reserved for accepted obstetric indications.
Cytotoxic therapy
Remote from term, it may be necessary to commence cytotoxic therapy while allowing gestation to progress.
Cyclophosphamide
This is the preferred agent with respect to efficacy, especially for management of glomerulonephritis.9 Unlike steroid therapy, which may show effects within 24 hours, cyclophosphamide therapy may take from 2 to 3 weeks to several months to achieve a satisfactory clinical response.
Warn of potential ovarian failure. It is important that the patient be informed that prolonged therapy with cyclophosphamide might lead to premature ovarian failure and subsequent infertility.
Azathioprine
An alternative, less toxic immunosuppressive agent that can be used is azathioprine. However, it is also less efficacious in treating severe nephritis. In pregnancy, the preferred role for azathioprine may be in the management of an initial, mild flare. Like cyclophosphamide, azathioprine may take several weeks to be effective.
The combination of glucocorticoids and azathioprine may be more effective than glucocorticoids alone in preventing recurrence of lupus flares, data indicate.
Methotrexate
Although this agent has also been used to manage lupus flares, it is generally effective in treating symptoms of arthritis and dermatitis, with little or no efficacy for life-threatening forms of SLE flares.
Thrombosis requires swift anticoagulation
In patients with SLE and antiphospholipid antibodies, the risk of thrombosis is increased. The ideal management during pregnancy is debatable, if the patient has no history of thrombosis. But in the setting of a lupus flare with either arterial or venous thrombosis, there is no debate. These patients require swift anticoagulation with either unfractionated or low molecular weight heparin. (Long-term therapy with a combination of heparin and glucocorticoids increases the risk of maternal osteoporosis.10)
1. Repke JT. Hypertensive disorders of pregnancy: differentiating preeclampsia from active systemic lupus erythematosus. J Reprod Med. 1998;43:350-354.
2. Petri M. Hopkins Lupus Pregnancy Center: 1987–1996. Rheum Dis Clin North Am. 1997;23:1-13.
3. Clowse MEB, Magder LS, Witter F, Petri M. Early risk factors for pregnancy loss in lupus. Obstet Gynecol. 2006;107:293-299.
4. Moroni G, Quaglini S, Banfi G, et al. Pregnancy in lupus nephritis. Am J Kid Dis. 2002;40:713-720.
5. Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy: the Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991;34:1538-1545.
6. Khamashta MA, Ruiz-Irastorza G, Hughes GRV. Systemic lupus erythematosus flares during pregnancy. Rheum Dis Clin North Am. 1997;23:15-30.
7. Mascola MA, Repke JT. Obstetric management of the high risk lupus pregnancy. Rheum Dis Clin North Am. 1997;23:119-132.
8. Williams WW, Ecker JL, Thadhani RI, Rahemtullah A. Case 38-2005: a 29-year-old pregnant woman with the nephritic syndrome and hypertension. N Engl J Med. 2005;353:2590-2600.
9. Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-2131.
10. Hahn BH. Systemic lupus erythematosus. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005:1960-1967.
The author reports no financial relationships relevant to this article.
1. Repke JT. Hypertensive disorders of pregnancy: differentiating preeclampsia from active systemic lupus erythematosus. J Reprod Med. 1998;43:350-354.
2. Petri M. Hopkins Lupus Pregnancy Center: 1987–1996. Rheum Dis Clin North Am. 1997;23:1-13.
3. Clowse MEB, Magder LS, Witter F, Petri M. Early risk factors for pregnancy loss in lupus. Obstet Gynecol. 2006;107:293-299.
4. Moroni G, Quaglini S, Banfi G, et al. Pregnancy in lupus nephritis. Am J Kid Dis. 2002;40:713-720.
5. Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy: the Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991;34:1538-1545.
6. Khamashta MA, Ruiz-Irastorza G, Hughes GRV. Systemic lupus erythematosus flares during pregnancy. Rheum Dis Clin North Am. 1997;23:15-30.
7. Mascola MA, Repke JT. Obstetric management of the high risk lupus pregnancy. Rheum Dis Clin North Am. 1997;23:119-132.
8. Williams WW, Ecker JL, Thadhani RI, Rahemtullah A. Case 38-2005: a 29-year-old pregnant woman with the nephritic syndrome and hypertension. N Engl J Med. 2005;353:2590-2600.
9. Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-2131.
10. Hahn BH. Systemic lupus erythematosus. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005:1960-1967.
The author reports no financial relationships relevant to this article.


